Abstract
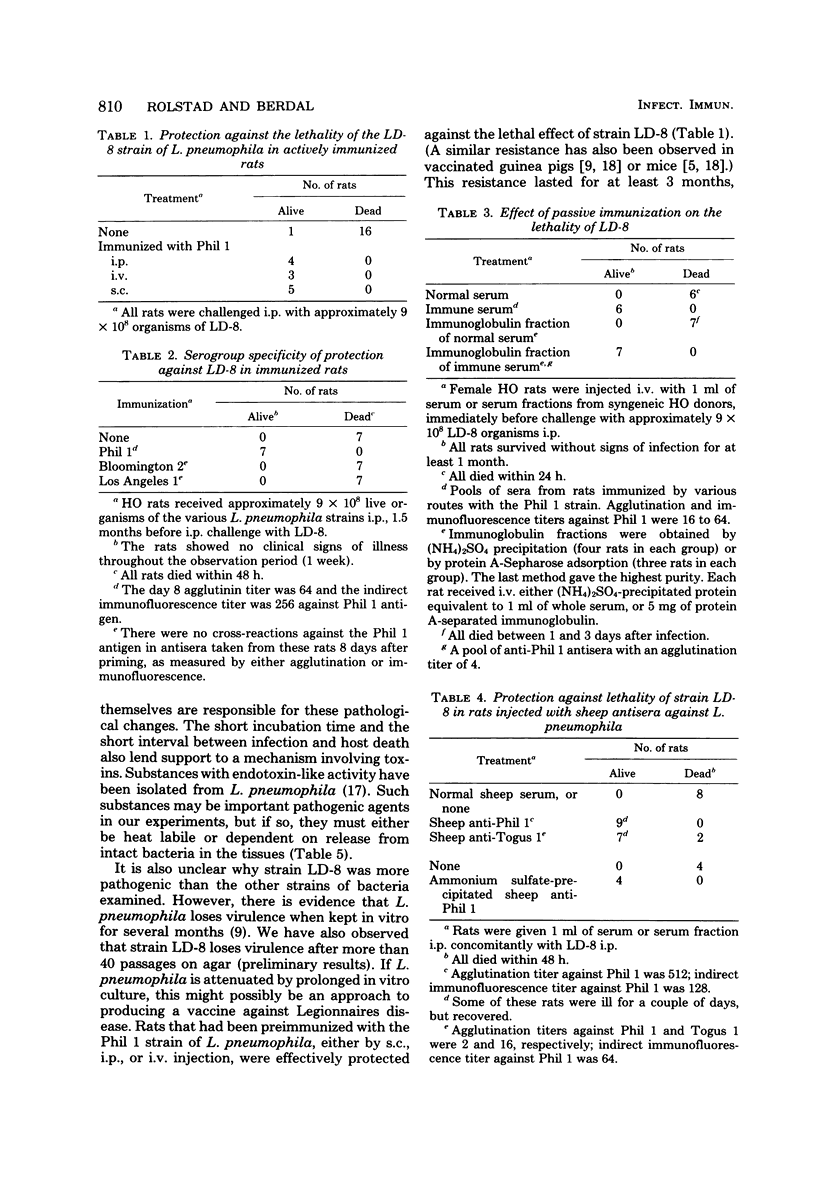
Rats were examined for their susceptibility to infection with Legionella pneumophila. Different strains of L. pneumophila representing serogroups 1 to 4 were tested (Philadelphia 1 and 2, Togus 1, Bloomington 2, Los Angeles 1, and the recently isolated LD-8 from the epidemics in Västeraas, Sweden). When about 9 × 108 bacteria, or 6 × 108 colony-forming units, were injected intraperitoneally, only rats infected with the LD-8 strain showed signs of illness. They became ill within 4 to 6 h and usually died within 24 h with septicemia and signs of circulatory collapse. Bacteria killed by heating at 100°C for 15 min lost their pathogenicity. Rats that were preimmunized with the Philadelphia 1 strain (same serogroup as LD-8), regardless of the route of immunization (subcutaneous, intraperitoneal, or intravenous), developed resistance to subsequent infection with LD-8. This resistance was serogroup specific and could be passively transferred to nonimmune animals with syngeneic immune serum or immunoglobulin fractions of serum. Antiserum or gamma globulin-enriched serum fractions from sheep with specificity for the Philadelphia 1 or Togus 1 strains also protected rats from the lethal effect of strain LD-8. The kinetics of the antibody and blood leukocyte response to immunization with the Philadelphia 1 strain of L. pneumophila in normal rats was investigated. During week 1 after intravenous injection the rats developed a marked lymphocytosis, which lasted for at least 1 week. The antibody response, as measured by indirect immunofluorescence or by a modification of the microagglutination method which we describe here, reached a peak during week 2 after immunization. Splenectomized rats were also resistant to infection with the Philadelphia 1 strain, but their antibody responses were substantially lower than those of their sham-operated littermates.
Full text
PDF

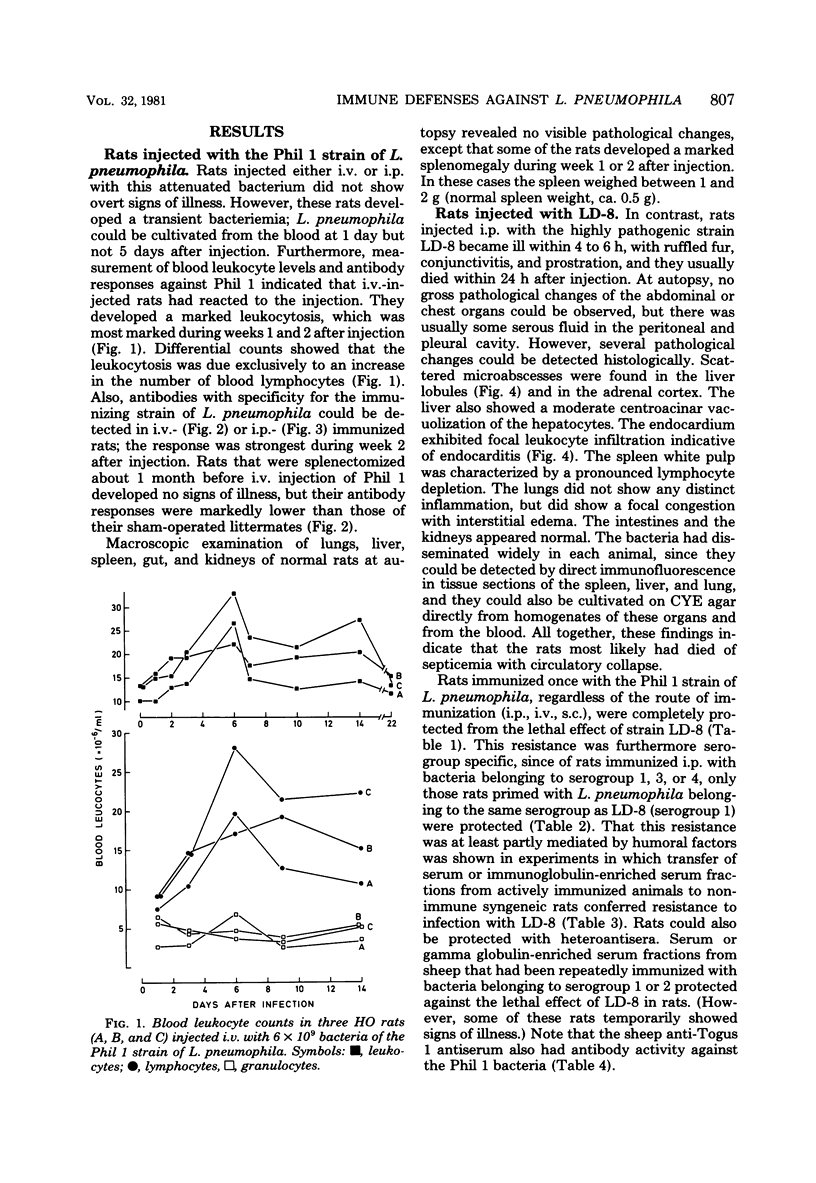
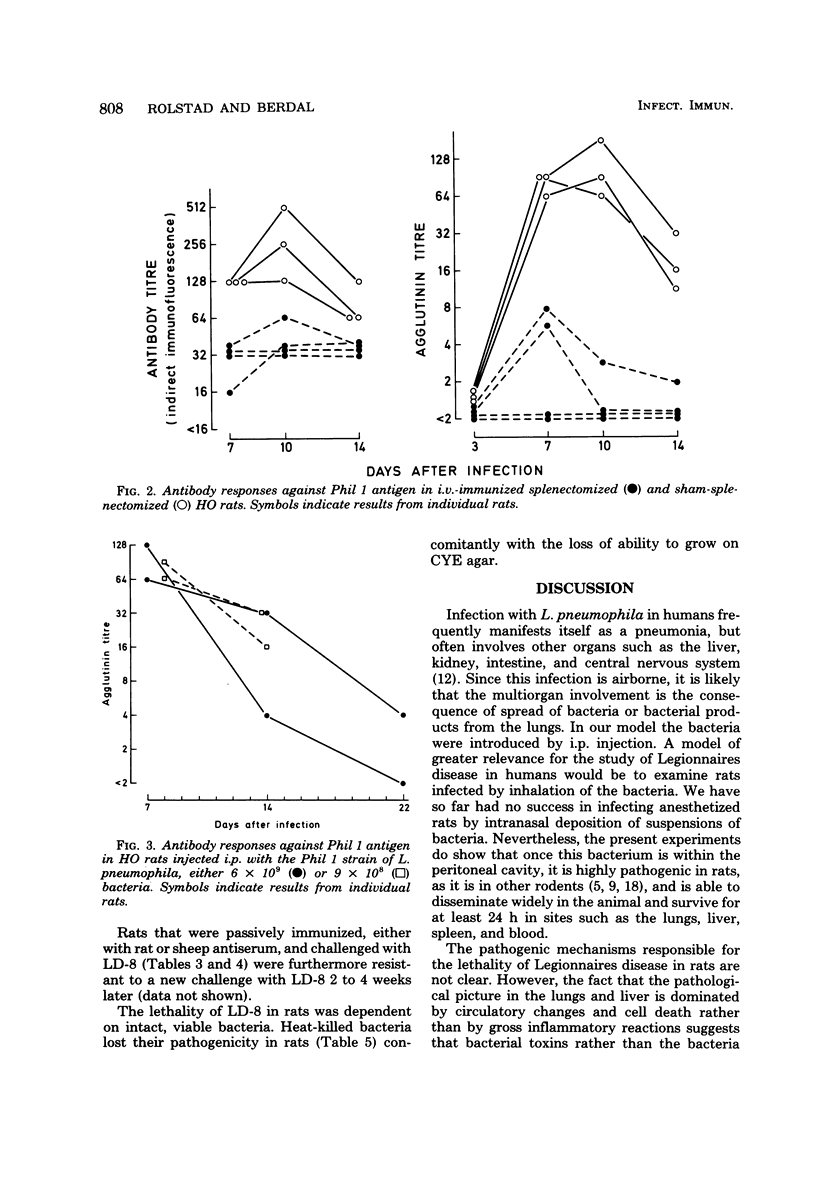
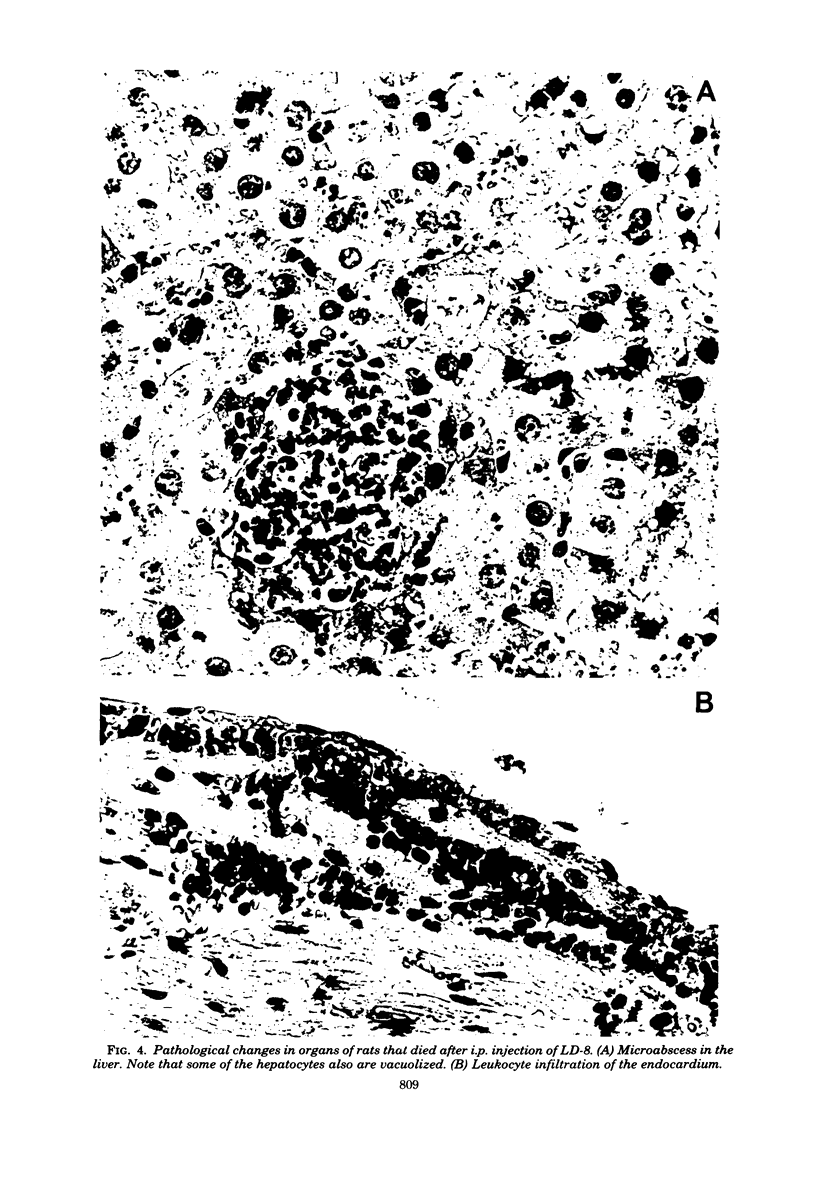


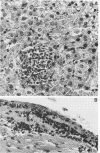
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arko R. J., Wong K. H., Feeley J. C. Immunologic factors affecting the in-vivo and in-vitro survival of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):680–683. doi: 10.7326/0003-4819-90-4-680. [DOI] [PubMed] [Google Scholar]
- Farshy C. E., Klein G. C., Feeley J. C. Detection of antibodies to legionnaires disease organism by microagglutination and micro-enzyme-linked immunosorbent assay tests. J Clin Microbiol. 1978 Apr;7(4):327–331. doi: 10.1128/jcm.7.4.327-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert G. A., Pelham P. L., Pittman B. Determination of the optimal ammonium sulfate concentration for the fractionation of rabbit, sheep, horse, and goat antisera. Appl Microbiol. 1973 Jan;25(1):26–36. doi: 10.1128/am.25.1.26-36.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund K. W., McGann V. G., Copeland D. S., Little S. F., Allen R. G. Immunologic protection against the Legionnaires' disease bacterium in the AKR/J mouse. Ann Intern Med. 1979 Apr;90(4):676–679. doi: 10.7326/0003-4819-90-4-676. [DOI] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- McDade J. E., Shepard C. C. Virulent to avirulent conversion of Legionnaires' disease bacterium (Legionella pneumophila)--its effect on isolation techniques. J Infect Dis. 1979 Jun;139(6):707–711. doi: 10.1093/infdis/139.6.707. [DOI] [PubMed] [Google Scholar]
- McKinney R. M., Thacker L., Harris P. P., Lewallen K. R., Hebert G. A., Edelstein P. H., Thomason B. M. Four serogroups of Legionnaires' disease bacteria defined by direct immunofluorescence. Ann Intern Med. 1979 Apr;90(4):621–624. doi: 10.7326/0003-4819-90-4-621. [DOI] [PubMed] [Google Scholar]
- Meyer R. D., Finegold S. M. Legionnaires' disease. Annu Rev Med. 1980;31:219–232. doi: 10.1146/annurev.me.31.020180.001251. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- Rolstad B., Williams A. F., Ford W. L. The alloantibody response to a strong transplantation antigen (Ag-B). Quantitative aspects and thymus dependence of the response. Transplantation. 1974 Apr;17(4):416–423. doi: 10.1097/00007890-197404000-00013. [DOI] [PubMed] [Google Scholar]
- Smalley D. L., Ourth D. D. Comparison of the microagglutination test with bactericidal response to Legionella pneumophila (Legionnaires disease bacterium). J Clin Microbiol. 1980 Feb;11(2):200–201. doi: 10.1128/jcm.11.2.200-201.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Moss C. W., Hochstein D. H., Arko R. J., Schalla W. O. "Endotoxicity" of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):624–627. doi: 10.7326/0003-4819-90-4-624. [DOI] [PubMed] [Google Scholar]
- Wong K. H., Schalla W. O., Arko R. J., Bullard J. C., Feeley J. C. Immunochemical, serologic, and immunologic properties of major antigens isolated from the Legionnaires' disease bacterium. Observations bearing on the feasibility of a vaccine. Ann Intern Med. 1979 Apr;90(4):634–638. doi: 10.7326/0003-4819-90-4-634. [DOI] [PubMed] [Google Scholar]