Abstract
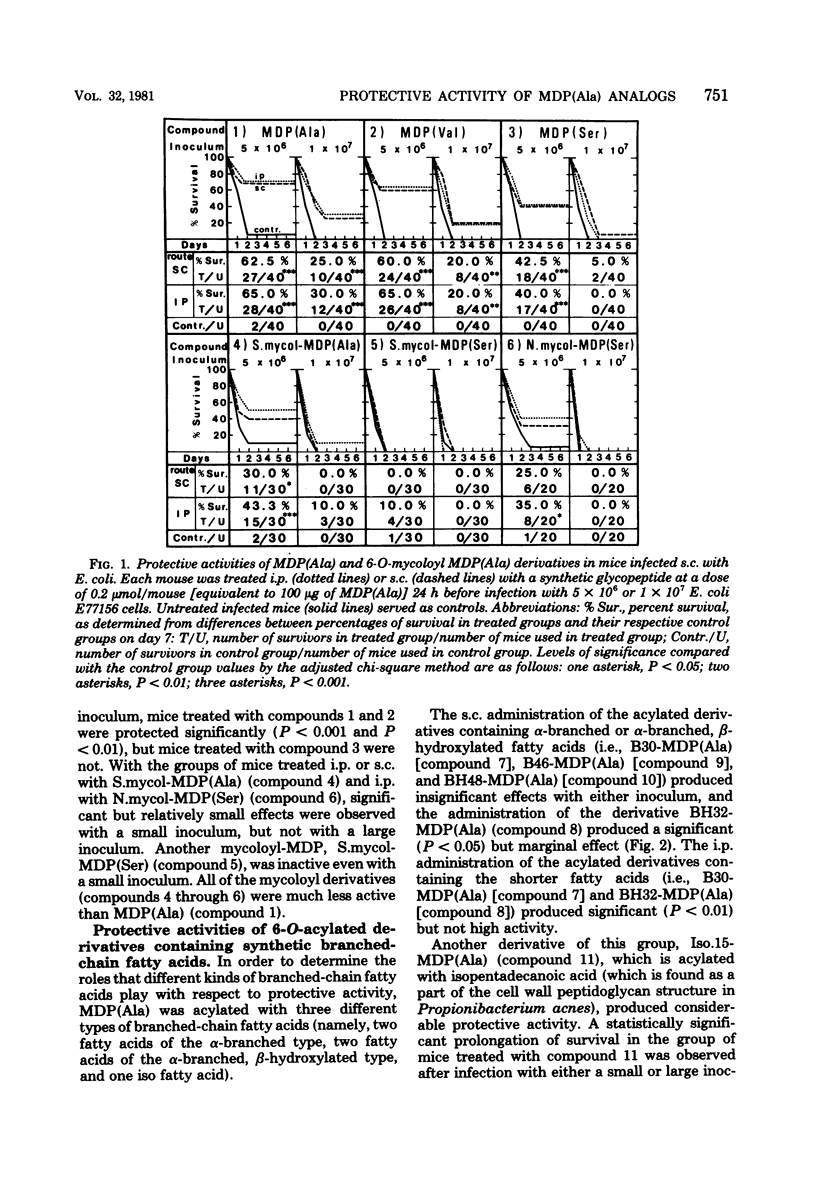
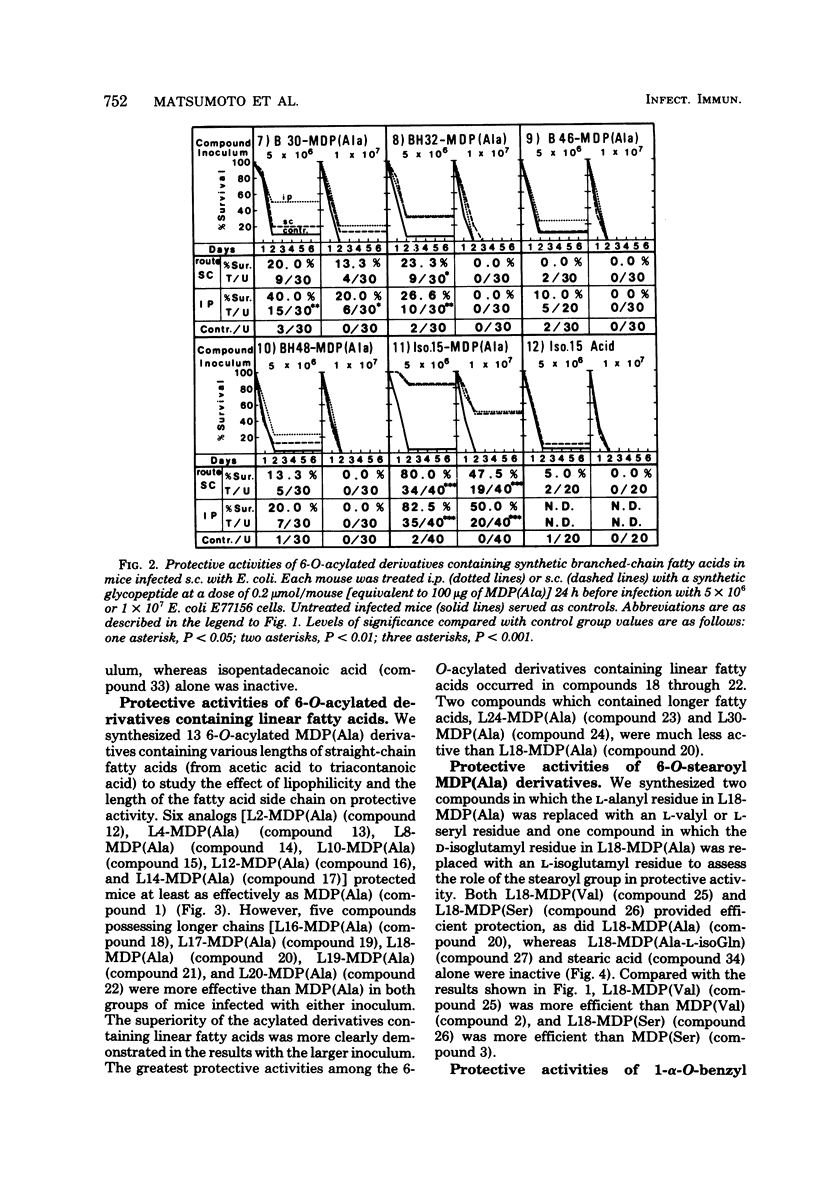
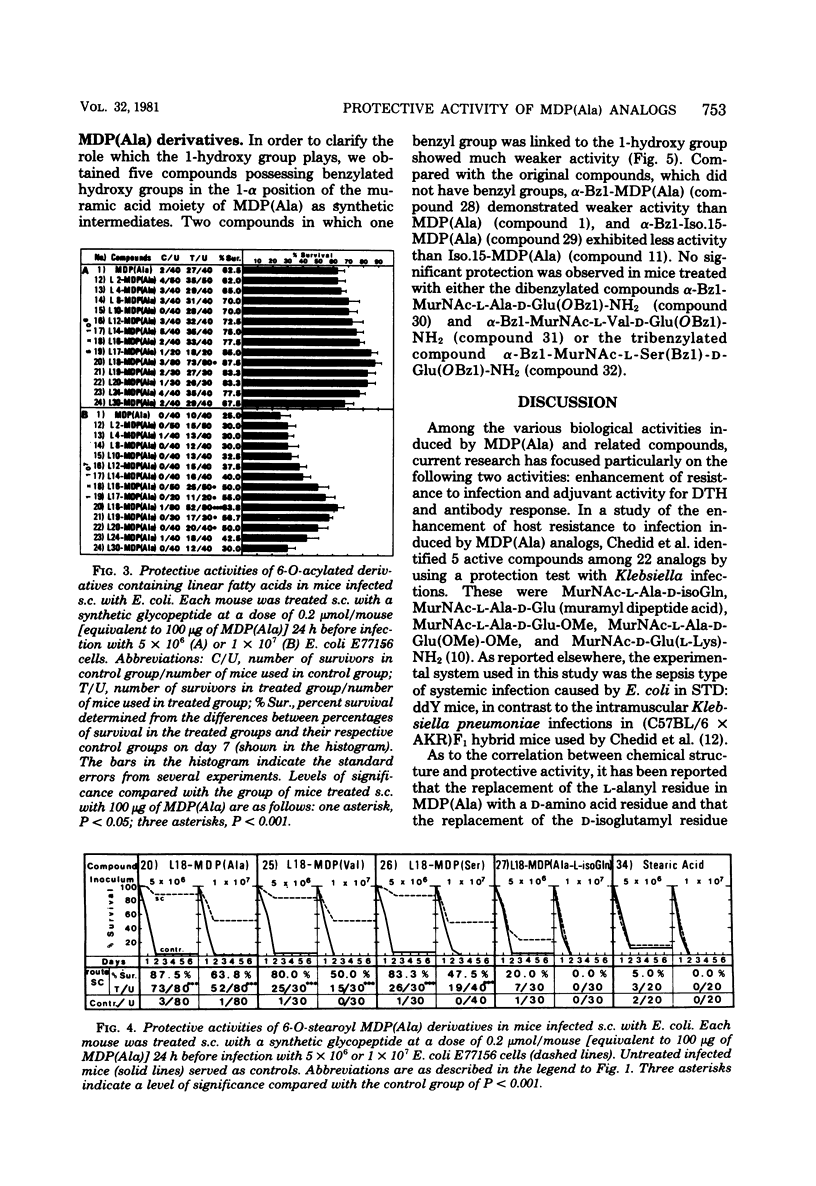
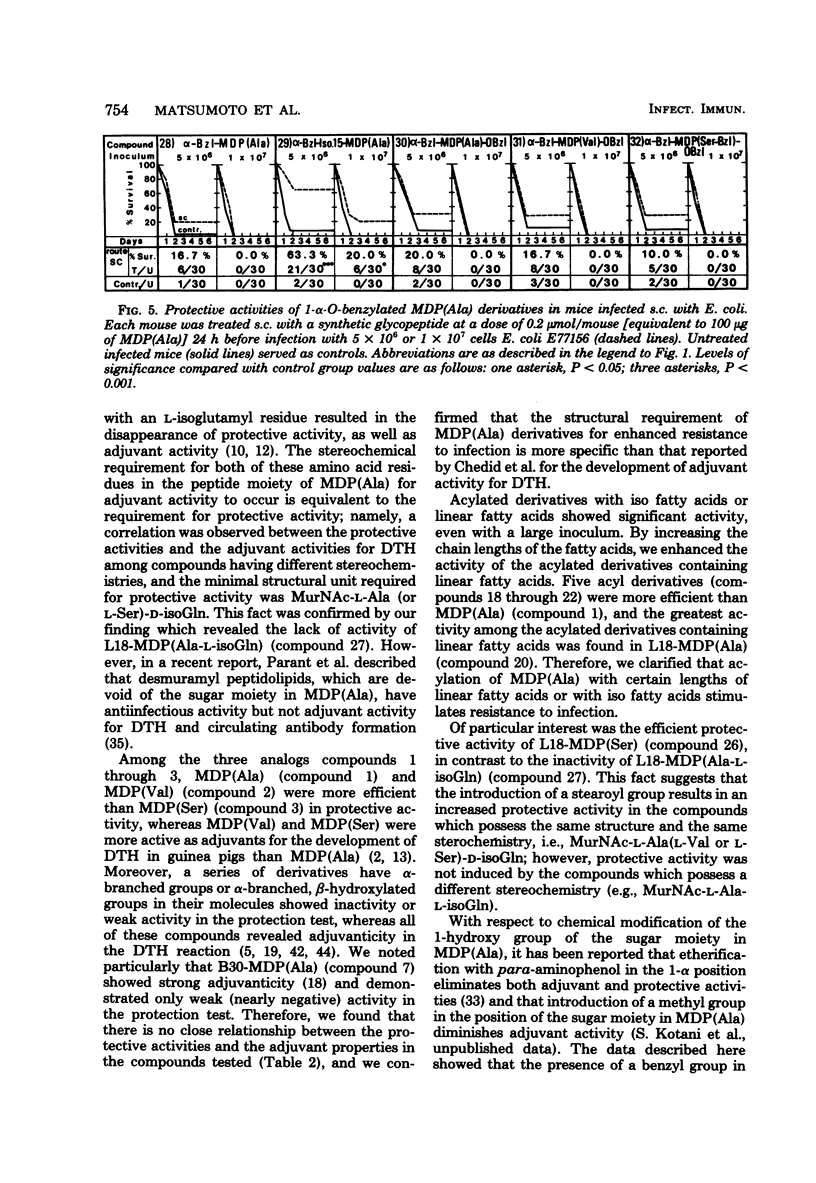
The experimental system utilized in investigating the correlation between the chemical structures of muramyl peptides and their protective activities in the sepsis type of systemic infections caused by Escherichia coli was applied in evaluating the enhancement of resistance to infection induced by 32 synthetic glycopeptide analogs, including 6-O-acyl derivatives and 1-alpha-O-benzyl derivatives of muramyl dipeptide (N-acetyl muramyl-L-alanyl-D-isoglutamine). In assessing the 6-O-acyl derivatives of muramyl dipeptide, we found that the degree of protective activity was attributable to the kinds of fatty acids introduced. Acylation of the 6-hydroxy group on the muramic acid moiety in muramyl dipeptide with natural mycolic acid or a synthetic fatty acid possessing either an alpha-branched or an alpha-branched, beta-hydroxylated group resulted in a decrease in or a disappearance of the protective activity of muramyl dipeptide. Acylation with a normal fatty acid or an iso fatty acid resulted in a retention or enhancement of muramyl dipeptide activity. The activity of acylated derivatives containing linear fatty acids was stimulated by increasing the chain length up to 18 carbon atoms. The highest degree of protective activity occurred with the derivatives acylated with straight-chain fatty acids, particularly with the derivatives acylated with palmitic acid and arachidic acid. Benzylation of the 1-hydroxy group of muramyl dipeptide resulted in a decrease in or a loss of protective activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Devys M., Souvannavong V., Lefrancier P., Choay J., Lederer E. Correlation of structure and adjuvant activity of N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP), its derivatives and analogues. Anti-adjuvant and competition properties of stereoisomers. Biochem Biophys Res Commun. 1976 Sep 7;72(1):339–346. doi: 10.1016/0006-291x(76)90999-2. [DOI] [PubMed] [Google Scholar]
- Azuma I., Kamisango K. I., Saiki I., Tanio Y., Kobayashi S., Yamamura Y. Adjuvant activity of N-acetyl muramyl dipeptides for the induction of delayed-type hypersensitivity to azobenzenearsonate-N-acetyl-L-tyrosine in guinea pigs. Infect Immun. 1980 Sep;29(3):1193–1196. doi: 10.1128/iai.29.3.1193-1196.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma I., Sugimura K., Taniyama T., Yamawaki M., Yamamura Y. Adjuvant activity of mycobacterial fractions: adjuvant activity of synthetic N-acetylmuramyl-dipeptide and the related compounds. Infect Immun. 1976 Jul;14(1):18–27. doi: 10.1128/iai.14.1.18-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma I., Sugimura K., Yamamura Y., Kusumoto S., Tarumi Y. Adjuvant activity of synthetic cell-wall peptidoglycan subunits on monoazobenzenearsonate-N-acetyl-L-tyrosine and bacterial alpha-amylase in guinea pigs. Jpn J Microbiol. 1976 Feb;20(1):63–66. doi: 10.1111/j.1348-0421.1976.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Azuma I., Sugimura K., Yamawaki M., Uemiya M., Kusumoto S., Okada S., Shiba T., Yamamura Y. Adjuvant activity of synthetic 6-O-"mycoloyl"-N-acetylmuramyl-L-alanyl-D-isoglutamine and related compounds. Infect Immun. 1978 Jun;20(3):600–607. doi: 10.1128/iai.20.3.600-607.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Lederer E. Past, present and future of the synthetic immunoadjuvant MDP and its analogs. Biochem Pharmacol. 1978;27(18):2183–2186. doi: 10.1016/0006-2952(78)90074-6. [DOI] [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Lefrancher P., Choay J., Lederer E. Enhancement of nonspecific immunity to Klebsiella pneumoniae infection by a synthetic immunoadjuvant (N-acetylmuramyl-L-alanyl-D-isoglutamine) and several analogs. Proc Natl Acad Sci U S A. 1977 May;74(5):2089–2093. doi: 10.1073/pnas.74.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Elin R. J., Chedid L., Wolff S. M. The pyrogenicity of the synthetic adjuvant muramyl dipeptide and two structural analogues. J Infect Dis. 1978 Dec;138(6):760–767. doi: 10.1093/infdis/138.6.760. [DOI] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Igarashi T., Okada M., Azuma I., Yamamura Y. Adjuvant activity of synthetic N-acetylmuramyl-L-alanyl-D-isoglutamine and related compounds on cell-mediated cytotoxicity in syngeneic mice. Cell Immunol. 1977 Dec;34(2):270–278. doi: 10.1016/0008-8749(77)90249-0. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S., Koga T. Structural requirements for arthritogenicity of peptidoglycans from Staphylococcus aureus and Lactobacillus plant arum and analogous synthetic compounds. J Immunol. 1976 Jun;116(6):1635–1639. [PubMed] [Google Scholar]
- Kotani S., Kinoshita F., Morisaki I., Shimono T., Okunaga T., Takada H., Tsujimoto M., Watanabe Y., Kato K., Shiba T. Immunoadjuvant activities of synthetic 6-O-acyl-N-acetylmuramyl-L-alanyl-D-isoglutamine with special reference to the effect of its administration with liposomes. Biken J. 1977 Dec;20(3-4):95–103. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Kato K., Harada K. Gelation of the amoebocyte lysate of Tachypleus tridentatus by cell wall digest of several gram-positive bacteria and synthetic peptidoglycan subunits of natural and unnatural configurations. Biken J. 1977 Mar;20(1):5–10. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Morisaki I., Kato K. The effect of replacement of L-alanine residue by glycine, L-serine or D-alanine in an N-acetylmuramyl-L-alanyl-D-isoglutamine on immunoadjuvancies of molecules. Biken J. 1977 Jun;20(2):39–45. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975 Jun;18(2):105–111. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Harada K., Shiba T. Correlation between the immunoadjuvant activities and pyrogenicities of synthetic N-acetylmuramyl-peptides or -amino acids. Biken J. 1976 Mar;19(1):9–13. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Kinoshita F., Narita T. Immunoadjuvant activities of peptidoglycan subunits from the cell walls of Staphyloccus aureus and Lactobacillus plantarum. Biken J. 1975 Jun;18(2):93–103. [PubMed] [Google Scholar]
- Merser C., Sinay P., Adam A. Total synthesis and adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1316–1322. doi: 10.1016/0006-291x(75)90503-3. [DOI] [PubMed] [Google Scholar]
- Nagao S., Tanaka A. Muramyl dipeptide-induced adjuvant arthritis. Infect Immun. 1980 May;28(2):624–626. doi: 10.1128/iai.28.2.624-626.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Togawa A., Chedid L., Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cell Immunol. 1980 Mar 1;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Cummings N. P., Shiba T., Kusumoto S., Kotani S. Lipophilic derivative of muramyl dipeptide is more active than muramyl dipeptide in priming macrophages to release superoxide anion. Infect Immun. 1980 Aug;29(2):617–622. doi: 10.1128/iai.29.2.617-622.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant M. A., Audibert F. M., Chedid L. A., Level M. R., Lefrancier P. L., Choay J. P., Lederer E. Immunostimulant activities of a lipophilic muramyl dipeptide derivative and of desmuramyl peptidolipid analogs. Infect Immun. 1980 Mar;27(3):826–831. doi: 10.1128/iai.27.3.826-831.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L. Enhancement of the neonate's nonspecific immunity to Klebsiella infection by muramyl dipeptide, a synthetic immunoadjuvant. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3395–3399. doi: 10.1073/pnas.75.7.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specter S., Friedman H., Chedid L. Dissociation between the adjuvant vs mitogenic activity of a synthetic muramyl dipeptide for murine splenocytes. Proc Soc Exp Biol Med. 1977 Jul;155(3):349–352. doi: 10.3181/00379727-155-39804. [DOI] [PubMed] [Google Scholar]
- Takada H., Kotani S., Kusumoto S., Tarumi Y., Ikenaka K. Mitogenic activity of adjuvant-active N-acetylmuramyl-L-alanyl-D-isoglutamine and its analogues. Biken J. 1977 Jun;20(2):81–85. [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kato K., Kotani S., Kusumoto S., Inage M., Shiba T., Yano I., Kawata S., Yokogawa K. Macrophage activation by bacterial cell walls and related synthetic compounds. Infect Immun. 1979 Jul;25(1):48–53. doi: 10.1128/iai.25.1.48-53.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Nagao R., Kotani S., Shiba T., Kusumoto S. Stimulation of the reticuloendothelial system of mice by muramyl dipeptide. Infect Immun. 1979 May;24(2):302–307. doi: 10.1128/iai.24.2.302-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Saito R., Kotani S., Kusumoto S., Shiba T. Correlation of stereochemically specific structure in muramyl dipeptide between macrophage activation and adjuvant activity. Biochem Biophys Res Commun. 1977 Jul 25;77(2):621–627. doi: 10.1016/s0006-291x(77)80024-7. [DOI] [PubMed] [Google Scholar]
- Uemiya M., Sugimura K., Kusama T., Saiki I., Yamawaki M., Azuma I., Yamamura Y. Adjuvant activity of 6-O-mycoloyl derivatives of N-acetylmuramyl-L-seryl-D-isoglutamine and related compounds in mice and guinea pigs. Infect Immun. 1979 Apr;24(1):83–89. doi: 10.1128/iai.24.1.83-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzien H. U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979 Aug 20;559(2-3):259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Nagao S., Tanaka A., Koga T., Onoue K. Inhibition of macrophage migration by synthetic muramyl dipeptide. Biochem Biophys Res Commun. 1978 Feb 28;80(4):923–928. doi: 10.1016/0006-291x(78)91333-5. [DOI] [PubMed] [Google Scholar]
- Yamamura Y., Azuma I., Sugimura K., Yamawaki M., Uemiya M. Adjuvant activity of 6-O-mycoloyl-N-acetylmuramuyl-L-alanyl-D-isoglutamine. Gan. 1976 Dec;67(6):867–877. [PubMed] [Google Scholar]


