Abstract
For a better understanding of Al inhibition of root elongation, knowledge of the morphological and functional organization of the root apex is a prerequisite. We developed a polyvinyl chloride-block technique to supply Al (90 μm monomeric Al) in a medium containing agarose to individual 1-mm root zones of intact seedlings of maize (Zea mays L. cv Lixis). Root elongation was measured during a period of 5 h. After Al treatment, callose (5 h) and Al (1 h) contents of individual 1-mm apical root segments were determined. For comparison, callose and Al levels were also measured in root segments after uniform Al supply in agarose blocks to the 10-mm root apex. Only applying Al to the three apical 1-mm root zones inhibited root elongation after 1 h. The order of sensitivity was 1 to 2 > 0 to 1 > 2 to 3 mm. In the 1- to 2-mm root zone high levels of Al-induced callose formation and accumulation of Al was found, independently of whether Al was applied to individual apical root zones or uniformly to the whole-root apex. We conclude from these results that the distal part of the transition zone of the root apex, where the cells are undergoing a preparatory phase for rapid elongation (F. Baluška, D. Volkmann, P.W. Barlow [1996] Plant Physiol 112: 3–4), is the primary target of Al in this Al-sensitive maize cultivar.
It is now generally accepted that the root apex plays a major role in the Al-perception and -response mechanisms (for recent reviews, see Delhaize and Ryan, 1995; Horst, 1995; Kochian, 1995; Taylor, 1995; Rengel, 1996). This is especially well demonstrated by the fact that Al sensitivity is characterized by enhanced accumulation of Al in the root apex (Delhaize et al., 1993a; Llugany et al., 1994; Samuels et al., 1997); Al-resistance mechanisms, such as the release of Al-complexing organic compounds, are confined mainly to the root apex (Horst et al., 1982; Delhaize et al., 1993b; Pellet et al., 1995); and callose formation, a sensitive marker of Al toxicity, is primarily induced in apical root cortical cells (Wissemeier et al., 1987; Zhang et al., 1994; Wissemeier and Horst, 1995).
In the past the root apex has been divided into three different zones: the root cap, the MZ, and the EZ. Bennet and Breen (1991a) attributed a major role to the root cap in the perception of Al toxicity in maize (Zea mays L.). However, Ryan et al. (1993) concluded from their divided-chamber experiments that the MZ (0–3 mm behind the root cap), and not the root cap, was the most Al-sensitive site in maize. Based on short-term studies of Al effects on cell division and cell elongation in root apices of soybean, Horst and Klotz (1990) attributed the inhibition of root elongation by Al to the inhibition of cell elongation rather than cell division. However, whether Al primarily interferes with processes related to cell division or cell elongation has not yet been unequivocally clarified.
In recent years our knowledge of the morphological and functional organization of the root apex has grown substantially. On the basis of morphological, cytological, and physiological characteristics, Ishikawa and Evans (1993, 1995, and refs. therein) and Baluška et al. (1996, and refs. therein) classified different zones of the maize root apex on a millimeter scale (the subdivision of EZ into apical EZ and central EZ is arbitrarily defined by Ishikawa and Evans [1993]) for maize plants grown in humid air under similar conditions, as in this study: MZ, 0 to 1.7; distal EZ or TZ, 1.7 to 3.4; apical EZ, 3.4 to 3.9; central EZ, 3.9 to 5.6; and EZ, >5.6. It appeared to us that for a better understanding of the inhibition of root elongation by Al and Al resistance, consideration of this spatial organization of the root apex is a prerequisite. In this report we have tested the responses of the first 5 mm of the maize root apex individually to study the differential responses of these zones to Al.
The presented experiments are based on the study of Ryan et al. (1993). However, we tried to avoid some of the shortcomings of that study by: (a) using agarose instead of agar as the culture medium (Calba et al., 1996); (b) increasing the spatial resolution by applying Al to 1-mm intact root segments; and (c) keeping the seedlings in a vertical position during the treatment to avoid interference with the physiological responses to gravity and hormones (Hasenstein et al., 1988; Ishikawa and Evans, 1993). In addition to growth, induction of callose formation, the most sensitive indicator of Al toxicity (Wissemeier et al., 1987), and accumulation of Al in 1-mm sections along the root apex were measured.
MATERIALS AND METHODS
Growth Conditions
Seeds of maize (Zea mays L. cv Lixis) that has been classified as Al sensitive (Llugany et al., 1994) were soaked in warm tap water for 8 h. Then the water was decanted and the seeds were held at 4 to 6°C for 20 h in darkness to induce synchronized germination (Shen-Miller et al., 1978). The seeds were germinated between filter paper-Styrofoam sandwiches for 6 d in a growth chamber under controlled environmental conditions with 70% RH, 30°C day and night temperature, and 300 μmol m−2 s−1 photon flux density during the 16-h d.
Uniform seedlings were then transferred to the PVC blocks especially designed to fit the experimental requirements (Fig 1A). Five seedlings were mounted on the PVC block with different apical root positions placed into the horizontal 1-mm slit, which was vertically sealed with petroleum jelly (Fig. 1B). Low-gelling agarose (0.6%) dissolved in NS by heating was poured into the horizontal slit using a fine-tipped Pasteur pipette just before solidification to completely embed the 1-mm root segments. For the Al treatments, 300 μm Al from an Al atomic spectroscopy standard stock solution (1000 mg L−1, Fluka) was added to the cooled agarose solution to achieve a final monomeric Al concentration (aluminon method, according to Kerven et al. [1989]) of 90 μm, measured after filtration through a 0.4-μm cellulose acetate filter (Sartorius, Hayward, CA). This agarose solution was eluted from the solidified agarose gel (see above), which was incubated for 2 d under room temperature, frozen at −20°C for 120 min, and then centrifuged for 3 min at 12,000g.
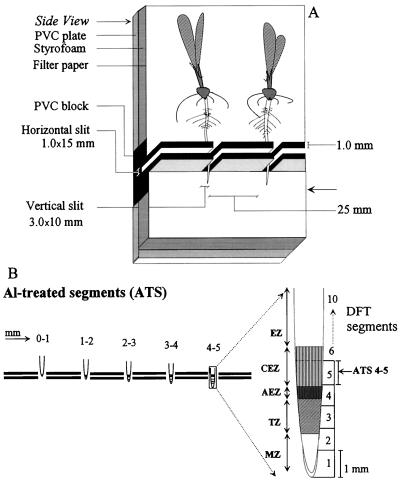
Figure 1.
The experimental setup of the PVC-block technique used in the present study (A). One PVC block consisted of five vertical slits, allowing us to mount five seedlings simultaneously at five different ATS positions (B). The horizontal slits were filled with agarose medium after positioning the roots and sealing the vertical slits with petroleum jelly. The setup was immersed in an upright position into NS up to the level indicated by the arrow. Once the seedlings were in place, the roots were covered with filter paper and kept moistened by frequent spraying with NS. ATS in relation to described morphological and physiological organization of the root apex (see introduction) are shown in B. AEZ, Apical EZ; CEZ, central EZ.
Great care was taken to keep the whole-root system moist during all manipulations and the subsequent treatment period. This was achieved by soaking the plant-supporting Styrofoam and filter paper in NS, covering the roots with NS-moistened filter paper, and spraying profusely with NS using a hand sprayer. The NS used for the preparation of the agarose medium had the following composition (μm): CaSO4, 250; KNO3, 400; MgSO4, 100; MnSO4, 1; ZnSO4, 0.1; CuSO4, 0.2; KH2PO4, 10; H3BO3, 8; (NH4)6Mo7O24, 0.1; and NH4NO3, 200. The pH of the NS was adjusted to 4.3 with 0.1 n HCl. The mounting of the plants on one horizontally positioned PVC block never took more than 5 to 10 min. Afterward, the PVC block was placed in an upright position into plastic troughs containing NS to the level marked by an arrow in Figure 1A and placed in a growth chamber under the conditions described above. At the end of the treatment period, 1 h for Al uptake and 5 h for the determination of root-elongation rate and callose formation, 5-cm-long root tips were dissected under double-distilled water and then fixed in 96% ethanol. In addition to the PVC-block technique, which allowed the Al treatment of 1-mm root segments, parallel experiments were performed in which the whole apical 10-mm root tips of intact seedlings were inserted in agarose blocks (50 mm × 50 mm) in small plastic trays with or without Al under conditions similar to those described above.
Determination of Root-Elongation Rate
Root-elongation rate was calculated from measurements of root lengths at 1-h intervals under 40× magnification against a scale, and by having the horizontal slit of the PVC block as the reference line. The precision of every measurement was 125 μm. Because the roots were positioned vertically, gravity-induced curvature did not complicate the measurements.
Determination of Callose in Root Segments
Roots fixed in 96% ethanol to avoid formation of wound callose were briefly washed and kept in a Petri dish containing double-distilled water. Under a stereomicroscope (Stemi SV8, Zeiss) with 20× magnification, roots were cut into 10 consecutive 1-mm segments starting at the apex, including the cap, using a razor blade. Two (Al treatment of individual 1-mm root segments) or 10 (Al treatment of the entire root apex) root segments from the identical DFT positions were pooled together to increase the precision of callose determination. After dissection, the segments were carefully blotted dry and transferred immediately to Eppendorf tubes containing 1 m NaOH. Callose levels were estimated following the method of Köhle et al. (1985), which was modified slightly to increase its sensitivity. Briefly, each sample containing the similarly treated root segments in NaOH was ultrasonicated directly (Bandalin Sonopuls, Bandalin Electronics, Berlin, Germany) for 1 min. Subsequently, the samples were placed in a water bath (80°C) for 30 min to solubilize the callose and then centrifuged for 15 min at 12,000g at room temperature. Callose concentration in the supernatant was quantified fluorimetrically (f-2000, excitation at 393 nm and emission at 484 nm; Hitachi, Tokyo, Japan) with decolorized aniline blue using Pachyman (250–690 polymerization grade, Calbiochem) as a reference. The callose contents of 1-mm Al treatments were expressed as Al-induced callose, which means that the callose levels of respective control segments (−Al) were subtracted from the Al-treated root segments. Callose contents are expressed as Pachyman equivalents on a millimeter basis.
Determination of Al in Root Segments
After a brief rinse of the excised roots in upw (18.2 MΩ, E-pure, D4642, Barnstead, Dubuque, IA), individual 1-mm root segments were dissected under upw from fresh root tips within 30 min after the treatment period. The root segments were individually placed into Eppendorf cups containing 250 μL of upw, frozen, and kept at −20°C until analysis. For the Al analysis the root segments including the upw were transferred to 5-mL Teflon cups and heated on a hot plate to 120°C to evaporate the upw. Then 500 μL of concentrated, double-distilled, ultra-pure HNO3 was added, and the temperature increased to 240°C for the digestion of the root segments until the acid was completely evaporated. Because of the low Al concentrations in the samples (from 0.002 to 0.35 nmol), great care had to be taken to avoid Al contamination while preparing the samples. This was achieved by using upw and 1:30 (v/v) HNO3:upw-washed Teflon labware. The ash was dissolved in 2 mL of 1:30 (v/v) HNO3:upw. Samples were analyzed for Al using a UNICAM 939 QZ graphite furnace atomic absorption spectrophotometer (Analytical Technology Inc., Cambridge, UK) with Zeeman background compensation. Instrumental adjustments were optimized for sensitivity. Al contents of Al-treated root segments were corrected for mean Al contents from blanks and root segments not treated with Al (control).
Statistical Analysis
Results are presented from a representative of six (root elongation), two (Al content), and two (callose content) experiments. Root-elongation experiments consisted of five independent replicates for each ATS and control position. All other experiments had three to six independent replicates. The statistical package SAS (version 6.11, SAS Institute, Cary, NC) was used for the calculation of the se and comparisons of the means (Tukey's test).
RESULTS
Root Elongation
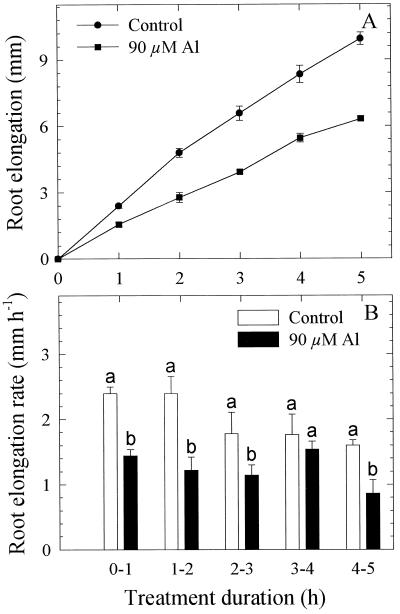
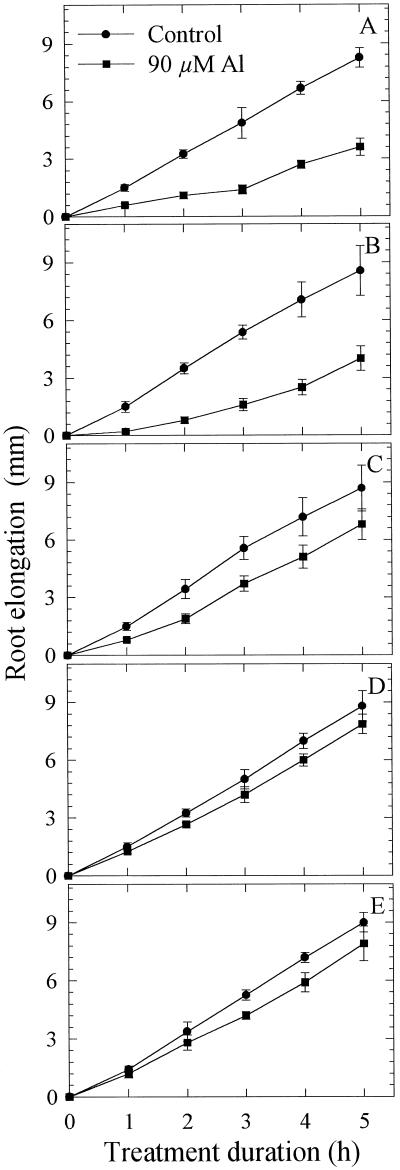
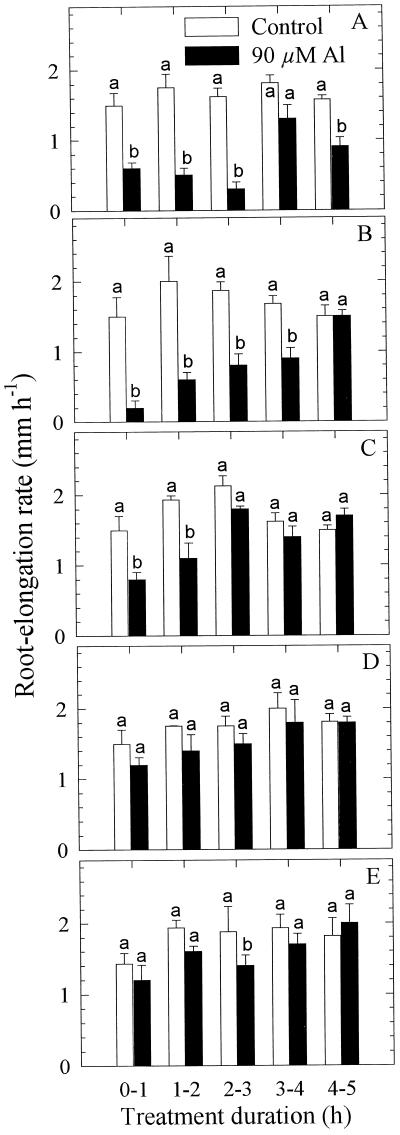
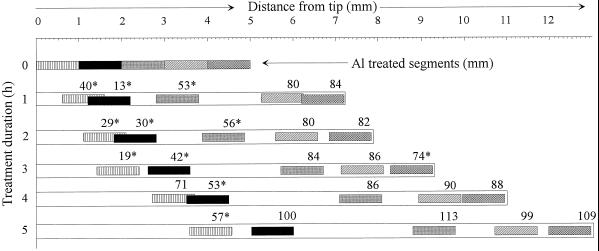
In the first approach Al was applied to the entire root apex (10 mm) using the agarose-block technique. In the presence of 90 μm Al, root elongation was inhibited as early as 1 h after the commencement of the Al treatment. Root-elongation rate was reduced to about 50% of the control over the entire 5-h Al-treatment duration (Fig. 2, A and B). Application of Al to individual 1-mm segments of the root apex led to inhibition of root elongation especially when the first 3-mm apical root segments were exposed to Al (Fig. 3). It appeared that the immediate (0–2 h) comparative sensitivity to Al between the root segments was 1 to 2 > 0 to 1 > 2 to 3 mm. With longer duration of the Al treatment, the roots seem to resume elongation growth. The immediate response and recovery from the initial Al response becomes distinct when root-elongation rates after 1 h are compared (Fig. 4). A differential response pattern between the root segments is apparent. Application of Al to the 0- to 1-mm segment becomes increasingly inhibitory to root elongation, whereas the inhibition is initially (after 1 h) maximum when Al is applied to the 1- to 2-mm segment (significant at P < 0.05 for the comparison of comparable root segments between ATS positions). With longer Al treatment duration, root-elongation rates nearly completely recovered: after 5 h of Al supply, root-elongation rate was no longer significantly different from the untreated control. Although there was a tendency toward decreased root-elongation rates when Al was applied to the 3- to 4-mm and 4- to 5-mm root segments, this difference was generally insignificant compared with the untreated controls.
Figure 2.
Effect of Al supply (5 h) in agarose medium to the entire (10 mm) root apex on the root elongation (A) and elongation rate (B) of seedlings of cv Lixis. Means ± se are of five independent replicates. Means with different letters are significantly different (P < 0.05, Tukey test) with regard to the Al effect.
Figure 3.
Effect of Al supply (5 h) in agarose medium (PVC-block technique) to individual 1-mm apical root segments on the root elongation of seedlings of cv Lixis. A, ATS 0 to 1 mm; B, 1 to 2 mm; C, 2 to 3 mm; D, 3 to 4 mm; and E, 4 to 5 mm. Means ± se are of five independent replicates.
Figure 4.
Effect of Al supply (5 h) in agarose medium (PVC-block technique) to individual 1-mm apical root segments on the root-elongation rate of seedlings of cv Lixis. Means ± se are of five independent replicates. Means with different letters are significantly different (P < 0.05, Tukey test) with regard to the Al effect.
Because the roots continued to grow during the 5-h Al treatment period, the Al-treatment zone moved along the root. Therefore, in Figure 5 root-elongation rates are presented in relation to the root segment in contact with Al after every 1 h of Al treatment. This presentation clearly shows that the root becomes increasingly sensitive to Al when the treatment zone moved into the 1- to 2.5-mm section and increasingly recovered when it moved out of this zone. Only one value (0- to 1-mm Al-treated segment after 4 h) does not fit this pattern. It is further confirmed that application of Al, not only to the 3- to 5-mm root segments but also up to 20 mm, did not lead to significant inhibition of root elongation.
Figure 5.
Effect of Al supply (5 h) in agarose medium (PVC-block technique) to individual 1-mm apical root segments on the root-elongation rate of seedlings of cv Lixis. Root length was measured at 1-h intervals and root-elongation rates were calculated. Initially (time 0) Al was applied to the 1- to 5-mm apical root segments. The position of the ATS after every 1 to 5 h of treatment is visualized by the hatched areas. The numbers represent mean elongation rates expressed as percent over control for the previous 1-h period. Numbers with asterisks (*) are significantly different (P < 0.05, Tukey test).
Callose Formation
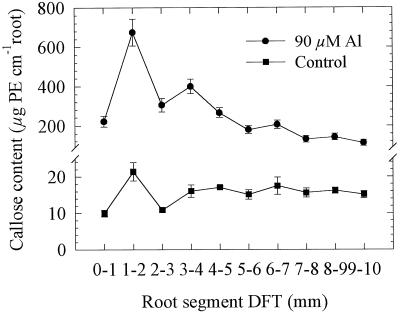
Callose formation is a sensitive indicator of Al injury to roots. In a preliminary approach Al was applied by incubating the whole 10-mm root apex using agarose blocks (Fig. 6). Callose contents were higher in Al-treated roots by a factor of about 10. They showed a sharp maximum (factor 3) in the root segment for 1 to 2 mm and then declined steadily toward more basal parts of the root. Also, in the controls without Al a slight peak of callose content could be seen.
Figure 6.
Effect of Al supply (5 h) in agarose medium (agarose blocks) to the 10-mm root apex on callose contents (Pachyman equivalents [PE]) of individual 1-mm root segments of seedlings of cv Lixis. Means ± se are of three independent replicates, each comprising 10 1-mm root segments of identical DFTs.
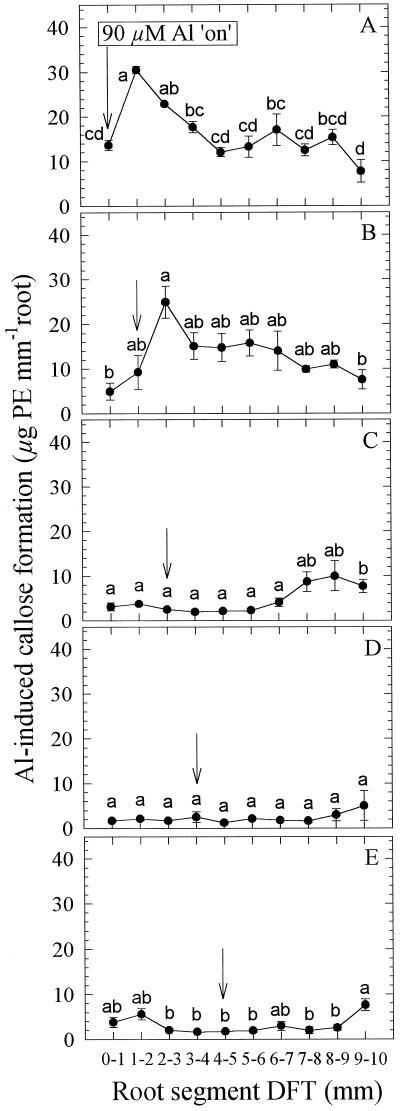
When Al was applied for 5 h to individual 1-mm root segments, the pattern of Al-induced callose formation showed a similar pattern along the root apex only when Al was applied to the first two apical root segments (Fig. 7). Generally, the callose content was lower by a factor of 2, probably because of the fact that the individual root segments of the growing root were in contact with Al for a shorter period of time, compared with the preliminary experiment. Application of Al to root segment 1 (Fig. 7A) and segment 2 (Fig. 7B) led to maximum callose formation in root segments 2 and 3, respectively. Al-induced callose formation gradually decreased toward more basal root segments but remained significantly different from zero. Application of Al to more basal apical root segments (Fig. 7, C–E) did not induce callose formation in the treated or adjacent root segments. There was a tendency for enhanced callose formation only in the basal root segments.
Figure 7.
Effect of Al supply (5 h) in agarose medium (PVC-block technique) to 1-mm apical root segments on Al-induced callose contents (expressed as Pachyman equivalents [PE]) of individual 1-mm root segments of seedlings of cv Lixis. A, ATS 0 to 1 mm; B, 1 to 2 mm; C, 2 to 3 mm; D, 3 to 4 mm; and E, 4 to 5 mm. Means ± se are of six independent replicates, each comprising four 1-mm root segments of identical DFTs. Means with different letters are significantly different (P < 0.05, Tukey test) with regard to the Al effect.
Al Content
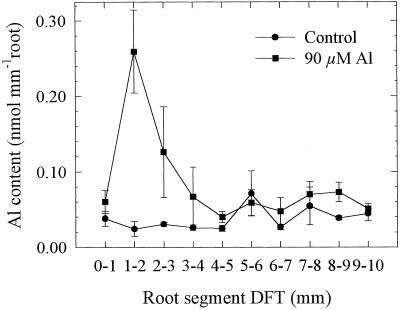
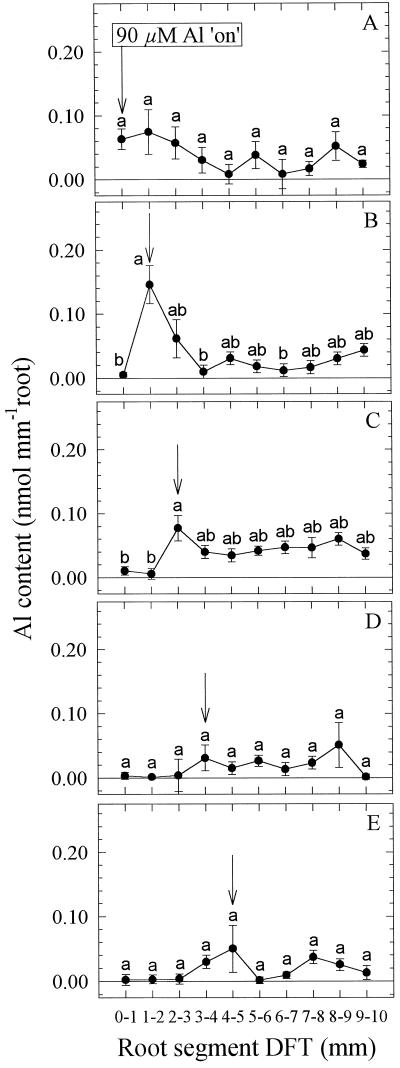
In contrast to the experiments described above, to focus on the initial Al effects on root elongation Al was applied only for 1 h (compare Figs. 3 and 4). In a first approach, Al was uniformly applied to the whole 10-mm apex using agarose blocks (Fig. 8). There was a maximum Al content in the root segment at 1 to 2 mm, sharply declining to control (without Al) levels toward the apex and more-basal root segments. The same pattern of distribution of Al between the root segments was found when Al was individually applied only to the 1- to 2-mm segment (Fig. 9B). Treatment of the other root segments did not lead to significantly higher Al contents in the treated compared with the untreated root segments, although a slight increase in the treated 3- to 5-mm segments (Fig. 9, C–E) and a broader peak in the three apical segments could be noticed when Al was applied to the 0- to 1-mm segment (Fig. 9A).
Figure 8.
Effect of Al supply (1 h) in agarose medium (agarose blocks) to the 10-mm root apex on Al contents of individual 1-mm root segments of seedlings of cv Lixis. Means ± se are of six independent replicates, each comprising one 1-mm root segment.
Figure 9.
Effect of Al supply (1 h) in agarose medium (PVC-block technique) to 1-mm apical root segments on Al contents of individual 1-mm root segments of seedlings of cv Lixis. A, ATS 0 to 1 mm; B, 1 to 2 mm; C, 2 to 3 mm; D, 3 to 4 mm; and E, 4 to 5 mm. Means ± se are of six independent replicates each comprising one 1-mm root segment. Means with different letters are significantly different (P < 0.05, Tukey test) with regard to the Al effect.
DISCUSSION
With the present experimental setup, we have tried to avoid some of the shortcomings of the previous study by Ryan et al. (1993) on the spatial sensitivity of maize roots to Al. First, the seedlings were kept in a vertical position to avoid any disturbance of the root response to gravity, such as modification of the pH gradients (Monshausen et al., 1996), Ca, and auxin flow (Hasenstein and Evans, 1988; Ishikawa and Evans, 1992, 1993), which might interfere with the response of the roots to Al. Second, agarose was used instead of agar, because the phytotoxic level of Al in the agarose, but not in the agar solution, is comparable to NS (Calba et al., 1996). Third, the total Al supply to individual root segments was increased considerably by the greater agarose volume in contact with the root zone. Nevertheless, a much higher Al concentration had to be applied for rapid inhibition of maize root elongation than in NS (Hasenstein and Evans, 1988; Ryan et al., 1993), because in NS, a much larger pool of Al is in direct contact with the root. Fourth, Al could be applied to 1-mm root segments, which allowed us to increase the resolution of the Al sensitivity of the root apex, which was necessary to relate the Al response corresponding to the spatial organization of the root apex (Fig. 1B) (Ishikawa and Evans, 1993, 1995; Baluška et al., 1994, 1996).
We are aware of the fact that the length of the different developmental apical root zones may vary according to the experimental conditions, as was shown by Ishikawa and Evans (1995). Therefore, we placed our emphasis on the creation of experimental conditions similar to those described by Ishikawa and Evans (1993). It is out of the scope of the present study to verify the accuracy of the spatial organization of the maize root apex for our experimental conditions, because the conclusions drawn by us do not require accuracy at the 0.1 mm level.
A shortcoming of the technique applied here might be the fact that the site of Al treatment moved during the course of the experiment because of root growth. However, as shown in Figure 5, the dynamics of the Al/root-zone interaction provided further support to our suggestion that the distal part of the EZ is the most Al-sensitive site of the root apex: (a) application of Al to the 1- to 2-mm, and not the 0- to 1-mm, root segment led to the most severe inhibition of root elongation; (b) movement of the Al-treated segment from the root tip into the 1- to 2.5-mm position enhanced inhibition of root elongation, and movement out of this zone led to alleviation of this inhibition; and (c) recovery from Al injury was faster after Al exposure to the 2- to 3-mm root segment compared with the 1- to 2-mm root segment.
This suggestion is strongly supported by the following results of the present study:
(a) The maximum induction of callose formation by Al occurs in this root zone (Figs. 6 and 7). Induction of callose formation has been shown to be a sensitive response to Al in roots and indicative of genotypic differences in Al sensitivity in soybean (Wissemeier and Horst, 1995), wheat (Zhang et al., 1994), and maize (Llugany et al., 1994; Horst et al., 1997).
(b) The high Al sensitivity of the DTZ is related to preferential Al accumulation in this root zone (1–2 mm) when Al is supplied uniformly to the whole-root apex (Fig. 8) or specifically to this root zone (Fig. 9) as well. Accumulation of Al in the apical 0- to 2-mm root zone has recently been shown to be indicative of genotypic Al sensitivity in wheat (Samuels et al., 1997). This is in agreement with the classification of the maize cultivar used in this study as Al sensitive (Llugany et al., 1994; Horst et al., 1997). Al resistance seems to be related to the plant's capacity to protect this sensitive root zone from Al, e.g. by the release of organic acids such as malate in wheat (Delhaize et al., 1993b; Ryan et al., 1995) and citrate in maize (Pellet et al., 1995).
These authors have demonstrated that the root apex is the most important site of organic acid excretion. However, based on the results presented in this study it is desirable to differentiate between the different apical root zones of the root apex. The reason for preferential Al accumulation in the DTZ compared with the main MZ (0–1 mm) and EZ is not known. Reduced Al uptake by the main MZ might be attributable to excretion of mucilage, which strongly binds Al (Archambault et al., 1996) and protects the root apex from Al injury (Horst et al., 1982). In the EZ, in contrast to the DTZ and TZ, an enhanced release of protons, leading to acidification of the apoplast (Monshausen et al., 1996) and thereby facilitating root growth (Weisenseel et al., 1992; Zieschang et al., 1993), could reduce the binding of Al to negative charges (Grauer and Horst, 1992; Kinraide, 1993).
The results presented do not support the hypothesis put forward by Bennet and Breen (1991b) that the root cap is the most sensitive perceptor of the Al signal. In contrast, they are in agreement with the results of Ryan et al. (1993), who showed that in horizontally growing maize roots the removal of the root cap did not modify the response of the roots to Al, and concluded that the root zone 0 to 3 mm behind the root cap is most sensitive to Al. In our study we could localize the most Al-sensitive root zone even more precisely: following the proposed spatial organization of the root apex (Fig. 1B) the most Al-sensitive zone is the DTZ (Baluška et al., 1996) or distal part of the distal EZ (Ishikawa and Evans, 1993, 1995). Particularly, this zone (DTZ) is characterized by a switch from cell division to cell elongation by the presence of cells that are changing their mitotic mode and undergoing a preparatory phase for rapid elongation. It is noteworthy that the TZ is the zone most responsive to a variety of environmental stimuli other than Al, such as gravitropism and auxin (Meuwly and Pilet, 1991; Ishikawa and Evans, 1993), thigmotropism (Ishi-kawa and Evans, 1992), mechanical impedance (Ishikawa and Evans, 1990), and drought stress (Sharp et al., 1988). Although our results indicate a special role of the distal part of the TZ in the expression of Al toxicity, we do not exclude that the proximal part of the MZ may also be involved.
Al accumulation in the DTZ led to a rapid inhibition of root elongation within less than 1 h (Figs. 4B and 9). This is difficult to reconcile with a direct effect of Al on rapid cell elongation in the EZ. Therefore, it is necessary to envisage interference of Al in the DTZ with the signaling system involved in the regulation of cell elongation. Earlier work by Hasenstein et al. (1988) also points to the same conclusion, although it must be interpreted with care because of the extremely high Al concentration and the pH buffer used. They showed that application of Al to one side of the cap of a vertically growing maize root induced curvature away from the Al source. In a subsequent study Hasenstein and Evans (1988) presented evidence that this Al effect was related to localized inhibitory effects of Al on basipetal auxin transport in the root cortex.
In conclusion, on the basis of the presented results we suggest that the DTZ of the root apex is the primary target of Al in an Al-sensitive maize cultivar. Further elucidation of Al toxicity and resistance requires focus to be placed on this root zone and on the signaling pathways between it and the EZ.
ACKNOWLEDGMENTS
We sincerely thank Hock Werner (Institute for Horticultural Engineering, University of Hannover) for his patience in manufacturing the PVC blocks to our specifications, Lutz Collet for his support during the refinement of the Al analysis, and the anonymous reviewers for their critical comments on the manuscript.
Abbreviations:
- ATS
Al-treated segment
- DFT
distance from tip (including cap)
- DTZ
distal part of the transition zone
- EZ
elongation zone
- MZ
meristematic zone
- NS
nutrient solution
- PVC
polyvinyl chloride
- TZ
transition zone
- upw
ultra-pure water
Footnotes
This research was supported by a grant from the German Research Foundation to W.J.H., and from an Indo (Ministry of Human Resource Development, Department of Education, Government of India)-German postdoctoral fellowship awarded by the German Academic Exchange Service, Bonn, to M.S.
LITERATURE CITED
- Archambault DJ, Zhang G, Taylor GJ. Accumulation of Al in root mucilage of an Al-resistant and an Al-sensitive cultivar of wheat. Plant Physiol. 1996;112:1471–1478. doi: 10.1104/pp.112.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Barlow PW, Kubica S. Importance of the post-mitotic isodiametric growth (PIG) region for growth and development of roots. Plant Soil. 1994;167:31–42. [Google Scholar]
- Baluška F, Volkmann D, Barlow PW. Specialized zones of development in roots. View from the cellular level. Plant Physiol. 1996;112:3–4. doi: 10.1104/pp.112.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet RJ, Breen CM. The recovery of the roots of Zea mays L. from various aluminum treatments: towards elucidating the regulatory processes that underlie root growth control. Environ Exp Bot. 1991a;157:447–451. [Google Scholar]
- Bennet RJ, Breen CM. The aluminium signal: new dimensions to mechanisms of aluminium tolerance. Plant Soil. 1991b;134:153–166. [Google Scholar]
- Calba H, Jaillard B, Fallavier C, Arvieu J-C. Agarose as a suitable substrate for use in the study of Al dynamics in the rhizosphere. Plant Soil. 1996;178:67–74. [Google Scholar]
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.). I. Uptake and distribution of aluminum in root apices. Plant Physiol. 1993a;103:685–693. doi: 10.1104/pp.103.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993b;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauer UE, Horst WJ. Modelling cation amelioration of aluminum phytotoxicity. Soil Sci Soc Am J. 1992;56:166–172. [Google Scholar]
- Hasenstein KH, Evans ML. Effects of cations on hormone transport in primary roots of Zea mays. Plant Physiol. 1988;86:890–894. doi: 10.1104/pp.86.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein KH, Evans ML, Stinemetz CL, Moore R, Fondren WM, Koon EC, Higby MA, Smucker AJM. Comparative effectiveness of metal ions in inducing curvature of primary roots of Zea mays. Plant Physiol. 1988;86:885–889. doi: 10.1104/pp.86.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ. The role of the apoplast in aluminum toxicity and resistance of higher plants: a review. Z Pflanzenernaehr Bodenkd. 1995;158:419–428. [Google Scholar]
- Horst WJ, Klotz F. Screening soybean for aluminum tolerance and adaptation to acid soils. In: El Bassam N, Dambroth M, Loughman BC, editors. Genetic Aspects of Plant Mineral Nutrition. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 355–360. [Google Scholar]
- Horst WJ, Püschel AK, Schmohl N. Induction of callose formation is a sensitive marker for genotypic aluminium sensitivity in maize. Plant Soil. 1997;192:23–30. [Google Scholar]
- Horst WJ, Wagner A, Marschner H. Mucilage protects root meristems from aluminium injury. Z Pflanzenphysiol. 1982;105:435–444. 192: 23–30. [Google Scholar]
- Ishikawa H, Evans ML. Electrotropism of maize roots: role of the root cap and relationship to gravitropism. Plant Physiol. 1990;94:913–918. doi: 10.1104/pp.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML. Introduction of curvature of maize roots by calcium or by thigmostimulation. Role of the post mitotic isodiametric growth zone. Plant Physiol. 1992;100:762–768. doi: 10.1104/pp.100.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML. The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol. 1993;102:1203–1210. doi: 10.1104/pp.102.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML. Specialized zones of development in roots. Plant Physiol. 1995;109:725–727. doi: 10.1104/pp.109.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerven GL, Edwards DG, Asher CJ, Hallman PS, Kokot S. Aluminium determination in soil solution. II. Short-term colorimetric procedures for the measurement of inorganic monomeric aluminium in the presence of organic acid ligands. Aust J Soil Res. 1989;27:91–102. [Google Scholar]
- Kinraide TB. Aluminum enhancement of plant growth in acid rooting media: a case of reciprocal alleviation of toxicity by two toxic cations. Physiol Plant. 1993;88:619–625. doi: 10.1111/j.1399-3054.1993.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Köhle H, Jeblick W, Poten W, Blashek W, Kauss H. Chitosan-elicited callose synthesis in soybean cells as a Ca2+-dependent process. Plant Physiol. 1985;77:544–551. doi: 10.1104/pp.77.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llugany M, Massot N, Wissemeier AH, Poschenrieder C, Horst WJ, Barcelo J. Aluminum tolerance of maize cultivars as assessed by callose production and root elongation. Z Pflanzenernaehr Bodenkd. 1994;157:447–451. [Google Scholar]
- Meuwly P, Pilet PE. Local treatment with indole-3-acetic acid influences differential growth responses in Zea mays L. roots. Planta. 1991;185:58–64. doi: 10.1007/BF00194515. [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Zieschang HE, Sievers A. Differential proton secretion in the apical elongation zone caused by gravistimulation is induced by a signal from the root cap. Plant Cell Environ. 1996;19:1408–1414. doi: 10.1111/j.1365-3040.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.) Planta. 1995;196:788–795. [Google Scholar]
- Rengel Z. Uptake of aluminium by plant cells. New Phytol. 1996;134:389–406. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterization of Al-stimulated efflux of malate from apices of Al-tolerant wheat roots. Planta. 1995;196:103–110. [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot. 1993;44:437–446. [Google Scholar]
- Samuels TD, Kücükakyüz K, Rincon M. Al partitioning patterns and root growth as related to Al sensitivity and Al tolerance in wheat. Plant Physiol. 1997;113:527–534. doi: 10.1104/pp.113.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, Silk WK, Hsiao TC. Growth of the maize primary root at low water potentials. I. Spatial distribution of expansive growth. Plant Physiol. 1988;87:50–57. doi: 10.1104/pp.87.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J, McNitt RE, Wojciechowski M. Regions of differential cell elongation and mitosis, and root meristem morphology in different tissues of geotropically stimulated maize root apices. Plant Physiol. 1978;61:7–12. doi: 10.1104/pp.61.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ. Overcoming barriers to understanding the cellular basis of aluminum resistance. Plant Soil. 1995;171:89–103. [Google Scholar]
- Weisenseel MH, Becker HF, Ehlgötz JG. Growth, gravitropism, and endogenous ion currents of cress roots (Lepidium sativum L.). Measurements using a novel three-dimensional recording probe. Plant Physiol. 1992;100:16–25. doi: 10.1104/pp.100.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissemeier AH, Horst WJ. Effect of calcium supply on aluminium-induced callose formation, its distribution and persistence in roots of soybean (Glycine max (L.) Merr.) J Plant Physiol. 1995;145:470–476. [Google Scholar]
- Wissemeier AH, Klotz F, Horst WJ. Aluminium induced callose synthesis in roots of soybean (Glycine max L.) J Plant Physiol. 1987;129:487–492. [Google Scholar]
- Zhang G, Hoddinott J, Taylor GJ. Characterization of 1,3-β-D-glucan (callose) synthesis in roots of Triticum aestivum in response to aluminum toxicity. J Plant Physiol. 1994;144:229–234. [Google Scholar]
- Zieschang HE, Köhler K, Sievers A. Changing proton concentrations at the surfaces of gravistimulated Phleum roots. Planta. 1993;190:546–554. [Google Scholar]