Abstract
We examined seven enterotoxigenic Escherichia coli strains which produced colonization factor antigen I (CFA/I). Four of these strains were from South Africa (three serotype O78:H12 and one serotype O63:H-), one was from Ethiopia (O78:H12), and two were from Bangladesh (O78:H11 and O78:H12). Plasmids coding for CFA/I were mobilized from six of these strains by using resistance or enterotoxin factors. No plasmid was mobilized from the serotype O78:H12 Bangladesh strain. The transconjugants obtained from crosses with the O78 strains also produced heat-stable enterotoxin (ST), and additional investigations showed that CFA/I and ST were coded for by a single non-autotransferring plasmid. These plasmids were fertility inhibition negative, did not restrict any of the coliphages with which they were tested, and were incompatible with each other. Four had molecular weights of approximately 60 X 10(6), and one had a molecular weight of 52 X 10(6). Like the other CFA/I plasmids, the CFA/I plasmid transferred from the O63:H- strain coded for ST, but this plasmid also coded for heat-labile enterotoxin. In most other respects the properties of this plasmid were similar to those of the CFA/I-ST plasmids previously described. The molecular weight of this plasmid was 65 X 10(6). The IncT R-factor Rtsl was marked with a transposon for tetracycline resistance and then transferred into the two Bangladesh wild-type strains. Plasmids which coded for tetracycline resistance, CFA/I, and ST were transferred from these strains. These plasmids were incompatible with Rtsl and with the CFA/I-ST plasmids described above and were recombinants between Rtsl and a CFA/I-ST plasmid. Their properties are also described.
Full text
PDF



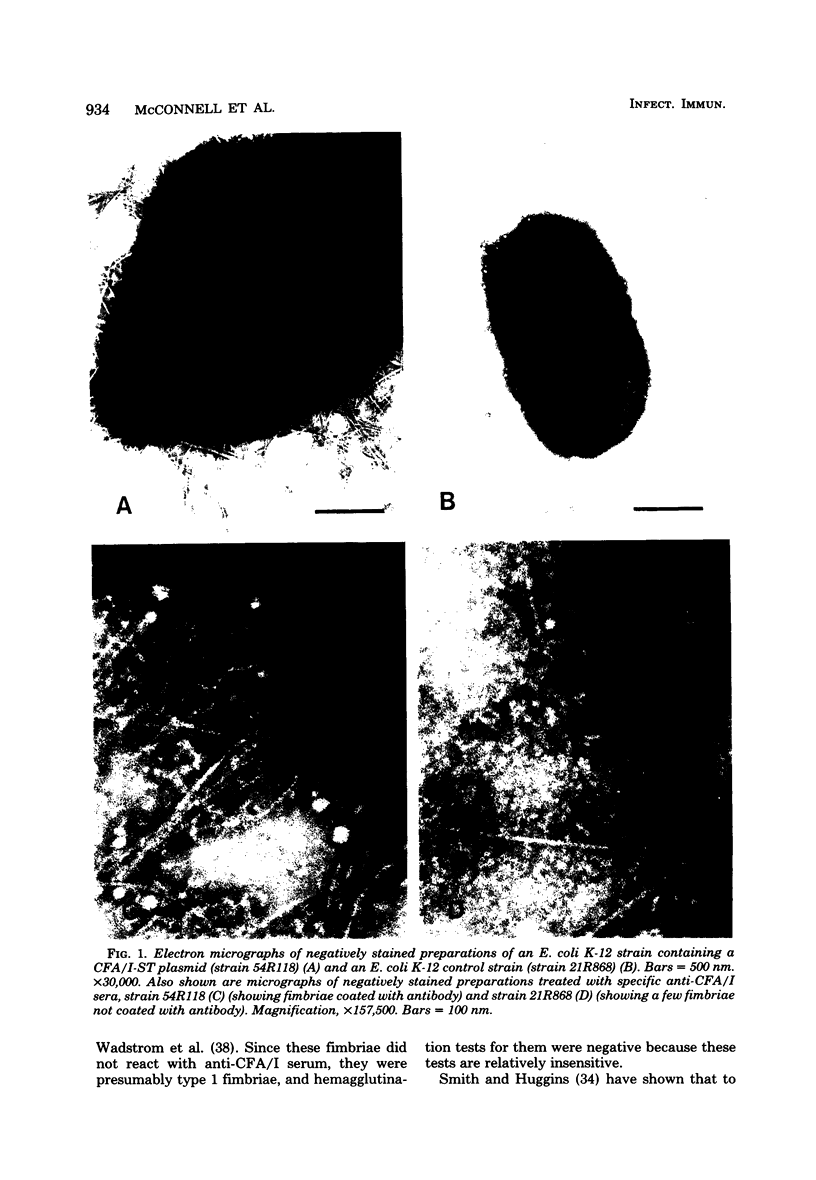


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S., Lewis M. J. Drug resistance and its transfer in Salmonella typhimurium. Nature. 1965 May 8;206(984):579–583. doi: 10.1038/206579a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Threlfall E. J., Carr J. M., McConnell M. M., Smith H. R. Clonal distribution of resistance plasmid-carrying Salmonella typhimurium, mainly in the Middle East. J Hyg (Lond) 1977 Dec;79(3):425–448. doi: 10.1017/s0022172400053286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Moon H. W., Whipp S. C. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science. 1974 Jan 25;183(4122):334–336. doi: 10.1126/science.183.4122.334. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. S., DuPont H. L. Detection and characterization of colonization factor of enterotoxigenic Escherichia coli isolated from adults with diarrhea. Infect Immun. 1978 Feb;19(2):727–736. doi: 10.1128/iai.19.2.727-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Grindley J. N., Smith H. R., Anderson E. S. Characterisation of derepressed mutants of an F-like R factor. Mol Gen Genet. 1973 Jan 18;120(1):27–34. doi: 10.1007/BF00332982. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J Gen Microbiol. 1974 Sep;84(1):135–144. doi: 10.1099/00221287-84-1-135. [DOI] [PubMed] [Google Scholar]
- McConnell M. M., Smith H. R., Willshaw G. A., Scotland S. M., Rowe B. Plasmids coding for heat-labile enterotoxin production isolated from Escherichia coli O78: comparison of properties. J Bacteriol. 1980 Jul;143(1):158–167. doi: 10.1128/jb.143.1.158-167.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M. M., Willshaw G. A., Smith H. R., Scotland S. M., Rowe B. Transposition of ampicillin resistance to an enterotoxin plasmid in an Escherichia coli strain of human origin. J Bacteriol. 1979 Aug;139(2):346–355. doi: 10.1128/jb.139.2.346-355.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine small intestine by Escherichia coli: ileal colonization and adhesion by pig enteropathogens that lack K88 antigen and by some acapsular mutants. Infect Immun. 1976 Apr;13(4):1214–1220. doi: 10.1128/iai.13.4.1214-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., SOJKA W. J., WITTIG W. K ANTIGENS K88AB(L) AND K88AC(L) IN E. COLI. A NEW O ANTIGEN: 0147 AND A NEW K ANTIGEN: K89(B). Acta Pathol Microbiol Scand. 1964;62:439–447. doi: 10.1111/apm.1964.62.3.439. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Special O:K:H serotypes among enterotoxigenic E. coli strains from diarrhea in adults and children. Occurrence of the CF (colonization factor) antigen and of hemagglutinating abilities. Med Microbiol Immunol. 1977 Jul 18;163(2):99–110. doi: 10.1007/BF02121825. [DOI] [PubMed] [Google Scholar]
- Orskov I., Sharma V., Orskov F. Genetic mapping of the K1 and K4 antigens (L) of Escherichia coli. Non-allelism of K(L) antigens with K antigens of O8:K27(A), O8:K8(L) and O9:K57(B). Acta Pathol Microbiol Scand B. 1976 Jun;84(3):125–131. [PubMed] [Google Scholar]
- Reis M. H., Heloiza M., Affonso T., Trabulsi L. R., Mazaitis A. J., Maas R., Maas W. K. Transfer of a CFA/I-ST plasmid promoted by a conjugative plasmid in a strain of Escherichia coli of serotype O128ac:H12. Infect Immun. 1980 Jul;29(1):140–143. doi: 10.1128/iai.29.1.140-143.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite T. K., Evans D. G., DuPont H. L., Evans D. J., Jr Role of Escherichia coli colonisation factor antigen in acute diarrhoea. Lancet. 1978 Jul 22;2(8082):181–184. doi: 10.1016/s0140-6736(78)91921-9. [DOI] [PubMed] [Google Scholar]
- Scotland S. M., Day N. P., Cravioto A., Thomas L. V., Rowe B. Production of heat-labile or heat-stable enterotoxins by strains of Escherichia coli belonging to serogroups O44, O114, and O128. Infect Immun. 1981 Jan;31(1):500–503. doi: 10.1128/iai.31.1.500-503.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. R., Humphreys G. O., Anderson E. S. Genetic and molecular characterisation of some non-transferring plasmids. Mol Gen Genet. 1974 Mar 27;129(3):229–242. doi: 10.1007/BF00267915. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The influence of plasmid-determined and other characteristics of enteropathogenic Escherichia coli on their ability to proliferate in the alimentary tracts of piglets, calves and lambs. J Med Microbiol. 1978 Nov;11(4):471–492. doi: 10.1099/00222615-11-4-471. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Grant R. B. Incompatibility and bacteriophage inhibition properties of N-1, a plasmid belonging to the H2 incompatibility group. Mol Gen Genet. 1977 May 20;153(1):5–10. doi: 10.1007/BF01035990. [DOI] [PubMed] [Google Scholar]
- Terawaki Y., Takayasu H., Akiba T. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967 Sep;94(3):687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willshaw G. A., Smith H. R., Anderson E. S. Application of agarose gel electrophoresis to the characterization of plasmid DNA in drug-resistant enterobacteria. J Gen Microbiol. 1979 Sep;114(1):15–25. doi: 10.1099/00221287-114-1-15. [DOI] [PubMed] [Google Scholar]