Abstract
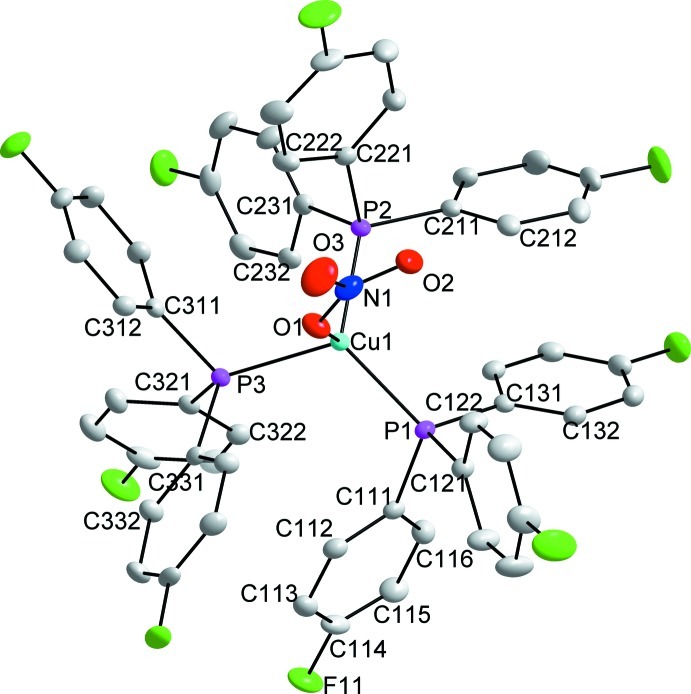
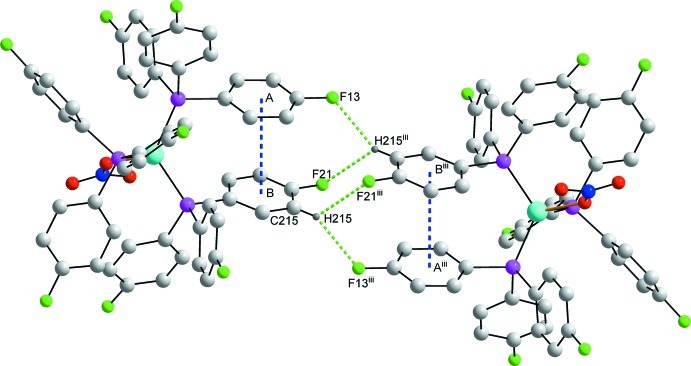
In the title complex, [Cu(NO3)(C18H12F3P)3], the ligating atoms define a distorted tetrahedon with the three tris(4-fluorophenyl)phosphane ligands in the basal positions and the nitrate ligand in the axial position. The intramolecular π–π interaction [centroid–centroid distance = 3.6113 (11) Å] between two of the 4-fluorophenyl groups is complemented by both C—H⋯F and C—H⋯O interactions with distances in the range 2.51–2.60 Å, resulting in a tight head-to-tail packing.
Related literature
For related complexes, see: Hanna et al. (2005 ▶); Steyl (2009 ▶); Saravanabharathi et al. (2002 ▶); Dyason et al. (1986 ▶); Matthew et al. (1971 ▶). 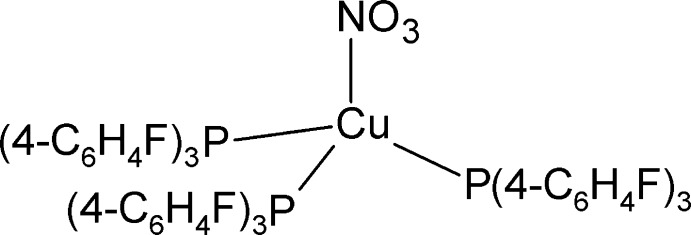
Experimental
Crystal data
[Cu(NO3)(C18H12F3P)3]
M r = 1074.29
Triclinic,
a = 9.3861 (3) Å
b = 12.2552 (4) Å
c = 21.4820 (7) Å
α = 85.274 (2)°
β = 86.843 (1)°
γ = 74.954 (1)°
V = 2376.76 (13) Å3
Z = 2
Mo Kα radiation
μ = 0.64 mm−1
T = 100 K
0.38 × 0.11 × 0.08 mm
Data collection
Bruker X8 APEXII 4K KappaCCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2004 ▶) T min = 0.792, T max = 0.950
28458 measured reflections
11760 independent reflections
9439 reflections with I > 2σ(I)
R int = 0.030
Refinement
R[F 2 > 2σ(F 2)] = 0.035
wR(F 2) = 0.085
S = 1.05
11760 reflections
640 parameters
H-atom parameters constrained
Δρmax = 0.40 e Å−3
Δρmin = −0.41 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT-Plus (Bruker, 2004 ▶); data reduction: SAINT-Plus and XPREP (Bruker, 2004 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg & Putz, 2005 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812043346/mw2090sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812043346/mw2090Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| O1—Cu1 | 2.1182 (12) |
| P1—Cu1 | 2.2901 (5) |
| P2—Cu1 | 2.2840 (5) |
| P3—Cu1 | 2.3256 (5) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C122—H122⋯O2 | 0.95 | 2.30 | 3.224 (2) | 163 |
| C336—H336⋯O1 | 0.95 | 2.17 | 3.037 (2) | 151 |
| C126—H126⋯F22i | 0.95 | 2.40 | 3.281 (2) | 154 |
| C136—H136⋯O3ii | 0.95 | 2.53 | 3.223 (2) | 130 |
| C215—H215⋯F13iii | 0.95 | 2.51 | 3.301 (2) | 141 |
| C315—H315⋯F33iv | 0.95 | 2.50 | 3.403 (2) | 159 |
| C332—H332⋯F32v | 0.95 | 2.48 | 3.131 (2) | 125 |
| C326—H326⋯F33vi | 0.95 | 2.36 | 3.150 (2) | 141 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii) 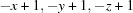 ; (iv)
; (iv)  ; (v)
; (v) 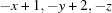 ; (vi)
; (vi) 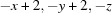 .
.
Acknowledgments
Financial assistance from the University of the Free State is gratefully acknowledged. We also express our gratitude towards SASOL and the South African National Research Foundation (SA-NRF/THRIP) for financial support of this project. Part of this material is based on work supported by the SA-NRF/THRIP under grant No. GUN 2068915. Opinions, findings, conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the SA-NRF.
supplementary crystallographic information
Comment
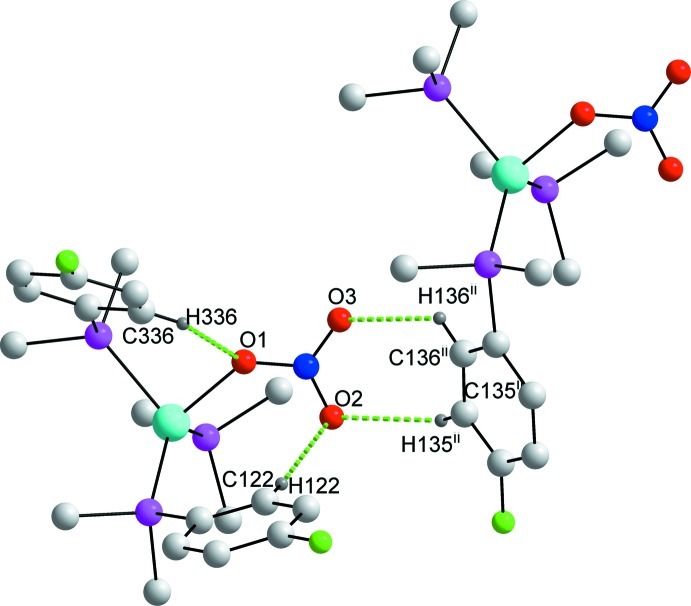
The title compound (I) has a copper(I) metal center co-ordinated by three tris-4-fluorophenylphosphane ligands ((p-FPh)3P) and a nitrato ligand. The ligating atoms define a distorted trigonal pyramid which is similar to what was found for [Cu(PPh3)3(X)] X = ClO4-, BF4-,NO3-, HCO2- (Hanna et al., 2005) complexes, where the average P—Cu—P angles are in the range 112.29 (4)° - 121.37 (6)°. While markedly different, the P—Cu—P bond angles for for I (Table 1) fall within this range with an average of 116 (2)°. The dissimilarity observed for the O—Cu—P bond angles (Table 1) are as a result of C—H···O (Figure 4) and O2···π (centroid C221-C226) interactions contributing to the non-linearity of the N1—O1—Cu1 angle and the deviation (14.39 (5)°) of the nitrato ligand from the axial position. The average Cu—P bond lengths for [Cu(PPh3)3(NO3)].EtOH (Dyason et al., 1986), [Cu(PPh3)3(NO3)].MeOH (Steyl, 2009) and I were observed to be 2.329 (9) Å, 2.326 (10) Å and 2.300 (13) Å respectively.
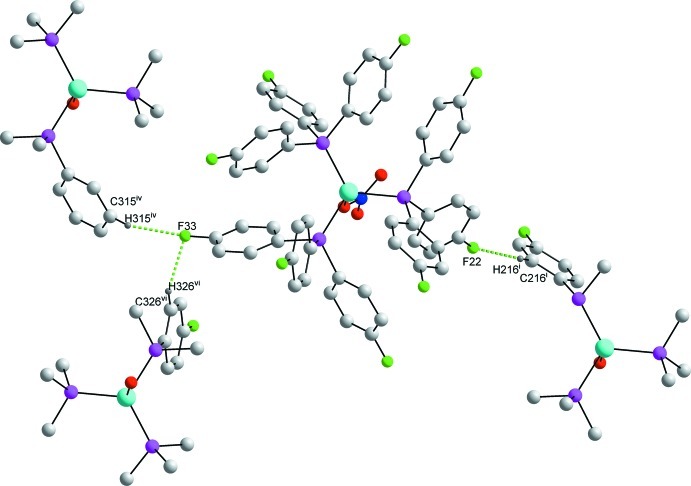
An intermolecular π–π interaction is observed for A (centroid C131—C136)···B (centroid C211—C216) with a distance of 3.6113 (11) Å and is stabilized by the bifurcated hydrogen fluorine interaction C215—H215···F21iii and C215—H215···F13iii with H···F distances of 2.61 Å and 2.51 Å respectively (see Figure 2). An additional stabilizing effect arises from the C135ii—H135ii···O2 and C136ii—H136ii···O3 interactions (Figure 4) with H···O distances of 2.58 Å and 2.53 Å. Additional C—H···F interactions are illustrated in Figure 3. All of these interactions contribute to tight packing of I.
Experimental
tris-4-fluorophenylphosphane (2 mmol) was added to a solution of CuNO3 (1 mmol) in warm MeOH (15 ml, 70 °C) and the resulting solution was stirred for c.a. 1 h. The solution was filtered and allowed to cool slowly. Crystals suitable for single-crystal X-ray diffraction were obtained from the slow evaporation of the solution.
Refinement
All H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C—H = 0.95 Å and Uiso(H) = 1.2 Ueq(C) for aromatic H atoms.
Figures
Fig. 1.
View of I (50% probability displacement ellipsoids). Phenyl rings are numbered Cxyz where x represents the phosphane to which the ring is attached, y represents the ring number and z the atom number in the ring. Only the first phenyl ring is completely numbered for illustrative purpose Hydrogen atoms have been omitted for clarity.
Fig. 2.
Intermolecular C—H···F and intramolecular π–π interactions (dashed bonds) for I. Symmetry code (iii) -x + 1, -y + 1, -z + 1. Non-relavent hydrogen atoms and phenyl rings have been omitted for clarity.
Fig. 3.
Intermolecular C—H···F (dashed bonds) for I. Symmetry codes (i) x - 1, y + 1, z, (iv) x, y - 1, z and (vi) -x + 2, -y + 2, -z. Non-relevant hydrogen atoms and phenyl rings have been omitted for clarity.
Fig. 4.
Inter- and intramolecular C—H···O interactions (dashed bonds) for I. Symmetry code (iii) x - 1, y, z. Non-relevant hydrogen atoms and phenyl rings have been omitted for clarity.
Crystal data
| [Cu(NO3)(C18H12F3P)3] | Z = 2 |
| Mr = 1074.29 | F(000) = 1092 |
| Triclinic, P1 | Dx = 1.501 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.3861 (3) Å | Cell parameters from 9076 reflections |
| b = 12.2552 (4) Å | θ = 2.5–28.2° |
| c = 21.4820 (7) Å | µ = 0.64 mm−1 |
| α = 85.274 (2)° | T = 100 K |
| β = 86.843 (1)° | Column, colourless |
| γ = 74.954 (1)° | 0.38 × 0.11 × 0.08 mm |
| V = 2376.76 (13) Å3 |
Data collection
| Bruker X8 APEXII 4K KappaCCD diffractometer | 11760 independent reflections |
| Radiation source: sealed tube | 9439 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.030 |
| Detector resolution: 512 pixels mm-1 | θmax = 28.5°, θmin = 2.4° |
| φ and ω scans | h = −9→12 |
| Absorption correction: multi-scan (SADABS; Bruker, 2004) | k = −16→16 |
| Tmin = 0.792, Tmax = 0.950 | l = −28→27 |
| 28458 measured reflections |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.035 | w = 1/[σ2(Fo2) + (0.0333P)2 + 0.8637P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.085 | (Δ/σ)max = 0.001 |
| S = 1.05 | Δρmax = 0.40 e Å−3 |
| 11760 reflections | Δρmin = −0.41 e Å−3 |
| 640 parameters |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C111 | 0.64094 (18) | 1.01573 (14) | 0.27195 (8) | 0.0156 (3) | |
| C112 | 0.68685 (19) | 1.07502 (15) | 0.21964 (9) | 0.0188 (4) | |
| H112 | 0.7894 | 1.0632 | 0.2097 | 0.023* | |
| C113 | 0.5860 (2) | 1.15064 (16) | 0.18194 (9) | 0.0214 (4) | |
| H113 | 0.6179 | 1.1905 | 0.1463 | 0.026* | |
| C114 | 0.4384 (2) | 1.16645 (16) | 0.19755 (9) | 0.0224 (4) | |
| C115 | 0.3879 (2) | 1.11255 (17) | 0.24917 (9) | 0.0236 (4) | |
| H115 | 0.2851 | 1.1269 | 0.2592 | 0.028* | |
| C116 | 0.49013 (19) | 1.03644 (16) | 0.28659 (9) | 0.0200 (4) | |
| H116 | 0.4569 | 0.9981 | 0.3225 | 0.024* | |
| C121 | 0.88511 (18) | 0.99336 (15) | 0.35054 (8) | 0.0164 (4) | |
| C122 | 1.0241 (2) | 0.93895 (17) | 0.37273 (9) | 0.0235 (4) | |
| H122 | 1.0636 | 0.8604 | 0.3679 | 0.028* | |
| C123 | 1.1054 (2) | 0.99874 (18) | 0.40190 (10) | 0.0302 (5) | |
| H123 | 1.2002 | 0.9621 | 0.4172 | 0.036* | |
| C124 | 1.0452 (2) | 1.11196 (18) | 0.40800 (10) | 0.0300 (5) | |
| C125 | 0.9084 (2) | 1.16862 (18) | 0.38719 (11) | 0.0338 (5) | |
| H125 | 0.8696 | 1.2471 | 0.3925 | 0.041* | |
| C126 | 0.8282 (2) | 1.10793 (16) | 0.35806 (10) | 0.0261 (4) | |
| H126 | 0.7333 | 1.1454 | 0.3431 | 0.031* | |
| C131 | 0.67292 (18) | 0.86418 (15) | 0.38106 (8) | 0.0155 (3) | |
| C132 | 0.67507 (19) | 0.89769 (15) | 0.44123 (8) | 0.0183 (4) | |
| H132 | 0.7372 | 0.9443 | 0.4497 | 0.022* | |
| C133 | 0.5868 (2) | 0.86338 (16) | 0.48895 (9) | 0.0217 (4) | |
| H133 | 0.5892 | 0.885 | 0.5303 | 0.026* | |
| C134 | 0.4963 (2) | 0.79770 (16) | 0.47510 (9) | 0.0214 (4) | |
| C135 | 0.49013 (19) | 0.76214 (15) | 0.41629 (9) | 0.0202 (4) | |
| H135 | 0.4258 | 0.717 | 0.4082 | 0.024* | |
| C136 | 0.58129 (19) | 0.79473 (15) | 0.36951 (9) | 0.0177 (4) | |
| H136 | 0.5817 | 0.7695 | 0.3288 | 0.021* | |
| C211 | 0.84201 (19) | 0.55517 (15) | 0.36385 (8) | 0.0169 (4) | |
| C212 | 0.88652 (19) | 0.60020 (16) | 0.41492 (9) | 0.0203 (4) | |
| H212 | 0.956 | 0.6446 | 0.4086 | 0.024* | |
| C213 | 0.8305 (2) | 0.58076 (18) | 0.47462 (9) | 0.0259 (4) | |
| H213 | 0.861 | 0.6106 | 0.5095 | 0.031* | |
| C214 | 0.7292 (2) | 0.51683 (18) | 0.48172 (9) | 0.0286 (5) | |
| C215 | 0.6808 (2) | 0.47230 (17) | 0.43309 (10) | 0.0296 (5) | |
| H215 | 0.6102 | 0.429 | 0.4399 | 0.036* | |
| C216 | 0.7382 (2) | 0.49225 (16) | 0.37315 (9) | 0.0225 (4) | |
| H216 | 0.706 | 0.4626 | 0.3385 | 0.027* | |
| C221 | 1.10916 (18) | 0.48680 (15) | 0.29250 (8) | 0.0161 (4) | |
| C222 | 1.2149 (2) | 0.49938 (16) | 0.24626 (9) | 0.0225 (4) | |
| H222 | 1.1869 | 0.5508 | 0.2108 | 0.027* | |
| C223 | 1.3602 (2) | 0.43753 (17) | 0.25149 (11) | 0.0288 (5) | |
| H223 | 1.4323 | 0.4455 | 0.2198 | 0.035* | |
| C224 | 1.3979 (2) | 0.36487 (17) | 0.30303 (10) | 0.0269 (4) | |
| C225 | 1.2974 (2) | 0.34649 (18) | 0.34868 (10) | 0.0297 (5) | |
| H225 | 1.3265 | 0.2931 | 0.3832 | 0.036* | |
| C226 | 1.1516 (2) | 0.40819 (17) | 0.34301 (9) | 0.0249 (4) | |
| H226 | 1.0797 | 0.3967 | 0.374 | 0.03* | |
| C231 | 0.83035 (18) | 0.52013 (15) | 0.23431 (8) | 0.0155 (3) | |
| C232 | 0.70057 (19) | 0.58837 (16) | 0.20949 (8) | 0.0182 (4) | |
| H232 | 0.6622 | 0.6624 | 0.2232 | 0.022* | |
| C233 | 0.6262 (2) | 0.55066 (17) | 0.16533 (9) | 0.0236 (4) | |
| H233 | 0.5381 | 0.5978 | 0.1482 | 0.028* | |
| C234 | 0.6842 (2) | 0.44263 (18) | 0.14708 (9) | 0.0238 (4) | |
| C235 | 0.8096 (2) | 0.37125 (17) | 0.17122 (10) | 0.0263 (4) | |
| H235 | 0.8451 | 0.2964 | 0.1582 | 0.032* | |
| C236 | 0.8839 (2) | 0.41083 (16) | 0.21519 (9) | 0.0224 (4) | |
| H236 | 0.9716 | 0.363 | 0.2322 | 0.027* | |
| C311 | 1.04880 (18) | 0.69465 (14) | 0.10744 (8) | 0.0140 (3) | |
| C312 | 1.18958 (18) | 0.70551 (15) | 0.08908 (8) | 0.0168 (4) | |
| H312 | 1.2172 | 0.7721 | 0.0972 | 0.02* | |
| C313 | 1.2899 (2) | 0.62024 (16) | 0.05907 (9) | 0.0214 (4) | |
| H313 | 1.3852 | 0.6283 | 0.0459 | 0.026* | |
| C314 | 1.2481 (2) | 0.52395 (16) | 0.04881 (9) | 0.0212 (4) | |
| C315 | 1.1117 (2) | 0.50835 (16) | 0.06709 (9) | 0.0224 (4) | |
| H315 | 1.0865 | 0.4404 | 0.0598 | 0.027* | |
| C316 | 1.0117 (2) | 0.59482 (15) | 0.09661 (9) | 0.0192 (4) | |
| H316 | 0.9168 | 0.5858 | 0.1096 | 0.023* | |
| C321 | 0.74569 (18) | 0.81865 (14) | 0.10980 (8) | 0.0144 (3) | |
| C322 | 0.61174 (19) | 0.85818 (15) | 0.14206 (9) | 0.0177 (4) | |
| H322 | 0.6108 | 0.8702 | 0.1852 | 0.021* | |
| C323 | 0.4793 (2) | 0.88015 (16) | 0.11152 (10) | 0.0244 (4) | |
| H323 | 0.3876 | 0.9072 | 0.1332 | 0.029* | |
| C324 | 0.4848 (2) | 0.86173 (16) | 0.04930 (10) | 0.0241 (4) | |
| C325 | 0.6133 (2) | 0.82046 (17) | 0.01569 (9) | 0.0248 (4) | |
| H325 | 0.6123 | 0.8071 | −0.0272 | 0.03* | |
| C326 | 0.7452 (2) | 0.79875 (16) | 0.04667 (8) | 0.0196 (4) | |
| H326 | 0.8361 | 0.7701 | 0.0246 | 0.024* | |
| C331 | 0.96604 (18) | 0.93534 (14) | 0.12332 (8) | 0.0137 (3) | |
| C332 | 0.8948 (2) | 1.00834 (15) | 0.07499 (9) | 0.0200 (4) | |
| H332 | 0.8237 | 0.9868 | 0.0522 | 0.024* | |
| C333 | 0.9262 (2) | 1.11221 (16) | 0.05949 (9) | 0.0225 (4) | |
| H333 | 0.8762 | 1.1625 | 0.027 | 0.027* | |
| C334 | 1.0311 (2) | 1.13997 (15) | 0.09233 (9) | 0.0190 (4) | |
| C335 | 1.1049 (2) | 1.07060 (16) | 0.14004 (9) | 0.0210 (4) | |
| H335 | 1.1774 | 1.0924 | 0.1618 | 0.025* | |
| C336 | 1.07114 (19) | 0.96782 (15) | 0.15582 (9) | 0.0182 (4) | |
| H336 | 1.1201 | 0.9191 | 0.1891 | 0.022* | |
| N1 | 1.24915 (16) | 0.72517 (14) | 0.29043 (8) | 0.0232 (4) | |
| O1 | 1.14700 (13) | 0.76757 (11) | 0.25203 (6) | 0.0228 (3) | |
| O2 | 1.21663 (16) | 0.68829 (12) | 0.34380 (7) | 0.0288 (3) | |
| O3 | 1.37844 (15) | 0.71966 (15) | 0.27340 (8) | 0.0413 (4) | |
| F11 | 0.33854 (12) | 1.23879 (10) | 0.16002 (6) | 0.0319 (3) | |
| F12 | 1.12332 (15) | 1.17149 (11) | 0.43652 (7) | 0.0458 (4) | |
| F13 | 0.40845 (13) | 0.76620 (10) | 0.52191 (6) | 0.0330 (3) | |
| F21 | 0.67367 (15) | 0.49784 (12) | 0.54008 (6) | 0.0447 (4) | |
| F22 | 1.54253 (12) | 0.30798 (11) | 0.30944 (7) | 0.0418 (3) | |
| F23 | 0.61304 (14) | 0.40369 (11) | 0.10379 (6) | 0.0355 (3) | |
| F31 | 1.34503 (13) | 0.44073 (10) | 0.01912 (6) | 0.0320 (3) | |
| F32 | 0.35528 (12) | 0.88451 (11) | 0.01908 (6) | 0.0364 (3) | |
| F33 | 1.06177 (12) | 1.24204 (9) | 0.07802 (5) | 0.0261 (3) | |
| P1 | 0.78177 (5) | 0.90846 (4) | 0.31532 (2) | 0.01394 (9) | |
| P2 | 0.92541 (5) | 0.58263 (4) | 0.28780 (2) | 0.01375 (9) | |
| P3 | 0.91661 (5) | 0.80309 (4) | 0.15007 (2) | 0.01231 (9) | |
| Cu1 | 0.92130 (2) | 0.766649 (17) | 0.258012 (10) | 0.01294 (6) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C111 | 0.0158 (8) | 0.0129 (9) | 0.0179 (9) | −0.0022 (7) | −0.0035 (7) | −0.0036 (7) |
| C112 | 0.0167 (8) | 0.0160 (9) | 0.0234 (10) | −0.0028 (7) | −0.0020 (7) | −0.0035 (7) |
| C113 | 0.0265 (10) | 0.0166 (9) | 0.0217 (10) | −0.0066 (8) | −0.0040 (8) | 0.0000 (7) |
| C114 | 0.0231 (9) | 0.0154 (9) | 0.0281 (11) | −0.0017 (8) | −0.0144 (8) | 0.0006 (8) |
| C115 | 0.0145 (8) | 0.0253 (11) | 0.0304 (11) | −0.0027 (8) | −0.0071 (8) | −0.0021 (8) |
| C116 | 0.0172 (8) | 0.0226 (10) | 0.0202 (10) | −0.0046 (8) | −0.0029 (7) | −0.0012 (7) |
| C121 | 0.0139 (8) | 0.0164 (9) | 0.0190 (9) | −0.0034 (7) | −0.0020 (7) | −0.0020 (7) |
| C122 | 0.0201 (9) | 0.0185 (10) | 0.0297 (11) | 0.0007 (8) | −0.0072 (8) | −0.0029 (8) |
| C123 | 0.0207 (9) | 0.0300 (12) | 0.0405 (13) | −0.0047 (9) | −0.0131 (9) | −0.0029 (9) |
| C124 | 0.0306 (11) | 0.0278 (11) | 0.0375 (12) | −0.0154 (9) | −0.0142 (9) | −0.0013 (9) |
| C125 | 0.0368 (12) | 0.0165 (10) | 0.0497 (14) | −0.0053 (9) | −0.0207 (10) | −0.0040 (9) |
| C126 | 0.0228 (9) | 0.0181 (10) | 0.0370 (12) | −0.0013 (8) | −0.0143 (9) | −0.0036 (8) |
| C131 | 0.0134 (8) | 0.0139 (9) | 0.0173 (9) | −0.0002 (7) | −0.0016 (7) | 0.0001 (7) |
| C132 | 0.0196 (9) | 0.0149 (9) | 0.0199 (9) | −0.0026 (7) | −0.0021 (7) | −0.0027 (7) |
| C133 | 0.0258 (10) | 0.0186 (10) | 0.0177 (10) | 0.0002 (8) | −0.0014 (7) | −0.0020 (7) |
| C134 | 0.0200 (9) | 0.0182 (10) | 0.0231 (10) | −0.0021 (8) | 0.0053 (7) | 0.0026 (7) |
| C135 | 0.0162 (8) | 0.0158 (9) | 0.0276 (10) | −0.0029 (7) | −0.0026 (7) | 0.0012 (7) |
| C136 | 0.0167 (8) | 0.0150 (9) | 0.0204 (9) | −0.0012 (7) | −0.0023 (7) | −0.0025 (7) |
| C211 | 0.0161 (8) | 0.0134 (9) | 0.0179 (9) | 0.0010 (7) | 0.0020 (7) | 0.0012 (7) |
| C212 | 0.0165 (8) | 0.0199 (10) | 0.0211 (10) | 0.0007 (7) | −0.0007 (7) | 0.0019 (7) |
| C213 | 0.0228 (9) | 0.0292 (11) | 0.0185 (10) | 0.0056 (8) | −0.0010 (8) | 0.0001 (8) |
| C214 | 0.0302 (11) | 0.0268 (11) | 0.0197 (10) | 0.0042 (9) | 0.0103 (8) | 0.0067 (8) |
| C215 | 0.0323 (11) | 0.0214 (11) | 0.0338 (12) | −0.0083 (9) | 0.0142 (9) | 0.0013 (9) |
| C216 | 0.0258 (10) | 0.0150 (9) | 0.0253 (10) | −0.0041 (8) | 0.0049 (8) | −0.0005 (8) |
| C221 | 0.0153 (8) | 0.0129 (9) | 0.0197 (9) | −0.0024 (7) | −0.0007 (7) | −0.0022 (7) |
| C222 | 0.0188 (9) | 0.0181 (10) | 0.0278 (11) | −0.0018 (8) | 0.0018 (8) | 0.0034 (8) |
| C223 | 0.0175 (9) | 0.0226 (11) | 0.0441 (13) | −0.0038 (8) | 0.0080 (9) | −0.0001 (9) |
| C224 | 0.0145 (9) | 0.0202 (10) | 0.0429 (13) | 0.0040 (8) | −0.0076 (8) | −0.0083 (9) |
| C225 | 0.0301 (11) | 0.0255 (11) | 0.0249 (11) | 0.0083 (9) | −0.0057 (9) | 0.0002 (8) |
| C226 | 0.0248 (10) | 0.0229 (11) | 0.0212 (10) | 0.0030 (8) | 0.0021 (8) | 0.0011 (8) |
| C231 | 0.0146 (8) | 0.0160 (9) | 0.0162 (9) | −0.0052 (7) | 0.0026 (7) | −0.0002 (7) |
| C232 | 0.0187 (8) | 0.0163 (9) | 0.0204 (9) | −0.0068 (7) | 0.0009 (7) | 0.0014 (7) |
| C233 | 0.0231 (9) | 0.0255 (11) | 0.0241 (10) | −0.0110 (8) | −0.0043 (8) | 0.0044 (8) |
| C234 | 0.0273 (10) | 0.0337 (12) | 0.0169 (10) | −0.0189 (9) | −0.0003 (8) | −0.0031 (8) |
| C235 | 0.0235 (9) | 0.0238 (11) | 0.0344 (12) | −0.0089 (8) | 0.0054 (8) | −0.0143 (9) |
| C236 | 0.0167 (9) | 0.0191 (10) | 0.0305 (11) | −0.0021 (7) | 0.0020 (8) | −0.0073 (8) |
| C311 | 0.0144 (8) | 0.0148 (9) | 0.0121 (8) | −0.0030 (7) | −0.0020 (6) | 0.0013 (6) |
| C312 | 0.0154 (8) | 0.0153 (9) | 0.0196 (9) | −0.0040 (7) | −0.0021 (7) | 0.0003 (7) |
| C313 | 0.0146 (8) | 0.0218 (10) | 0.0266 (10) | −0.0028 (7) | 0.0005 (7) | −0.0012 (8) |
| C314 | 0.0225 (9) | 0.0176 (10) | 0.0202 (10) | 0.0013 (8) | 0.0009 (7) | −0.0039 (7) |
| C315 | 0.0290 (10) | 0.0154 (9) | 0.0242 (10) | −0.0087 (8) | 0.0022 (8) | −0.0028 (7) |
| C316 | 0.0199 (9) | 0.0174 (9) | 0.0214 (10) | −0.0077 (7) | 0.0023 (7) | −0.0005 (7) |
| C321 | 0.0147 (8) | 0.0135 (8) | 0.0164 (9) | −0.0067 (7) | −0.0028 (6) | 0.0019 (7) |
| C322 | 0.0167 (8) | 0.0159 (9) | 0.0213 (10) | −0.0052 (7) | −0.0024 (7) | −0.0015 (7) |
| C323 | 0.0131 (8) | 0.0223 (10) | 0.0379 (12) | −0.0049 (8) | −0.0032 (8) | −0.0008 (8) |
| C324 | 0.0190 (9) | 0.0205 (10) | 0.0358 (12) | −0.0108 (8) | −0.0165 (8) | 0.0094 (8) |
| C325 | 0.0312 (10) | 0.0270 (11) | 0.0207 (10) | −0.0155 (9) | −0.0107 (8) | 0.0048 (8) |
| C326 | 0.0200 (9) | 0.0232 (10) | 0.0177 (9) | −0.0098 (8) | −0.0030 (7) | 0.0020 (7) |
| C331 | 0.0133 (8) | 0.0128 (8) | 0.0152 (9) | −0.0039 (7) | 0.0030 (6) | −0.0024 (6) |
| C332 | 0.0233 (9) | 0.0187 (10) | 0.0199 (10) | −0.0087 (8) | −0.0051 (7) | 0.0013 (7) |
| C333 | 0.0300 (10) | 0.0178 (10) | 0.0191 (10) | −0.0063 (8) | −0.0041 (8) | 0.0051 (7) |
| C334 | 0.0224 (9) | 0.0125 (9) | 0.0225 (10) | −0.0066 (7) | 0.0099 (7) | −0.0033 (7) |
| C335 | 0.0178 (9) | 0.0188 (10) | 0.0290 (11) | −0.0086 (8) | −0.0003 (7) | −0.0044 (8) |
| C336 | 0.0155 (8) | 0.0170 (9) | 0.0226 (10) | −0.0049 (7) | −0.0034 (7) | 0.0003 (7) |
| N1 | 0.0156 (7) | 0.0231 (9) | 0.0315 (10) | −0.0032 (7) | −0.0046 (7) | −0.0089 (7) |
| O1 | 0.0144 (6) | 0.0267 (7) | 0.0269 (7) | −0.0059 (6) | −0.0067 (5) | 0.0062 (6) |
| O2 | 0.0338 (8) | 0.0245 (8) | 0.0250 (8) | −0.0007 (6) | −0.0099 (6) | 0.0000 (6) |
| O3 | 0.0131 (7) | 0.0582 (11) | 0.0545 (11) | −0.0075 (7) | −0.0034 (7) | −0.0180 (9) |
| F11 | 0.0277 (6) | 0.0272 (7) | 0.0392 (7) | −0.0039 (5) | −0.0192 (5) | 0.0091 (5) |
| F12 | 0.0462 (8) | 0.0346 (8) | 0.0653 (10) | −0.0196 (6) | −0.0318 (7) | −0.0038 (7) |
| F13 | 0.0361 (7) | 0.0334 (7) | 0.0298 (7) | −0.0134 (6) | 0.0123 (5) | 0.0017 (5) |
| F21 | 0.0517 (8) | 0.0537 (9) | 0.0229 (7) | −0.0095 (7) | 0.0156 (6) | 0.0076 (6) |
| F22 | 0.0165 (6) | 0.0341 (8) | 0.0685 (10) | 0.0086 (5) | −0.0121 (6) | −0.0083 (7) |
| F23 | 0.0444 (7) | 0.0440 (8) | 0.0280 (7) | −0.0263 (6) | −0.0070 (6) | −0.0078 (6) |
| F31 | 0.0287 (6) | 0.0238 (6) | 0.0409 (7) | −0.0006 (5) | 0.0087 (5) | −0.0140 (5) |
| F32 | 0.0242 (6) | 0.0418 (8) | 0.0470 (8) | −0.0159 (6) | −0.0236 (5) | 0.0143 (6) |
| F33 | 0.0366 (6) | 0.0156 (6) | 0.0289 (6) | −0.0135 (5) | 0.0089 (5) | −0.0020 (5) |
| P1 | 0.0130 (2) | 0.0125 (2) | 0.0160 (2) | −0.00207 (17) | −0.00181 (16) | −0.00245 (17) |
| P2 | 0.0129 (2) | 0.0121 (2) | 0.0153 (2) | −0.00204 (17) | −0.00006 (16) | 0.00020 (17) |
| P3 | 0.01112 (19) | 0.0127 (2) | 0.0135 (2) | −0.00410 (17) | −0.00106 (16) | 0.00032 (16) |
| Cu1 | 0.01211 (10) | 0.01249 (11) | 0.01381 (11) | −0.00253 (8) | −0.00091 (8) | −0.00025 (8) |
Geometric parameters (Å, º)
| C111—C116 | 1.394 (2) | C225—H225 | 0.95 |
| C111—C112 | 1.397 (3) | C226—H226 | 0.95 |
| C111—P1 | 1.8372 (18) | C231—C236 | 1.391 (2) |
| C112—C113 | 1.384 (3) | C231—C232 | 1.392 (2) |
| C112—H112 | 0.95 | C231—P2 | 1.8209 (18) |
| C113—C114 | 1.375 (3) | C232—C233 | 1.383 (3) |
| C113—H113 | 0.95 | C232—H232 | 0.95 |
| C114—F11 | 1.359 (2) | C233—C234 | 1.375 (3) |
| C114—C115 | 1.369 (3) | C233—H233 | 0.95 |
| C115—C116 | 1.391 (3) | C234—F23 | 1.359 (2) |
| C115—H115 | 0.95 | C234—C235 | 1.370 (3) |
| C116—H116 | 0.95 | C235—C236 | 1.392 (3) |
| C121—C126 | 1.386 (3) | C235—H235 | 0.95 |
| C121—C122 | 1.392 (2) | C236—H236 | 0.95 |
| C121—P1 | 1.8249 (17) | C311—C312 | 1.395 (2) |
| C122—C123 | 1.389 (3) | C311—C316 | 1.396 (2) |
| C122—H122 | 0.95 | C311—P3 | 1.8340 (18) |
| C123—C124 | 1.370 (3) | C312—C313 | 1.388 (3) |
| C123—H123 | 0.95 | C312—H312 | 0.95 |
| C124—F12 | 1.359 (2) | C313—C314 | 1.374 (3) |
| C124—C125 | 1.371 (3) | C313—H313 | 0.95 |
| C125—C126 | 1.390 (3) | C314—F31 | 1.355 (2) |
| C125—H125 | 0.95 | C314—C315 | 1.375 (3) |
| C126—H126 | 0.95 | C315—C316 | 1.391 (3) |
| C131—C132 | 1.391 (2) | C315—H315 | 0.95 |
| C131—C136 | 1.401 (2) | C316—H316 | 0.95 |
| C131—P1 | 1.8263 (18) | C321—C322 | 1.392 (2) |
| C132—C133 | 1.389 (3) | C321—C326 | 1.398 (2) |
| C132—H132 | 0.95 | C321—P3 | 1.8236 (17) |
| C133—C134 | 1.371 (3) | C322—C323 | 1.391 (2) |
| C133—H133 | 0.95 | C322—H322 | 0.95 |
| C134—F13 | 1.360 (2) | C323—C324 | 1.370 (3) |
| C134—C135 | 1.379 (3) | C323—H323 | 0.95 |
| C135—C136 | 1.385 (2) | C324—F32 | 1.363 (2) |
| C135—H135 | 0.95 | C324—C325 | 1.371 (3) |
| C136—H136 | 0.95 | C325—C326 | 1.392 (2) |
| C211—C216 | 1.389 (2) | C325—H325 | 0.95 |
| C211—C212 | 1.397 (3) | C326—H326 | 0.95 |
| C211—P2 | 1.8166 (18) | C331—C332 | 1.392 (2) |
| C212—C213 | 1.385 (3) | C331—C336 | 1.394 (2) |
| C212—H212 | 0.95 | C331—P3 | 1.8385 (17) |
| C213—C214 | 1.376 (3) | C332—C333 | 1.390 (2) |
| C213—H213 | 0.95 | C332—H332 | 0.95 |
| C214—F21 | 1.357 (2) | C333—C334 | 1.368 (3) |
| C214—C215 | 1.367 (3) | C333—H333 | 0.95 |
| C215—C216 | 1.396 (3) | C334—F33 | 1.3624 (19) |
| C215—H215 | 0.95 | C334—C335 | 1.372 (3) |
| C216—H216 | 0.95 | C335—C336 | 1.388 (2) |
| C221—C226 | 1.393 (3) | C335—H335 | 0.95 |
| C221—C222 | 1.393 (2) | C336—H336 | 0.95 |
| C221—P2 | 1.8199 (18) | N1—O3 | 1.234 (2) |
| C222—C223 | 1.383 (3) | N1—O2 | 1.249 (2) |
| C222—H222 | 0.95 | N1—O1 | 1.2757 (19) |
| C223—C224 | 1.364 (3) | O1—Cu1 | 2.1182 (12) |
| C223—H223 | 0.95 | P1—Cu1 | 2.2901 (5) |
| C224—F22 | 1.364 (2) | P2—Cu1 | 2.2840 (5) |
| C224—C225 | 1.370 (3) | P3—Cu1 | 2.3256 (5) |
| C225—C226 | 1.387 (3) | ||
| C116—C111—C112 | 118.43 (16) | C231—C232—H232 | 119.3 |
| C116—C111—P1 | 123.28 (14) | C234—C233—C232 | 117.67 (18) |
| C112—C111—P1 | 118.20 (13) | C234—C233—H233 | 121.2 |
| C113—C112—C111 | 121.34 (17) | C232—C233—H233 | 121.2 |
| C113—C112—H112 | 119.3 | F23—C234—C235 | 118.14 (18) |
| C111—C112—H112 | 119.3 | F23—C234—C233 | 118.74 (18) |
| C114—C113—C112 | 118.08 (18) | C235—C234—C233 | 123.11 (17) |
| C114—C113—H113 | 121 | C234—C235—C236 | 118.50 (18) |
| C112—C113—H113 | 121 | C234—C235—H235 | 120.8 |
| F11—C114—C115 | 118.73 (17) | C236—C235—H235 | 120.8 |
| F11—C114—C113 | 118.43 (17) | C231—C236—C235 | 120.30 (18) |
| C115—C114—C113 | 122.83 (17) | C231—C236—H236 | 119.8 |
| C114—C115—C116 | 118.59 (17) | C235—C236—H236 | 119.8 |
| C114—C115—H115 | 120.7 | C312—C311—C316 | 118.70 (16) |
| C116—C115—H115 | 120.7 | C312—C311—P3 | 121.87 (13) |
| C115—C116—C111 | 120.70 (18) | C316—C311—P3 | 119.29 (13) |
| C115—C116—H116 | 119.7 | C313—C312—C311 | 120.86 (16) |
| C111—C116—H116 | 119.7 | C313—C312—H312 | 119.6 |
| C126—C121—C122 | 119.30 (16) | C311—C312—H312 | 119.6 |
| C126—C121—P1 | 122.57 (13) | C314—C313—C312 | 118.43 (16) |
| C122—C121—P1 | 118.08 (14) | C314—C313—H313 | 120.8 |
| C123—C122—C121 | 120.47 (18) | C312—C313—H313 | 120.8 |
| C123—C122—H122 | 119.8 | F31—C314—C313 | 118.77 (16) |
| C121—C122—H122 | 119.8 | F31—C314—C315 | 118.34 (17) |
| C124—C123—C122 | 118.28 (18) | C313—C314—C315 | 122.88 (17) |
| C124—C123—H123 | 120.9 | C314—C315—C316 | 118.14 (17) |
| C122—C123—H123 | 120.9 | C314—C315—H315 | 120.9 |
| F12—C124—C123 | 118.94 (18) | C316—C315—H315 | 120.9 |
| F12—C124—C125 | 117.93 (19) | C315—C316—C311 | 120.98 (16) |
| C123—C124—C125 | 123.13 (18) | C315—C316—H316 | 119.5 |
| C124—C125—C126 | 118.07 (19) | C311—C316—H316 | 119.5 |
| C124—C125—H125 | 121 | C322—C321—C326 | 119.14 (16) |
| C126—C125—H125 | 121 | C322—C321—P3 | 118.73 (13) |
| C121—C126—C125 | 120.75 (18) | C326—C321—P3 | 122.01 (13) |
| C121—C126—H126 | 119.6 | C323—C322—C321 | 120.44 (17) |
| C125—C126—H126 | 119.6 | C323—C322—H322 | 119.8 |
| C132—C131—C136 | 118.98 (16) | C321—C322—H322 | 119.8 |
| C132—C131—P1 | 123.15 (13) | C324—C323—C322 | 118.23 (17) |
| C136—C131—P1 | 117.86 (13) | C324—C323—H323 | 120.9 |
| C133—C132—C131 | 120.39 (16) | C322—C323—H323 | 120.9 |
| C133—C132—H132 | 119.8 | F32—C324—C323 | 118.35 (18) |
| C131—C132—H132 | 119.8 | F32—C324—C325 | 117.95 (18) |
| C134—C133—C132 | 118.55 (17) | C323—C324—C325 | 123.70 (17) |
| C134—C133—H133 | 120.7 | C324—C325—C326 | 117.62 (18) |
| C132—C133—H133 | 120.7 | C324—C325—H325 | 121.2 |
| F13—C134—C133 | 118.11 (17) | C326—C325—H325 | 121.2 |
| F13—C134—C135 | 118.56 (16) | C325—C326—C321 | 120.84 (17) |
| C133—C134—C135 | 123.33 (17) | C325—C326—H326 | 119.6 |
| C134—C135—C136 | 117.47 (16) | C321—C326—H326 | 119.6 |
| C134—C135—H135 | 121.3 | C332—C331—C336 | 118.71 (16) |
| C136—C135—H135 | 121.3 | C332—C331—P3 | 122.55 (13) |
| C135—C136—C131 | 121.24 (17) | C336—C331—P3 | 118.56 (13) |
| C135—C136—H136 | 119.4 | C333—C332—C331 | 121.02 (16) |
| C131—C136—H136 | 119.4 | C333—C332—H332 | 119.5 |
| C216—C211—C212 | 119.37 (17) | C331—C332—H332 | 119.5 |
| C216—C211—P2 | 123.31 (14) | C334—C333—C332 | 118.15 (17) |
| C212—C211—P2 | 117.31 (13) | C334—C333—H333 | 120.9 |
| C213—C212—C211 | 120.73 (18) | C332—C333—H333 | 120.9 |
| C213—C212—H212 | 119.6 | F33—C334—C333 | 118.72 (17) |
| C211—C212—H212 | 119.6 | F33—C334—C335 | 118.29 (16) |
| C214—C213—C212 | 117.87 (19) | C333—C334—C335 | 122.97 (17) |
| C214—C213—H213 | 121.1 | C334—C335—C336 | 118.45 (17) |
| C212—C213—H213 | 121.1 | C334—C335—H335 | 120.8 |
| F21—C214—C215 | 118.41 (19) | C336—C335—H335 | 120.8 |
| F21—C214—C213 | 118.1 (2) | C335—C336—C331 | 120.68 (17) |
| C215—C214—C213 | 123.51 (18) | C335—C336—H336 | 119.7 |
| C214—C215—C216 | 118.15 (19) | C331—C336—H336 | 119.7 |
| C214—C215—H215 | 120.9 | O3—N1—O2 | 121.54 (17) |
| C216—C215—H215 | 120.9 | O3—N1—O1 | 118.99 (17) |
| C211—C216—C215 | 120.35 (19) | O2—N1—O1 | 119.45 (15) |
| C211—C216—H216 | 119.8 | N1—O1—Cu1 | 129.87 (11) |
| C215—C216—H216 | 119.8 | C121—P1—C131 | 103.52 (8) |
| C226—C221—C222 | 118.76 (17) | C121—P1—C111 | 102.89 (8) |
| C226—C221—P2 | 122.63 (14) | C131—P1—C111 | 101.99 (8) |
| C222—C221—P2 | 118.38 (14) | C121—P1—Cu1 | 115.18 (6) |
| C223—C222—C221 | 120.54 (18) | C131—P1—Cu1 | 116.00 (6) |
| C223—C222—H222 | 119.7 | C111—P1—Cu1 | 115.38 (6) |
| C221—C222—H222 | 119.7 | C211—P2—C221 | 102.44 (8) |
| C224—C223—C222 | 118.67 (18) | C211—P2—C231 | 103.80 (8) |
| C224—C223—H223 | 120.7 | C221—P2—C231 | 104.60 (8) |
| C222—C223—H223 | 120.7 | C211—P2—Cu1 | 117.00 (6) |
| F22—C224—C223 | 118.50 (18) | C221—P2—Cu1 | 114.73 (6) |
| F22—C224—C225 | 118.46 (19) | C231—P2—Cu1 | 112.79 (6) |
| C223—C224—C225 | 123.04 (18) | C321—P3—C311 | 102.69 (8) |
| C224—C225—C226 | 118.02 (19) | C321—P3—C331 | 101.81 (8) |
| C224—C225—H225 | 121 | C311—P3—C331 | 103.93 (8) |
| C226—C225—H225 | 121 | C321—P3—Cu1 | 119.88 (6) |
| C225—C226—C221 | 120.88 (18) | C311—P3—Cu1 | 113.47 (6) |
| C225—C226—H226 | 119.6 | C331—P3—Cu1 | 113.18 (6) |
| C221—C226—H226 | 119.6 | O1—Cu1—P2 | 103.96 (4) |
| C236—C231—C232 | 118.97 (16) | O1—Cu1—P1 | 112.11 (4) |
| C236—C231—P2 | 123.58 (14) | P2—Cu1—P1 | 119.537 (18) |
| C232—C231—P2 | 117.38 (13) | O1—Cu1—P3 | 87.93 (4) |
| C233—C232—C231 | 121.42 (18) | P2—Cu1—P3 | 112.289 (18) |
| C233—C232—H232 | 119.3 | P1—Cu1—P3 | 115.765 (18) |
| C116—C111—C112—C113 | −1.6 (3) | C332—C331—C336—C335 | −0.4 (3) |
| P1—C111—C112—C113 | 174.93 (14) | P3—C331—C336—C335 | −175.65 (14) |
| C111—C112—C113—C114 | 0.3 (3) | O3—N1—O1—Cu1 | 168.52 (13) |
| C112—C113—C114—F11 | −178.64 (16) | O2—N1—O1—Cu1 | −10.1 (2) |
| C112—C113—C114—C115 | 1.4 (3) | C126—C121—P1—C131 | −86.16 (17) |
| F11—C114—C115—C116 | 178.39 (16) | C122—C121—P1—C131 | 91.29 (16) |
| C113—C114—C115—C116 | −1.6 (3) | C126—C121—P1—C111 | 19.76 (18) |
| C114—C115—C116—C111 | 0.2 (3) | C122—C121—P1—C111 | −162.80 (15) |
| C112—C111—C116—C115 | 1.4 (3) | C126—C121—P1—Cu1 | 146.16 (15) |
| P1—C111—C116—C115 | −175.00 (14) | C122—C121—P1—Cu1 | −36.39 (17) |
| C126—C121—C122—C123 | −0.2 (3) | C132—C131—P1—C121 | 2.17 (17) |
| P1—C121—C122—C123 | −177.78 (16) | C136—C131—P1—C121 | −178.98 (14) |
| C121—C122—C123—C124 | −0.1 (3) | C132—C131—P1—C111 | −104.43 (15) |
| C122—C123—C124—F12 | 179.89 (19) | C136—C131—P1—C111 | 74.42 (15) |
| C122—C123—C124—C125 | 0.6 (4) | C132—C131—P1—Cu1 | 129.34 (14) |
| F12—C124—C125—C126 | −179.9 (2) | C136—C131—P1—Cu1 | −51.81 (15) |
| C123—C124—C125—C126 | −0.6 (4) | C116—C111—P1—C121 | −115.02 (15) |
| C122—C121—C126—C125 | 0.2 (3) | C112—C111—P1—C121 | 68.61 (15) |
| P1—C121—C126—C125 | 177.63 (17) | C116—C111—P1—C131 | −7.93 (17) |
| C124—C125—C126—C121 | 0.2 (3) | C112—C111—P1—C131 | 175.70 (14) |
| C136—C131—C132—C133 | −0.5 (3) | C116—C111—P1—Cu1 | 118.70 (14) |
| P1—C131—C132—C133 | 178.38 (14) | C112—C111—P1—Cu1 | −57.67 (15) |
| C131—C132—C133—C134 | −1.1 (3) | C216—C211—P2—C221 | −104.51 (16) |
| C132—C133—C134—F13 | −178.86 (16) | C212—C211—P2—C221 | 75.32 (15) |
| C132—C133—C134—C135 | 1.2 (3) | C216—C211—P2—C231 | 4.16 (18) |
| F13—C134—C135—C136 | −179.62 (16) | C212—C211—P2—C231 | −176.01 (14) |
| C133—C134—C135—C136 | 0.4 (3) | C216—C211—P2—Cu1 | 129.11 (14) |
| C134—C135—C136—C131 | −2.0 (3) | C212—C211—P2—Cu1 | −51.06 (15) |
| C132—C131—C136—C135 | 2.0 (3) | C226—C221—P2—C211 | 6.02 (17) |
| P1—C131—C136—C135 | −176.86 (14) | C222—C221—P2—C211 | −168.41 (14) |
| C216—C211—C212—C213 | 1.3 (3) | C226—C221—P2—C231 | −102.03 (16) |
| P2—C211—C212—C213 | −178.51 (14) | C222—C221—P2—C231 | 83.53 (15) |
| C211—C212—C213—C214 | −0.6 (3) | C226—C221—P2—Cu1 | 133.86 (14) |
| C212—C213—C214—F21 | −179.82 (17) | C222—C221—P2—Cu1 | −40.58 (16) |
| C212—C213—C214—C215 | −0.3 (3) | C236—C231—P2—C211 | −94.43 (16) |
| F21—C214—C215—C216 | 179.91 (17) | C232—C231—P2—C211 | 88.54 (14) |
| C213—C214—C215—C216 | 0.4 (3) | C236—C231—P2—C221 | 12.62 (17) |
| C212—C211—C216—C215 | −1.2 (3) | C232—C231—P2—C221 | −164.42 (13) |
| P2—C211—C216—C215 | 178.61 (15) | C236—C231—P2—Cu1 | 137.95 (14) |
| C214—C215—C216—C211 | 0.4 (3) | C232—C231—P2—Cu1 | −39.08 (15) |
| C226—C221—C222—C223 | −2.1 (3) | C322—C321—P3—C311 | 155.05 (14) |
| P2—C221—C222—C223 | 172.58 (15) | C326—C321—P3—C311 | −28.92 (16) |
| C221—C222—C223—C224 | −0.5 (3) | C322—C321—P3—C331 | −97.54 (14) |
| C222—C223—C224—F22 | −176.99 (17) | C326—C321—P3—C331 | 78.49 (16) |
| C222—C223—C224—C225 | 2.9 (3) | C322—C321—P3—Cu1 | 28.18 (16) |
| F22—C224—C225—C226 | 177.41 (18) | C326—C321—P3—Cu1 | −155.80 (12) |
| C223—C224—C225—C226 | −2.5 (3) | C312—C311—P3—C321 | 135.09 (15) |
| C224—C225—C226—C221 | −0.3 (3) | C316—C311—P3—C321 | −49.29 (16) |
| C222—C221—C226—C225 | 2.5 (3) | C312—C311—P3—C331 | 29.31 (16) |
| P2—C221—C226—C225 | −171.92 (16) | C316—C311—P3—C331 | −155.07 (14) |
| C236—C231—C232—C233 | −1.5 (3) | C312—C311—P3—Cu1 | −94.04 (14) |
| P2—C231—C232—C233 | 175.64 (14) | C316—C311—P3—Cu1 | 81.58 (15) |
| C231—C232—C233—C234 | 0.6 (3) | C332—C331—P3—C321 | −10.69 (17) |
| C232—C233—C234—F23 | 179.96 (16) | C336—C331—P3—C321 | 164.33 (14) |
| C232—C233—C234—C235 | 1.0 (3) | C332—C331—P3—C311 | 95.75 (16) |
| F23—C234—C235—C236 | 179.45 (17) | C336—C331—P3—C311 | −89.23 (15) |
| C233—C234—C235—C236 | −1.6 (3) | C332—C331—P3—Cu1 | −140.71 (14) |
| C232—C231—C236—C235 | 0.9 (3) | C336—C331—P3—Cu1 | 34.31 (15) |
| P2—C231—C236—C235 | −176.07 (15) | N1—O1—Cu1—P2 | −47.17 (15) |
| C234—C235—C236—C231 | 0.6 (3) | N1—O1—Cu1—P1 | 83.31 (15) |
| C316—C311—C312—C313 | 1.7 (3) | N1—O1—Cu1—P3 | −159.63 (15) |
| P3—C311—C312—C313 | 177.38 (14) | C211—P2—Cu1—O1 | 115.58 (8) |
| C311—C312—C313—C314 | −1.1 (3) | C221—P2—Cu1—O1 | −4.47 (8) |
| C312—C313—C314—F31 | 179.49 (16) | C231—P2—Cu1—O1 | −124.11 (7) |
| C312—C313—C314—C315 | −0.2 (3) | C211—P2—Cu1—P1 | −10.33 (7) |
| F31—C314—C315—C316 | −178.93 (17) | C221—P2—Cu1—P1 | −130.38 (6) |
| C313—C314—C315—C316 | 0.7 (3) | C231—P2—Cu1—P1 | 109.98 (6) |
| C314—C315—C316—C311 | 0.0 (3) | C211—P2—Cu1—P3 | −150.88 (7) |
| C312—C311—C316—C315 | −1.2 (3) | C221—P2—Cu1—P3 | 89.07 (7) |
| P3—C311—C316—C315 | −176.91 (14) | C231—P2—Cu1—P3 | −30.58 (6) |
| C326—C321—C322—C323 | −1.5 (3) | C121—P1—Cu1—O1 | −3.63 (8) |
| P3—C321—C322—C323 | 174.63 (14) | C131—P1—Cu1—O1 | −124.75 (7) |
| C321—C322—C323—C324 | 0.1 (3) | C111—P1—Cu1—O1 | 116.10 (7) |
| C322—C323—C324—F32 | −179.21 (16) | C121—P1—Cu1—P2 | 118.34 (7) |
| C322—C323—C324—C325 | 1.5 (3) | C131—P1—Cu1—P2 | −2.78 (7) |
| F32—C324—C325—C326 | 179.20 (16) | C111—P1—Cu1—P2 | −121.93 (6) |
| C323—C324—C325—C326 | −1.5 (3) | C121—P1—Cu1—P3 | −102.42 (7) |
| C324—C325—C326—C321 | 0.0 (3) | C131—P1—Cu1—P3 | 136.47 (6) |
| C322—C321—C326—C325 | 1.5 (3) | C111—P1—Cu1—P3 | 17.32 (6) |
| P3—C321—C326—C325 | −174.51 (14) | C321—P3—Cu1—O1 | −179.52 (8) |
| C336—C331—C332—C333 | −0.6 (3) | C311—P3—Cu1—O1 | 58.78 (7) |
| P3—C331—C332—C333 | 174.43 (14) | C331—P3—Cu1—O1 | −59.34 (7) |
| C331—C332—C333—C334 | 1.2 (3) | C321—P3—Cu1—P2 | 76.24 (7) |
| C332—C333—C334—F33 | −179.35 (16) | C311—P3—Cu1—P2 | −45.46 (6) |
| C332—C333—C334—C335 | −0.7 (3) | C331—P3—Cu1—P2 | −163.59 (6) |
| F33—C334—C335—C336 | 178.36 (15) | C321—P3—Cu1—P1 | −65.89 (7) |
| C333—C334—C335—C336 | −0.2 (3) | C311—P3—Cu1—P1 | 172.40 (6) |
| C334—C335—C336—C331 | 0.8 (3) | C331—P3—Cu1—P1 | 54.28 (6) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C122—H122···O2 | 0.95 | 2.30 | 3.224 (2) | 163 |
| C336—H336···O1 | 0.95 | 2.17 | 3.037 (2) | 151 |
| C126—H126···F22i | 0.95 | 2.40 | 3.281 (2) | 154 |
| C136—H136···O3ii | 0.95 | 2.53 | 3.223 (2) | 130 |
| C215—H215···F13iii | 0.95 | 2.51 | 3.301 (2) | 141 |
| C315—H315···F33iv | 0.95 | 2.50 | 3.403 (2) | 159 |
| C332—H332···F32v | 0.95 | 2.48 | 3.131 (2) | 125 |
| C326—H326···F33vi | 0.95 | 2.36 | 3.150 (2) | 141 |
Symmetry codes: (i) x−1, y+1, z; (ii) x−1, y, z; (iii) −x+1, −y+1, −z+1; (iv) x, y−1, z; (v) −x+1, −y+2, −z; (vi) −x+2, −y+2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: MW2090).
References
- Brandenburg, K. & Putz, H. (2005). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2004). SAINT-Plus and SADABS Bruker AXS Inc., Madison, Wisconsin. USA.
- Bruker (2005). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
- Dyason, J. C., Engelhardt, L. M., Healy, P. C., Klich, H. L. & White, A. H. (1986). Aust. J. Chem. 39, 2003–2011.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Hanna, J. V., Boyd, S. E., Healy, P. C., Bowmaker, G. A., Skelton, B. W. & White, A. H. (2005). J. Chem. Soc. Dalton Trans. pp. 2547–2556. [DOI] [PubMed]
- Matthew, M., Palenik, G. J. & Carty, A. J. (1971). Can. J. Chem. 49, 4119–4121.
- Saravanabharathi, D., Monika, Venugopalan, P. & Samuelson, A. G. (2002). Polyhedron, 21, 2433–2443.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Steyl, G. (2009). Acta Cryst. E65, m272. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812043346/mw2090sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812043346/mw2090Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report