Abstract
Based on evidence suggesting that deep brain stimulation (DBS) may promote certain cognitive processes, we have been interested in developing DBS as a means of mitigating memory and learning impairments in Alzheimer’s disease (AD). In this study we used an animal model of AD (TgCRND8 mice) to determine the effects of high-frequency stimulation (HFS) on non-amyloidogenic α-secretase activity and DBS in short-term memory. We tested our hypothesis using hippocampal slices (in vitro studies) from TgCRND8 mice to evaluate whether HFS increases α-secretase activity (non-amyloidogenic pathway) in the CA1 region. In a second set of experiments, we performed in vivo studies to evaluate whether DBS in midline thalamic region re-establishes hippocampal dependent short-term memory in TgCRND8 mice. The results showed that application of HFS to isolated hippocampal slices significantly increased synaptic plasticity in the CA1 region and promoted a 2-fold increase of non-amyloidogenic α-secretase activity, in comparison to low frequency stimulated controls from TgCRND8 mice. In the in vivo studies, DBS treatment facilitated acquisition of object recognition memory in TgCRND8 mice, in comparison to their own baseline before treatment. These results provide evidence that DBS could enhance short-term memory in the CA1 region of hippocampus in a mouse model of AD.
Keywords: Alzheimer’s disease, Deep brain stimulation, Hippocampus, LTP, Neuronal transmission Synaptic plasticity, Memory, TgCRND8 mice, Thalamus
1. Introduction
Clinical applications of Deep Brain Stimulation (DBS) for the treatment of essential tremor, dystonia or motor dysfunction (e.g., resting tremor, rigidity and bradykinesia) in patients with Parkinson’s disease have provided very encouraging results to expand its use to other neurologic and psychiatric disorders (e.g., pain, depression, epilepsy, traumatic brain injury and obsessive compulsive disorder). The use of DBS in these disorders has provided an effective clinical technique to ameliorate symptoms that have not responded to pharmacological treatments. As a result of the therapeutic effectiveness of DBS on motor disorders, preclinical and clinical studies in humans have been developed to understand its mechanisms of action on cognitive processes [1,2].
Recent studies have investigated the effects of central thalamic stimulation to increase exploratory motor behaviors and grooming activity, consistent with a global increase in arousal in normal rats. It has also been reported that electrical stimulation of the central thalamus may enhance memory and learning through neocortical and hippocampal neuronal activation by specific regulation of zif268 gene expression [3]. Thus it has been proposed that central thalamic simulation could be a viable treatment for remediation of learning and memory deficits. Anatomical and physiological evidence in rat brain show direct connections from the midline thalamic nuclei (MTN) to the hippocampus, these well-described thalamohippocampal connections allow the direct and powerful excitation of the CA1 region [4,5].
Based on this evidence, our study established an in vivo pre-clinical model of DBS to determine its effectiveness in alleviating learning and memory deficits in an animal model of Alzheimer’s disease (AD). To date, AD is the sixth-leading cause of death in the United States and is the most common cause of dementia in the world characterized by memory loss, difficulty in performing familiar tasks, behavioral and language alterations, and learning deficits, among others. Current therapy which is predominantly pharmacological, aims at decreasing the cognitive, behavioral and psychiatric symptoms but the effectiveness of these treatments is low and there are major side effects. In this study we intended to explore a new therapeutic strategy with the goal of slowing down the progression of the disease.
2. Experimental design and methods
2.1 Animals
B6C3F1 wild type (WT) and TgCRND8 (Tg) mice were housed with food and water available ad libitum, and maintained on a 12:12-h light/dark cycle with lights on at 07:00 h in a temperature-controlled (20 ± 2°C) vivarium. All procedures and protocols were approved by The Mount Sinai School of Medicine’s Institutional Animal Care and Use Committee (IACUC) through The Center for Comparative Medicine and Surgery. Offspring were produced by mating TgCRND8 males (carrying the double mutation in APP transgene) with WT females (Charles River, Wilmington, MA, USA). The offspring of the WT and heterozygous Tg were genotyped at 30 day-old using a colorimetric-ELISA-Kit (Invitrogen), according to the manufacture’s protocol. This immunoassay makes a quantitative determination of amyloid (Aβ1-40 or Aβ1-42) fragments in blood samples.
2.2 Brain slice preparation
Hippocampal brain slices were obtained from 5 week-old WT (n=8–10) and Tg (n=8–10) male mice. The animals were decapitated and the brains quickly removed, hemisected and placed into ice-cold oxygenated artificial cerebrospinal fluid (ACSF) for 1 min. Both hippocampi were dissected and sliced (350 μm) using a tissue chopper (McIllwain, the Mickle Laboratory Engineering Co, United Kingdom). All slices were incubated in oxygenated-ACSF at room temperature for a minimum of 2 h to acclimatize. The composition of the ACSF (mM) was: 10 D-glucose, 124 NaCl, 1.25 NaH2PO4, 26 NaHCO3, 4.9 KCL, 1 CaCl2 and 4 MgCl2; and was saturated with 95% O2 + 5% CO2.
2.3 Electrophysiological recordings in hippocampal slices
Slices were transferred to a recording chamber and perfused continuously with oxygenated-ACSF. An automatic temperature control unit maintained the ACSF at 32°C. Slices were perfused at a rate of 1 ml/min and humidified with 95% O2 + 5% CO2 gas during the entire length of the experiment. For extracellular recordings, Schaffer collateral projections from the CA3 region were stimulated with a monopolar stainless steel electrode (100 μm diameter) for activation of CA1 neurons. A recording borosilicate glass electrode, filled with 3M NaCl with 1–2 MΩ resistance was placed in the CA1 region (within 150–200 μm) to record field excitatory postsynaptic potentials (fEPSP). Constant current pulses (150μs, 10–100μA) were delivered using a stimulus isolation unit (A310 Accupulser, World Precision Instruments, FL) and evoked fEPSPs amplified (A-M Systems, WA), monitored on a digital oscilloscope and a computer screen. Responses were elicited every 1 min and digitized, stored and analyzed using an Apple Macintosh computer and custom built software based on Lab-View 5.1 software (National Instruments, Tx). After an initial mapping of the area of interest on the slices for the most optimal recording, basic synaptic transmission was assessed by determining the input/output function of EPSP slope and population spike amplitude by increasing the stimulation intensity at small steps and recording the fEPSPs at each level. A test-stimulus was chosen at approximately 50% of the maximum evoked responses. Baseline responses were then recorded for 10 min followed with HFS (4 trains of 50Hz, 100Hz or 200Hz, 1sec duration, 20 sec inter-train intervals) and the fEPSPs were monitored for 15 min to assess the magnitude of potentiation.
2.4 APP processing and α- and β-secretase activity
Hippocampal slices obtained at the end of electrophysiological recordings were stored at −80°C and processed for the detection of α- and β-secretase activity using commercially available kits (R&D Systems, MN). Briefly, brain samples were homogenized in supplied buffers and then added to secretase-specific APP peptide conjugated to the reporter molecules EDANS and DABCYL. In the uncleaved form, fluorescent emissions from EDANS were quenched by the physical proximity of the DABCYL moiety. Cleavage of APP peptide by secretase physically separates the EDANS and DABCYL reporter molecules, allowing for the release of a fluorescent signal. The level of secretase enzymatic activity is proportional to the fluorometric reaction in the homogenate.
2.5 Brain surgery
8 week-old TgCRND8 mice were bilaterally implanted with chronic stainless steel stimulating and recording electrodes (cross-section diameter, 100 μm) in the midline thalamic nuclei (MTN) and CA1 region of hippocampus respectively under deep sodium pentobarbital anesthesia. The animals were placed in a stereotaxic frame and the skull was exposed and cleaned. The electrodes were lowered into the brain through holes made in the skull at the following coordinates: MTN, 1.1 mm posterior to bregma, 0.3 mm lateral to midline and 2.5–3.5 mm dorsal-ventral; and CA1 region, 2.1 mm posterior to bregma, 0.5 mm lateral to midline and 1.2 mm dorsal-ventral according to the mouse brain atlas [6]. The final positions of the electrodes were determined by audio monitoring of unit firing and recording of evoked responses elicited after test stimulation of the MTN (120 μA, 250 msec, 0.05 Hz). A depth profile was taken for each animal by first positioning the stimulating electrode and moving the recording electrode to obtain the highest response. One screw positioned on the frontal bone served as reference for recording and a second above the parietal cortex served as the stimulus indifferent. The electrodes were assembled into a nano-connector (Omnetics, MN) which was cemented to the skull. All animals were allowed at least 5 days to recover from surgery before starting the experiment.
2.6 Deep Brain Stimulation
Brain stimulation was performed in a chamber, which consisted of an illuminated wooden box (45 × 45 × 80 cm) with a small fan fixed to one side of the chamber to provide both ventilation and constant low intensity white noise to muffle external sounds. The chamber was completely enclosed, and viewing of the animals was possible through a one-way mirror set on the chamber wall. Electrical stimulation was applied using a stimulus isolation unit (ML180, PowerLab) in MTN bilaterally, in each animal during 3 consecutive days (8 trains of HFS; each train of 25 Hz, 1 sec duration, 300 μA, and 10 sec inter-train interval); this was based on preliminary studies from our laboratory. Animals were evaluated for memory in the object recognition task immediately after electrical stimulation during three consecutive days.
2.7 Behavioral analysis
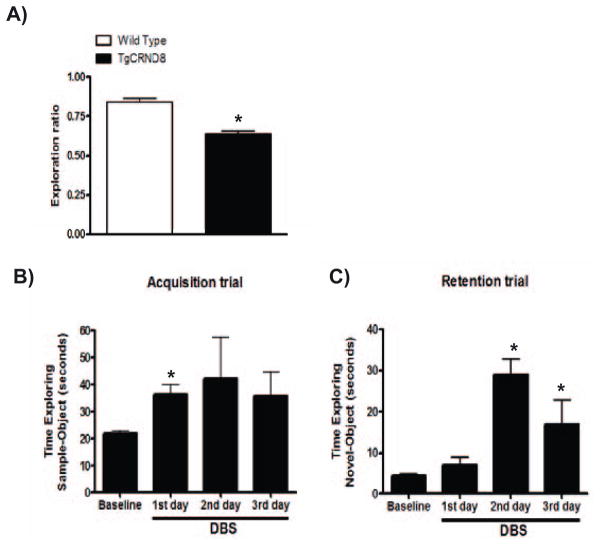
The object recognition task was performed as previously described [7]. Briefly, mice were habituated for 1 h to a Plexiglas open-field box (52 × 52 × 40 cm) with white vertical walls. The next day, the animals were placed in the same box and submitted to a 10 min acquisition trial. During this trial mice were placed individually in the open field in the presence of object A (marble or dice), and the time spent exploring object A (when the animal’s snout was directed toward the object at a distance <1 cm) was measured. During a 10 min retention trail (second day), which was performed 1 h later, a novel object (object B) was placed together with the familiar object (object A) in the open field. The time (tA and tB) the animal spent exploring the two objects was recorded. The recognition index (RI), defined as the ratio of time spent exploring the novel object over the time spent exploring both objects was used as a measure of short-term memory.
2.8 Histological and Immunohistochemical analysis
After the last behavioral assessment, the animals were perfused under pentobarbital anesthesia (1 mg/100 g body weight) via the ascending aorta with saline solution, followed by 50 ml of ice-cold 4% paraformaldehyde at pH 7.6. The brains were removed and postfixed in the perfusion solution for 2–3 h and then stored overnight in potassium PBS with 10% sucrose at 4°C. Serial, 40 μm coronal sections were cut throughout the MTN and hippocampus regions on a microtome. Serial sections for each region were collected into 1-in-4 series, placed in tissue-cultured wells containing cryoprotectant solution (ethylene glycol) and maintained at −20°C until cresyl violet staining or immunohistochemical analysis was performed.
For immunohistochemical analysis, FosB immunoreactivity was detected using a conventional avidin–biotin immunoperoxidase protocol [8]. Briefly, free-floating sections were first pretreated with hydrogen peroxide for 10 min to quench endogenous peroxidase activity, followed by two rinses in PBS, and then incubated in 1.0% sodium borohydrate to reduce free aldehydes. The tissue sections were then incubated with the primary antibody (FosB, Santa Cruz Biotechnology, Santa Cruz, CA) raised in rabbit at a dilution 1:500 at 4°C for 48 h. Overnight incubation with secondary antibody was performed using a 1:200 dilution at 4°C. The primary antibody was detected with the avidin–DH-biotinylated horseradish peroxidase–H-complex (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA); the diaminobenzidine reaction product was developed using a nickel-enhanced glucose oxidase method. As negative control, the primary antibody was omitted.
2.9 Statistical Analysis
Data were compiled and statistical analysis was performed using GraphPad Prism software (V4.03, GraphPad Software, Inc, San Diego, CA). Data are presented as mean ± SEM and analyzed using a Two-Way ANOVA (source of variation: interaction, treatment and time) in a within-subjects design for the fEPSPs and α- and β-secretase activity in the hippocampal slices. When significant interactions were detected, the Bonferroni posthoc test was used to make pair-wise comparisons. A student’s t-test was used for the data obtained in the object recognition task. Significant differences were set at p<0.05, p<0.01 and p<0.001.
3. Results
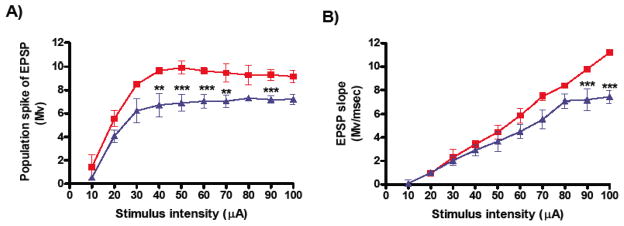
In the in vitro studies, we examined the effects of electrical stimulation on basal neuronal transmission and synaptic plasticity from 5 week-old TgCRND8 mice and their WT age-matched controls. fEPSPs were recorded in the CA1 region of the hippocampus following HFS at different frequencies (50Hz, 100Hz or 200Hz), while controls received low-frequency stimulation (LFS) or were not stimulated (NS). Differences in input-output function and short-term potentiation were assessed. We observed significant differences in basal synaptic transmission between TgCRND8 and WT controls. The maximum amplitude (mean ± SEM) of the population spike was (9.91 ± 0.51) for the WT group (n=10 mice) and (7.21 ± 0.45) for the TgCRND8 group (n=10 mice) (***p<0.01, Figure 1A). The maximum fEPSP slope was (11.20 ± 0.06) for the WT group and (7.42 ± 0.42) for TgCRND8 group (***p<0.001, Figure 1B).
Figure 1.
Basal synaptic transmission in hippocampal slices from 5 weeks-old TgCRND8 and WT control mice. Input–Output curves, as measured by the population spike (A) and EPSP slope (B), were constructed in the CA1 region with stimulation of the Schaffer collaterals. The data represent mean ± S.E.M. and were analyzed using a 2-way ANOVA in a within-subjects design, followed by Bonferroni’s post-hoc tests for pair-wise comparisons. Significant differences were designated as follows: **p<0.01; and ***p<0.001. The results show that in slices from TgCRND8 group (blue triangles, n=20 slices per group) there was a significant deficit in basal synaptic transmission compared to WT group (red squares, n=23 slices per group).
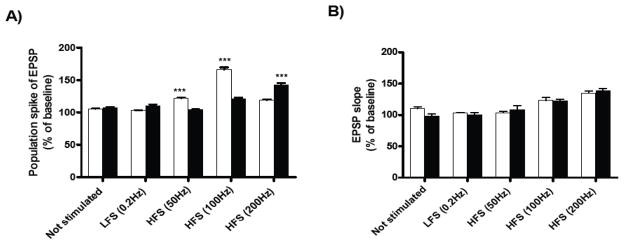
Short-term potentiation (15 min after tetanus) was measured in the pyramidal cell layer of the CA1 region following HFS of the Schaffer collaterals. In slices from WT mice, short-term potentiation was significantly increased after HFS at 50Hz and 100Hz in comparison with slices from TgCRND8 mice, as measured by the population spike (Figure 2A). In contrast, slices from TgCRND8 mice showed a significant increase in short-term potentiation only after HFS at 200Hz (Figure 2A), compared with WT group. For the EPSP slope, we did not observed significant differences between groups (Figure 2B).
Figure 2.
Effects of HFS on short-term potentiation in hippocampal slices from 5-weeks-old TgCRND8 mice and their age-matched WT controls. Short-term- potentiation was measured in the pyramidal cell layer of the CA1 region following high-frequency stimulation (50Hz, 100Hz or 200Hz) of the Schaffer collaterals. Values of the amplitude of the population spike (A) and fEPSP slope (B) are expressed as percentages compared to their own baselines normalized to 100%. Each graph shows the mean ± S.E.M. of the first 15 min of recording after tetanus. The data were analyzed using a Two-way ANOVA in a within-subjects design, followed by Bonferroni’s post-hoc tests for pair-wise comparisons. Significant difference was designated as ***p<0.001. The results show a significant increase in synaptic plasticity at 50Hz and 100Hz of slices from WT mice (white bar, n=4 slices per group) and for TgCRND8 mice at 200Hz (black bar, n=4 slices per group) in the amplitude of the population spike. There were no significant differences in the fEPSP slope between groups.
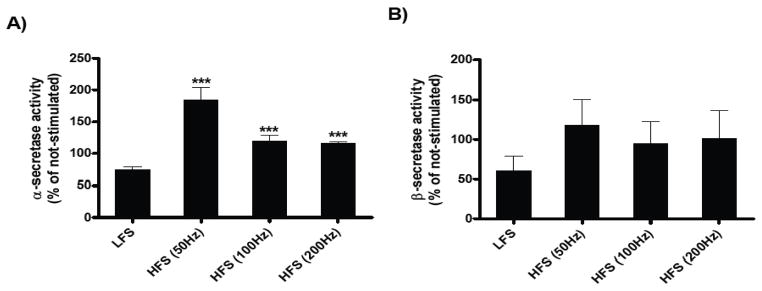
Since changes in synaptic activity could modulate secretase activity, we explored changes in secretase activity following electrical stimulation in hippocampal slices from TgCRND8 and WT animals. After recordings, the tissue was processed for α- and β-secretase activity. The data showed an increase in the α-secretase activity after HFS at 50Hz, 100Hz and 200Hz, in slices from TgCRND8 mice (***p<0.001, Figure 3A). These results show that HFS was associated with selective enhancement in α, but not β-secretase activity in the brain (Figure 3B).
Figure 3.
Effect of HFS on α- and β-secretase activity in hippocampal slices of TgCRND8 mice. Values of the α-secretase activity (A) and β-secretase activity (B) are expressed as percentages compared to their own non-stimulated slices normalized to 100%. Each graph shows the mean ± S.E.M. The data were analyzed using a Two-way ANOVA in a within-subjects design, followed by Bonferroni’s post-hoc tests for pair-wise comparisons. Significant difference was designated as ***p<0.001. The results show that HFS stimulation was associated with selective enhancement in α, but not β-secretase activity in the CA1 region of the hippocampus.
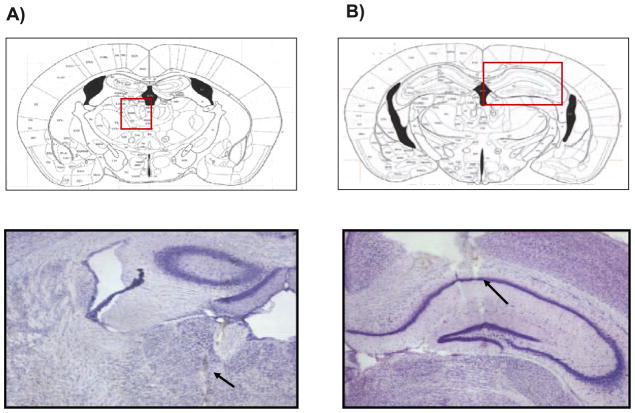
In the in vivo studies, the position of stimulating and recording electrodes in the region of MTN (Figure 4A) and in the CA1 region of the hippocampus (Figure 4B) were verified histologically by Nissl staining in all of the brains for the animals used in these experiments. We observed that TgCRND8 mice presented a deficit in short-term memory, in comparison to WT group, as measured by the novel object recognition test without DBS (Figure 5A). DBS treatment in TgCRND8 mice facilitated acquisition memory (*p<0.05, 2-tailed Student’s t-test, Figure 5B) and short-term memory, as measured by time exploring of the novel-object during retention trial (Figure 5C).
Figure 4.
Histological sections showing placement of stimulating and recording electrodes in the region of midline thalamic nuclei (A) and in the CA1 region of the hippocampus (B) respectively. Position of the electrode for electrical stimulation in the midline thalamic nuclei was −1.1 mm posterior to bregma and the position of the electrode for recording in the CA1 region of the hippocampus was −2.1 mm posterior to bregma.
Figure 5.
Effects of chronic DBS on short-term memory from TgCRND8 mice. Short-term working memory (1 hour) measured by novel object recognition test in WT and TgCRND8 mice without DBS (A). DBS treatment facilitateed acquisition memory in TgCRND8 mice. Time exploring of the samples (familiar)-objects during acquisition trial (training session) 10 min after DBS treatment (B) and time exploring of the novel-object during retention trial (testing session (C), 1 hour after acquisition trial in TgCRND8 mice. Values represent group mean ± SEM, n=3 per group. *p<0.05, 2-tailed Student’s t-test.
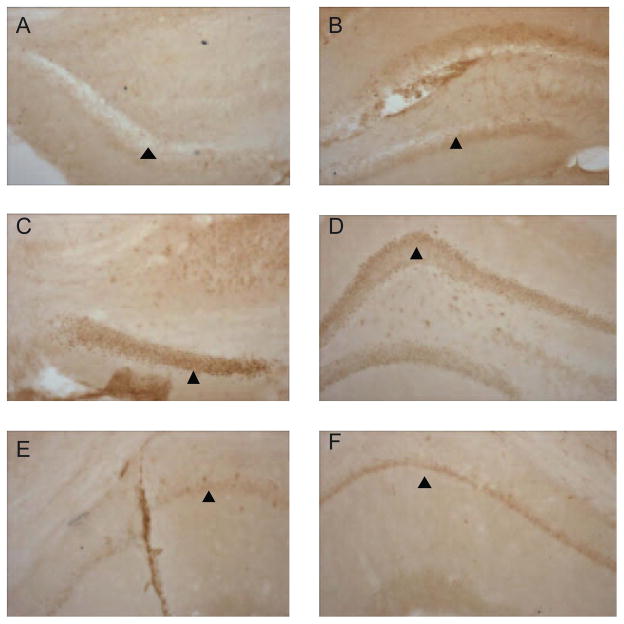
We assessed neuronal-functional activation associated with electrical stimulation in the MTN by using a well known neuronal activity maker, FosB protein expression pattern in the CA1 region of hippocampus from stimulated TgCRN8 mice. Bilateral electrical stimulation of MTN produced up-regulation of FosB protein expression in the dentate gyrus (Figure 6C and D) and CA1 region of the hippocampus (Figure 6E and F) from TgCRND8 mice treated with DBS in the MTN. We did not observe upregulation of FosB protein in TgCRND8 mice without DBS (Figure 6A and B).
Figure 6.
Changes in FosB immunoreactive cells in the hippocampus of TgCRND8 mice without (A and B) and with (C–F) electrical stimulation in the MTN. Representative photomicrographs of brain sections were incubated with specific anti-FosB antibody and processed for immunohistochemistry (see Materials and Methods). Anterior (A) and mid level (B) of the dentate gyrus of the hippocampus from TgCRND8 mice without electrical stimulation. Anterior (C) and mid level (D) of the dentate gyrus and CA1 region of the hippocampus (E–F) from TgCRND8 mice with electrical stimulation in the MTN.
4. Discussion
Our results indicate a significant reduction in overall CA1 basal synaptic transmission in young TgCRND8 mice prior to the onset of amyloid neuropathology (see Figure 1A and B). These findings confirm and extend previous reports regarding synaptic dysfunction in the hippocampus from TgCRND8 mice, which appears to have a strong correlation with progressive memory and learning impairment in this mouse model of AD [9,10]. We found that the application of HFS to isolated hippocampal slices from TgCRND8 mice improves synaptic transmission and short-term potentiation (see Figure 2A). This improvement correlated with a selective increase in α-secretase activity (see Figure 3A) in the CA1 region. The fact that basal neuronal transmission is decreased in the CA1 region of the hippocampal slices strongly suggests these neurons are less excitable in TgCRND8 mice. Further, our in vivo results suggest that DBS treatment in TgCRND8 mice facilitates acquisition and short-term memory, as measured by time exploring the novel-object during retention trial (Figure 5B and C). Previous in vivo studies have investigated the effects of central thalamic stimulation to enhance memory and learning function in rats [3], generating the possibility of exploring therapeutic effects of HFS in AD dementia and for the treatment of impaired cognitive function. Neurons within the intralaminar nuclei and paralaminar regions of the central thalamus link brainstem arousal systems to cerebral cortical and basal ganglia networks, crucial to the organization of wake behaviors [11]. Detailed electrophysiological studies and clinical observations further indicate a key role of these neuronal populations in maintaining an alert wakeful state [12]. Further, the relevance of activation of these circuits as a therapeutic strategy for acquired cognitive disabilities associated with non-progressive brain injuries has been proposed [3].
HFS enhances synaptic plasticity and at the same time changes cellular secretase activity (e.g. β-secretase) critical for Aβ generation. For example, electrical depolarization of rat hippocampal slices has been shown to release amino-terminal amyloid precursor protein (APP) derivatives with increasing stimulation frequencies, suggesting that neuronal activity regulates APP processing in the mammalian brain and that the net effect of depolarization involves several subtypes of acetylcholine receptors [13]. Recent evidence suggests that appropriate expression and activity of β-secretase in hippocampal slices in β-secretase knock-out mice regulates memory and synaptic plasticity which depends on prior synaptic activity in the hippocampus [14].
A key molecule that may perturb network activity in AD is the Aβ peptide, which is derived from the APP through specific proteolytic cleavages by β- and γ-secretase. APP can also be processed by a non-amyloidogenic α-secretase activity that cleaves APP at the Aβ peptide sites and thereby precludes the generation of Aβ peptides. The excessive accumulation of pathogenic Aβ peptide assemblies in the brain appears to play a causal role in AD [15]. In strong support of this notion, neuronal expression of human APP and Aβ peptides in transgenic mice elicits several AD-like abnormalities, including amyloid plaques, neuritic dystrophy, aberrant sprouting of axon terminals, functional and structural synaptic deficits, impairments in learning and memory and other behavioral alterations [16]. These transgenic models have made the mechanistic study of AD more feasible. The potential therapeutic role of HFS in AD dementia may occur via indirect mechanisms (e.g., the possibility that HFS can improve overall cognitive status) as well as direct mechanisms (the possibility that HFS may modulate Aβ accumulation in the brain).
The present findings provide the first steps in establishing a mouse model of DBS in which we can determine specific stimulation parameters and their effects on excitatory and inhibitory projections in the thalamocortical-hippocampal circuit and further its effects on learning and memory. Future experiments must be addressed to determine which mechanism(s) might be involved in the enhancement of short-term memory by DBS in the thalamic region.
Acknowledgments
This work was supported by discretionary funding to GMP.
References
- 1.Kringelbach ML, Jenkinson N, Owen S, Aziz TZ. Translational principles of deep brain stimulation. Nature Rev Neuroscience. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 2.Hu R, Eskandar E, Williams Z. Role of deep brain stimulation in modulating memory formation and recall. Neurosurg Focus. 2009;27:1–5. doi: 10.3171/2009.4.FOCUS0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirvalkar P, Seth M, Schiff ND, Herrera DG. Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A. 2006;103:17007–12. doi: 10.1073/pnas.0604811103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience. 1999;92:15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- 5.Kiss ZHT, Mooney DM, Renaud L, Hu B. Neuronal response to local electrical stimulation in rat thalamus: physiological implications for mechanism of deep brain stimulation. Neuroscience. 2002;113:137–143. doi: 10.1016/s0306-4522(02)00122-7. [DOI] [PubMed] [Google Scholar]
- 6.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; 2001. [Google Scholar]
- 7.Mitchell AS, Dalrymple-Alford JC. Lateral and anterior thalamic lesions impair independent memory systems. Learn Mem. 2006;13:388–96. doi: 10.1101/lm.122206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrieta I, Díaz-Ibáñez LB, Morales T, Mendoza-Garcés L, Morimoto S, Moreno-Mendoza N, Cerbón MA. Progesterone receptor gene and protein expression in the anterior preoptic area and hypothalamus of defeminized rats. J Neurobiol. 2003;56:338–46. doi: 10.1002/neu.10241. [DOI] [PubMed] [Google Scholar]
- 9.Bellucci A, Luccarini I, Scali C, Prosperi C, Giovannini MG, Pepeu G, Casamenti F. Cholinergic dysfunction, neuronal damage and axonal loss in TgCRND8 mice. Neurobiol Dis. 2006;23:260–272. doi: 10.1016/j.nbd.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Ye H, Jalini S, Mylvaganam S, Carlen P. Activation of large-conductance Ca(2+)-activated K(+) channels depresses basal synaptic transmission in the hippocampal CA1 area in APP (swe/ind) TgCRND8 mice. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Steriade M. In: Thalamus. Steriade M, Jones E, McCormick D, editors. Elsevier; Amsterdam: 1997. pp. 721–742. [Google Scholar]
- 12.Schiff ND, Purpura KP. Towards a neurophysiological foundation for cognitive neuromodulation through deep brain stimulation. Thalamus and Related Systems. 2002;2:55–69. [Google Scholar]
- 13.Farber SA, Nitsch RM, Schulz JG, Wurtman RJ. Regulated secretion of beta-amyloid precursor protein in rat brain. J Neurosci. 1995;15:7442–51. doi: 10.1523/JNEUROSCI.15-11-07442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–70. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 16.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–6. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]