The structure of the putative aminoacrylate peracid reductase RutC of the rut operon, a member of the YjgF family, is reported.
Keywords: YjgF superfamily, rut operon, peroxy-aminoacrylate, aminoacrylate, RutC
Abstract
RutC is the third enzyme in the Escherichia coli rut pathway of uracil degradation. RutC belongs to the highly conserved YjgF family of proteins. The structure of the RutC protein was determined and refined to 1.95 Å resolution. The crystal belonged to space group P21212 and contained six molecules in the asymmetric unit. The structure was solved by SAD phasing and was refined to an R work of 19.3% (R free = 21.7%). The final model revealed that this protein has a Bacillus chorismate mutase-like fold and forms a homotrimer with a hydrophobic cavity in the center of the structure and ligand-binding clefts between two subunits. A likely function for RutC is the reduction of peroxy-aminoacrylate to aminoacrylate as a part of a detoxification process.
1. Introduction
The rut pathway, a novel pathway among α- and γ-proteobacteria, degrades pyrimidines to produce nitrogen at suboptimal temperatures (Kim et al., 2010 ▶). The rut operon in Escherichia coli is composed of six enzymes that are responsible for reducing/degrading the uracil ring to 3-hydroxypropionic acid (RutA–F), the uracil transporter RutG and the TetR-family repressor RutR (Shimada et al., 2007 ▶).
RutC is a member of the highly conserved YjgF/YER057c/UK114 family of proteins. This family (henceforth abbreviated in this paper as the YjgF family) is known to be widely distributed among eubacteria, archaea and eukaryotes. Although various studies have suggested that YjgF-family members may be involved in removing potentially toxic secondary products that result from normal metabolism (Volz, 1999 ▶; Parsons et al., 2002 ▶), only YjgF has had its cellular role revealed. YjgF is required in Salmonella enterica for isoleucine biosynthesis when grown on pyruvate medium (Lambrecht et al., 2010 ▶). It has recently been shown that it catalyzes the hydrolysis of enamines and imines, possibly as a means of liberating ammonia, although the extrapolation of this to RutC requires further investigation (Lambrecht et al., 2012 ▶).
Several other family members have been shown to be physiologically important. TdcF from E. coli is involved in 2-ketobutyrate metabolism (Burman et al., 2007 ▶), and YabJ is involved in the regulation of purine biosynthesis (Rappu et al., 1999 ▶; Sinha et al., 1999 ▶) and isoleucine biosynthesis (Kim et al., 2001 ▶). YabJ has ribonuclease activity (Morishita et al., 1999 ▶) or translation-inhibition activity (Oka et al., 1995 ▶).
Interestingly, rutC has been identified as a gene that is induced during infection by avian pathogenic E. coli (APEC), which has a significant economic impact in the poultry industry throughout the world (Tuntufye et al., 2012 ▶).
YjgF-family proteins are ∼14 kDa proteins that form homotrimeric assemblies. The homotrimer has a Bacillus chorismate mutase fold. Despite high fold similarity to other chorismate mutase fold-containing proteins, there is no obvious sequence similarity or functional connection among those proteins. Most proteins in the YjgF family have a characteristic globular shape with a large hydrophobic cavity of unknown function in the middle of the trimeric quaternary structure. Additionally, three clefts are formed at the interfaces of adjacent monomers, which have been identified as putative ligand-binding sites (Sinha et al., 1999 ▶).
Of 36 known YjgF structures, only one has a ligand bound which could be of biological significance. This is TdcF, an E. coli protein (Burman et al., 2007 ▶; PDB entry 2uyn), in complex with 2-ketobutyrate. TdcF belongs to the tdc operon responsible for anaerobic degradation of l-threonine and l-serine, in which l-threonine is deaminated to 2-ketobutyrate (Burman et al., 2007 ▶). The ketobutyrate molecule is bound in the putative active site formed by two adjacent monomers.
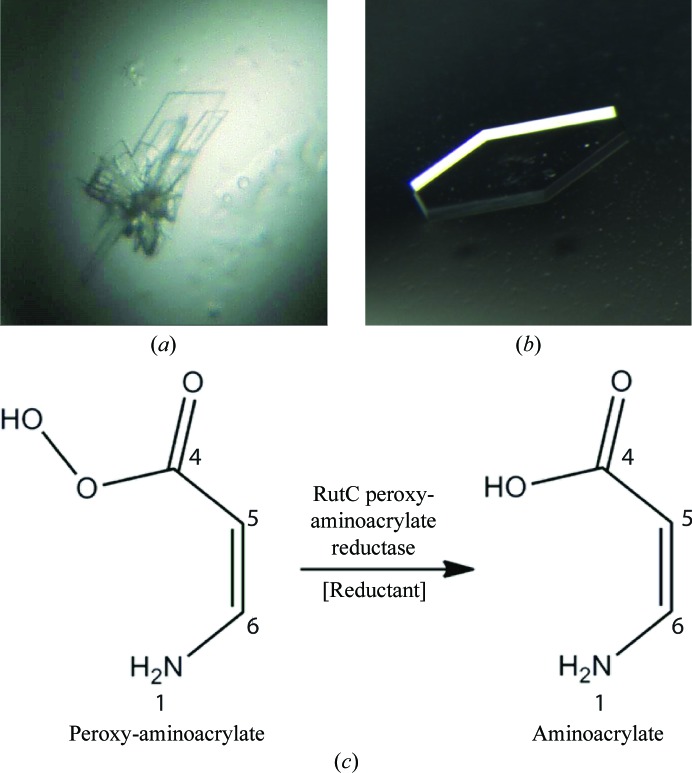
RutC is part of the uracil-degrading rut operon. In vitro, only three enzymes (RutA, RutB and RutF) of the rut pathway are needed to release both N atoms from the uracil ring in the form of ammonium ions (Mukherjee et al., 2010 ▶). However, the in vivo production of toxic intermediates requires a detoxification mechanism in the living cell. This function has been proposed to be provided by both the RutC and RutD proteins by Kim et al. (2010 ▶). RutC could reduce peroxy-aminoacrylate to aminoacrylate, inhibiting the spontaneous hydrolysis of the amino group, so that in the next step RutD can increase the rate of hydrolysis of aminoacrylate (Lambrecht et al., 2010 ▶; Knapik et al., 2012 ▶). A schematic of the reaction catalyzed by RutC is shown in Fig. 1 ▶(c).
Figure 1.
(a) Initial and (b) optimized crystals of RutC protein. (c) Schematic of the RutC-catalyzed reaction. The reductant that participates in the conversion of peroxyaminoacrylate to aminoacrylate is unknown.
2. Materials and methods
2.1. Cloning, expression and purification
The rutC gene was extracted and amplified by PCR on E. coli genomic DNA using the following primers: 5′-GAATTCCATATGCCAAAATCCGTAATTATTCCC-3′ (forward) and 5′-ATCTCGAGTTACTTGGCGATATGCGCATTTG-3′ (reverse). Each primer confers a restriction-enzyme site at either end of the gene; these sites are NdeI and XhoI, respectively. The amplified gene was cloned into a modified pET15b vector where a linker was cloned between the NdeI and NcoI restriction sites and replaced the thrombin-cleavage site with a tobacco etch virus (TEV) protease site. This linker was made with the following primers: 5′-CATGGGCTCTTCTCATCATCATCATCATCATTCTTCTGGTCGTGAGAACTTGTACTTTCAAGGCCA-3′ (forward) and 5′-TATGGCCTTGAAAGTACAAGTTCTCACGACCAGAAGAATGATGATGATGATGATGAGAAGAGCC-3′ (reverse). The clones were verified by sequencing and transformed into E. coli strain BL21 (DE3) CodonPlus RIL (Stratagene). The cells were grown in M9 minimal medium with selenomethionine (SeMet) at 310 K until the OD600 reached 1.2, followed by the induction of overexpression of recombinant RutC with 1 mM IPTG (isopropyl β-d-1-thiogalactopyranoside) in the presence of a cocktail of inhibitory amino acids and SeMet according to the manufacturer’s specifications (Shanghai Medicilon Inc.). Induction was performed at 289 K overnight. Cell pellets were resuspended in lysis buffer (50 mM HEPES pH 7.5, 500 mM NaCl, 5% glycerol) with protease-inhibitor cocktail tablets (Roche), lysed by sonication and spun down in an ultracentrifuge. The supernatant was loaded onto an Ni–NTA affinity column (Qiagen). The column was washed with wash buffer (50 mM HEPES pH 7.5, 500 mM NaCl, 5% glycerol, 30 mM imidazole). RutC protein was eluted with elution buffer (50 mM HEPES pH 7.5, 500 mM NaCl, 5% glycerol, 250 mM imidazole). The eluted protein was incubated with polyhistidine-tagged recombinant TEV protease to remove the N-terminal affinity tag while dialyzing against 50 mM HEPES pH 7.5, 500 mM NaCl, 5% glycerol buffer at 277 K overnight. To remove cleaved polyhistidine tag and TEV protease, the sample was passed through a second Ni–NTA column. The collected flowthrough was concentrated and further purified using a size-exclusion column in 10 mM HEPES pH 7.5, 500 mM NaCl. The protein was concentrated to a final concentration of 10 mg ml−1.
2.2. Crystallization
RutC protein was crystallized by vapor diffusion in hanging drops at 293 K. Initial crystals were obtained using The PACT Suite (Qiagen) in two conditions: (i) 0.1 M MMT pH 4.0, 30% PEG 1500 and (ii) 0.1 M SPG pH 5.0, 25% PEG 1500. Both conditions gave rise to thin clustered plates that diffracted to 2.8 Å resolution (Fig. 1 ▶ a). Crystals were optimized by the addition of a cocktail of xylitol, potassium/sodium tartrate, 2,6-pyridinecarboxylic acid, 2,4-pyridinecarboxylic acid and 3-sulfobenzoic acid. This resulted in single ‘coffin-shaped’ crystals (elongated hexagonal prisms) that diffracted to 1.95 Å resolution (Fig. 1 ▶ b) and led to the final structure of the RutC protein. For data collection, crystals were flash-cooled in liquid nitrogen using a 1:1 mixture of mother liquor and 50% glycerol for cryoprotection.
2.3. Data collection and processing, structure solution and refinement
X-ray diffraction data were collected from RutC protein crystals cooled to 100 K on the 21ID-G beamline of the Advanced Photon Source (APS) at Argonne National Laboratory. The data were collected at the selenium absorbance peak for phasing. Data collection and reduction were performed with the HKL-2000 package (Otwinowski & Minor, 1997 ▶). Solution of the structure by single-wavelength anomalous diffraction (SAD) phasing and building of the initial models were performed using HKL-3000 (Minor et al., 2006 ▶), which is integrated with SHELXC/D/E (Sheldrick, 2008 ▶), MLPHARE (Otwinowski, 1991 ▶), DM (Cowtan & Zhang, 1999 ▶), ARP/wARP (Perrakis et al., 1999 ▶), CCP4 (Winn et al., 2011 ▶), SOLVE (Terwilliger & Berendzen, 1999 ▶) and RESOLVE (Terwilliger, 2002 ▶). Model refinement was performed with REFMAC5 (Murshudov et al., 1997 ▶, 2011 ▶; Winn et al., 2011 ▶) and Coot (Emsley & Cowtan, 2004 ▶). MolProbity (Chen et al., 2010 ▶) and ADIT (Yang et al., 2004 ▶) were used to validate the structure. Data-collection and refinement statistics are presented in Table 1 ▶. The model was deposited in the PDB with accession code 3v4d.
Table 1. Data-collection and refinement statistics for RutC from E. coli .
Values in parentheses are for the highest resolution shell.
| Data collection | |
| Wavelength () | 0.9787 |
| Unit-cell parameters (, ) | a = 92.3, b = 179.2, c = 45.2, = = = 90.00 |
| Space group | P21212 |
| Resolution range () | 501.95 (1.981.95) |
| No. of observations | 534145 |
| No. of unique reflections | 55640 |
| Data completeness (%) | 99.9 (100) |
| R merge | 0.094 (0.633) |
| I/(I) | 34.3 (3.0) |
| Refinement | |
| No. of protein atoms | 5666 |
| Mean B value for protein atoms (2) | 43.3 |
| No. of water atoms | 342 |
| Mean B value for water atoms (2) | 45.0 |
| R work (%) | 19.5 |
| R free (%) | 21.7 |
| R.m.s.d. bond lengths () | 0.018 |
| R.m.s.d. bond angles () | 1.7 |
| Geometry | |
| Ramachandran outliers (%) | 0 |
| Ramachandran favored (%) | 98.8 |
| Residues with bad bonds (%) | 0 |
| Residues with bad angles (%) | 0.13 |
| Clashscore | 5.65 |
| Clashscore percentile | 96 |
More detailed structure analyses (calculation of the cavity volumes and superposition) were performed using the Internal Coordinate Mechanics Professional (ICM-PRO) software from Molsoft LLC (Abagyan & Totrov, 1997 ▶). The figures were created using PyMOL (The PyMOL Molecular Graphics System v.1.2r3pre, Schrödinger LLC) and ICM-PRO. R.m.s.d.s and percentage identities were calculated using the DALI sever (Holm & Rosenström, 2010 ▶). The sequence alignment was rendered using PySHADE (Porebski et al., manuscript in preparation).
3. Results and discussion
3.1. Overall structure and multimeric assembly of the RutC protein
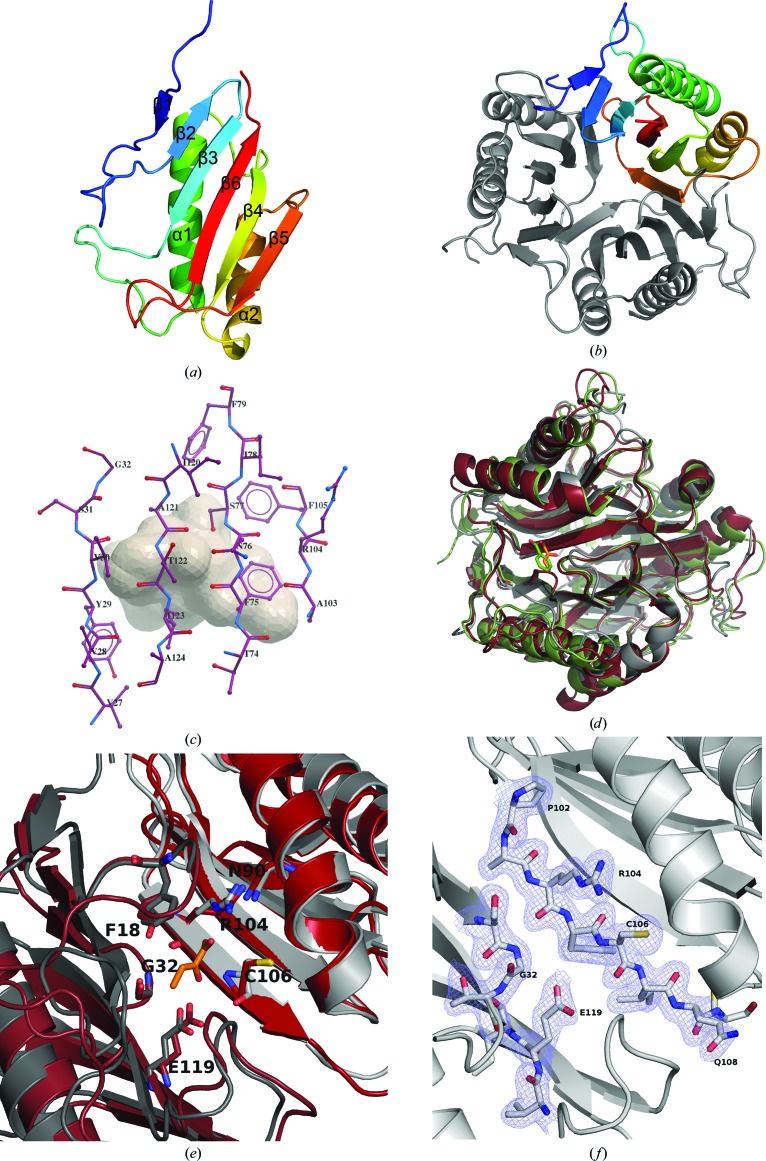
The structure of E. coli RutC was determined by the SAD technique and refined to 1.95 Å resolution. A RutC monomer is a single-domain, 13.7 kDa, 128-residue-long polypeptide chain with a Bacillus chorismate-mutase-like fold. The monomer consists of two α-helices and six β-strands. The β-strands form a flat sheet, with strands β1 and β5 twisted. The helices are packed tightly adjacent to the sheet, parallel to the plane of the sheet and to each other (Fig. 2 ▶ a).
Figure 2.
Cartoon representation of (a) a monomer and (b) the trimeric form of the RutC protein. (c) A model of the tubular cavity and the residues composing its surface (represented as sticks), as calculated by ICM-PRO. (d) Superposition of RutC (gray) and its two structural homologs TdcF (burgundy) and hp14.5 (green). Two ligands are visible in the putative active-site cavity of TdcF (2-ketobutyrate; orange) and hp14.5 (benzoate; lime green). (e) Close-up of the active sites of RutC and TdcF. Only RutC residues are labelled. (f) A 2F o − F c electron-density map of the RutC active site. Some residues that would obscure the view have been omitted.
Gel-filtration chromatography shows that the RutC protein forms a trimeric assembly in solution. In the crystal lattice, there are two trimers in the asymmetric unit. The RutC homotrimer forms a globular structure with a tubular inner cavity (Fig. 2 ▶ b). Six helices (two from each monomer) form the outer rim of the trimer. The barrel-like core of the structure is formed by 12 β-strands (four from each monomer). One β-strand from each monomer (β5) is twisted with respect to the others and this twist causes these strands from each monomer to close off one end of the large (400 Å3 in volume) tubular cavity inside the trimer structure. The surface of the cavity (Fig. 2 ▶ c) consists mostly of hydrophobic residues, some of which are well conserved among the YjgF family of proteins. The nonconserved amino acids involved in the formation of the cavity are similar in hydrophobicity to the consensus residues present in other RutC orthologs (Burman et al., 2007 ▶; Volz, 1999 ▶). Some of the residues involved in cavity formation are also responsible for formation of the quaternary structure. They are well conserved and form hydrophobic (Leu15, Pro17, Tyr86, Tyr93, Phe97 and Pro102) and polar patches organized in two rings. One is formed by nine phenylalanines, three from each chain (Phe75, Phe79 and Phe105); the second, which is more polar, is made up of Tyr29, His125 and Val27 from all chains.
3.2. Homologs of RutC and the putative active site
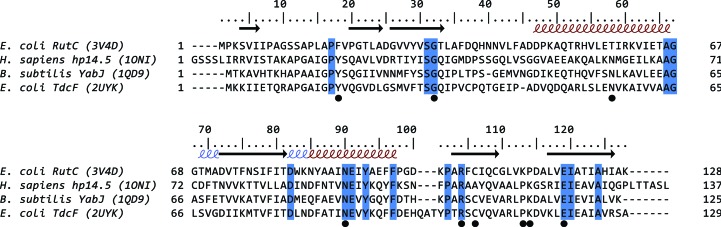
The cleft located between two monomers has been shown to bind various ligands: 2-ketobutyrate, ethylene glycol or serine in E. coli TdcF (Burman et al., 2007 ▶), acetate in YabJ (Sinha et al., 1999 ▶) and benzoate in the human protein hp14.5 (Manjasetty et al., 2004 ▶). Previous analysis (Thakur et al., 2010 ▶) has shown that those clefts vary in volume, charge distribution and amino-acid composition among YjgF-family representatives, and it was proposed that the cavity on the border of two monomers forms a ligand-binding site (Fig. 2 ▶ d). The putative active site in the YjgF family is characterized by 7–9 residues which are highly conserved among YjgF proteins and are all located within the cleft (Volz, 1999 ▶). Residues from two monomers participate in the construction of one active site. These residues are also conserved in RutC and are listed in Table 2 ▶.
Table 2. Residue compositions of the putative active sites of RutC and three analyzed homologs.
Residues which are 100% conserved are shown in bold.
| RutC | TdcF | hp14.5 | YabJ |
|---|---|---|---|
| Phe18 | Tyr17 | Tyr21 | Tyr17 |
| Gly32 | Gly31 | Gly35 | Gly31 |
| Thr58 | Asn56 | Asn62 | Asn56 |
| Asn90 | Asn88 | Asn93 | Asn88 |
| Arg104 | Arg105 | Arg107 | Arg102 |
| Cys106 | Cys107 | Ala109 | Cys104 |
| Glu119 | Glu120 | Glu122 | Glu117 |
To map a putative ligand-binding site in the RutC protein, we superimposed its structure on those of other YjgF structures with bound ligands. The structure of the TdcF protein from E. coli bound with its physiological ligand 2-ketobutyrate and the structure of hp14.5 (Homo sapiens) with its ligand benzoate were both used for superposition. The putative binding site in RutC superposes with the TdcF 2-ketobutyrate binding site, which suggests that this cleft could be a physiological binding site (Figs. 2 ▶ e and 2 ▶ f). The β1–β2 loop forms a lid for this binding site in two of the six copies in the asymmetric unit, but is disordered in the other four chains.
RutC and TdcF share 32% sequence identity and have an r.m.s.d. of 1.6 Å for 128 aligned Cα atoms, while RutC and hp14.5 share 24% sequence identity and have an r.m.s.d. of 1.6 Å for 112 aligned Cα atoms. A sequence alignment of the RutC, TdcF, hp14.5 and YabJ proteins is presented in Fig. 3 ▶. The residues that are directly involved in binding ligands are identical in all three of the aligned proteins (Arg104 and Glu119 in RutC). The invariant arginine creates hydrogen bonds to the ligands of TdcF and hp14.5. In the case of 2-ketobutyrate, its carboxyl group creates a bidentate salt bridge with the Arg105 side chain.
Figure 3.
Alignment of YjgF-family members with RutC. The amino-acid sequences of the YjgF-family members assayed in this study were aligned using ClustalW (Thompson et al., 1994 ▶) and manually edited using BioEdit (Hall, 1999 ▶). The numbering of the residues is based on the E. coli RutC sequence. Residues that are 100% conserved are highlighted; residues that are involved in active-site formation are marked with a dot. The secondary structure was calculated using PROMOTIF (Hutchinson & Thornton, 1996 ▶) on the basis of the RutC structure. Red and blue helices represent α-helices and 310-helices, respectively.
The second important residue is glutamic acid (Glu119 in RutC), which is present in all three aligned proteins and in TdcF creates hydrogen bonds to 2-ketobutyrate and other ligands. It has been suggested by Kim et al. (2010 ▶) that the XC106 XXC109 motif could participate in the reduction of the peracid form of aminoacrylate. The conserved residue Cys107 in TdcF also creates hydrogen bonds to 2-ketobutyrate. If RutC catalyzes this proposed reaction, Cys106 in RutC could be in an analogous position. Another cysteine residue in the vicinity of the putative active site in RutC is Cys109. It is located 9 Å away from Cys106 and is not conserved among members of the YjgF family. In the homologous protein YjgF from E. coli (Volz, 1999 ▶), it was reported that a conserved cysteine residue (Cys107) underwent an unusual post-translational modification, which was identified as a thiosulfate or thiophosphate group with an unknown function (Feng et al., 2008 ▶). The corresponding cysteine residue in RutC does not show this modification. The importance of this possible modification is still unknown, but it suggests that this residue may be of importance to the function and regulation of this protein.
4. Conclusions
The described RutC structure is the third solved structure of a protein from the rut operon. It has a characteristic trimeric Bacillus chorismate mutase fold. The rut operon is a recently discovered novel biochemical pathway and its enzymatic components might be direct targets for further drug development.
Supplementary Material
PDB reference: RutC, 3v4d
Acknowledgments
The authors would like thank Dr Matthew D. Zimmerman for valuable discussions and Rachel Vigour for proofreading the manuscript. The work described in this paper was supported by NIH grant GM094662 to the New York Structural Genomics Consortium, and GM53163. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor for the support of this research program (Grant 085P1000817).
References
- Abagyan, R. & Totrov, M. (1997). Proteins, 29, Suppl. 1, 215–220. [DOI] [PubMed]
- Burman, J. D., Stevenson, C. E., Sawers, R. G. & Lawson, D. M. (2007). BMC Struct. Biol. 7, 30. [DOI] [PMC free article] [PubMed]
- Chen, V. B., Arendall, W. B., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S. & Richardson, D. C. (2010). Acta Cryst. D66, 12–21. [DOI] [PMC free article] [PubMed]
- Cowtan, K. D. & Zhang, K. Y. J. (1999). Prog. Biophys. Mol. Biol. 72, 245–270. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Feng, J., Zhu, M., Schaub, M. C., Gehrig, P., Roschitzki, B., Lucchinetti, E. & Zaugg, M. (2008). Cardiovasc. Res. 80, 20–29. [DOI] [PubMed]
- Hall, T. A. (1999). Nucleic Acids Symp. Ser. 41, 95–98
- Holm, L. & Rosenström, P. (2010). Nucleic Acids Res. 38, W545–W549. [DOI] [PMC free article] [PubMed]
- Hutchinson, E. G. & Thornton, J. M. (1996). Protein Sci. 5, 212–220. [DOI] [PMC free article] [PubMed]
- Kim, J.-M., Yoshikawa, H. & Shirahige, K. (2001). Genes Cells, 6, 507–517. [DOI] [PubMed]
- Kim, K.-S., Pelton, J. G., Inwood, W. B., Andersen, U., Kustu, S. & Wemmer, D. E. (2010). J. Bacteriol. 192, 4089–4102. [DOI] [PMC free article] [PubMed]
- Knapik, A. A., Petkowski, J. J., Otwinowski, Z., Cymborowski, M. T., Cooper, D. R., Majorek, K. A., Chruszcz, M., Krajewska, W. M. & Minor, W. (2012). Proteins, 80, 2359–2368. [DOI] [PMC free article] [PubMed]
- Lambrecht, J. A., Browne, B. A. & Downs, D. M. (2010). J. Biol. Chem. 285, 34401–34407. [DOI] [PMC free article] [PubMed]
- Lambrecht, J. A., Flynn, J. M. & Downs, D. M. (2012). J. Biol. Chem. 287, 3454–3461. [DOI] [PMC free article] [PubMed]
- Manjasetty, B. A., Delbrück, H., Pham, D. T., Mueller, U., Fieber-Erdmann, M., Scheich, C., Sievert, V., Büssow, K., Niesen, F. H., Weihofen, W., Loll, B., Saenger, W., Heinemann, U. & Neisen, F. H. (2004). Proteins, 54, 797–800. [DOI] [PubMed]
- Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. (2006). Acta Cryst. D62, 859–866. [DOI] [PubMed]
- Morishita, R., Kawagoshi, A., Sawasaki, T., Madin, K., Ogasawara, T., Oka, T. & Endo, Y. (1999). J. Biol. Chem. 274, 20688–20692. [DOI] [PubMed]
- Mukherjee, T., Zhang, Y., Abdelwahed, S., Ealick, S. E. & Begley, T. P. (2010). J. Am. Chem. Soc. 132, 5550–5551. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997). Acta Cryst. D53, 240–255. [DOI] [PubMed]
- Oka, T., Tsuji, H., Noda, C., Sakai, K., Hong, Y., Suzuki, I., Muñoz, S. & Natori, Y. (1995). J. Biol. Chem. 270, 30060–30067. [DOI] [PubMed]
- Otwinowski, Z. (1991). Proceedings of the CCP4 Study Weekend. Isomorphous Replacement and Anomalous Scattering, edited by W. Wolf, P. R. Evans & A. G. W. Leslie, pp. 80–86. Warrington: Daresbury Laboratory.
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Parsons, L., Bonander, N., Eisenstein, E., Gilson, M., Kairys, V. & Orban, J. (2002). Biochemistry, 42, 80–89. [DOI] [PubMed]
- Perrakis, A., Morris, R. & Lamzin, V. S. (1999). Nature Struct. Biol. 6, 458–463. [DOI] [PubMed]
- Rappu, P., Shin, B. S., Zalkin, H. & Mäntsälä, P. (1999). J. Bacteriol. 181, 3810–3815. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shimada, T., Hirao, K., Kori, A., Yamamoto, K. & Ishihama, A. (2007). Mol. Microbiol. 66, 744–757. [DOI] [PubMed]
- Sinha, S., Rappu, P., Lange, S. C., Mäntsälä, P., Zalkin, H. & Smith, J. L. (1999). Proc. Natl Acad. Sci. USA, 96, 13074–13079. [DOI] [PMC free article] [PubMed]
- Terwilliger, T. C. (2002). Acta Cryst. D58, 1937–1940. [DOI] [PubMed]
- Terwilliger, T. C. & Berendzen, J. (1999). Acta Cryst. D55, 849–861. [DOI] [PMC free article] [PubMed]
- Thakur, K. G., Praveena, T. & Gopal, B. (2010). Proteins, 78, 773–778. [DOI] [PMC free article] [PubMed]
- Thompson, J. D., Higgins, D. G. & Gibson, T. J. (1994). Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed]
- Tuntufye, H. N., Lebeer, S., Gwakisa, P. S. & Goddeeris, B. M. (2012). Appl. Environ. Microbiol. 78, 3343–3351. [DOI] [PMC free article] [PubMed]
- Volz, K. (1999). Protein Sci. 8, 2428–2437. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Yang, H., Guranovic, V., Dutta, S., Feng, Z., Berman, H. M. & Westbrook, J. D. (2004). Acta Cryst. D60, 1833–1839. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: RutC, 3v4d