Abstract
The title molecule, C23H26N2O8, was synthesized in three steps starting from m-nitrocinnamic acid. The central oxazolidine ring adopts an almost perfect envelope conformation with the O atom as the flap [puckering parameter ϕ = 0.3 (6)°]. The dihedral angle formed by the benzene rings is 61.81 (9)°. In the crystal, molecules are connected into double chains parallel to [010] by C—H⋯O hydrogen bonds. The absolute configuration was assigned from the synthetic procedure.
Related literature
For the Sharpless asymmetric aminohydroxylation, see: Rudolph et al. (1996 ▶). For the synthesis of the phenylisoserine precursor of the title molecule, see: Montiel-Smith et al. (2002 ▶). For the stereocontrolled formation of the oxazolidine in the title molecule, see: Denis et al. (1994 ▶). For the structure of a related chiral N-Boc-protected oxazolidine, see: Tinant et al. (1996 ▶). For puckering parameters, see: Cremer & Pople (1975 ▶).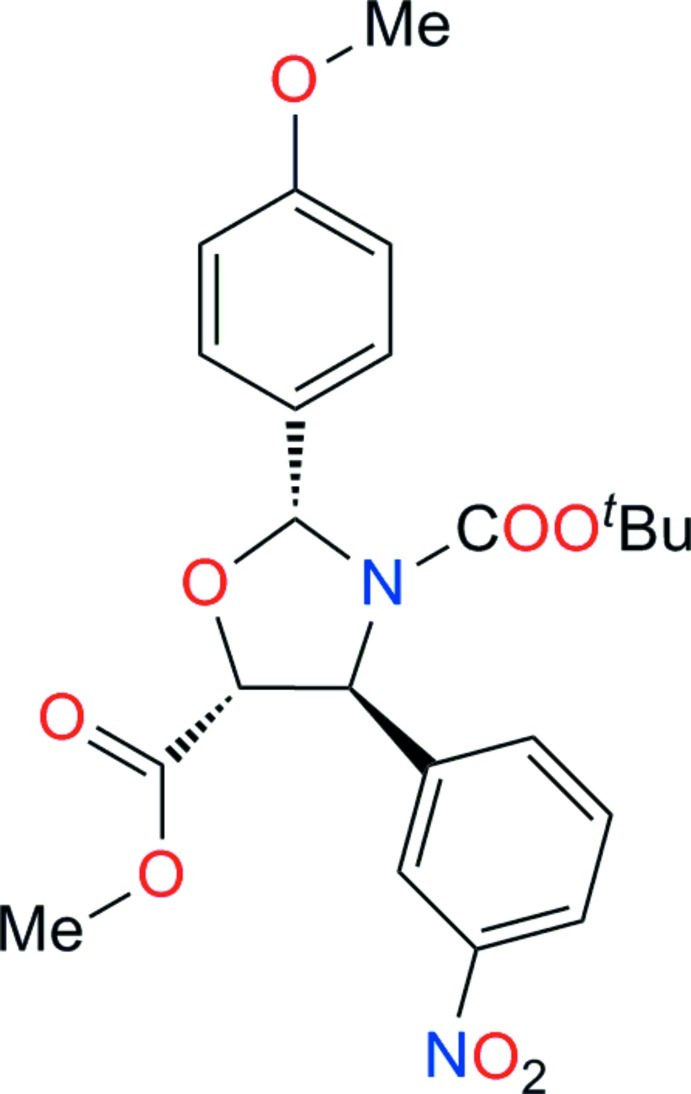
Experimental
Crystal data
C23H26N2O8
M r = 458.46
Monoclinic,

a = 10.383 (1) Å
b = 6.0303 (6) Å
c = 18.7366 (17) Å
β = 95.591 (4)°
V = 1167.57 (19) Å3
Z = 2
Mo Kα radiation
μ = 0.10 mm−1
T = 298 K
0.60 × 0.16 × 0.16 mm
Data collection
Siemens P4 diffractometer
3169 measured reflections
2275 independent reflections
1628 reflections with I > 2σ(I)
R int = 0.024
3 standard reflections every 97 reflections intensity decay: 0.5%
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.095
S = 1.02
2275 reflections
304 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.12 e Å−3
Δρmin = −0.13 e Å−3
Data collection: XSCANS (Siemens, 1996 ▶); cell refinement: XSCANS; data reduction: XSCANS; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S160053681204192X/rz5008sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681204192X/rz5008Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681204192X/rz5008Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2A⋯O15i | 0.98 | 2.50 | 3.400 (4) | 153 |
| C5—H5A⋯O28ii | 0.98 | 2.59 | 3.387 (4) | 138 |
| C26—H26A⋯O1iii | 0.93 | 2.59 | 3.252 (4) | 128 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
This work was supported by the France–Mexico ECOS-ANUIES (M97–E02) agreement.
supplementary crystallographic information
Comment
The title compound is related to a project about new synthetic routes to obtain isoserines (α-hydroxy-β-amino acids). The stereocontrol of the synthesis is a key point, since chiral isoserines are found in bioactive substances, as in the side chain of the emblematic anti-cancer agent Paclitaxel, initially marketed under the brand name Taxol. We focused our efforts toward the synthesis of (2R,3S)-N-Boc-β-phenylisoserines (Montiel-Smith et al., 2002). Starting from commercially available m-nitrocinnamic acid, which was esterified in a first step, we probed various conditions for an asymmetric aminohydroxylation (Rudolph et al., 1996), and the best results were obtained by using tert-butyl-N-chlorocarbamate as the nitrogen source, (DHQ)2PHAL (hydroquinine 1,4-phthalazinediyl diether) as chiral ligand, and K2OsO2(OH)4 as catalyst. The desired phenylisoserine was eventually obtained with 81% ee (see compound 2c in Montiel-Smith et al., 2002). The title compound resulted from the protection of the amine and hydroxyl groups, via the formation of an oxazolidine (Fig. 1).
The molecular structure (Fig. 2) allowed to check for the configuration of chiral atoms C2 and C3 in the precursor isoserine 3, confirming that the chiral inductor (DHQ)2PHAL affords the (2R,3S) isomer predominantly, as expected. The deduced configuration of the third stereocenter in the oxazolidine I, 2R, also agrees with literature data for related reactions (Denis et al., 1994). The substituents in the oxazolidine skeleton are arranged in such a way that steric hindrance is avoided. The oxazolidine exhibits a conformation very close to the ideal envelope conformation on O1, the puckering parameters (Cremer & Pople, 1975) being φ = 0.3 (6)° and q2 = 0.331 (3) Å. The ring conformation is related to the substituents distribution. For instance, the X-ray structure for another N-Boc protected oxazolidine with a different absolute configuration, (2R,4R,5S), showed a twisted oxazolidine ring (Tinant et al., 1996).
The crystal structure (Fig. 3) is dominated by the stacking of bulky Boc groups, which are oriented along [100], with the molecules linked into double chains parallel to [010] by C—H···O hydrogen bonds (Table 1).
Experimental
The synthesis starting from commercially available m-nitrocinnamic acid 1 is depicted in Fig. 1. The two steps preparation of the phenylisoserine 3 has been published (Montiel-Smith et al., 2002; see compound 2c therein). The enantiospecific aminohydroxylation reaction was carried out using tBuOCONHCl as nitrogen source, reagent previously prepared in situ by reacting tBuOCONH2 and tBuOCl in a NaOH solution. The last step to afford the title compound is a protection of the amine and hydroxyl groups of 3, via the formation of an oxazolidine, carried out by reacting 3 with 1-(dimethoxymethyl)-4-methoxybenzene in presence of pyridinium p-toluenesulfonate, in refluxing toluene. The isolated compound I was crystallized from AcOEt/heptane.
Refinement
The assignment of the absolute configuration of the three chiral centers was based on the stereospecificity of the synthetic pathway. The second synthetic step (see Fig. 1) allows to fix the stereochemistry for C4 and C5 centers. The last chiral center on C2, formed in the third step, is assigned as 2R relatively to the (4S,5R) stereoisomer. The reaction afforded a single stereoisomer. The formation of the chiral center 2R in I is in agreement with reports for related compounds (Denis et al., 1994). Measured Friedel pairs are not suitable for checking this assignation, and were merged (425 pairs). All H atoms were placed in idealized positions, with C—H bond lengths fixed to 0.98 (methine CH), 0.96 (methyl CH3, rigid groups free to rotate about the C—C bonds) or 0.93 Å (aromatic CH). Isotropic displacement parameters for H atoms were calculated as Uiso(H) = xUeq(carrier C), where x = 1.5 (methyl groups) or 1.2 (aromatic and methine CH).
Figures
Fig. 1.
The 3-steps synthesis of the title molecule, (I). i) SOCl2, MeOH, reflux; ii) (DHQ)2PHAL, n-PrOH/tBuOCONHCl; K2OsO2(OH)4, 0 °C; iii) p-MeOC6H4CH(OMe)2, PPTS, toluene, 80 °C.
Fig. 2.
ORTEP-like view of the title molecule, showing 30% displacement ellipsoids for non-H atoms.
Fig. 3.
Part of the crystal structure of the title compound, viewed along the b axis. In four molecules, the Boc substituents are shown using a spacefill representation, in order to emphasize the stacking for these groups in the crystal.
Crystal data
| C23H26N2O8 | F(000) = 484 |
| Mr = 458.46 | Dx = 1.304 Mg m−3 |
| Monoclinic, P21 | Melting point = 388–391 K |
| Hall symbol: P 2yb | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.383 (1) Å | Cell parameters from 58 reflections |
| b = 6.0303 (6) Å | θ = 3.9–11.9° |
| c = 18.7366 (17) Å | µ = 0.10 mm−1 |
| β = 95.591 (4)° | T = 298 K |
| V = 1167.57 (19) Å3 | Block, colourless |
| Z = 2 | 0.60 × 0.16 × 0.16 mm |
Data collection
| Siemens P4 diffractometer | Rint = 0.024 |
| Radiation source: fine-focus sealed tube | θmax = 25.0°, θmin = 2.0° |
| Graphite monochromator | h = −12→1 |
| ω scans | k = −7→1 |
| 3169 measured reflections | l = −22→22 |
| 2275 independent reflections | 3 standard reflections every 97 reflections |
| 1628 reflections with I > 2σ(I) | intensity decay: 0.5% |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.039 | H-atom parameters constrained |
| wR(F2) = 0.095 | w = 1/[σ2(Fo2) + (0.0467P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max < 0.001 |
| 2275 reflections | Δρmax = 0.12 e Å−3 |
| 304 parameters | Δρmin = −0.13 e Å−3 |
| 1 restraint | Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 constraints | Extinction coefficient: 0.017 (3) |
| Primary atom site location: structure-invariant direct methods |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.7821 (2) | 0.5989 (4) | 0.18897 (11) | 0.0582 (7) | |
| C2 | 0.8184 (3) | 0.5427 (6) | 0.26275 (16) | 0.0467 (8) | |
| H2A | 0.7769 | 0.6446 | 0.2942 | 0.056* | |
| N3 | 0.7630 (3) | 0.3207 (5) | 0.26723 (13) | 0.0474 (7) | |
| C4 | 0.7412 (3) | 0.2150 (6) | 0.19661 (14) | 0.0457 (8) | |
| H4A | 0.7970 | 0.0844 | 0.1947 | 0.055* | |
| C5 | 0.7866 (3) | 0.3983 (7) | 0.14856 (17) | 0.0543 (9) | |
| H5A | 0.7241 | 0.4104 | 0.1061 | 0.065* | |
| C6 | 0.9643 (3) | 0.5490 (5) | 0.28030 (15) | 0.0440 (8) | |
| C7 | 1.0343 (3) | 0.3772 (6) | 0.31379 (17) | 0.0508 (9) | |
| H7A | 0.9914 | 0.2492 | 0.3258 | 0.061* | |
| C8 | 1.1675 (3) | 0.3915 (7) | 0.32989 (17) | 0.0549 (9) | |
| H8A | 1.2129 | 0.2732 | 0.3520 | 0.066* | |
| C9 | 1.2322 (3) | 0.5807 (7) | 0.31311 (16) | 0.0519 (9) | |
| C10 | 1.1655 (4) | 0.7536 (7) | 0.27930 (19) | 0.0628 (10) | |
| H10A | 1.2090 | 0.8805 | 0.2668 | 0.075* | |
| C11 | 1.0321 (4) | 0.7370 (6) | 0.26391 (19) | 0.0603 (10) | |
| H11A | 0.9870 | 0.8558 | 0.2419 | 0.072* | |
| O12 | 1.3643 (2) | 0.5782 (5) | 0.33188 (12) | 0.0708 (8) | |
| C13 | 1.4373 (4) | 0.7685 (8) | 0.3159 (2) | 0.0840 (14) | |
| H13A | 1.5271 | 0.7437 | 0.3311 | 0.126* | |
| H13B | 1.4071 | 0.8949 | 0.3406 | 0.126* | |
| H13C | 1.4269 | 0.7953 | 0.2651 | 0.126* | |
| C14 | 0.7368 (3) | 0.2149 (6) | 0.32801 (17) | 0.0446 (8) | |
| O15 | 0.7056 (2) | 0.0214 (4) | 0.32967 (12) | 0.0564 (6) | |
| O16 | 0.7505 (2) | 0.3553 (4) | 0.38389 (10) | 0.0502 (6) | |
| C17 | 0.7426 (3) | 0.2749 (6) | 0.45810 (16) | 0.0556 (10) | |
| C18 | 0.7608 (4) | 0.4849 (8) | 0.5014 (2) | 0.0852 (14) | |
| H18A | 0.6974 | 0.5923 | 0.4834 | 0.128* | |
| H18B | 0.8460 | 0.5428 | 0.4975 | 0.128* | |
| H18C | 0.7506 | 0.4536 | 0.5507 | 0.128* | |
| C19 | 0.6121 (4) | 0.1736 (10) | 0.4654 (2) | 0.0896 (16) | |
| H19A | 0.5457 | 0.2681 | 0.4428 | 0.134* | |
| H19B | 0.6000 | 0.1575 | 0.5152 | 0.134* | |
| H19C | 0.6072 | 0.0307 | 0.4427 | 0.134* | |
| C20 | 0.8534 (4) | 0.1162 (10) | 0.4763 (2) | 0.0901 (14) | |
| H20A | 0.8410 | −0.0143 | 0.4471 | 0.135* | |
| H20B | 0.8566 | 0.0757 | 0.5260 | 0.135* | |
| H20C | 0.9333 | 0.1864 | 0.4674 | 0.135* | |
| C21 | 0.6011 (3) | 0.1515 (6) | 0.17565 (15) | 0.0439 (8) | |
| C22 | 0.4983 (3) | 0.2716 (7) | 0.19742 (18) | 0.0613 (10) | |
| H22A | 0.5140 | 0.3881 | 0.2296 | 0.074* | |
| C23 | 0.3719 (3) | 0.2199 (8) | 0.17162 (19) | 0.0719 (12) | |
| H23A | 0.3036 | 0.3021 | 0.1866 | 0.086* | |
| C24 | 0.3470 (3) | 0.0468 (8) | 0.12382 (18) | 0.0637 (11) | |
| H24A | 0.2627 | 0.0117 | 0.1060 | 0.076* | |
| C25 | 0.4499 (3) | −0.0711 (7) | 0.10355 (15) | 0.0494 (9) | |
| C26 | 0.5756 (3) | −0.0233 (6) | 0.12914 (15) | 0.0465 (9) | |
| H26A | 0.6432 | −0.1089 | 0.1150 | 0.056* | |
| N27 | 0.4263 (3) | −0.2579 (6) | 0.05319 (14) | 0.0626 (9) | |
| O28 | 0.3196 (3) | −0.2710 (6) | 0.01928 (13) | 0.0901 (11) | |
| O29 | 0.5151 (3) | −0.3873 (6) | 0.04674 (14) | 0.0893 (9) | |
| C30 | 0.9204 (4) | 0.3588 (8) | 0.12370 (18) | 0.0605 (11) | |
| O31 | 0.9865 (3) | 0.1999 (6) | 0.13651 (16) | 0.0899 (10) | |
| O32 | 0.9520 (3) | 0.5284 (7) | 0.08509 (16) | 0.1015 (11) | |
| C33 | 1.0810 (4) | 0.5279 (14) | 0.0607 (3) | 0.137 (3) | |
| H33A | 1.0921 | 0.6582 | 0.0326 | 0.206* | |
| H33B | 1.0913 | 0.3983 | 0.0321 | 0.206* | |
| H33C | 1.1446 | 0.5267 | 0.1015 | 0.206* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0616 (14) | 0.0538 (16) | 0.0564 (13) | −0.0020 (14) | −0.0088 (11) | 0.0128 (14) |
| C2 | 0.0548 (19) | 0.042 (2) | 0.0421 (17) | −0.0031 (18) | −0.0010 (14) | 0.0022 (17) |
| N3 | 0.0571 (17) | 0.0470 (17) | 0.0376 (14) | −0.0109 (15) | 0.0019 (12) | 0.0019 (14) |
| C4 | 0.0458 (18) | 0.052 (2) | 0.0385 (16) | −0.0010 (18) | −0.0007 (13) | −0.0027 (17) |
| C5 | 0.054 (2) | 0.063 (3) | 0.0437 (17) | −0.008 (2) | −0.0041 (15) | 0.005 (2) |
| C6 | 0.0543 (18) | 0.036 (2) | 0.0411 (16) | −0.0041 (18) | 0.0005 (15) | 0.0003 (16) |
| C7 | 0.053 (2) | 0.046 (2) | 0.0542 (19) | −0.0040 (19) | 0.0073 (16) | 0.0064 (18) |
| C8 | 0.057 (2) | 0.054 (2) | 0.054 (2) | 0.004 (2) | 0.0032 (16) | 0.0095 (19) |
| C9 | 0.051 (2) | 0.066 (3) | 0.0385 (16) | −0.007 (2) | 0.0045 (15) | −0.0062 (19) |
| C10 | 0.067 (2) | 0.051 (2) | 0.069 (2) | −0.016 (2) | 0.0007 (18) | 0.006 (2) |
| C11 | 0.063 (2) | 0.042 (2) | 0.073 (2) | −0.004 (2) | −0.0075 (18) | 0.005 (2) |
| O12 | 0.0519 (15) | 0.085 (2) | 0.0746 (15) | −0.0143 (17) | 0.0006 (12) | 0.0006 (17) |
| C13 | 0.062 (2) | 0.091 (4) | 0.100 (3) | −0.033 (3) | 0.014 (2) | −0.018 (3) |
| C14 | 0.0409 (18) | 0.047 (2) | 0.045 (2) | −0.0020 (19) | 0.0015 (14) | −0.0019 (19) |
| O15 | 0.0693 (15) | 0.0461 (16) | 0.0547 (14) | −0.0124 (14) | 0.0099 (11) | −0.0018 (12) |
| O16 | 0.0613 (14) | 0.0495 (14) | 0.0393 (11) | −0.0080 (13) | 0.0026 (10) | −0.0022 (11) |
| C17 | 0.065 (2) | 0.064 (3) | 0.0383 (17) | −0.011 (2) | 0.0088 (16) | 0.0024 (19) |
| C18 | 0.108 (3) | 0.092 (4) | 0.055 (2) | −0.026 (3) | 0.006 (2) | −0.017 (2) |
| C19 | 0.091 (3) | 0.113 (4) | 0.071 (2) | −0.035 (3) | 0.038 (2) | −0.016 (3) |
| C20 | 0.104 (3) | 0.103 (4) | 0.061 (2) | 0.011 (3) | −0.006 (2) | 0.022 (3) |
| C21 | 0.0427 (18) | 0.049 (2) | 0.0398 (15) | −0.0022 (17) | 0.0029 (14) | 0.0001 (17) |
| C22 | 0.055 (2) | 0.070 (3) | 0.059 (2) | 0.005 (2) | 0.0047 (17) | −0.012 (2) |
| C23 | 0.049 (2) | 0.096 (3) | 0.072 (2) | 0.014 (2) | 0.0076 (18) | −0.011 (3) |
| C24 | 0.045 (2) | 0.096 (3) | 0.0491 (18) | −0.007 (2) | 0.0001 (16) | −0.004 (2) |
| C25 | 0.052 (2) | 0.063 (2) | 0.0326 (15) | −0.0074 (19) | 0.0009 (14) | −0.0010 (17) |
| C26 | 0.047 (2) | 0.054 (2) | 0.0382 (16) | −0.0030 (17) | 0.0044 (14) | 0.0000 (17) |
| N27 | 0.068 (2) | 0.080 (3) | 0.0386 (15) | −0.023 (2) | −0.0005 (15) | −0.0020 (18) |
| O28 | 0.0673 (17) | 0.139 (3) | 0.0621 (15) | −0.040 (2) | −0.0047 (13) | −0.0214 (19) |
| O29 | 0.105 (2) | 0.087 (2) | 0.0720 (17) | 0.006 (2) | −0.0127 (16) | −0.0255 (19) |
| C30 | 0.061 (3) | 0.077 (3) | 0.0432 (19) | −0.016 (3) | 0.0004 (17) | 0.007 (2) |
| O31 | 0.079 (2) | 0.099 (3) | 0.098 (2) | 0.005 (2) | 0.0398 (16) | 0.007 (2) |
| O32 | 0.0703 (18) | 0.138 (3) | 0.0973 (19) | −0.023 (2) | 0.0104 (15) | 0.049 (2) |
| C33 | 0.066 (3) | 0.223 (8) | 0.127 (4) | −0.030 (4) | 0.031 (3) | 0.062 (5) |
Geometric parameters (Å, º)
| O1—C5 | 1.430 (4) | C17—C18 | 1.506 (5) |
| O1—C2 | 1.437 (4) | C17—C20 | 1.510 (6) |
| C2—N3 | 1.462 (5) | C18—H18A | 0.9600 |
| C2—C6 | 1.519 (4) | C18—H18B | 0.9600 |
| C2—H2A | 0.9800 | C18—H18C | 0.9600 |
| N3—C14 | 1.356 (4) | C19—H19A | 0.9600 |
| N3—C4 | 1.466 (4) | C19—H19B | 0.9600 |
| C4—C21 | 1.518 (4) | C19—H19C | 0.9600 |
| C4—C5 | 1.529 (5) | C20—H20A | 0.9600 |
| C4—H4A | 0.9800 | C20—H20B | 0.9600 |
| C5—C30 | 1.526 (5) | C20—H20C | 0.9600 |
| C5—H5A | 0.9800 | C21—C26 | 1.377 (4) |
| C6—C7 | 1.381 (4) | C21—C22 | 1.384 (5) |
| C6—C11 | 1.385 (5) | C22—C23 | 1.390 (5) |
| C7—C8 | 1.389 (5) | C22—H22A | 0.9300 |
| C7—H7A | 0.9300 | C23—C24 | 1.383 (6) |
| C8—C9 | 1.376 (5) | C23—H23A | 0.9300 |
| C8—H8A | 0.9300 | C24—C25 | 1.368 (5) |
| C9—C10 | 1.372 (5) | C24—H24A | 0.9300 |
| C9—O12 | 1.383 (4) | C25—C26 | 1.376 (4) |
| C10—C11 | 1.391 (5) | C25—N27 | 1.474 (5) |
| C10—H10A | 0.9300 | C26—H26A | 0.9300 |
| C11—H11A | 0.9300 | N27—O29 | 1.223 (4) |
| O12—C13 | 1.423 (5) | N27—O28 | 1.224 (4) |
| C13—H13A | 0.9600 | C30—O31 | 1.189 (5) |
| C13—H13B | 0.9600 | C30—O32 | 1.313 (5) |
| C13—H13C | 0.9600 | O32—C33 | 1.456 (5) |
| C14—O15 | 1.212 (4) | C33—H33A | 0.9600 |
| C14—O16 | 1.343 (4) | C33—H33B | 0.9600 |
| O16—C17 | 1.483 (4) | C33—H33C | 0.9600 |
| C17—C19 | 1.504 (5) | ||
| C5—O1—C2 | 106.9 (2) | C19—C17—C18 | 111.1 (3) |
| O1—C2—N3 | 101.7 (2) | O16—C17—C20 | 107.9 (3) |
| O1—C2—C6 | 111.4 (2) | C19—C17—C20 | 113.3 (4) |
| N3—C2—C6 | 113.6 (3) | C18—C17—C20 | 111.0 (3) |
| O1—C2—H2A | 110.0 | C17—C18—H18A | 109.5 |
| N3—C2—H2A | 110.0 | C17—C18—H18B | 109.5 |
| C6—C2—H2A | 110.0 | H18A—C18—H18B | 109.5 |
| C14—N3—C2 | 126.3 (3) | C17—C18—H18C | 109.5 |
| C14—N3—C4 | 121.8 (3) | H18A—C18—H18C | 109.5 |
| C2—N3—C4 | 111.9 (3) | H18B—C18—H18C | 109.5 |
| N3—C4—C21 | 113.8 (2) | C17—C19—H19A | 109.5 |
| N3—C4—C5 | 100.8 (3) | C17—C19—H19B | 109.5 |
| C21—C4—C5 | 111.9 (2) | H19A—C19—H19B | 109.5 |
| N3—C4—H4A | 110.0 | C17—C19—H19C | 109.5 |
| C21—C4—H4A | 110.0 | H19A—C19—H19C | 109.5 |
| C5—C4—H4A | 110.0 | H19B—C19—H19C | 109.5 |
| O1—C5—C30 | 111.8 (3) | C17—C20—H20A | 109.5 |
| O1—C5—C4 | 105.8 (2) | C17—C20—H20B | 109.5 |
| C30—C5—C4 | 114.1 (3) | H20A—C20—H20B | 109.5 |
| O1—C5—H5A | 108.3 | C17—C20—H20C | 109.5 |
| C30—C5—H5A | 108.3 | H20A—C20—H20C | 109.5 |
| C4—C5—H5A | 108.3 | H20B—C20—H20C | 109.5 |
| C7—C6—C11 | 117.3 (3) | C26—C21—C22 | 118.7 (3) |
| C7—C6—C2 | 123.3 (3) | C26—C21—C4 | 118.5 (3) |
| C11—C6—C2 | 119.4 (3) | C22—C21—C4 | 122.6 (3) |
| C6—C7—C8 | 121.4 (3) | C21—C22—C23 | 120.6 (4) |
| C6—C7—H7A | 119.3 | C21—C22—H22A | 119.7 |
| C8—C7—H7A | 119.3 | C23—C22—H22A | 119.7 |
| C9—C8—C7 | 120.0 (3) | C24—C23—C22 | 120.4 (4) |
| C9—C8—H8A | 120.0 | C24—C23—H23A | 119.8 |
| C7—C8—H8A | 120.0 | C22—C23—H23A | 119.8 |
| C10—C9—C8 | 120.0 (3) | C25—C24—C23 | 118.0 (3) |
| C10—C9—O12 | 124.7 (4) | C25—C24—H24A | 121.0 |
| C8—C9—O12 | 115.3 (4) | C23—C24—H24A | 121.0 |
| C9—C10—C11 | 119.2 (4) | C24—C25—C26 | 122.3 (3) |
| C9—C10—H10A | 120.4 | C24—C25—N27 | 119.2 (3) |
| C11—C10—H10A | 120.4 | C26—C25—N27 | 118.5 (3) |
| C6—C11—C10 | 122.0 (4) | C25—C26—C21 | 119.9 (3) |
| C6—C11—H11A | 119.0 | C25—C26—H26A | 120.0 |
| C10—C11—H11A | 119.0 | C21—C26—H26A | 120.0 |
| C9—O12—C13 | 118.2 (3) | O29—N27—O28 | 124.0 (4) |
| O12—C13—H13A | 109.5 | O29—N27—C25 | 118.1 (3) |
| O12—C13—H13B | 109.5 | O28—N27—C25 | 117.9 (4) |
| H13A—C13—H13B | 109.5 | O31—C30—O32 | 124.7 (4) |
| O12—C13—H13C | 109.5 | O31—C30—C5 | 126.1 (4) |
| H13A—C13—H13C | 109.5 | O32—C30—C5 | 109.3 (4) |
| H13B—C13—H13C | 109.5 | C30—O32—C33 | 117.2 (5) |
| O15—C14—O16 | 126.5 (3) | O32—C33—H33A | 109.5 |
| O15—C14—N3 | 123.4 (3) | O32—C33—H33B | 109.5 |
| O16—C14—N3 | 110.0 (3) | H33A—C33—H33B | 109.5 |
| C14—O16—C17 | 120.9 (3) | O32—C33—H33C | 109.5 |
| O16—C17—C19 | 110.5 (3) | H33A—C33—H33C | 109.5 |
| O16—C17—C18 | 102.4 (3) | H33B—C33—H33C | 109.5 |
| C5—O1—C2—N3 | −34.8 (3) | C4—N3—C14—O15 | 7.2 (5) |
| C5—O1—C2—C6 | 86.6 (3) | C2—N3—C14—O16 | 9.3 (4) |
| O1—C2—N3—C14 | −160.3 (3) | C4—N3—C14—O16 | −172.8 (3) |
| C6—C2—N3—C14 | 80.0 (4) | O15—C14—O16—C17 | 7.2 (5) |
| O1—C2—N3—C4 | 21.7 (3) | N3—C14—O16—C17 | −172.8 (2) |
| C6—C2—N3—C4 | −98.1 (3) | C14—O16—C17—C19 | −60.6 (4) |
| C14—N3—C4—C21 | 60.8 (4) | C14—O16—C17—C18 | −179.0 (3) |
| C2—N3—C4—C21 | −121.0 (3) | C14—O16—C17—C20 | 63.8 (4) |
| C14—N3—C4—C5 | −179.3 (3) | N3—C4—C21—C26 | −152.8 (3) |
| C2—N3—C4—C5 | −1.1 (3) | C5—C4—C21—C26 | 93.8 (4) |
| C2—O1—C5—C30 | −89.2 (3) | N3—C4—C21—C22 | 31.9 (4) |
| C2—O1—C5—C4 | 35.6 (3) | C5—C4—C21—C22 | −81.6 (4) |
| N3—C4—C5—O1 | −20.3 (3) | C26—C21—C22—C23 | −1.4 (5) |
| C21—C4—C5—O1 | 100.9 (3) | C4—C21—C22—C23 | 174.0 (3) |
| N3—C4—C5—C30 | 103.0 (3) | C21—C22—C23—C24 | 0.1 (6) |
| C21—C4—C5—C30 | −135.8 (3) | C22—C23—C24—C25 | 0.5 (6) |
| O1—C2—C6—C7 | −129.1 (3) | C23—C24—C25—C26 | 0.1 (5) |
| N3—C2—C6—C7 | −15.0 (4) | C23—C24—C25—N27 | 179.4 (3) |
| O1—C2—C6—C11 | 52.5 (4) | C24—C25—C26—C21 | −1.4 (5) |
| N3—C2—C6—C11 | 166.7 (3) | N27—C25—C26—C21 | 179.3 (3) |
| C11—C6—C7—C8 | −0.5 (5) | C22—C21—C26—C25 | 2.0 (4) |
| C2—C6—C7—C8 | −178.9 (3) | C4—C21—C26—C25 | −173.6 (3) |
| C6—C7—C8—C9 | 0.7 (5) | C24—C25—N27—O29 | −165.8 (3) |
| C7—C8—C9—C10 | −1.2 (5) | C26—C25—N27—O29 | 13.5 (5) |
| C7—C8—C9—O12 | 179.6 (3) | C24—C25—N27—O28 | 15.7 (5) |
| C8—C9—C10—C11 | 1.5 (5) | C26—C25—N27—O28 | −164.9 (3) |
| O12—C9—C10—C11 | −179.3 (3) | O1—C5—C30—O31 | 122.5 (4) |
| C7—C6—C11—C10 | 0.9 (5) | C4—C5—C30—O31 | 2.5 (5) |
| C2—C6—C11—C10 | 179.3 (3) | O1—C5—C30—O32 | −57.8 (4) |
| C9—C10—C11—C6 | −1.4 (5) | C4—C5—C30—O32 | −177.8 (3) |
| C10—C9—O12—C13 | 0.6 (5) | O31—C30—O32—C33 | −4.6 (6) |
| C8—C9—O12—C13 | 179.8 (3) | C5—C30—O32—C33 | 175.7 (4) |
| C2—N3—C14—O15 | −170.7 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2A···O15i | 0.98 | 2.50 | 3.400 (4) | 153 |
| C5—H5A···O28ii | 0.98 | 2.59 | 3.387 (4) | 138 |
| C26—H26A···O1iii | 0.93 | 2.59 | 3.252 (4) | 128 |
Symmetry codes: (i) x, y+1, z; (ii) −x+1, y+1/2, −z; (iii) x, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RZ5008).
References
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Denis, J.-N., Kanazawa, A. M. & Green, A. E. (1994). Tetrahedron Lett. 35, 105–108.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Montiel-Smith, S., Cervantes-Mejía, V., Dubois, J., Guénard, D., Guéritte, F. & Sandoval-Ramírez, J. (2002). Eur. J. Org. Chem. pp. 2260–2264.
- Rudolph, J., Sennhenn, P. C., Vlaar, C. P. & Sharpless, K. B. (1996). Angew. Chem. Int. Ed. 35, 2810–2813.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1996). XSCANS Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Tinant, B., Declercq, J. P. & Cagnon, J. R. (1996). Bull. Soc. Chim. Belg. 105, 325–328.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S160053681204192X/rz5008sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681204192X/rz5008Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681204192X/rz5008Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report





