Abstract
The PI3K/AKT and RAF/MEK/ERK signaling pathways are activated in a wide range of human cancers. In many cases, concomitant inhibition of both pathways is necessary to block proliferation and induce cell death and tumor shrinkage. Several feedback systems have been described in which inhibition of one intracellular pathway leads to activation of a parallel signaling pathway, thereby decreasing the effectiveness of single-agent targeted therapies. In this study we describe a feedback mechanism in which MEK inhibition leads to activation of PI3K/AKT signaling in EGFR and HER2-driven cancers. We found that MEK inhibitor-induced activation of PI3K/AKT resulted from hyperactivation of ERBB3 as a result of the loss of an inhibitory threonine phosphorylation in the conserved juxtamembrane (JM) domains of EGFR and HER2. Mutation of this amino acid led to increased ERBB receptor activation and up-regulation of the ERBB3/PI3K/AKT signaling pathway, which was no longer responsive to MEK inhibition. Taken together, these results elucidate an important, dominant feedback network regulating central oncogenic pathways in human cancer.
Keywords: cancer, feedback, ERBB receptors, PI3K, MEK
INTRODUCTION
The phosphatidylinositol 3-kinase (PI3K), RAF/MEK/ERK mitogen-activated protein kinase (MAPK), and mammalian target of rapamycin complex 1 (mTORC1) pathways transmit signals from receptor tyrosine kinases (RTKs) to downstream effector networks regulating cell growth, metabolism, survival, and proliferation (1–3). Numerous feedback systems regulating these oncogenic pathways have been described, and can potentially impact the sensitivity of cancers to kinase inhibitors. For example, inhibition of mTORC1 relieves proteasomal degradation of IRS-1 leading to feedback up-regulation of IRS-1/PI3K/AKT, reducing the efficacy of mTORC1 inhibitors as single agents and prompting the use of combination therapies (4–6). PI3K and AKT inhibitors relieve a negative feedback on ERBB receptors and other RTKs leading to partial re-activation of PI3K/AKT signaling, MEK/ERK signaling, and other downstream pathways, potentially limiting the utility of PI3K inhibitors as single agents (7–9).
Targeted therapies, such as the EGFR inhibitors gefitinib and erlotinib, are highly effective when cells are “addicted”, and inhibition of the target leads to down-regulation of critical growth and survival signaling pathways, especially PI3K/AKT and MEK/ERK (10–12). We recently found that treatment with a combination of a MEK inhibitor and a PI3K inhibitor led to significant apoptosis in EGFR-driven cancers, similar to that induced by an EGFR TKI, whereas treatment with either pathway inhibitor alone did not induce marked cell death (11). In those studies, treatment with a single-agent MEK inhibitor led to increased AKT phosphorylation. Indeed, several other studies have shown that MEK inhibition leads to increased AKT activation, often resulting in reduced efficacy of MEK inhibitors as single agents (11, 13–15). However, the molecular mechanisms underlying this feedback remain unknown.
Several mechanisms for MEK feedback regulation of AKT signaling have been suggested. For example, ERK-mediated serine phosphorylation of the GAB1 adaptor has been shown to negatively regulate GAB1-PI3K binding and downstream AKT signaling (16–18). MEK inhibition can also down-regulate mTORC1 signaling, relieving negative feedback on IGF-IR/IRS-1 and activating PI3K/AKT signaling (19). ERK has also been shown to directly regulate ERBB tyrosine phosphorylation (20, 21). However, it remains unclear which mechanisms, if any, are dominant in MEK inhibitor-induced activation of AKT signaling in EGFR or HER2-driven cancers. As multiple MEK and BRAF inhibitors, including the highly selective allosteric MEK1/2 inhibitor, AZD6244 (22), are being developed, understanding the signaling feedbacks induced by MEK inhibitors that may ultimately impact their utility will become increasingly important.
In this study, we examined the molecular mechanism by which MEK inhibition leads to increased AKT phosphorylation in EGFR and HER2-driven cancers. We provide evidence suggesting that this feedback occurs at the level of increased phosphatidylinositol 3,4,5-trisphosphate (PIP3) induced by an increased association between ERBB3 and PI3K. Increased ERBB3 activation results from loss of an inhibitory ERK-dependent threonine phosphorylation in the conserved JM domains of EGFR and HER2, previously found to regulate to EGFR auto-phosphorylation (21). Elucidation of this mechanism provides a greater understanding of the feedback systems regulating key pathways that drive human cancers.
MATERIALS AND METHODS
Cell culture reagents and Western analyses
Cell lines, inhibitors, and growth conditions are described in Supplemental Materials and Methods. Cells were lysed in an NP-40 containing buffer, separated by SDS/PAGE, and transferred to PVDF membranes. Antibody binding was detected using enhanced chemiluminescence (PerkinElmer).
Biotin labeling and Immunoprecipitation
HCC827 cells were washed with PBS and labeled for 1hr at 4degC in 0.5ug/mL Sulfo-NHS-LC-Biotin (Thermo Scientific) re-suspended in PBS +/-AZD6244. Labeling was quenched with 100mM glycine. Cells were then returned to media at 37degC before lysis. Biotin-labeled cell surface proteins were immunoprecipitated with NeutrAvidin Agarose Resins (Thermo Scientific), separated by SDS page, and immunoblotted to detect the indicated proteins. Transferrin receptor was used as a loading control.
Xenograft Studies
Xenograft studies were performed in accordance with the standards of the Institutional Animal Care and Use Committee (IACUC) under a protocol approved by the Animal Care and Use Committee of Massachusetts General Hospital. Nude mice (nu/nu; 6–8 weeks old; Charles River Laboratories) were injected with a suspension of 5×106 H1975 cells subcutaneously into the flank of each mouse. Once the mean tumor volume reached ~500mm3 AZD6244 was administered by oral gavage in 3 doses of 25mg/kg over 30 hours.
PIP2/PIP3 Quantification
Phospholipids were isolated from cells and PIP3 and PI(4,5)P2 levels were measured using ELISA kits (Echelon, K-2500s and K4500) according to the manufacturer's instructions. Statistical significance was calculated using a t-test.
qRT-PCR
RNA was isolated using the RNEasy kit (Qiagen) and cDNA was transcribed with Superscript II Reverse Transcriptase (Invitrogen) and used as a template for PCR amplifications. The relative copy number for ERBB3 and HRG was determined using q-RT-PCR using a light-cycler 480 (Roche) as previously described (11). The PCR primers and conditions are available upon request.
siRNA and Transient Transfections
HCC827 and BT-474 cells were transfected with 50nM ERBB3s4779 silencer select validated siRNA or negative control (Ambion) with HiPerFect Transfection Reagent (Qiagen) according to manufacturer’s instructions.
Transient transfections of CHO-KI cells were performed with TransIT®-LT1 Transfection Reagent (MirusBio LLC) according to the manufacturer’s recommendations. Wild-type ERBB3 was co-transfected with an equal ratio of GFP or wild-type or mutant EGFR or HER2.
shRNA, DNA Constructs and Lentiviral Production
HCC827 cells were infected with shERBB3 as previously described (23, 24) with tet-on PLKO shERBB3 or scramble shRNA knockdown vectors (Addgene) and selected in puromycin. shRNA hairpin sequences are provided in the Supplemental Materials and Methods.
Human EGFR (wild-type and exon 19del) and HER2 cDNA coding regions were cloned into the pENTR/D-TOPO vector (Invitrogen) and mutants were constructed with Quick Change Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s instructions. All constructs were confirmed by DNA sequencing. Constructs were cloned into the plenti-IRES-GFP lentiviral vector (Addgene) and infections were performed as described previously (25).
Flow Cytometry
BT-474 cells were transfected with ERBB3 siRNA (Ambion) for 48hrs, then treated with AZD6244 or GDC-0941 for 72hrs. Cells were collected and stained with propidium iodide (PI) and AnnexinV as described previously (26). Cells were analyzed using a BD LSR3 analytical flow cytometer (BD Biosciences). Apoptosis was calculated using the sum of AnnexinV positive and PI/AnnexinV double-positive cells.
Tandem mass spectrometry (LC/MS/MS)
EGFR or HER2 was immunoprecipitated from cells treated with AZD6244 using anti-EGFR antibody (Santa Cruz) or an anti-HER2 antibody, separated by SDS/PAGE, stained with Coomassie blue. Bands were excised and samples were prepared and analyzed by reversed-phase microcapillary/tandem mass spectrometry (LC/MS/MS) as described previously (27–29) and further detailed in the Supplemental Materials and Methods.
RESULTS
MEK inhibition leads to activation of ERBB3/PI3K/AKT
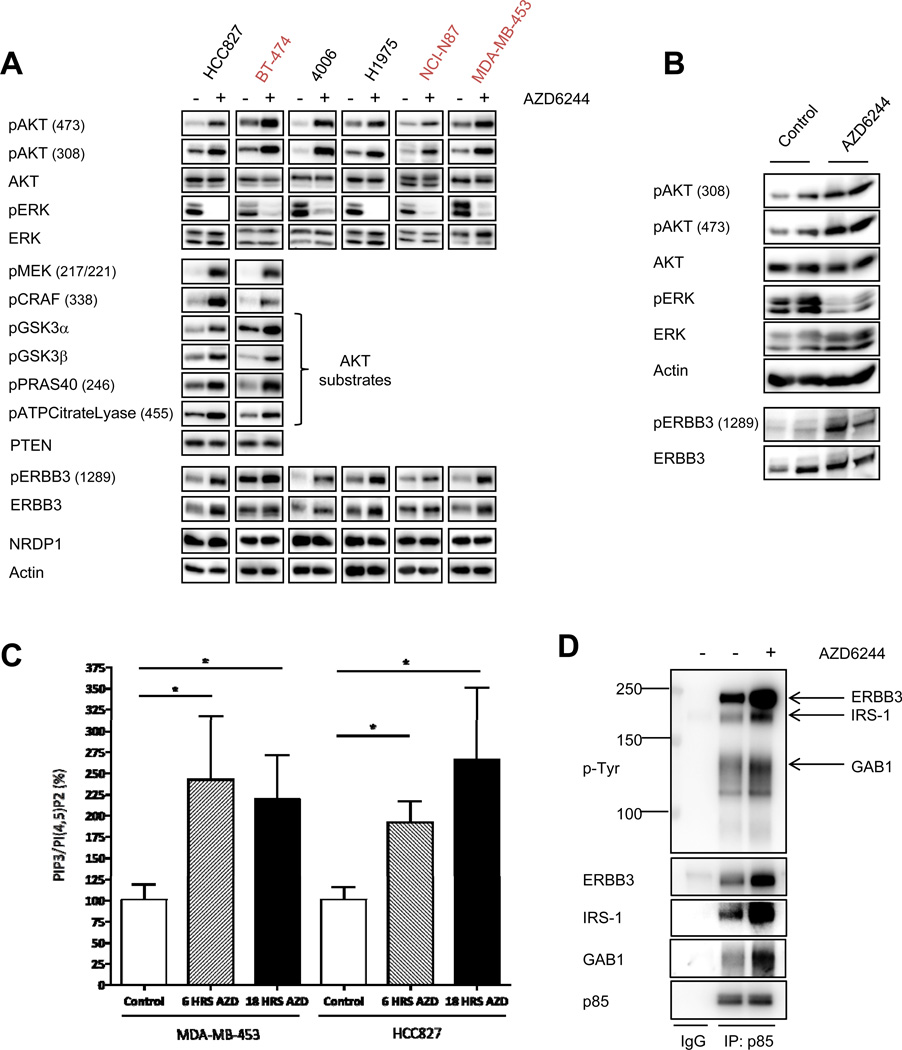
We previously observed that AKT phosphorylation increased in response to MEK inhibition in HER2-amplified and EGFR-mutant cancer cells (11). To determine whether this potential feedback is observed in multiple EGFR or HER2-addicted cancer models, we treated HER2-amplified or EGFR-mutant cell lines with the highly selective allosteric MEK1/2 inhibitor, AZD6244. This MEK inhibitor was used at a concentration of 2µM, which sufficiently inhibited ERK1/2 phosphorylation in the HCC827 cell line (Supplemental Figure 1). Similar results were observed using two distinct allosteric MEK inhibitors, GSK212 and PD0325901 (Supplemental Figure 1). In each cell line, we observed increased AKT phosphorylation at both S473 and T308 following AZD6244 treatment, as well as increased phosphorylation of several AKT targets including GSK3α/β, ATP citrate lyase, and PRAS40 (Figure 1A). We confirmed that these proteins were AKT substrates, as co-treatment with an allosteric AKT inhibitor blocked their phosphorylation (Supplemental Figure 2). MEK inhibition also led to up-regulation of phospho-CRAF and phospho-MEK (Figure 1A), suggesting activation of a common upstream signaling molecule. This feedback also occurred in vivo, as we observed increased phospho-AKT in an EGFR-mutant H1975 (L858R/T790M) xenograft model treated with AZD6244 (Figure 1B).
Figure 1. MEK inhibition leads to feedback activation of ERBB3/PI3K/AKT signaling.
(A) EGFR-addicted (HCC827, H4006, H1975) or HER2-addicted (BT-474, NCI-N87, MDA-MB-453) cell lines were treated with 2µM AZD6244 for 6 hours. Cell lysates were immunoblotted to detect the indicated proteins. (B) H1975 (EGFR L858R/T790M) xenografts were treated with AZD6244 25mg/kg BID. Mice were sacrificed 6hrs following treatment and tumors were harvested. Tumor cell lysates were immunoblotted to detect the indicated proteins. (C) MDA-MB-453 and HCC827 cells were treated for 6hrs or 18 hours with 2µM AZD6244. Phospholipids were isolated from cell lysates and relative PIP3 and PI(4,5)P2 levels were quantified by ELISA. Each data point represents the mean ±SEM of two independent experiments performed in triplicate. For normalization, the PIP3/PI(4,5)P2 ratio was set to 100% for untreated cells. Asterisks represent p<0.05. (D) HCC827 cells were treated for 6hrs with 2µM AZD6244. Cell extracts were immunoprecipitated with an anti-p85 antibody followed by Western blot with anti-p-Tyr and anti-p85 antibodies. Blots were stripped and re-probed with antibodies specific to ERBB3, GAB1, and IRS-1.
Increased AKT phosphorylation suggested a potential increase in the abundance of PIP3 (the lipid product of PI3K). Therefore, EGFR-driven HCC827 and HER2-driven MDA-MB-453 cells were treated with a MEK inhibitor, lipids were isolated, and PIP3 levels were quantified. In both cell lines, AZD6244 induced significant increases in PIP3 (Figure 1C). We did not observe any change in expression of the PTEN phosphatase responsible for de-phosphorylating PIP3, following MEK inhibition (Figure 1A). To determine if MEK inhibition led to activation of PI3K, we immunoprecipitated the p85 regulatory subunit of PI3K and assessed the abundance of bound adaptors. PI3K consists of a p110 catalytic subunit and a p85 regulatory subunit, and is activated when p85 SH2 domains bind to tyrosine-phosphorylated proteins with YXXM motifs. Treatment with AZD6244 increased the association between PI3K and tyrosine-phosphorylated adaptors, including ERBB3 and GAB1 (Figure 1D). These results suggest that MEK inhibition leads to an increase in the phospho-tyrosine signaling cascades that directly activate PI3K.
In EGFR and HER2-driven cancers, ERBB3 is a primary activator of PI3K/AKT (24, 30). We observed increased ERBB3 binding to PI3K following MEK inhibition (Figure 1D), and accordingly, MEK inhibition substantially increased tyrosine-phosphorylated ERBB3 levels (Figure 1A). In some cell lines, we observed an increase in total ERBB3 along with phospho-ERBB3 (Figure 1A). Of note, we did not observe a change in expression of the E3-ubiquitin ligase, neuregulin receptor degradation protein 1 (NRDP1), which can control the steady state levels of ERBB3 (31, 32) (Figure 1A). There was also no increase in ERBB3 mRNA levels following AZD6244 treatment (Supplemental Figure 3), suggesting that any increase in ERBB3 protein levels is post-transcriptional.
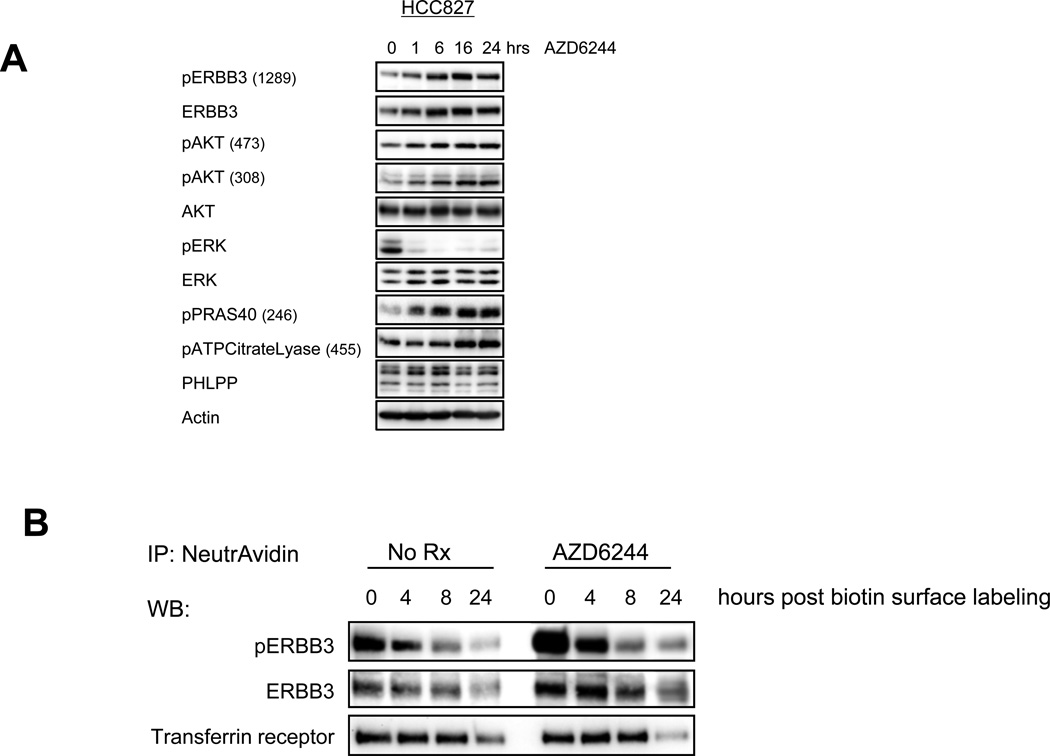
To assess the kinetics of this feedback response, we treated the HCC827 cells with AZD6244 over a time course. Phoshosphorylation of ERBB3 and AKT, as well as downstream substrates, increased after just one hour of MEK inhibition and continued to accumulate for 24 hours (Figure 2A). To determine if the feedback activation of ERBB3 occurs on the plasma membrane, we biotin-labeled the surface of HCC827 cells in the presence or absence of AZD6244 and immunoprecipitated the labeled proteins. After just one hour of MEK inhibition during biotin labeling, surface levels of the activated receptor were substantially elevated (Figure 2B). Total ERBB3 on the cell surface also increased following AZD6244 treatment. MEK inhibition did not seem to significantly affect the kinetics of loss of ERBB3 on the cell surface (Figure 2B), suggesting that receptor internalization or cycling was not significantly affected. These data demonstrate that feedback activation of ERBB3 occurs rapidly on the plasma membrane.
Figure 2. Feedback activation and increased surface localization of ERBB3 occurs within one hour of treatment with AZD6244.
(A) HCC827 cells were treated for the indicated number of hours with AZD6244 (2µM). Cell lysates were immunoblotted to detect the indicated proteins. (B) HCC827 cells were treated with DMSO (NoRx) or AZD6244 (2µM) for 1 hour at 4degC during biotin labeling of surface proteins. Following labeling, cells were returned to media (+/− AZD6244) and treated for the indicated number of hours before lysis. Biotin labeled surface proteins were immunoprecipitated with NeutrAvidin beads, separated by SDS page, and immunoblotted to detect the indicated proteins.
Knockdown of ERBB3 abrogates MEK/ERK feedback on AKT and downstream substrates
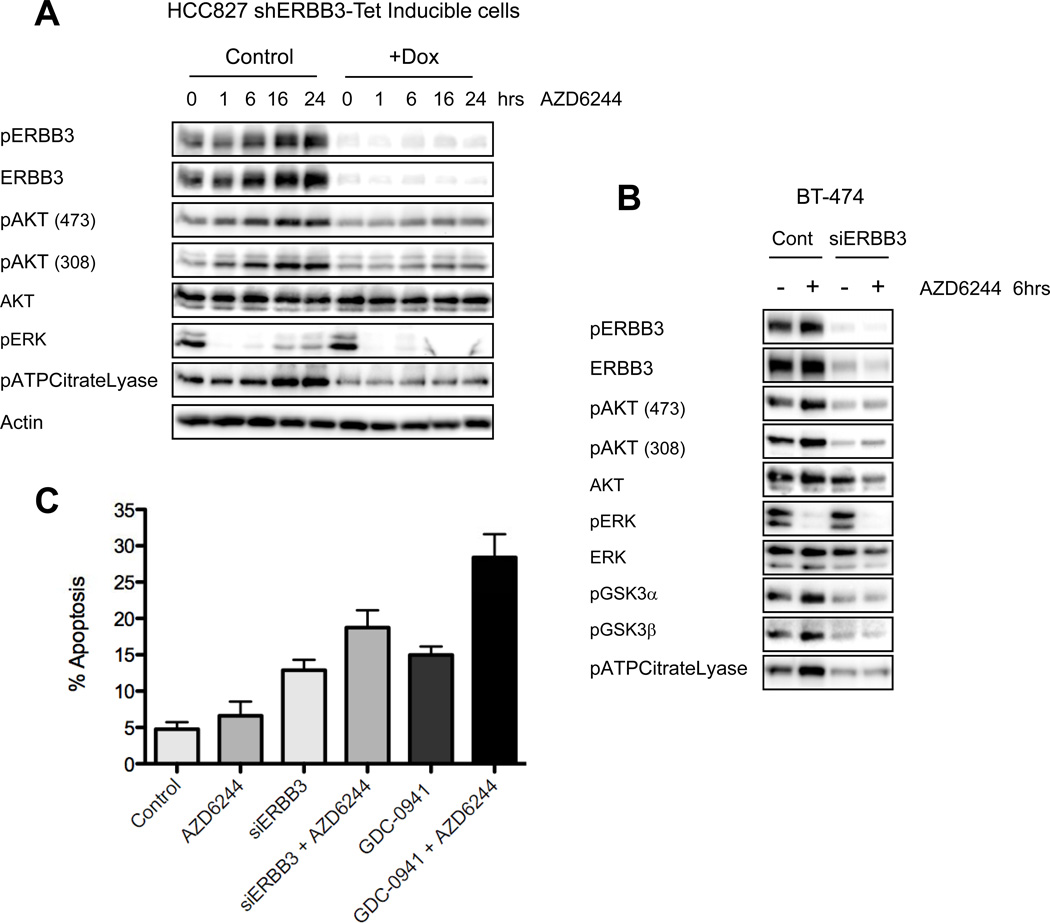
To determine if increased ERBB3 phosphorylation caused the increase in AKT phosphorylation following MEK inhibition, we suppressed expression of ERBB3 using a Tet-inducible shERBB3 hairpin construct. Following treatment with doxycycline there was effective knockdown of ERBB3, and this abrogated the increase in AKT signaling normally observed following MEK inhibition (Figure 3A). In HER2-amplified BT-474 cells with abrogated ERBB3 expression, the increase in AKT signaling following MEK inhibition was also attenuated (Figure 3B). In contrast to HCC827 cells, we observed significant down-regulation of basal AKT phosphorylation in BT-474 cells following ERBB3 knockdown (Figure 3B), indicating the sole reliance on ERBB3 for PI3K activation in this HER2-amplified cancer. In contrast, EGFR-mutant cancers also utilize GAB1 to activate PI3K (Figure 1D) (24, 33).
Figure 3. Suppression of ERBB3 expression abrogates MEK/ERK feedback on PI3K/AKT signaling.
(A) HCC827 cells infected with a lentivirus expressing a Tet-inducible shERBB3 hairpin were induced with 100ng/mL doxycycline (+Dox) for 48hrs. Following knockdown, cells were treated with AZD6244 (2µM) for the indicated number of hours. Cell lysates were immunoblotted to detect the indicated proteins. (B) BT-474 cells were transfected with control or ERBB3 targeted siRNA for 48hrs, followed by treatment with AZD6244 for 6hrs. Cell lysates were immunoblotted with the indicated proteins. (C) BT-474 cells were transfected with control or ERBB3 targeted siRNA for 48hrs, followed by treatment with 2µM AZD6244, 1µM of the PI3K inhibitor GDC-0941, or the combination for 72hrs. Cells were collected and stained for propidium iodide and AnnexinV to determine the percentage of apoptotic cells. Each data point represents the mean ±SD of three independent experiments.
We suspected that knockdown of ERBB3 may increase the efficacy of MEK inhibition by suppressing PI3K/AKT signaling. Treatment with ERBB3 siRNA induced similar levels of cell death compared to treatment with a PI3K inhibitor, GDC-0941 (Figure 3C). Indeed, combining ERBB3 siRNA with AZD6244 increased the cell death response, approaching the level of apoptosis achieved with GDC-0941 in combination with AZD6244 (Figure 3C). These data indicate that ERBB3 plays a significant role in MEK feedback on PI3K/AKT signaling in EGFR and HER2-driven cell lines, suggesting that combination therapies targeting MEK and ERBB3 or MEK and PI3K may block feedback activation of ERBB3/PI3K/AKT signaling and thus be more effective than treatment with a MEK inhibitor alone.
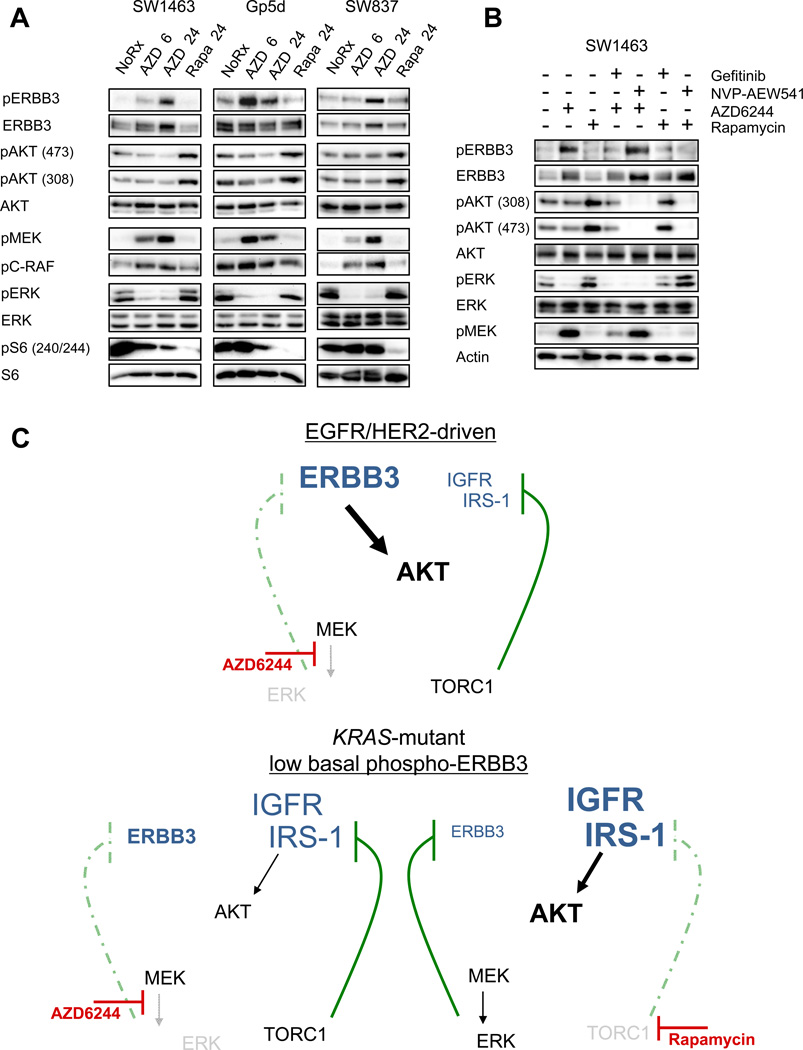
MEK inhibition results in feedback activation of ERBB3 in KRAS-mutant cell lines with low basal levels of phospho-ERBB3
We next determined whether MEK feedback on ERBB3 also occurs in cancers not addicted to EGFR or HER2. We treated a panel of KRAS-mutant cell lines, which have low basal levels of phospho-ERBB3, with AZD6244. Surprisingly, MEK inhibition led to significant activation of ERBB3, but in contrast to EGFR-mutant and HER2-amplified cancers, the increased ERBB3 activation did not translate to increased phospho-AKT (Figure 4A). Similar to the EGFR and HER2-driven models, we also observed up-regulation of phospho-CRAF and phospho-MEK following MEK inhibition. We suspect that increased ERBB3 phosphorylation did not drive PI3K in these KRAS-mutant cell lines because they express significantly less EGFR and HER2, resulting in markedly lower levels of phospho-ERBB3 compared to those observed in EGFR and HER2-driven models (Supplemental Figure 4). Indeed, we recently reported that IGF-IR/IRS signaling is the major PI3K input in these cells (19). Thus, the feedback from MEK inhibition to activation of ERBB3 appears to be conserved in all three of the models we tested, including EGFR-mutant, HER2-amplified, and KRAS-mutant cancers, but results in increased PI3K/AKT signaling only in cells that express sufficient absolute levels of phospho-ERBB3.
Figure 4. MEK/ERK feedback on ERBB3 occurs in KRAS-mutant cell lines and is distinct from TORC1 feedback on IRS-1.
(A) KRAS-mutant cell lines were treated with AZD6244 (2µM) or Rapamycin (50 nM) for 6 or 24hrs. Cell lysates were immunoblotted to detect the indicated proteins. (B) SW1463 cells were treated with 1µM of the EGFR inhibitor gefitinib or 1µM of the IGF-IR inhibitor NVP-AEW541, alone or in combination with 2µM AZD6244 or 50nM Rapamycin for 24 hours. Cell lysates were immunoblotted to detect the indicated proteins. (C) Model of MEK feedback on ERBB3 in EGFR/HER2-addicted cancers (top) and KRAS-mutant cancers with low phospho-ERBB3 (bottom).
The feedback observed in EGFR and HER2-driven cancers is distinct from a well-described feedback mechanism in which mTORC1 inhibition leads to increased IRS-1 expression and up-regulation of IGF-IR/IRS signaling (5, 6). In the KRAS-mutant cell lines that we analyzed, which primarily use IGF-1R/IRS to activate PI3K (19), treatment with the mTORC1 inhibitor rapamycin led to feedback activation of AKT signaling that was blocked by co-treatment with the IGF-IR/IR inhibitor, NVP-AEW541 (Figure 4A, B). In contrast, MEK inhibitor-induced activation of ERBB3 in the KRAS-mutant cancers was blocked by gefitinib, but not by NVP-AEW541 (Figure 4B). Accordingly, NVP-AEW541 failed to abrogate AZD6244-induced activation of phospho-AKT in EGFR and HER2-driven cell lines (Supplemental Figure 5). Of note, we have also previously observed cancers in which MEK inhibition leads to inhibition of downstream phospho-S6, resulting in feedback activation of IGF-IR/IRS-1/AKT signaling independent of ERBB3 in both KRAS wild-type and mutant cancers (19), suggesting that cancers not driven by EGFR or HER2 may have alternate, ERBB3-independent, mechanisms of MEK-inhibitor induced feedback activation of AKT. Our data suggest that the effect of MEK inhibition on ERBB3 is a novel feedback mechanism, distinct from mTORC1 feedback on IGF-IR/IRS-1. A model describing these findings is shown in Figure 4C.
MEK inhibition results in increased tyrosine phosphorylation of ERBB3 due to inhibition of ERK-mediated threonine phosphorylation of EGFR and HER2
We investigated the mechanism leading to increased ERBB3 phosphorylation following MEK inhibition. HRG ligand expression was not increased with AZD6244 (Supplemental Figure 6); however, MEK inhibitor-induced feedback activation of AKT required EGFR or HER2 kinase activity (Supplemental Figure 7). Indeed, even in KRAS-mutant SW1463 cells, MEK feedback on ERBB3 was still dependent on EGFR kinase activity (Figure 4B).
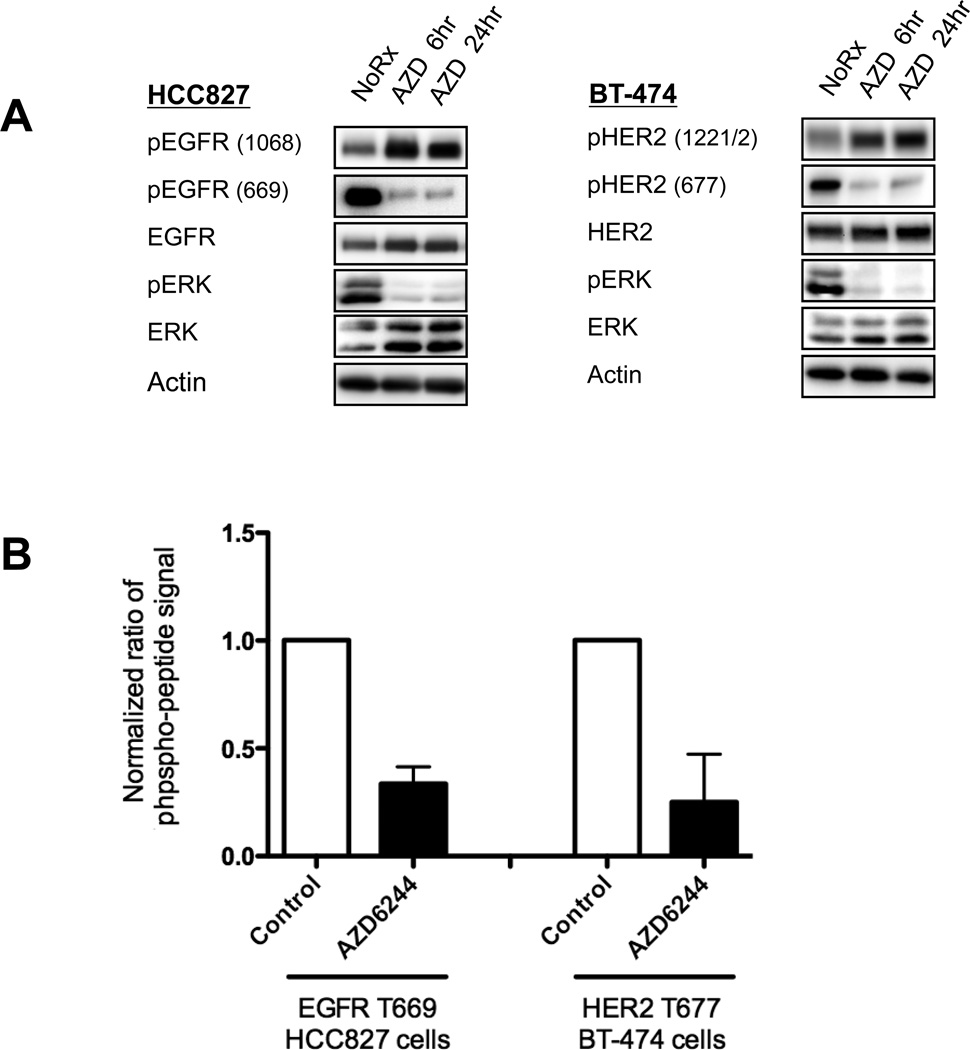
Because EGFR and HER2 inhibition blocked MEK feedback activation of ERBB3/PI3K/AKT, we investigated whether MEK inhibition affected the activation of these receptors. Treatment of HCC827 and BT-474 cells with AZD6244 resulted in increased tyrosine phosphorylation of both EGFR and HER2, indicative of receptor activation (Figure 5A). It has been reported that activation of EGFR involves the formation of an asymmetric dimer (34). Formation of the active RTK dimer is facilitated by stabilizing contacts made between the juxtamembrane (JM) domain of the “receiver/acceptor” kinase and the C-terminal lobe of the “activator/donor” kinase (21, 34, 35). Threonine 669 of EGFR, a putative MAPK target site, is conserved within the JM domains of EGFR, HER2, and ERBB4 (21). When overexpressed in CHO-KI cells, mutation of this threonine site has been shown to augment EGFR tyrosine phosphorylation (20, 21, 36, 37). However, the physiological consequences of EGFR T669 phosphorylation in cancer models and on ERBB3/PI3K/AKT signaling remained unknown.
Figure 5. MEK inhibition blocks phosphorylation of a direct ERK target site in the conserved JM domains of EGFR and HER2.
(A) HCC827 and BT-474 cells were treated with AZD6244 (2µM) for 6 or 24hrs. Cell lysates were immunoblotted to detect the indicated proteins. Phospho-HER2 (677) runs at 185kDa and is recognized by the anti-phospho-EGFR (669) antibody. (B) HCC827 or BT-474 cells were treated with AZD6244 (2µM) for 6hrs. EGFR or HER2 was immunoprecipitated, and coomassie blue bands were excised for quantitative mass spectrometry analysis of peptide phosphorylation. Each data point represents the mean ±SD of three independent experiments.
We hypothesized that the MEK/ERK pathway may suppress trans-phosphorylation of ERBB3 by directly phosphorylating the JM domains of EGFR and HER2, and that this could be a dominant MEK inhibitor-induced feedback leading to AKT activation in these cancers. We used tandem mass spectrometry to measure the effects of AZD6244 on phosphorylation of this JM domain threonine residue in both EGFR-mutant and HER2-amplified cancer models. Targeting both the phosphorylated and non-phosphorylated peptide forms, we detected a 66% average decrease in EGFR T669 phosphorylation and a 75% decrease in HER2 T677 phosphorylation upon treatment with AZD6244 (Figure 5B, Supplemental Figure 8). Phospho-specific antibodies confirmed that treatment with AZD6244 inhibited phosphorylation of T669 of EGFR and the analogous T677 of HER2 (Figure 5A). Together these data indicate that loss of this inhibitory threonine phosphorylation on the JM domains of EGFR and HER2 occurs in cancer cell lines following MEK inhibition.
Mutation of T669 and T677 abrogates MEK inhibitor-induced suppression of ERBB3 activation
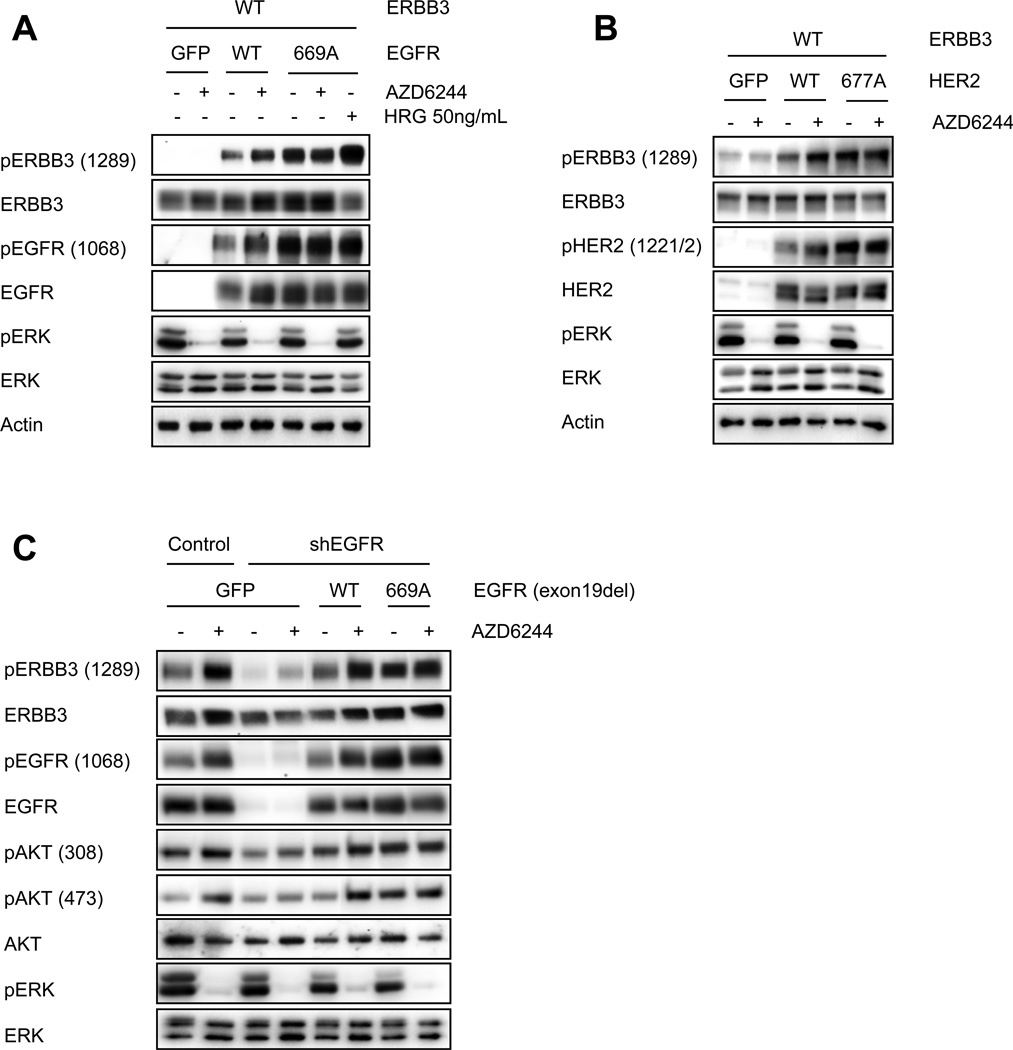
We hypothesized that MEK inhibition activates AKT by inhibiting ERK activity, which blocks an inhibitory threonine phosphorylation on the JM domains of EGFR and HER2, thereby increasing ERBB3 phosphorylation. To test this hypothesis, we transiently transfected CHO-KI cells, which do not express ERBB receptors endogenously, with wild-type ERBB3 with either wild-type EGFR or EGFR T669A. In cells transfected with wild-type EGFR, MEK inhibition led to feedback activation of phospho-ERBB3 and phosho-EGFR, recapitulating the results we had observed in our panel of cancer cell lines (Figure 6A). In contrast, the EGFR T669A mutant increased both basal EGFR and ERBB3 tyrosine phosphorylation that was not augmented by MEK inhibition. As a control, we treated CHO-KI cells expressing EGFR T669A with HRG ligand to induce maximal ERBB3 phosphorylation (Figure 6A), indicating that the lack of induction of phospho-ERBB3 in EGFR T669A expressing cells following MEK inhibition was not simply due to the saturation of the system with phospho-ERBB3. We observed analogous results in CHO-KI cells expressing wild-type ERBB3 in combination with wild-type or T677A mutant HER2 (Figure 6B). Together these results support the hypothesis that inhibition of ERK-mediated phosphorylation of a conserved JM domain threonine residue leads to feedback activation of EGFR, HER2, and ERBB3 (Figure 7).
Figure 6. T669A mutation of EGFR or T677A mutation of HER2 blocks MEK feedback activation of ERBB receptors.
(A, B) CHO-KI cells were transiently transfected to express wild-type ERBB3 in combination with a GFP control, or wild-type or mutant (A) EGFR or (B) HER2. 48hrs post transfection cells were treated with AZD6244 (2µM) for 90 minutes. Cell lysates were immunoblotted to detect indicated proteins. Cells expressing EGFR T669A were also treated with 50ng/mL HRG ligand for 30 minutes to achieve maximal ERBB3 phsophorylation. (C) HCC827 cells were infected with a control or shEGFR hairpin, followed by infection with lentiviral vectors expressing GFP, T669 wild-type EGFR (exon 19del), or EGFR T669A (exon 19del). Following knockdown and puro selection for 72hrs, cells were treated with AZD6244 (2µM) for 6hrs. Cell lysates were immunoblotted to detect the indicated proteins.
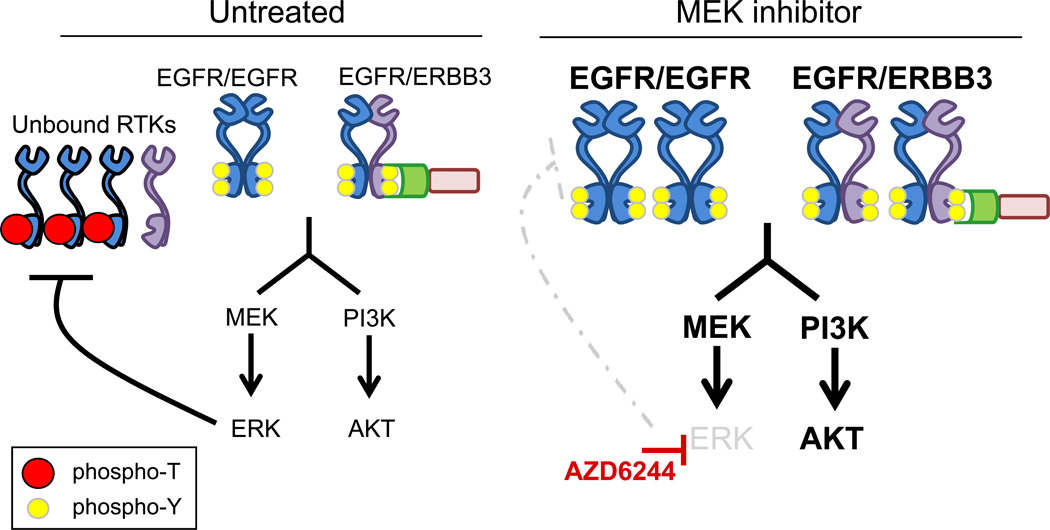
Figure 7. Model of MEK inhibitor-induced feedback on ERBB receptor signaling pathways.
In untreated cells EGFR is phosphorylated at T669 by MEK/ERK, which inhibits activation of EGFR and ERBB3. In the presence of AZD6244, ERK is inhibited and T669 phosphorylation is blocked, increasing EGFR and ERBB3 tyrosine phosphorylation and up-regulating downstream signaling.
To determine if this feedback model explains the activation of PI3K signaling in EGFR-mutant cancers, we used shRNA to knockdown endogenous EGFR (which carries an exon 19 deletion) in the HCC827 NSCLC cell line and replaced with either EGFR (exon 19del) wild-type at T669, or EGFR (exon 19del) carrying a T669A mutation. Of note, this is the same EGFR-mutant cell line in which we observed that EGFR T669 is phosphorylated in MEK-dependent manner (Figure 5, Supplemental Figure 8A). When endogenous EGFR was replaced with EGFR (exon19del) wild-type at T669, MEK inhibition led to significant feedback activation of ERBB3/PI3K/AKT signaling (Figure 6C). However, replacement with the EGFR (exon19 del) T669A mutant led to increased tyrosine phosphorylation of both EGFR and ERBB3, and activation of PI3K/AKT signaling, mimicking the effect of MEK inhibition (Figure 6C). As expected, addition of AZD6244 failed to further augment ERBB3 and AKT phosphorylation in cells expressing the 669A mutant. These results demonstrate that EGFR T669 phosphorylation is necessary for MEK/ERK to suppress EGFR-mediated activation of ERBB3. This supports the hypothesis that a dominant ERK feedback on ERBB3/PI3K/AKT is mediated though phosphorylation of T669 on EGFR (or T677 HER2).
DISCUSSION
RAF and MEK inhibitors are being developed as treatments for cancers with activation of RAF/MEK/ERK signaling. However, with the exception of BRAF-mutant melanomas, the efficacy of these drugs as single agents has been underwhelming to date. Although there are several potential reasons for this lack of efficacy, feedback activation of parallel oncogenic pathways including PI3K/AKT has been invoked (11, 13–15). This idea is analogous to findings that mTORC1 inhibitors are limited by feedback activation of PI3K signaling (4, 6). In this study, we observe that MEK-inhibitor induced activation of PI3K/AKT occurs in multiple ERBB-driven cancer models via loss of an inhibitory threonine phosphorylation in the conserved JM domains of EGFR and HER2. Phosphorylation of this threonine residue has been shown to impair EGFR activation, likely through disruption of receptor dimerization (21).
Our findings suggest that direct ERK-mediated phosphorylation of EGFR T669 and HER2 T677 suppresses activation of ERBB3. These findings agree with those by Li and colleagues who observed that MEK inhibition failed to increase phosphorylation of EGFR T669A homodimers expressed in CHO-KI cells (20). In this study, we extend previous findings by directly showing the effects of EGFR T669A on ERBB3/PI3K/AKT signaling in an EGFR-mutant cancer cell line. Furthermore, we show that although multiple mechanisms for MAPK feedback regulation of AKT signaling have been proposed, T669A mutation of EGFR is sufficient to block MEK inhibitor-induced feedback activation of PI3K/AKT, suggesting that the feedback we describe herein is one of the dominant mechanisms regulating AKT activation in EGFR and HER-driven cancers.
In addition to increased ERBB3 tyrosine phosphorylation, we also observe increased expression of total ERBB3 protein following MEK inhibition. This increase appears to be post-transcriptional as no change in ERBB3 mRNA levels was observed with AZD6244 (Supplemental Figure 3). We were unable to definitively determine the mechanism for increased expression of total ERBB3. However, we observed that increased ERBB3 expression was not solely a result of increased tyrosine phosphorylation of ERBB3 (Figure 4B). Interestingly, inhibition of ERK-mediated phosphorylation of the threonine JM domain sites appeared to be necessary for both increased phospho- and total ERBB3 levels. For example, expression of T669A EGFR in CHO-KI cells and HCC827 cells led to increased basal ERBB3 expression and phosphorylation, which was not further augmented by AZD6244 (Figure 6). This suggests that the increases in both phosho- and total ERBB3 are the result of increased dimer formation between EGFR and ERBB3, which results from loss of inhibitory threonine phosphorylation within the JM domain of EGFR. Although we believe that the data support such a model, it remains possible that phosphorylation of the EGFR JM domain affects tyrosine-phosphorylated and total ERBB3 levels via a mechanism not linked to heterodimer formation.
Overall, this study suggests that combining MEK inhibitors with either ERBB or PI3K inhibitors, may be effective strategies in the clinic. Although there are currently no approved therapies targeting ERBB3, development of anti-ERBB3 antibodies is underway and our data suggests the possible utility of combining these antibodies with MEK inhibitors to block feedback activation of AKT in multiple cancer models. Interestingly, we also observed feedback activation of ERBB3 following MEK inhibition in KRAS-mutant cancers that express low basal levels of phospho-ERBB3 and therefore do not use ERBB3 to activate PI3K. This observation suggests that MEK feedback on ERBB3 occurs in a range of cancers, regardless of dependence on ERBB signaling, and highlights another potential complication for patients treated exclusively with inhibitors of the RAF/MEK/ERK pathway. For example, in KRAS-mutant cancers that initially respond to single agent RAF/MEK inhibitors, chronic inhibition of this pathway may lead to persistent activation of EGFR or HER2. Therefore, these data suggest that activation of ERBB signaling may lead to resistance to single-agent RAF or MEK inhibitors.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Min Yuan and Susanne Breitkopf for help with mass spectrometry, Mike Rothernberg and Cyril Benes for helpful discussions, and Zachary Morris and Andrea McClatchey for assistance with biotin labeling experiments.
GRANT SUPPORT
Financial Support:
This study is supported by the NIH Gastrointestinal Cancer SPORE P50 CA127003 (J.A.E.), K08 grant CA120060-01 (J.A.E.), R01CA137008-01 (J.A.E.), R01CA140594 (J.A.E.), 1U01CA141457-01 (J.A.E.), Lung SPORE P50CA090578 (J.A.E.), the V Foundation (J.A.E.), American Cancer Society RSG-06-102-01-CCE (J.A.E.), the Ellison Foundation Scholar Award (J.A.E.), the American Lung Cancer Association Lung Cancer Discovery Award (J.A.E.), NIH 5P01CA120964-04 (J.M.A.), NIH DF/HCC Cancer Center Support Grant 5P30CA006516-46 (J.M.A.), R01CA80195 (C.L.A.) and Vanderbilt Breast Cancer SPORE P50 CA98131 (C. L.A.).
Footnotes
Conflict of Interest Statement: J.A.E receives research support from AstraZeneca and GalaxoSmithKline and consults for AstraZeneca, GalaxoSmithKline, Novartis and Genentech.
REFERENCES
- 1.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 2.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–134. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Sun S, LM R, X W, Z Z, P Y, H F, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Research. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 5.Carracedo A, Baselga J, Pandolfi PP. Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle. 2008;7:3805–3809. doi: 10.4161/cc.7.24.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Breast Cancer Special Feature: Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev. 2008;18:73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono M, Hirata A, Kometani T, Miyagawa M, Ueda S, Kinoshita H, et al. Sensitivity to gefitinib (Iressa, ZD1839) in non-small cell lung cancer cell lines correlates with dependence on the epidermal growth factor (EGF) receptor/extracellular signal-regulated kinase 1/2 and EGF receptor/Akt pathway for proliferation. Mol Cancer Ther. 2004;3:465–472. [PubMed] [Google Scholar]
- 13.Hoeflich KP, O'Brien C, Boyd Z, Cavet G, Guerrero S, Jung K, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15:4649–4664. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 14.Mirzoeva OK, Das D, Heiser LM, Bhattacharya S, Siwak D, Gendelman R, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–572. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon YK, Kim HP, Han SW, Hur HS, Oh do Y, Im SA, et al. Combination of EGFR and MEK1/2 inhibitor shows synergistic effects by suppressing EGFR/HER3-dependent AKT activation in human gastric cancer cells. Mol Cancer Ther. 2009;8:2526–2536. doi: 10.1158/1535-7163.MCT-09-0300. [DOI] [PubMed] [Google Scholar]
- 16.Lehr S, Kotzka J, Avci H, Sickmann A, Meyer HE, Herkner A, et al. Identification of major ERK-related phosphorylation sites in Gab1. Biochemistry. 2004;43:12133–12140. doi: 10.1021/bi049753e. [DOI] [PubMed] [Google Scholar]
- 17.Roshan B, Kjelsberg C, Spokes K, Eldred A, Crovello CS, Cantley LG. Activated ERK2 interacts with and phosphorylates the docking protein GAB1. J Biol Chem. 1999;274:36362–36368. doi: 10.1074/jbc.274.51.36362. [DOI] [PubMed] [Google Scholar]
- 18.Yu CF, Liu ZX, Cantley LG. ERK negatively regulates the epidermal growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase. J Biol Chem. 2002;277:19382–19388. doi: 10.1074/jbc.M200732200. [DOI] [PubMed] [Google Scholar]
- 19.Ebi H, Corcoran RB, Singh A, Chen Z, Song Y, Lifshits E, et al. Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. The Journal of clinical investigation. 2011;121:4311–4321. doi: 10.1172/JCI57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Huang Y, Jiang J, Frank SJ. ERK-dependent threonine phosphorylation of EGF receptor modulates receptor downregulation and signaling. Cell Signal. 2008;20:2145–2155. doi: 10.1016/j.cellsig.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Red Brewer M, Choi SH, Alvarado D, Moravcevic K, Pozzi A, Lemmon MA, et al. The juxtamembrane region of the EGF receptor functions as an activation domain. Mol Cell. 2009;34:641–651. doi: 10.1016/j.molcel.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 23.Rothenberg SM, Engelman JA, Le S, Riese DJ, 2nd, Haber DA, Settleman J. Modeling oncogene addiction using RNA interference. Proc Natl Acad Sci U S A. 2008;105:12480–12484. doi: 10.1073/pnas.0803217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelman JA, Mukohara T, Zejnullahu K, Lifshits E, Borras AM, Gale CM, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116:2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faber AC, Dufort FJ, Blair D, Wagner D, Roberts MF, Chiles TC. Inhibition of phosphatidylinositol 3-kinase-mediated glucose metabolism coincides with resveratrol-induced cell cycle arrest in human diffuse large B-cell lymphomas. Biochem Pharmacol. 2006;72:1246–1256. doi: 10.1016/j.bcp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng B, Jeong JH, Asara JM, Yuan YY, Granter SR, Chin L, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–247. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453–457. doi: 10.1038/sj.bjc.6603910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carraway KL., 3rd E3 ubiquitin ligases in ErbB receptor quantity control. Semin Cell Dev Biol. 2010;21:936–943. doi: 10.1016/j.semcdb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diamonti AJ, Guy PM, Ivanof C, Wong K, Sweeney C, Carraway KL., 3rd An RBCC protein implicated in maintenance of steady-state neuregulin receptor levels. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2866–2871. doi: 10.1073/pnas.052709799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hubbard SR. The juxtamembrane region of EGFR takes center stage. Cell. 2009;137:1181–1183. doi: 10.1016/j.cell.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, et al. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009;137:1293–1307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Northwood IC, Gonzalez FA, Wartmann M, Raden DL, Davis RJ. Isolation and characterization of two growth factor-stimulated protein kinases that phosphorylate the epidermal growth factor receptor at threonine 669. J Biol Chem. 1991;266:15266–15276. [PubMed] [Google Scholar]
- 37.Takishima K, Griswold-Prenner I, Ingebritsen T, Rosner MR. Epidermal growth factor (EGF) receptor T669 peptide kinase from 3T3-L1 cells is an EGF-stimulated"MAP" kinase. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2520–2524. doi: 10.1073/pnas.88.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.