Abstract
In the title compound C11H17NO4S, an intramolecular O—H⋯O hydrogen bond forms an S(8) ring and determines the conformation of the bis(2-hydroxyethyl) segment of the molecule, holding the two CH2CH2OH groups close to coplanar (r.m.s. deviation = 0.185 Å). In the crystal, O—H⋯O hydrogen bonds link the molecules into zigzag chains along the b axis. Weaker additional C—H⋯O and C—H⋯π contacts generate a three dimensional network, with molecules stacked along the b-axis direction.
Related literature
For pharmaceutical background to sulfonamides, see: Casini et al. (2002 ▶); Chambers & Jawetz (1998 ▶). For an alternative synthesis, see: Hori et al. (2011 ▶). For a related structure, see: Yoon et al. (2001 ▶). For standard bond lengths, see: Allen et al. (1987 ▶) and for hydrogen-bond motifs, see: Bernstein et al. (1995 ▶). 
Experimental
Crystal data
C11H17NO4S
M r = 259.32
Orthorhombic,

a = 17.9308 (5) Å
b = 7.1881 (2) Å
c = 19.8333 (6) Å
V = 2556.28 (13) Å3
Z = 8
Mo Kα radiation
μ = 0.26 mm−1
T = 296 K
0.16 × 0.12 × 0.10 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
3124 measured reflections
3124 independent reflections
2343 reflections with I > 2σ(I)
Refinement
R[F 2 > 2σ(F 2)] = 0.050
wR(F 2) = 0.161
S = 0.99
3124 reflections
161 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.45 e Å−3
Δρmin = −0.39 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: APEX2 and SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97, ORTEP-3 (Farrugia, 1997 ▶), enCIFer (Allen et al., 2004 ▶), PLATON (Spek, 2009 ▶) and publCIF (Westrip 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536812041682/nk2181sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812041682/nk2181Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the C1–C6 benzene ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O4—H4O⋯O3 | 0.76 (7) | 1.89 (7) | 2.634 (3) | 166 (7) |
| O3—H3O⋯O4i | 0.75 (4) | 1.92 (4) | 2.661 (3) | 172 (4) |
| C10—H10A⋯O1ii | 0.97 | 2.61 | 3.361 (3) | 135 |
| C3—H3⋯Cg1iii | 0.93 | 2.78 | 3.522 (2) | 138 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
The authors acknowledge Mr Ejaz for his kind assistance with the X-ray data collection.
supplementary crystallographic information
Comment
Sulfonamides are an important class of drugs with antibacterial, diuretic, hypoglycemic, antithyroid and antitumor action (Casini et al., 2002). Certain sulfonamides are used in combination with other drugs as antimicrobial agents and this paved the way for an antibiotic revolution in medicine. Sulfonamides also act as anti-metabolites and compete for the enzyme involved in the production of folic acid (Chambers & Jawetz, 1998). Most sulfonamides behave as weak acids and binding to basic amino acids can occur. The crystal structure of a tritosylate of diethanolamine has been reported in which the three tosyl groups are separated from one another as much as possible, to minimize steric repulsion (Yoon et al., 2001). The promising pharmaceutical potential of sulfonamides has prompted us to synthesize and characterize diethanolamine derived sulfonamides. Diethanolamine (DEA) itself has skin irritant characteristics; however, when DEA is transformed into new materials, the resulting compounds may become pharmaceutically useful. We therefore synthesized N,N-bis(2-hydroxyethyl)-4-methylbenzenesulfonamide (N-tosyl diethanolamine) (1) and report its molecular and crystal structure here.
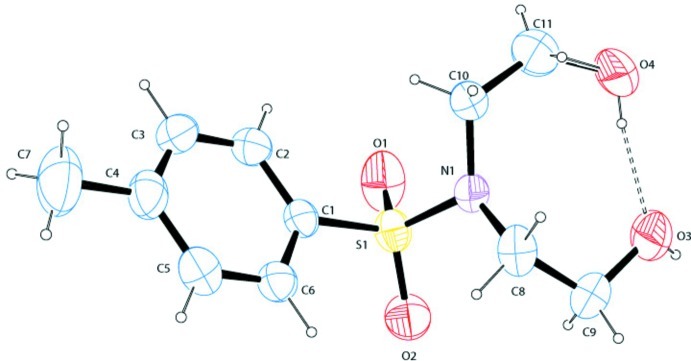
The molecular structure of (1) is shown in Fig. 1. The tolyl ring (C1···C6) is inclined at dihedral angles of 27.29 (0.16) ° and 37.78 (0.17) ° with respect to the N1/C8/C9 and N1/C10/C11 planes respectively. Bond distances (Allen et al., 1987) and angles within the molecule are normal and similar to those reported for N,N-bis(tosyloxyethyl)-p-toluenesulfonamide (Yoon et al., 2001). The nitrogen atom of the diethanolamine substituent is approximately sp3 hybridized with the geometry around the N1 atom close to pyramidal [C8—N1—S1 = 117.49 (14)°, C10—N1—S1 = 116.76 (14)° and C8—N1—C10 = 117.98 (18)°]. The two ethanol substituents are unsymmetrically oriented around the nitrogen atom with their conformations ultimately determined by an intramolecular O4—H4···O3 hydrogen bond that forms an S(8) ring (Bernstein et al., 1995).
In the crystal structure intermolecular O3—H3···O4 hydrogen bonds, Table 1, link molecules into zigzag chains along the b axis, Fig 2. C3—H3···π contacts, together with additional C10—H10A···O1 hydrogen bonds, further connect the chains to form a three-dimensional network structure, with molecules stacked along b, Fig 3.
Experimental
Tosyl chloride (0.836 g, 4.4 mmol) was added to a flask containing diethanolamine (0.42 g, 4 mmol) dissolved in CH2Cl2. Pyridine was added as a base (0.35 g, 4.4 mmol) and the solution refluxed for 4 hrs. On completion of the reaction, the pyridine was removed in vacuo and the product purified by crystallization. White crystals were obtained with an overall yield of 77%; Rf 0.13 (1:1 hexane and ethyl acetate). M.P: 95–98 0 C; 1H NMR 500 MHz, CDCl3: 7.70 (d, 2H), 7.33 (d, 2H), 3.87 (t, –CH2OH, 4H), 3.23 (t, –CH2N–, 4H), 2.43 (s, –CH3, 3H). The synthesis of (1) has also been reported using triethylamine rather than pyridine (Hori et al., 2011).
Refinement
H atoms of the OH groups were located in a difference Fourier map and their coordinates refined with Ueq = 1.5Ueq (O). Other H-atoms were refined using a riding model with d(C—H) = 0.93 Å for aromatic, 0.97 Å and for CH2 H atoms with Uiso = 1.2Ueq (C) and 0.96 Å, Uiso = 1.5Ueq (C) for CH3 H atoms.
Figures
Fig. 1.
The molecular structure of (1) showing the atom numbering scheme with displacement ellipsoids drawn at the 50% probability level. The intramolecular hydrogen bond is drawn as an open dashed line.
Fig. 2.
Zigzag chains of molecules formed along b by intermolecular O—H···O hydrogen bonds, shown as dashed lines.
Fig. 3.
Overall packing for (1) viewed along the b axis with hydrogen bonds drawn as dashed lines.
Crystal data
| C11H17NO4S | F(000) = 1104 |
| Mr = 259.32 | Dx = 1.348 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 4787 reflections |
| a = 17.9308 (5) Å | θ = 2.3–27.7° |
| b = 7.1881 (2) Å | µ = 0.26 mm−1 |
| c = 19.8333 (6) Å | T = 296 K |
| V = 2556.28 (13) Å3 | Block, colourless |
| Z = 8 | 0.16 × 0.12 × 0.10 mm |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 2343 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.000 |
| Graphite monochromator | θmax = 28.3°, θmin = 3.1° |
| φ and ω scans | h = 0→23 |
| 3124 measured reflections | k = 0→9 |
| 3124 independent reflections | l = 0→25 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.050 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.161 | H atoms treated by a mixture of independent and constrained refinement |
| S = 0.99 | w = 1/[σ2(Fo2) + (0.0948P)2 + 1.0844P] where P = (Fo2 + 2Fc2)/3 |
| 3124 reflections | (Δ/σ)max = 0.009 |
| 161 parameters | Δρmax = 0.45 e Å−3 |
| 0 restraints | Δρmin = −0.39 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.20186 (10) | 0.1722 (3) | 0.09437 (9) | 0.0733 (6) | |
| O2 | 0.08536 (10) | 0.0146 (2) | 0.06009 (8) | 0.0598 (4) | |
| S1 | 0.13232 (3) | 0.17523 (7) | 0.05993 (3) | 0.04344 (19) | |
| C1 | 0.15067 (10) | 0.2344 (3) | −0.02460 (10) | 0.0382 (4) | |
| C2 | 0.21585 (12) | 0.3277 (3) | −0.04138 (12) | 0.0501 (5) | |
| H2 | 0.2511 | 0.3542 | −0.0084 | 0.060* | |
| C3 | 0.22799 (14) | 0.3806 (3) | −0.10709 (13) | 0.0575 (6) | |
| H3 | 0.2716 | 0.4437 | −0.1181 | 0.069* | |
| C4 | 0.17630 (14) | 0.3417 (3) | −0.15754 (12) | 0.0529 (6) | |
| C7 | 0.1893 (2) | 0.4011 (5) | −0.22931 (15) | 0.0846 (9) | |
| H7A | 0.2302 | 0.3318 | −0.2478 | 0.127* | |
| H7B | 0.2008 | 0.5315 | −0.2305 | 0.127* | |
| H7C | 0.1452 | 0.3778 | −0.2554 | 0.127* | |
| C5 | 0.11233 (13) | 0.2452 (3) | −0.13986 (11) | 0.0501 (5) | |
| H5 | 0.0777 | 0.2156 | −0.1731 | 0.060* | |
| C6 | 0.09883 (11) | 0.1920 (3) | −0.07401 (10) | 0.0420 (4) | |
| H6 | 0.0553 | 0.1284 | −0.0630 | 0.050* | |
| N1 | 0.08353 (9) | 0.3445 (2) | 0.09165 (9) | 0.0429 (4) | |
| C8 | 0.00254 (12) | 0.3381 (3) | 0.08243 (11) | 0.0495 (5) | |
| H8A | −0.0087 | 0.2717 | 0.0411 | 0.059* | |
| H8B | −0.0162 | 0.4640 | 0.0777 | 0.059* | |
| C9 | −0.03700 (14) | 0.2449 (4) | 0.14009 (13) | 0.0635 (7) | |
| H9A | −0.0899 | 0.2359 | 0.1302 | 0.076* | |
| H9B | −0.0177 | 0.1200 | 0.1460 | 0.076* | |
| O3 | −0.02636 (12) | 0.3483 (3) | 0.19990 (9) | 0.0680 (6) | |
| H3O | −0.036 (2) | 0.286 (6) | 0.2288 (19) | 0.102* | |
| C10 | 0.11973 (15) | 0.5289 (3) | 0.09459 (12) | 0.0600 (7) | |
| H10A | 0.1681 | 0.5203 | 0.0729 | 0.072* | |
| H10B | 0.0899 | 0.6163 | 0.0689 | 0.072* | |
| C11 | 0.12997 (17) | 0.6026 (5) | 0.16224 (16) | 0.0775 (9) | |
| H11A | 0.1668 | 0.5252 | 0.1844 | 0.093* | |
| H11B | 0.1518 | 0.7254 | 0.1576 | 0.093* | |
| O4 | 0.07285 (19) | 0.6183 (4) | 0.20375 (14) | 0.1263 (13) | |
| H4O | 0.049 (3) | 0.532 (10) | 0.207 (3) | 0.190* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0538 (10) | 0.1121 (17) | 0.0539 (10) | 0.0251 (9) | −0.0090 (8) | −0.0026 (10) |
| O2 | 0.0809 (11) | 0.0353 (8) | 0.0632 (10) | 0.0006 (7) | 0.0116 (8) | 0.0062 (7) |
| S1 | 0.0454 (3) | 0.0439 (3) | 0.0411 (3) | 0.00854 (19) | 0.0022 (2) | −0.0011 (2) |
| C1 | 0.0384 (9) | 0.0358 (9) | 0.0404 (10) | 0.0034 (7) | 0.0051 (8) | −0.0069 (8) |
| C2 | 0.0441 (11) | 0.0514 (12) | 0.0548 (13) | −0.0088 (9) | 0.0050 (9) | −0.0136 (10) |
| C3 | 0.0587 (13) | 0.0475 (12) | 0.0664 (15) | −0.0128 (10) | 0.0219 (11) | −0.0084 (11) |
| C4 | 0.0680 (14) | 0.0399 (11) | 0.0508 (12) | 0.0061 (9) | 0.0193 (11) | −0.0007 (9) |
| C7 | 0.116 (3) | 0.080 (2) | 0.0577 (16) | 0.0089 (18) | 0.0318 (16) | 0.0138 (15) |
| C5 | 0.0536 (12) | 0.0520 (12) | 0.0447 (11) | 0.0054 (10) | −0.0009 (9) | −0.0071 (10) |
| C6 | 0.0383 (10) | 0.0427 (11) | 0.0449 (11) | −0.0004 (8) | 0.0026 (8) | −0.0057 (8) |
| N1 | 0.0449 (9) | 0.0390 (9) | 0.0447 (9) | −0.0018 (6) | 0.0129 (7) | −0.0056 (7) |
| C8 | 0.0488 (11) | 0.0580 (13) | 0.0418 (11) | 0.0102 (9) | 0.0038 (9) | −0.0003 (10) |
| C9 | 0.0537 (13) | 0.0734 (16) | 0.0634 (15) | −0.0154 (12) | 0.0206 (11) | −0.0090 (13) |
| O3 | 0.0879 (13) | 0.0678 (12) | 0.0484 (10) | −0.0134 (9) | 0.0284 (9) | 0.0010 (8) |
| C10 | 0.0765 (15) | 0.0487 (13) | 0.0548 (14) | −0.0185 (11) | 0.0248 (12) | −0.0147 (11) |
| C11 | 0.0856 (19) | 0.080 (2) | 0.0669 (18) | −0.0254 (15) | 0.0062 (14) | −0.0239 (15) |
| O4 | 0.171 (3) | 0.116 (2) | 0.0927 (17) | −0.0743 (19) | 0.0787 (18) | −0.0682 (16) |
Geometric parameters (Å, º)
| O1—S1 | 1.4220 (18) | C6—H6 | 0.9300 |
| O2—S1 | 1.4291 (18) | N1—C8 | 1.465 (3) |
| S1—N1 | 1.6252 (17) | N1—C10 | 1.477 (3) |
| S1—C1 | 1.761 (2) | C8—C9 | 1.503 (3) |
| C1—C2 | 1.388 (3) | C8—H8A | 0.9700 |
| C1—C6 | 1.385 (3) | C8—H8B | 0.9700 |
| C2—C3 | 1.375 (4) | C9—O3 | 1.413 (3) |
| C2—H2 | 0.9300 | C9—H9A | 0.9700 |
| C3—C4 | 1.392 (4) | C9—H9B | 0.9700 |
| C3—H3 | 0.9300 | O3—H3O | 0.75 (4) |
| C4—C5 | 1.385 (3) | C10—C11 | 1.454 (4) |
| C4—C7 | 1.504 (3) | C10—H10A | 0.9700 |
| C7—H7A | 0.9600 | C10—H10B | 0.9700 |
| C7—H7B | 0.9600 | C11—O4 | 1.319 (4) |
| C7—H7C | 0.9600 | C11—H11A | 0.9700 |
| C5—C6 | 1.382 (3) | C11—H11B | 0.9700 |
| C5—H5 | 0.9300 | O4—H4O | 0.76 (7) |
| O1—S1—O2 | 120.20 (12) | C8—N1—S1 | 117.52 (14) |
| O1—S1—N1 | 107.31 (11) | C10—N1—S1 | 116.78 (14) |
| O2—S1—N1 | 106.67 (10) | N1—C8—C9 | 112.75 (19) |
| O1—S1—C1 | 107.29 (10) | N1—C8—H8A | 109.0 |
| O2—S1—C1 | 107.90 (10) | C9—C8—H8A | 109.0 |
| N1—S1—C1 | 106.77 (9) | N1—C8—H8B | 109.0 |
| C2—C1—C6 | 120.13 (19) | C9—C8—H8B | 109.0 |
| C2—C1—S1 | 120.15 (17) | H8A—C8—H8B | 107.8 |
| C6—C1—S1 | 119.70 (15) | O3—C9—C8 | 109.9 (2) |
| C3—C2—C1 | 119.6 (2) | O3—C9—H9A | 109.7 |
| C3—C2—H2 | 120.2 | C8—C9—H9A | 109.7 |
| C1—C2—H2 | 120.2 | O3—C9—H9B | 109.7 |
| C2—C3—C4 | 121.3 (2) | C8—C9—H9B | 109.7 |
| C2—C3—H3 | 119.3 | H9A—C9—H9B | 108.2 |
| C4—C3—H3 | 119.3 | C9—O3—H3O | 107 (3) |
| C5—C4—C3 | 118.0 (2) | C11—C10—N1 | 114.8 (2) |
| C5—C4—C7 | 120.6 (3) | C11—C10—H10A | 108.6 |
| C3—C4—C7 | 121.3 (2) | N1—C10—H10A | 108.6 |
| C4—C7—H7A | 109.5 | C11—C10—H10B | 108.6 |
| C4—C7—H7B | 109.5 | N1—C10—H10B | 108.6 |
| H7A—C7—H7B | 109.5 | H10A—C10—H10B | 107.6 |
| C4—C7—H7C | 109.5 | O4—C11—C10 | 120.6 (3) |
| H7A—C7—H7C | 109.5 | O4—C11—H11A | 107.2 |
| H7B—C7—H7C | 109.5 | C10—C11—H11A | 107.2 |
| C6—C5—C4 | 121.5 (2) | O4—C11—H11B | 107.2 |
| C6—C5—H5 | 119.2 | C10—C11—H11B | 107.2 |
| C4—C5—H5 | 119.2 | H11A—C11—H11B | 106.8 |
| C5—C6—C1 | 119.36 (19) | C10—C11—H4O | 105 (2) |
| C5—C6—H6 | 120.3 | H11A—C11—H4O | 99.7 |
| C1—C6—H6 | 120.3 | H11B—C11—H4O | 129.5 |
| C8—N1—C10 | 117.96 (18) | C11—O4—H4O | 115 (5) |
| O1—S1—C1—C2 | −22.1 (2) | C2—C1—C6—C5 | −0.7 (3) |
| O2—S1—C1—C2 | −152.92 (17) | S1—C1—C6—C5 | 177.53 (16) |
| N1—S1—C1—C2 | 92.73 (18) | O1—S1—N1—C8 | −159.22 (16) |
| O1—S1—C1—C6 | 159.73 (17) | O2—S1—N1—C8 | −29.17 (18) |
| O2—S1—C1—C6 | 28.87 (19) | C1—S1—N1—C8 | 86.00 (16) |
| N1—S1—C1—C6 | −85.47 (17) | O1—S1—N1—C10 | 52.0 (2) |
| C6—C1—C2—C3 | 1.2 (3) | O2—S1—N1—C10 | −177.94 (17) |
| S1—C1—C2—C3 | −177.04 (17) | C1—S1—N1—C10 | −62.76 (19) |
| C1—C2—C3—C4 | −0.4 (3) | C10—N1—C8—C9 | −119.1 (2) |
| C2—C3—C4—C5 | −0.9 (3) | S1—N1—C8—C9 | 92.5 (2) |
| C2—C3—C4—C7 | 179.5 (2) | N1—C8—C9—O3 | 63.5 (3) |
| C3—C4—C5—C6 | 1.4 (3) | C8—N1—C10—C11 | 94.0 (3) |
| C7—C4—C5—C6 | −179.0 (2) | S1—N1—C10—C11 | −117.4 (2) |
| C4—C5—C6—C1 | −0.6 (3) | N1—C10—C11—O4 | −54.6 (4) |
Hydrogen-bond geometry (Å, º)
Cg1 is the centroid of the C1–C6 benzene ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| O4—H4O···O3 | 0.76 (7) | 1.89 (7) | 2.634 (3) | 166 (7) |
| O3—H3O···O4i | 0.75 (4) | 1.92 (4) | 2.661 (3) | 172 (4) |
| C10—H10A···O1ii | 0.97 | 2.61 | 3.361 (3) | 135 |
| C3—H3···Cg1iii | 0.93 | 2.78 | 3.522 (2) | 138 |
Symmetry codes: (i) −x, y−1/2, −z+1/2; (ii) −x+1/2, y+1/2, z; (iii) x, −y−1/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NK2181).
References
- Allen, F. H., Johnson, O., Shields, G. P., Smith, B. R. & Towler, M. (2004). J. Appl. Cryst. 37, 335–338.
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Casini, A., Scozzafava, A., Mastrolorenzo, A. & Supuran, L. T. (2002). Curr. Cancer Drug Targets, 2, 55–75. [DOI] [PubMed]
- Chambers, H. F. & Jawetz, E. (1998). Sulfonamides, trimethoprim, and quinolones, in basic and clinical pharmacology, edited by B. G. Katzung, pp. 761–763. Stamford: Appleton–Lange.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Hori, Y., Pei, N., Kumagai, R. & Sasanuma, Y. (2011). Polym. Chem. 2, 2183–2185.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Yoon, I., Park, K.-M. & Lee, S. S. (2001). Acta Cryst. C57, 321–322. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536812041682/nk2181sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812041682/nk2181Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report