Abstract
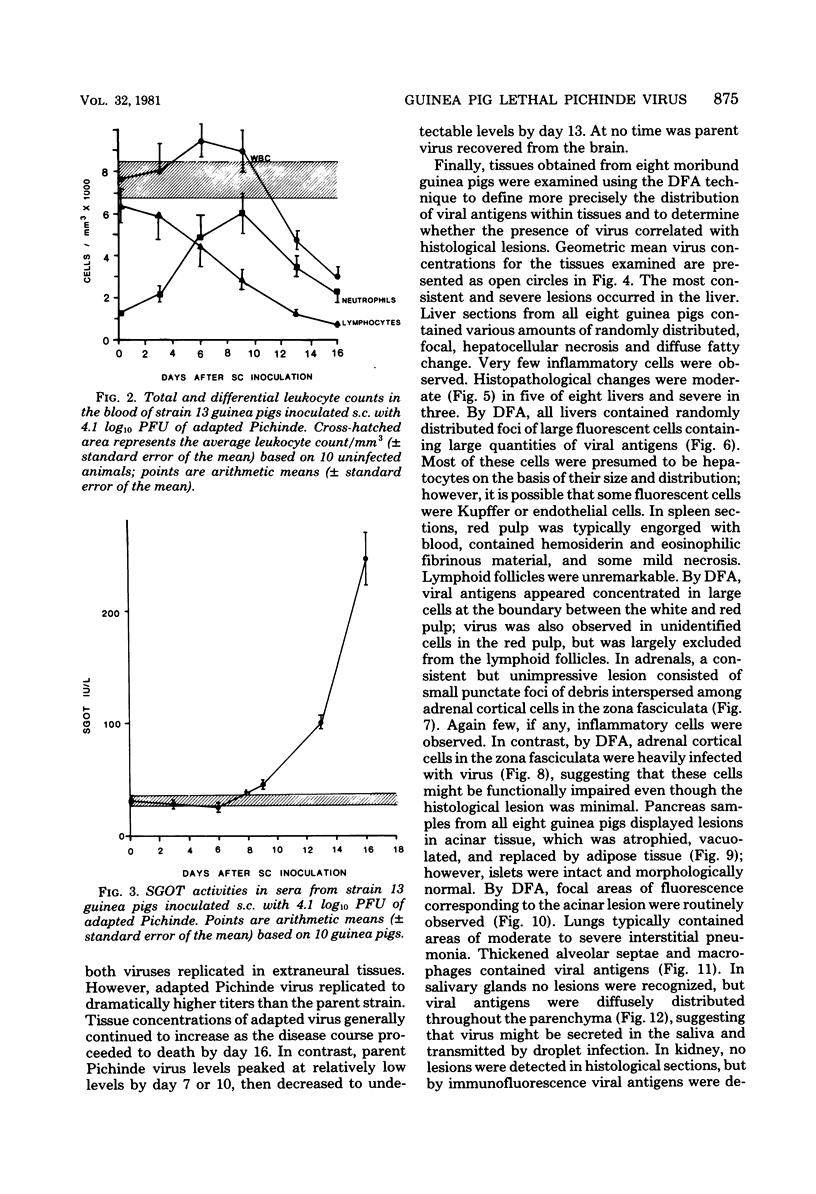
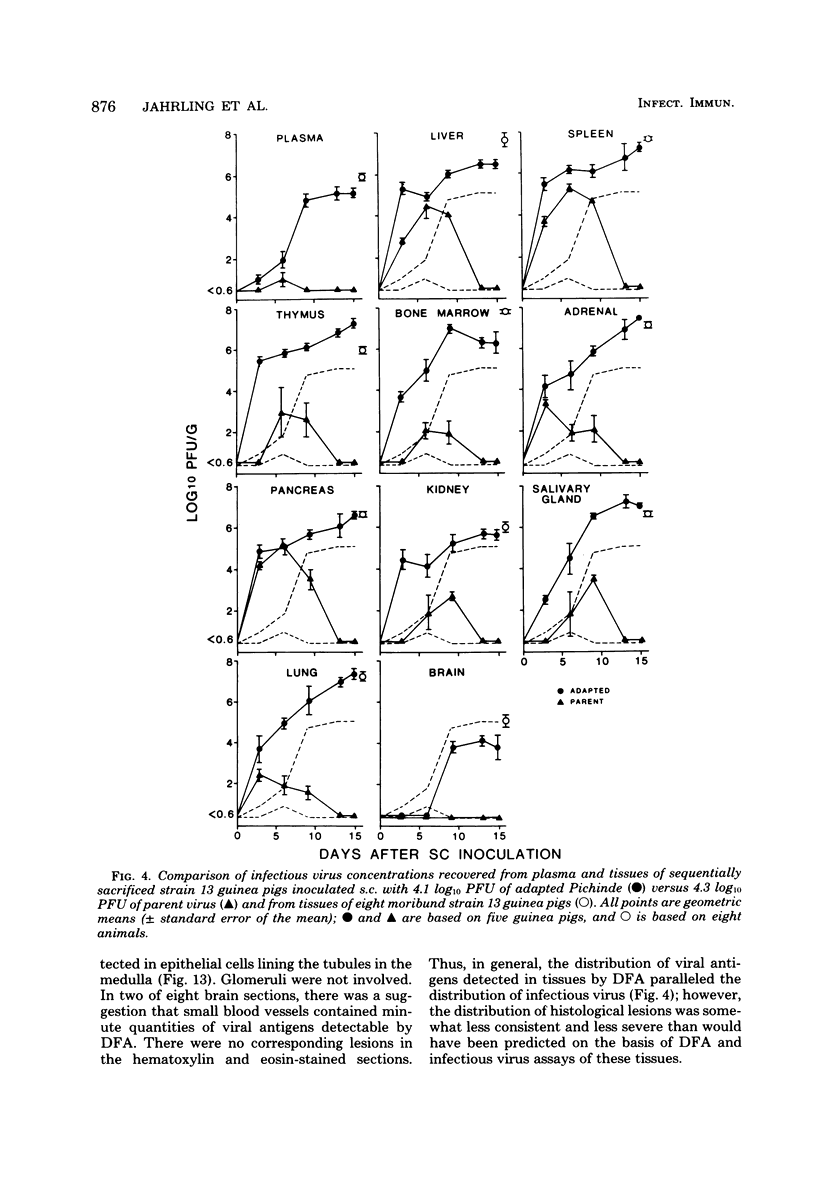
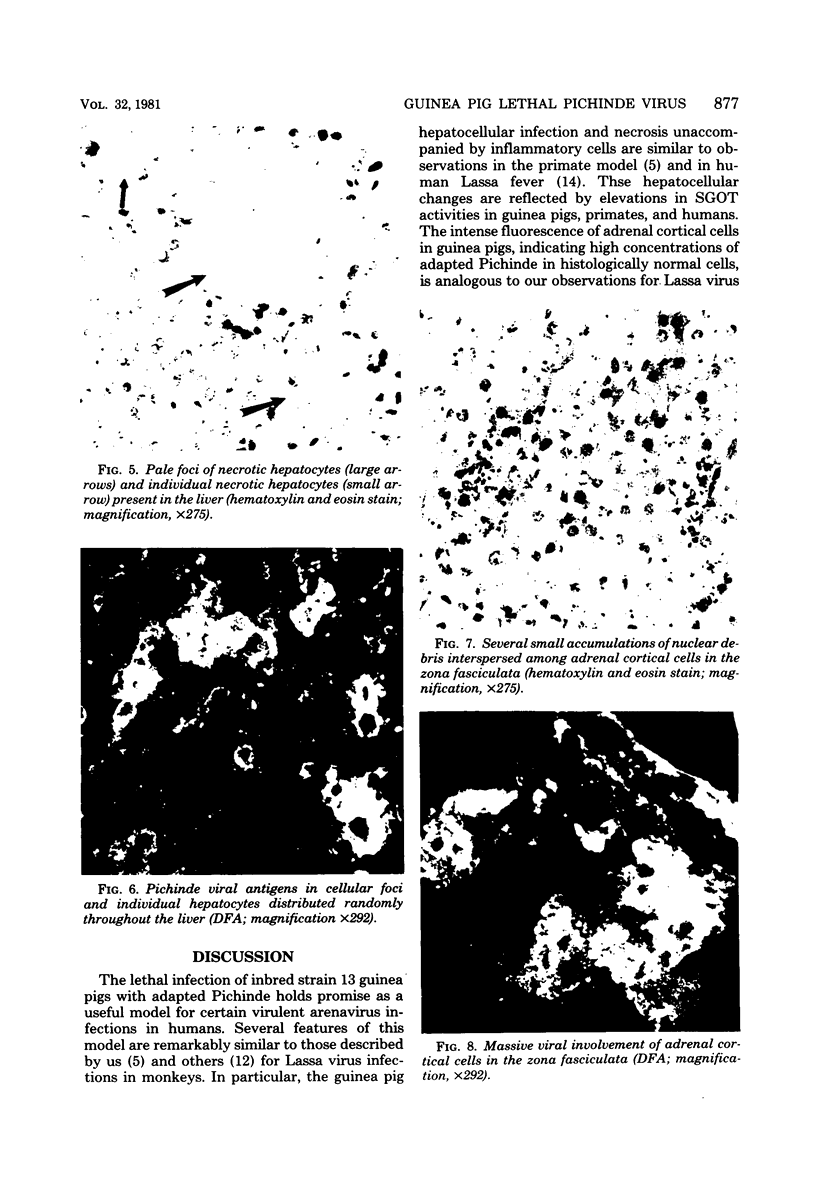
A model for studying the pathogenesis of virulent arenavirus infection was developed by adapting Pichinde virus to produce lethal infections of inbred guinea pigs. This adapted Pichinde virus retained low virulence for primates, thus potentially reducing the biohazard to investigators. Whereas all inbred (strain 13) guinea pigs were infected and killed by 3 plaque-forming units or more of adapted Pichinde virus injected subcutaneously, outbred (Hartley strain) guinea pigs were relatively resistant. All infected, inbred guinea pigs died at 13 to 19 days after inoculation, with viremias in excess of 5 log10 plaque-forming units/ml, severe lymphopenia (<1,000/mm3), and elevated serum glutamic oxaloacetic acid transaminase levels. Immunofluorescent antibody examination of tissues and infectivity titrations of tissue homogenates obtained at 3- to 4-day intervals demonstrated significant viral replication in all visceral tissues examined, but not in brain. Livers of all moribund guinea pigs contained moderate to severe hepatocellular necrosis and diffuse fatty change. Splenic red pulp and adrenal cortical tissues were engorged with blood and contained necrotic foci. Pancreatic acinar tissues were atrophied and vacuolated; lung sections typically contained areas of moderate to severe interstitial pneumonia. Inflammatory cells were conspicuously absent from all lesions. The virological and pathological features of adapted Pichinde infection in guinea pigs are remarkably similar to those described for Lassa virus infections in rhesus monkeys and humans, suggesting that this model might provide insight into the pathogenesis and treatment of Lassa fever in humans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila M. M., Samoilovich S. R., Weissenbacher M. C. Infección del cobayo con la cepa atenuada del virus Junin XJCl3. Medicina (B Aires) 1979 Sep-Oct;39(5):597–603. [PubMed] [Google Scholar]
- Buchmeier M. J., Rawls W. E. Variation between strains of hamsters in the lethality of Pichinde virus infections. Infect Immun. 1977 May;16(2):413–421. doi: 10.1128/iai.16.2.413-421.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy G. A., Scott S. K., Wagner F. S., Brand O. M. Pathogenesis of Machupo virus infection in primates. Bull World Health Organ. 1975;52(4-6):517–521. [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Hesse R. A., Eddy G. A., Johnson K. M., Callis R. T., Stephen E. L. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J Infect Dis. 1980 May;141(5):580–589. doi: 10.1093/infdis/141.5.580. [DOI] [PubMed] [Google Scholar]
- Maiztegui J. I. Clinical and epidemiological patterns of Argentine haemorrhagic fever. Bull World Health Organ. 1975;52(4-6):567–575. [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Buchmeier M. J., Rawls W. E. The reticuloendothelium as the target in a virus infection. Pichinde virus pathogenesis in two strains of hamsters. Lab Invest. 1977 Nov;37(5):502–515. [PubMed] [Google Scholar]
- Oldstone M. B., Holmstoen J., Welsh R. M., Jr Alterations of acetylcholine enzymes in neuroblastoma cells persistently infected with lymphocytic choriomeningitis virus. J Cell Physiol. 1977 Jun;91(3):459–472. doi: 10.1002/jcp.1040910316. [DOI] [PubMed] [Google Scholar]
- Peters C. J., Webb P. A., Johnson K. M. Measurement of antibodies to Machupo virus by the indirect fluorescent technique. Proc Soc Exp Biol Med. 1973 Feb;142(2):526–531. doi: 10.3181/00379727-142-37060. [DOI] [PubMed] [Google Scholar]
- Vezza A. C., Cash P., Jahrling P., Eddy G., Bishop D. H. Arenavirus recombination: the formation of recombinants between prototype pichinde and pichinde munchique viruses and evidence that arenavirus S RNA codes for N polypeptide. Virology. 1980 Oct 30;106(2):250–260. doi: 10.1016/0042-6822(80)90248-2. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Wulff H., Lange J. V., Murphy F. A. Comparative pathology of Lassa virus infection in monkeys, guinea-pigs, and Mastomys natalensis. Bull World Health Organ. 1975;52(4-6):523–534. [PMC free article] [PubMed] [Google Scholar]
- Winn W. C., Jr, Walker D. H. The pathology of human Lassa fever. Bull World Health Organ. 1975;52(4-6):535–545. [PMC free article] [PubMed] [Google Scholar]