Abstract
Background
Plasmacytoid dendritic cells (DC) play a crucial role in antiviral immunity and promoting Th1 polarization, possibly protecting against development of allergic disease.
Objective
Examination of the relationship between peripheral blood plasmacytoid DC levels and manifestations of asthma and atopy early in life.
Methods
We have isolated peripheral blood mononuclear cells (PBMC) from 73 children (mean age ± SD: 6.6 ± 0.5 years old) participating in the RSV Bronchiolitis in Early Life (RBEL) study. Flow cytometry was performed on PBMC detecting DC surface-markers: Blood Dendritic Cell Antigens (BDCA) 1, 3, and 2 which identify myeloid type 1, type 2, and plasmacytoid cells respectively. Total serum IgE, peripheral eosinophil count, and allergy skin tests were documented.
Results
45% (n=33) of study participants had physician-diagnosed asthma by 6 years of age. These children had significantly lower quantities (mean ± SD) of plasmacytoid DC than their non-asthmatic counterparts (1020 ± 921 vs. 1952 ± 1170 cells per 106 PBMC, p=0.003). We found significantly lower numbers of myeloid dendritic cells in children with asthma (3836 ± 2472 cells per 106 PBMC) compared with those without (4768 ± 2224 cells per 106 PBMC, p=0.02); however, this divergence was not significant after adjusting for covariates of age, gender, race, skin test reactivity, smoke exposure, and day care attendance. We did not identify any direct association between DC levels and markers of atopy: skin test reactivity, peripheral eosinophilia, and IgE level.
Conclusion
Children who are diagnosed with asthma after severe RSV bronchiolitis appear to have a relative deficiency of plasmacytoid DC in peripheral blood.
Keywords: dendritic cell, asthma, respiratory syncytial virus
Introduction
By age of 2 years, most children experience an infection with Respiratory syncytial virus (RSV), with approximately 2–3% developing RSV bronchiolitis severe enough to be hospitalized.1,2 Children with an RSV infection necessitating a hospitalization demonstrate skewed T helper 2 (Th2) cytokine responses during the acute illness and, by some reports, show increased prevalence of atopy after years of follow up.3,4 A substantial proportion of children who are hospitalized with RSV bronchiolitis go on to develop recurrent wheezing and asthma.3,5 The immunologic markers that predict the development of asthma after a severe RSV infection remain to be elucidated.
Since asthma is associated with inflammation characteristically dominated by Th2-dominated immune response, factors that promote T helper 1(Th1) cytokine production and inhibit Th2 response may protect from development of atopy and asthma.6,7 One candidate which influences the Th1/Th2 balance is the dendritic cell (DC). During antigen presentation to naïve T cells, myeloid dendritic cells (mDC) elaborate various signals that skew naive lymphocytes into either Th1 or Th2 cytokine producing cells.8,9 The plasmacytoid dendritic cell (pDC), on the other hand, specializes in Type I Interferon (IFN) production which enhances anti-viral immunity, inhibits Th2 cytokine responses, and promotes regulatory T cells.9 Upon viral infection, pDC migrate to peripheral lymph-nodes that drain the sites of inflammation and secrete IFN, augmenting the Th1 immune response.10,11 In a murine asthma model, depletion of pDC exacerbates the asthma phenotype and increases Th2 cell cytokine production, whereas, exogenous transfer of pDC diminished this response.12
If pDC are associated with an attenuation of Th2 related inflammation, we propose that following severe RSV bronchiolitis lower levels of pDC would be detected in children with asthma (a Th2-dominant condition) than those children without asthma.
Methods
Peripheral blood samples were obtained from a subset of 73 consecutive children that participated in the RSV Bronchiolitis in Early Life (RBEL) study - a prospective cohort of 206 children with a severe episode of RSV bronchiolitis in the first year of life. The details on enrollment and characterization of this cohort are described elsewhere.13 Briefly, all subjects were recruited as infants (≤1 year of age, mean age 4 months) during an initial episode of wheezing in a context of severe RSV bronchiolitis that necessitated hospitalization. This cohort has been prospectively followed for six years with clinic visits, telephone follow up, biannual blood collection, and pulmonary function tests. Upon reaching 6 years of age, the primary outcome – manifestation of asthma phenotype – was defined as parental affirmation to the specific inquiry “Has the doctor ever told you that your child had asthma since your last follow-up?”, with confirmation from the physician’s medical records. The children whose parents denied a previous diagnosis of were assigned to “non-asthma” category. The decision to initiate inhaled corticosteroids and dose/duration of therapy was left up to subjects’ primary care providers. Washington University Institutional Review Board approved the study protocol and parents provided written informed consent.
We used frozen peripheral blood mononuclear cells (PMBC) samples isolated from RBEL subjects blood samples drawn between 6 to 7 years of age (6.6 ± 0.5 years, mean ± SD). Briefly, the subjects’ PBMC were isolated from whole blood using Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ) centrifugation at 13.2 G for 30 minutes at 18o C. The cells harvested from the middle layer of Ficoll-Hypaque were washed with PBS and 10% inactivated FBS. The PBMC were incubated with 1 μL ionomycin 1 μg/ml, and 2.5 μL PMA, 20μg/ml, for 4 hours at 37° C to evaluate for cytokine expression. The cells were than washed, counted in the BD FACSCalibur system (BD biosciences, San Jose, CA), and re-suspended at 106 PBMC per 240 μl of PBS/10%FBS/0.5% bovine serum albumin buffer. The samples were combined with 20 μl of anti-Blood Dendritic Cell Antigen (BDCA) cocktail, dead cell discriminator, or isotype antibody controls from Blood Dendritic Cell Enumeration Kit as per manufacture’s instructions (Miltenyi Biotec Inc., Auburn, CA). pDC were identified by florescence from anti-BDCA-2 antibody, and type-1 and type-2 mDC (mDC1 and mDC2) were identified by anti-BDCA-1 and anti-BDCA-3 antibodies respectively using the gating strategies outlined by the manufacturer. These surface markers have been shown to identify the myeloid and plasmacytoid subsets with very high fidelity – without having to rely on CD11c/CD123 antibodies.14 We counted 200,000 cells and multiplied the frequency of each dendritic cell subset by the total number of cells in the sample, i.e. 106 PBMC. Thus, each DC subset is reported as a number of cells per 106 PBMC (see example in Figure 1).
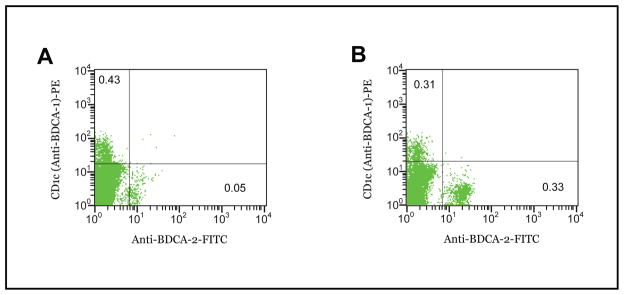
Figure 1. Representative Flow Cytometry Results for myeloid and plasmacytoid dendritic cell markers (BDCA-1 vs. BDCA-2, respectively) between asthma and nonasthma patients.
A. In the patient with asthma, only 0.05% of peripheral cells are identified with anti-BDCA-2 antibody yielding a total count of 500 plasmacytoid dendritic cells per 106 PBMC. B. In a nonasthmatic patient, the number of cells with BDCA-2 surface antigen is >6 fold greater, 0.33% of PBMC counted, or 3300 plasmacytoid dendritic cells per 106 PBMC.
Atopy characteristics were assessed by measuring total serum IgE and absolute peripheral eosinophil count at the time of follow up. In addition, at 3 years of age, the children underwent skin prick testing using the MultiTest II (Lincoln Laboratories, Lincoln, IL) with panel of 12 prevalent inhalant and food allergens, including dust mite mix, cat [standardized], dog [mixed breed], German Cockroach, Penicillium species mix, Aspergillus sp. mix, Timothy grass, short ragweed [standardized], eastern tree mix, egg, peanut, and milk (Greer Labs, Lenoir, NC) as described by Childhood Asthma Management Program (CAMP).15 The subjects were considered “skin test positive” if they had at least one allergen which produced a wheal 3 mm or greater than saline control. Life time prevalence of eczema was assessed quarterly by the following question: “Has doctor ever told you your child has eczema?”
The statistical analysis compared the quantities of pDC and mDC subsets, ratio of mDC1/pDC, mDC1 + mDC2, total serum IgE levels, absolute eosinophil counts, among subjects classified as asthmatic versus non-asthmatic counterparts using non-parametric analysis, Wilcoxon signed-rank test. Categorical variables, such as presence second hand smoke exposure or history of eczema were compared using Chi-square test. Logistic regression analysis was performed to adjust for age, gender, breast feeding history, daycare attendance, skin test reactivity at 3 years of age, pet exposure, income level, pre/postnatal smoke exposure, and therapy with inhaled corticosteroid using SAS software (version 9.1.2).
Results
Blood samples from 73 consecutive subjects age 6 were available for this sub-study. To date, in this sub-study, 33 children (45%) have been classified as having physician-diagnosed asthma and 40 children have never in their life been diagnosed with asthma. Table 1 compares baseline characteristics between asthma and non-asthma subjects, as well as, between this sub-study and the entire RBEL cohort. We found significantly greater prevalence of aeroallergen sensitization at age 3 years among children diagnosed with asthma by age 6 years compared to non-asthmatics (45.5% vs 17.5%, p=0.01). Food sensitization was demonstrated in 5 asthmatics and 1 non-asthmatics: all had positive skin tests to egg and none were sensitized to peanut or milk. All of egg-sensitive subjects exhibited sensitization to at least one inhalant allergen, so aeroallergen sensitivity alone identified all of the atopic subjects. We also observed that 42% of children with asthma were treated with inhaled corticosteroids vs. 11% of non-asthmatics (p=0.001). Otherwise, the cohorts were similar in the distribution of the baseline characteristics, including the age at which RSV infection took place, breast feeding, eczema, and second hand smoke exposure.
Table I.
Subject’s Characteristics and Selected Atopy Markers
| RBEL cohort† | Dendritic Cell Sub-study | |||
|---|---|---|---|---|
|
|
||||
| Asthma & Non-asthma Combined | Asthma | Non-asthma | ||
| N | 204 | 73 | 33 | 40 |
| Female, % | 42.6 | 38.4 | 48.5 | 30.0 |
| Age, month, at which RSV infection occurred, mean (SD) | 4.4 (3.2) | 4.5 (3.2) | 4.0 (3.0) | 5.1 (3.1) |
| Race, % | ||||
| Caucasian | 52.5 | 52.0 | 45.5 | 57.5 |
| African-American | 44.1 | 42.5 | 48.5 | 37.5 |
| Other | 3.4 | 5.5 | 6.0 | 5.0 |
| Smoking Exposure | ||||
| Prenatal, % | 24.5 | 27.4 | 21.2 | 32.5 |
| Postnatal, % | 63.7 | 67.1 | 66.7 | 67.5 |
| Asthma in First Degree Relative, % | 28.4 | 34.3 | 33.3 | 35.0 |
| Breast Feeding Prevalence, % | 50.5 | 43.8 | 51.5 | 37.5 |
| Pet, lifetime ownership | ||||
| Cat, % | 39.7 | 48.0 | 42.4 | 52.5 |
| Dog, % | 60.3 | 63.01 | 60.6 | 65.0 |
| Household income <$20,000, % | 42.7 | 41.1 | 45.5 | 37.5 |
| Lifetime Inhaled Corticosteroid Use, % (‡) | 42.4 | 11.1 | ||
| Atopy characteristics: | ||||
| Eczema, % | 32.8 | 32.9 | 36.6 | 30.0 |
| Skin test reactivity at 3 years of age, % (*) | 22.6 | 30.1 | 45.5 | 17.5 |
| Serum IgE level at 6 years of age, IU/ml, mean (SD) | 31.6 (5.0) | 41.5 (4.5) | 36.2 (4.9) | 46.3 (4.3) |
| Absolute Peripheral Eosinophil count at 6 years of age, cells/mm3, mean (SD) | 162.5 (2.3) | 155.8 (2.1) | 153.4 (2.4) | 157.7 (1.9) |
Asthma vs. Non-asthma: p = 0.01
Asthma vs. Non-asthma: p=0.001
RBEL = Respiratory syncytial virus bronchiolitis in early life cohort; RSV = Respiratory synctial virus; SD = standard deviation
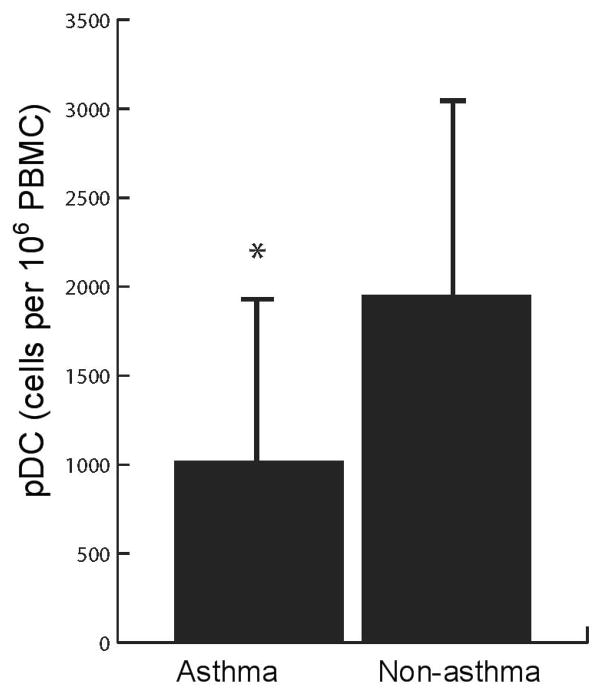
Subjects with asthma had a significantly lower mean quantity of peripheral pDC as compared with non-asthmatics (1020±921 vs. 1952±1170 cells per 106 PBMC, mean ± SD, p=0.0005) (Figure 2). Children with asthma also exhibited lower levels of mDC; however, their myeloid/plasmacytoid ratio remained significantly higher than that of non-asthmatics – indicative of a relative pDC deficiency (Table 2). In a logistic regression analysis, adjusting for the relevant covariates of age, gender, breast feeding history, daycare attendance, skin test reactivity at 3 years of age, pet exposure, parental marital status and income level, pre/postnatal smoke exposure and inhaled corticosteroid treatment, - the differences in pDC levels or mDC1/pDC ratios were independently associated with the diagnosis of asthma (Table 3). Not surprisingly, skin test reactivity and inhaled corticosteroid use were also associated with the diagnosis of asthma.
Figure 2. The levels of circulating plasmacytoid dendritic cells per 106 PBMC between asthma and non-asthma subjects.
*p<0.0003
Table II.
Levels of circulating dendritic cell subsets and ratios of myeloid to plasmacytoid lineages among subjects with and without asthma
| Dendritic Cells (cells per 106 PBMC) | Asthma | Non-asthma | p-value |
|---|---|---|---|
| Myeloid DC1 | 3836 (2474)* | 4768 (2224) | 0.02 |
| Myeloid DC2 | 145 (162) | 245 (198) | 0.02 |
| Myeloid DC1/plasmacytoid DC | 6.3 (6.2) | 5.1 (11.7) | 0.04 |
| Myeloid DC1 + DC2/plasmacytoid DC | 6.48 (6.2) | 5.29 (11.8) | 0.04 |
mean (standard deviation);
DC = dendritic cells, PBMC = peripheral blood mononuclear cells
Table III.
Factors associated with the diagnosis of asthma
| Parameter* | Estimated Odds Ratio for Asthma | p value | 95% CI | |
|---|---|---|---|---|
| log pDC level† | 0.45 | 0.02 | 0.23 | 0.88 |
| log mDC1/pDC† | 2.22 | 0.04 | 1.04 | 4.44 |
| Inhaled Corticosteroid Use | 5.90 | 0.01 | 1.47 | 23.69 |
| Skin test reactivity | 5.20 | 0.01 | 1.50 | 18.01 |
only variables with p < 0.05 are listed;
the DC levels were not normally distributed and, therefore, were logarithmically transformed to normalize the distribution prior to regression analysis.
The pDC level did not significantly correlate with serum IgE (r=−0.15, p=0.21) or with absolute eosinophil count (r=0.12, p=0.29). When the cohort was categorized by skin test reactivity at age 3 years, pDC levels among subjects with reactive skin tests were similar to those without (1355±997 vs. 1607±1220 cells per 106 PBMC, mean ± SD, p=0.61).
Discussion
In this prospective cohort of children with a history of severe RSV bronchiolitis, we found that children with a diagnosis of asthma by age 6 years exhibit lower quantity of pDC compared to those who were not diagnosed with asthma. The differences in pDC levels among children with asthma were not related to concomitant inhaled corticosteroid use.
On one hand, the relative decrease of pDC in children with asthma may have resulted from recruitment of pDC and mDC to the inflamed airways – a post-facto phenomenon of inflammation due to asthma. On the other hand, lower pDC level may be an a priori phenomenon that contributes to development of asthma. We propose that children diagnosed with asthma following severe RSV bronchiolitis have a relative deficiency of pDC, and thus, make less type I IFN to induce IL-10 secreting regulatory T cells9, promote Th1 immunity, and protect from the development of asthma. This inference parallels the reports that attenuated production of IFNγ, another pro-Th1 cytokine, by PBMC from cord blood correlated with a subsequent increased risk for atopy.19,20 This proposal is further supported by experiments with a murine model of asthma, where systemic depletion of pDC prior to ovalbumin challenge led to development of the asthma phenotype (peribronchial eosinophilia, goblet cell hyperplasia, and ovalbumin specific IgE) and adoptive transfer of pDC prior to ovalbumin sensitization protected against such response.12
To our knowledge, this is the first report on peripheral pDC levels in the aftermath of severe RSV bronchiolitis early in life. Our findings are consistent with previous studies comparing DC levels between asthmatics and normal subjects. 16,17 Hagendorens et al. observed lower quantity of pDC among children with allergic asthma as compared to healthy controls.16 Upham et al. recently reported an inverse relationship between number of circulating pDC at 6 and 12 month of age and the risk of subsequent wheezing and physician-diagnosed asthma at 5 years of age.17 Although the cited studies report pDC quantities in different units than our results, precluding a direct comparison of the number of cells detected, the general trend of lower levels of pDC in asthmatics is consistent with our findings.16,17 Intriguingly, a different association with DC numbers in adults was reported by Matsuda et al. among adults with asthma who had significantly elevated numbers of peripheral pDC as compared to healthy controls.18 The nature of the conflicting data among children and adults has not been elucidated but may be related to age difference or potential immunopathologic difference in childhood vs. adult onset asthma.
We did not find an association between the level of pDC and peripheral IgE level, eosinophilia, or eczema. Our study may have lacked adequate statistical power to demonstrate a statistically significant relationship. Alternatively, pDC may attenuate Th2 inflammation locally, in the lung, after migrating through peripheral circulation to mediastinal lymph nodes10 - thereby having little impact on the measures of atopy in periphery: peripheral IgE, peripheral eosinophil counts, and skin test reactivity.
We acknowledge several limitations to the current findings. The original design of the RBEL study did not incorporate a control group - children without asthma and RSV bronchiolitis – as all almost all children are exposed to RSV by two years of age. 1 Although our data cannot be directly extrapolated to the general asthma population, it offers an insight into immunological developments in children post-severe RSV bronchiolitis. In addition, the PMBC samples used for our analysis were initially stimulated by PMA and ionomycin – it is possible that different cytokine profiles were produced by stimulated PMBC cells from asthmatics vs. nonasthmatics resulting in a different viability of DC though we have not found this to be true (M. Castro, H. Yin-DeClue, unpublished communication). Furthermore, the results of our pilot study reflect previously published reports that employed non-stimulated PMBC – suggesting that are our observations are valid and not an artifact of differential pDC survival.
We do not have data on our cohort’s pDC levels in early life as many of the techniques for measurement of pDC were not available. Our next study will aim to prospectively follow pDC levels, ideally starting with cord blood and prior to development of RSV infection, to establish whether quantification of pDC carries a predictive value for development of asthma following severe RSV bronchiolitis. This may help to identify children with low pDC level at risk for asthma so that prophylaxis may be administered with anti-RSV immunoglobulin or antiviral medicines in an effort to avert development of severe bronchiolitis and subsequent asthma.21,22
In summary, among children with physician-diagnosed asthma following severe RSV bronchiolitis, there appears to be a relative deficiency of peripheral blood pDC. Further studies of pDC before the development of asthma may provide an indication of a valuable window of opportunity for future intervention and prevention of this common disease process.
Acknowledgments
Funding: National Institutes of Health HL61895
The authors gratefully thank Lisa Robertson, R.N., Lynette Tegtmeier, R.N., Amy Rahm, R.N. Michelle Jenkersen, R.N., R.R.T., JoAnn Bonfiglio, R.N., M.S.N., and Toni Schweiger, R.N. for their assistance in recruiting children and data collection for the RBEL study. We also would like to thank the children and their families that graciously provided their time and effort to participate in the RBEL study.
References
- 1.Hall C. Respiratory syncytial virus. In: Feigin R, Cherry J, editors. Pediatric Infectious Diseases. Philadelphia: WB Saunders; 1991. pp. 1633–1656. [Google Scholar]
- 2.Welliver R. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus infection. J Pediatr. 2003;143:S112–7. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 3.Sigurs N, Gustafsson PM, Bjarnson R, et al. Severe Respiratory Syncytial Virus Bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 4.Roman M, Galhoun WJ, Hinton KL, et al. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med. 1997;156:190–5. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 5.Henderson J, Hilliard TN, Sherriff A, et al. Hospitalization for RSV bronchiolitis before 12 month of age and subsequent asthma, atopy, and wheeze: A longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386–92. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma. Revision: Workshop Report, Global Strategy for Asthma Management and Prevention. 2006. [Google Scholar]
- 7.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344(5):350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 8.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 9.Grayson MH. Lung dendritic cells and inflammatory response. Ann Allergy Asthma Immunol. 2006;96(5):643–51. doi: 10.1016/S1081-1206(10)61061-7. [DOI] [PubMed] [Google Scholar]
- 10.Diacovo TG, Blasius AL, Mak TW, Cella M, Colonna M. Adhesive mechanisms governing interferon-producing cells recruitment into lymph nodes. J Exp Med. 2005;202:687–96. doi: 10.1084/jem.20051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Amakawa R, Inaba M, et al. Plasmacytoid Dendritic Cells Regulate Th Cell Responses through OX40 ligand and Type I IFNs. J of Immunol. 2004;172:4253–9. doi: 10.4049/jimmunol.172.7.4253. [DOI] [PubMed] [Google Scholar]
- 12.de Heer HJ, Hammad H, Soullie T, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200(1):89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley JP, Bacharier LB, Bonfiglio J, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:7–14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 14.Dzionek A, Fuchs A, Schmidt P, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 15.Weiss S, Horner A, Shapiro G, Sternberg A. The prevalence of environmental exposure to perceived asthma triggers in children with mild-to-moderate asthma: data from the Childhood Asthma Management Program (CAMP) J Allergy Clin Immunol. 2001;107(4):634–40. doi: 10.1067/mai.2001.113869. [DOI] [PubMed] [Google Scholar]
- 16.Hagendorens MM, Ebo DG, Schuerwegh AJ, et al. Differences in circulating dendritic cells subtypes in cord blood and peripheral blood of healthy and allergic children. Clin Exp Allergy. 2003;33:633–9. doi: 10.1046/j.1365-2222.2003.01649.x. [DOI] [PubMed] [Google Scholar]
- 17.Upham JW, Rate A, Kusel M, Sly PD, Johnston SL, Holt PG. The frequencies of plasmacytoid and myeloid dendritic cell subsets in infancy are differentially associated with risk for viral respiratory infections, allergic sensitization and asthma [abstract] Proceedings of the American Thoracic Society. 2006;3:A827. [Google Scholar]
- 18.Matsuda H, Suda T, Hashizume H, et al. Alteration of balance between myeloid dendritic cells and plasmacytoid dendritic cells in peripheral blood of patients with asthma. Am J Respir Crit Care Med. 2002;166:1050–4. doi: 10.1164/rccm.2110066. [DOI] [PubMed] [Google Scholar]
- 19.Holt PG, Clough JB, Holt BJ, et al. Genetic ‘risk’ for atopy is associated with delayed postnatal maturation of T-cell competence. Clin Exp Allergy. 1992;22(12):1093–9. doi: 10.1111/j.1365-2222.1992.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 20.Tang MLK, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-γ secretion in neonates and subsequent atopy. Lancet. 1994;344:983–6. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 21.Simoes EA, Groothuis JR, Cabonell-Estrany X, Rieger CHL, Mitchell I, Fredrick LM. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, Lin YT, Yang YH, Wang LC, Lee JH, Kao CL, et al. Ribavirin for respiratory syncytial virus bronchiolitis reduced the risk of asthma and allergen sensitization. Pediatr Allergy Immunol. 2008;19:166–72. doi: 10.1111/j.1399-3038.2007.00610.x. [DOI] [PubMed] [Google Scholar]