Abstract
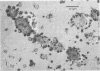
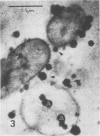
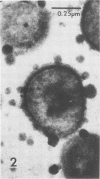
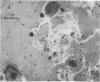
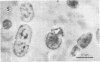
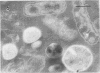
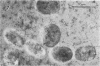
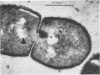
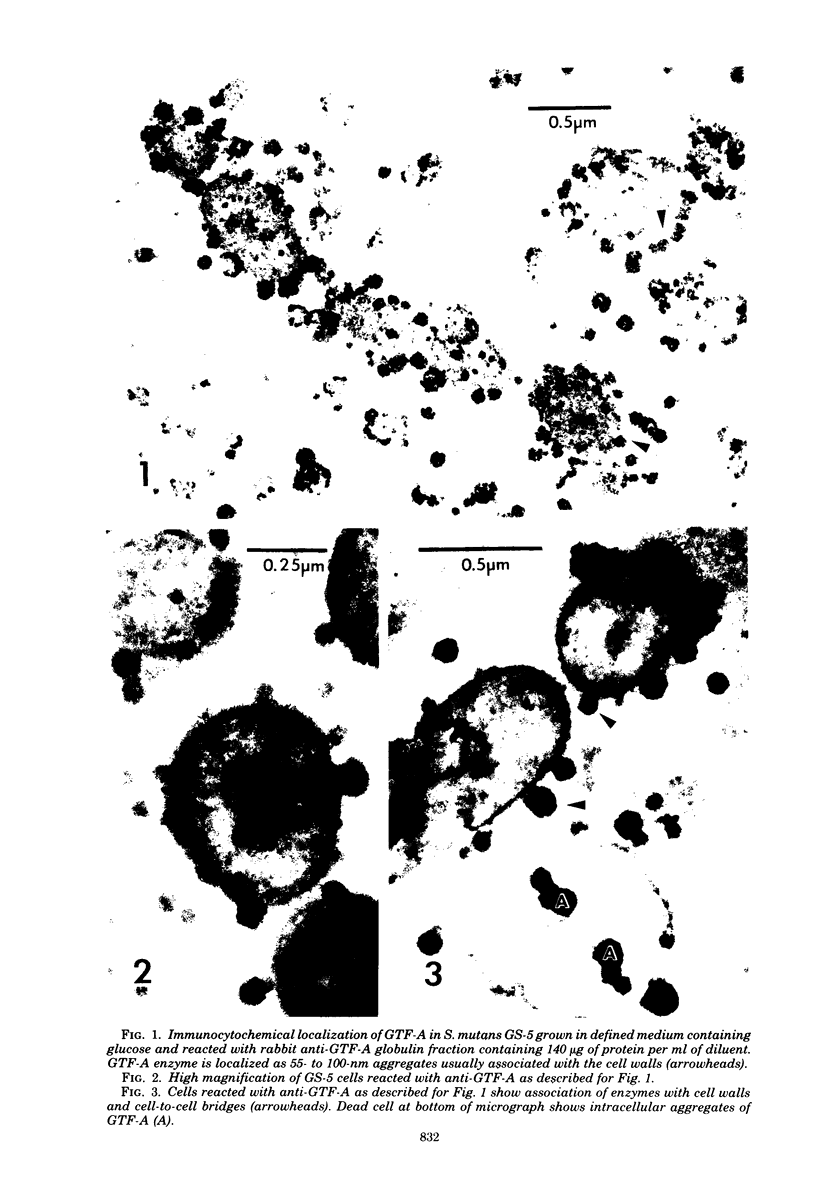
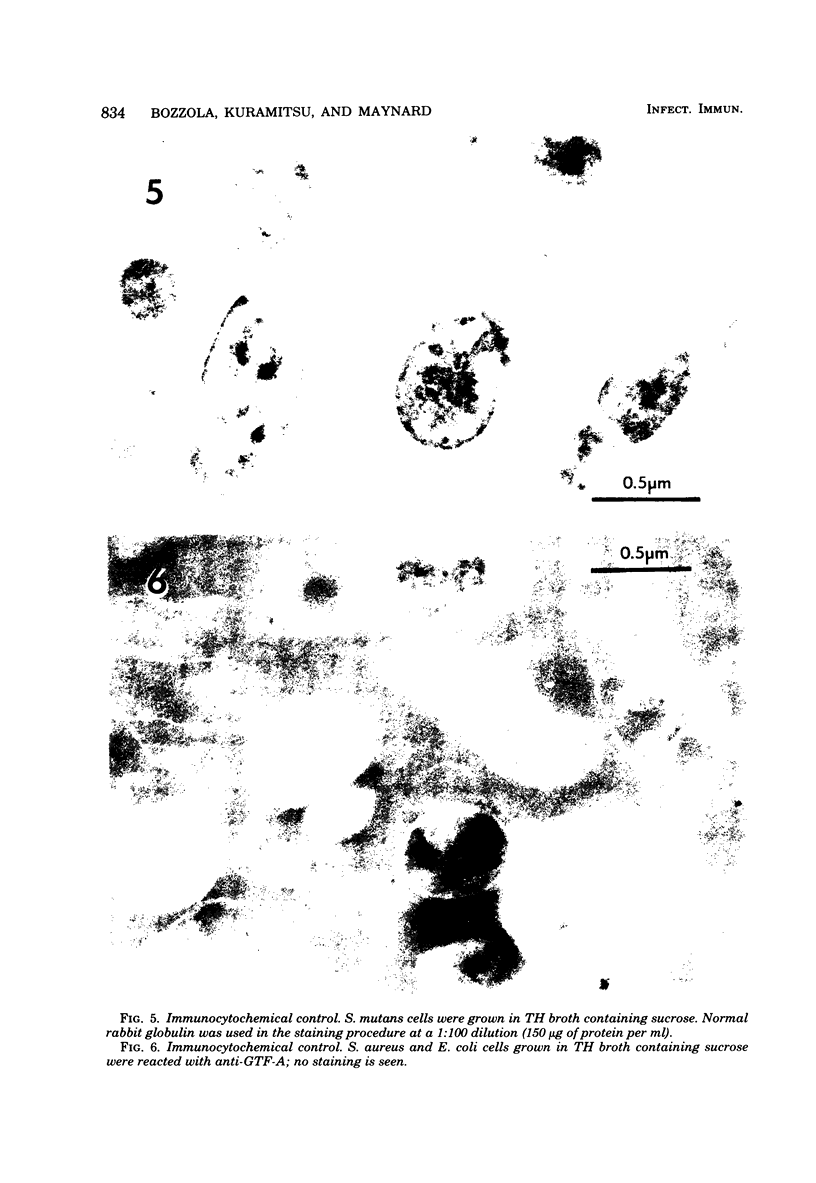
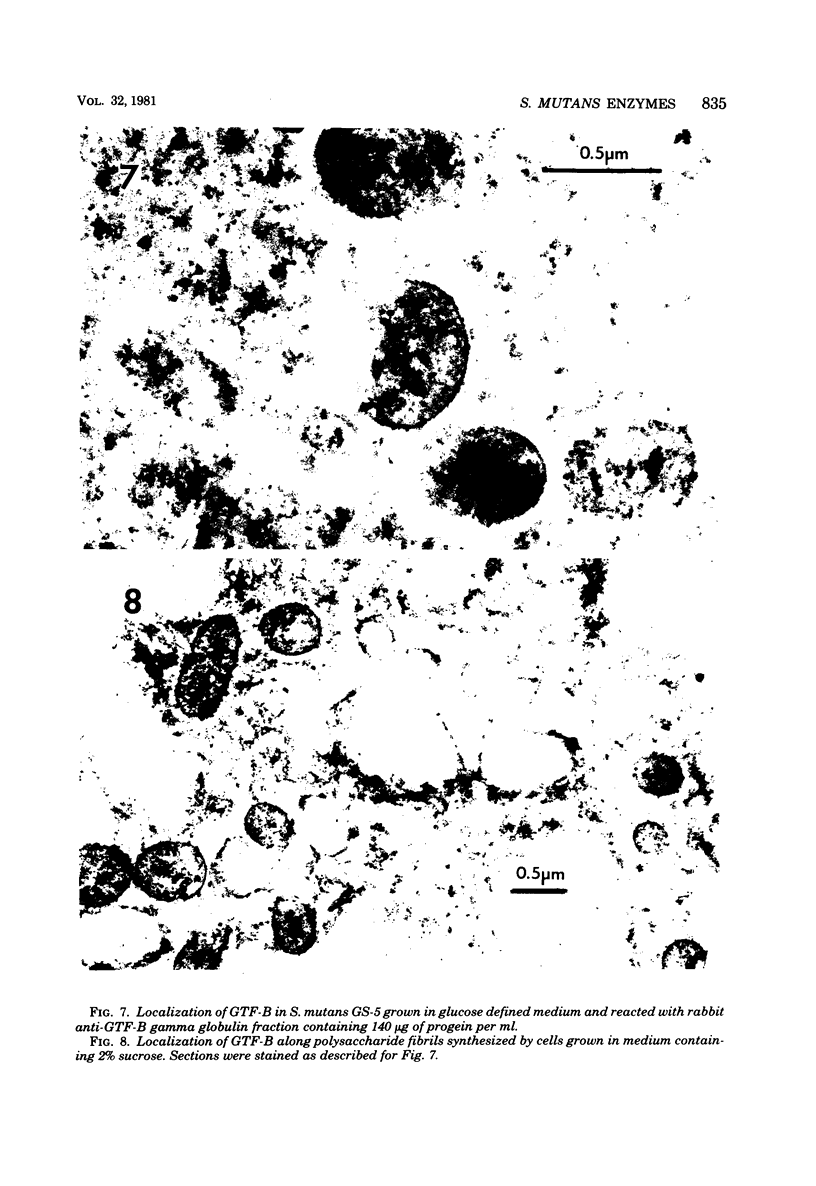
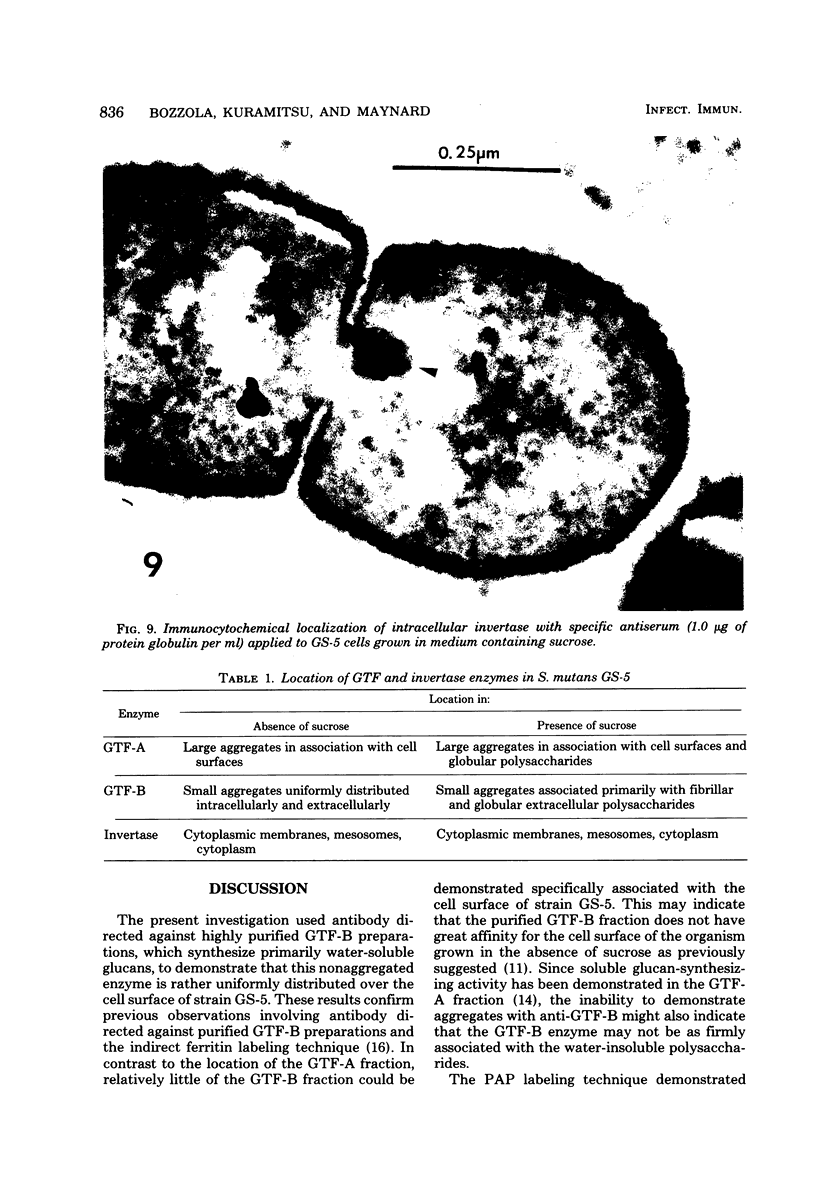
Antibodies directed against Streptococcus mutans GS-5 intracellular invertase and glucosyltransferase fractions capable of synthesizing primarily water-soluble or insoluble glucans were used to ultrastructurally localize the enzymes by means of the unlabeled antibody peroxidase-antiperoxidase method. This immunocytochemical procedure revealed that the intracellular invertase was associated primarily with the cytoplasmic membrane of the cariogenic organism. The glucosyltransferase complex responsible for insoluble glucan synthesis was localized as aggregates attached to the cell surface or extracellular polysaccharides of strain GS-5. In contrast, the glucosyltransferase activity synthesizing primarily water-soluble glucans was distributed uniformly over the cell surface or in association with extracellular polysaccharides. These results are discussed relative to the sucrose-metabolizing ability of Streptococcus mutans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozzola J. J., Johnson M. C., Shechmeister I. L. Ultrastructural localization of sucrases in Streptococcus mutans GS-5 and an extracellular polysaccharide mutant: a comparative cytochemical and immunocytochemical study. Infect Immun. 1977 Aug;17(2):447–457. doi: 10.1128/iai.17.2.447-457.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. A levansucrase from Streptococcus mutans. Caries Res. 1970;4(2):97–113. doi: 10.1159/000259632. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Porter E. V. Initial characterization of sucrose-6-phosphate hydrolase from Streptococcus mutans and its apparent identity with intracellular invertase. Biochem Biophys Res Commun. 1979 Jul 12;89(1):307–314. doi: 10.1016/0006-291x(79)90979-3. [DOI] [PubMed] [Google Scholar]
- Childs G. V., Cole D. E., Kubek M., Tobin R. B., Wilber J. F. Endogenous thyrotropin-releasing hormone in the anterior pituitary: sites of activity as identified by immunocytochemical staining. J Histochem Cytochem. 1978 Nov;26(11):901–908. doi: 10.1177/26.11.82570. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Janda W. M., Kuramitsu H. K. Regulation and extracellular glucosyltransferase production and the relationship between extracellular and cell-associated activities in Streptococcus mutans. Infect Immun. 1976 Jul;14(1):191–202. doi: 10.1128/iai.14.1.191-202.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L. Morphological study of Streptococcus mutans and two extracellular polysaccharide mutants. J Bacteriol. 1974 Apr;118(1):304–311. doi: 10.1128/jb.118.1.304-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of invertase activity from cariogenic Streptococcus mutans. J Bacteriol. 1973 Sep;115(3):1003–1010. doi: 10.1128/jb.115.3.1003-1010.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Ingersoll L. Interaction of Streptococcus mutans glucosyltransferases with antibodies. Adv Exp Med Biol. 1978;107:727–736. doi: 10.1007/978-1-4684-3369-2_82. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Immunological relationships between glucosyltransferases from Streptococcus mutans serotypes. Infect Immun. 1976 Sep;14(3):636–644. doi: 10.1128/iai.14.3.636-644.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard M. T., Kuramitsu H. K. Purification and antigenic properties of intracellular invertase from Streptococcus mutans. Infect Immun. 1979 Mar;23(3):873–883. doi: 10.1128/iai.23.3.873-883.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E., Cowman R. A. Invertase activity in Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1973 Apr;18(4):525–531. doi: 10.1016/0003-9969(73)90073-3. [DOI] [PubMed] [Google Scholar]
- Minah G. E., Loesche W. J. Sucrose metabolism by prominent members of the flora isolated from cariogenic and non-cariogenic dental plaques. Infect Immun. 1977 Jul;17(1):55–61. doi: 10.1128/iai.17.1.55-61.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty G. C., Moriarty C. M., Sternberger L. A. Ultrastructural immunocytochemistry with unlabeled antibodies and the peroxidase-antiperoxidase complex. A technique more sensitive than radioimmunoassay. J Histochem Cytochem. 1973 Sep;21(9):825–833. doi: 10.1177/21.9.825. [DOI] [PubMed] [Google Scholar]
- Petrali J. P., Hinton D. M., Moriarty G. C., Sternberger L. A. The unlabeled antibody enzyme method of immunocytochemistry. Quantitative comparison of sensitivities with and without peroxidase-antiperoxidase complex. J Histochem Cytochem. 1974 Aug;22(8):782–801. doi: 10.1177/22.8.782. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Brown A. T., McInerney M. F. Identification, preliminary characterization, and evidence for regulation of invertase in Streptococcus mutans. J Bacteriol. 1973 Oct;116(1):192–202. doi: 10.1128/jb.116.1.192-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]