Abstract
Wollastonia biflora (L.) DC. plants accumulate the osmoprotectant 3-dimethylsulfoniopropionate (DMSP), particularly when salinized. DMSP is known to be synthesized in the chloroplast from S-methylmethionine (SMM) imported from the cytosol, but the sizes of the chloroplastic and extrachloroplastic pools of these compounds are unknown. We therefore determined DMSP and SMM in mesophyll protoplasts and chloroplasts. Salinization with 30% (v/v) artificial seawater increased protoplast DMSP levels from 4.6 to 6.0 μmol mg−1 chlorophyll (Chl), and chloroplast levels from 0.9 to 1.9 μmol mg−1 Chl. The latter are minimum values because intact chloroplasts leaked DMSP during isolation. Correcting for this leakage, it was estimated that in vivo about one-half of the DMSP is chloroplastic and that stromal DMSP concentrations in control and salinized plants are about 60 and 130 mm, respectively. Such concentrations would contribute significantly to chloroplast osmoregulation and could protect photosynthetic processes from stress injury. SMM levels were measured using a novel mass-spectrometric method. About 40% of the SMM was located in the chloroplast in unsalinized W. biflora plants, as was about 80% in salinized plants; the chloroplastic pool in both cases was approximately 0.1 μmol mg−1 Chl. In contrast, ≥85% of the SMM was extrachloroplastic in pea (Pisum sativum L.) and spinach (Spinacia oleracea L.), which lack DMSP. DMSP synthesis may be associated with enhanced accumulation of SMM in the chloroplast.
Certain flowering plants accumulate the tertiary sulfonium compound DMSP, particularly under saline, low-nitrogen conditions (for review, see Hanson and Gage, 1996). These DMSP accumulators include the salt-tolerant plant Wollastonia biflora (Storey et al., 1993; Hanson et al., 1994), sugarcane (Paquet et al., 1994), and intertidal species of Spartina (Larher et al., 1977; Colmer et al., 1996). DMSP is also accumulated by many marine algae (Blunden and Gordon, 1986; Keller et al., 1989). DMSP is the main biogenic precursor of dimethylsulfide, which has major roles in the global sulfur cycle, in cloud formation, and in acid precipitation (Malin, 1996).
DMSP is a sulfur analog of a betaine and, like betaines, is compatible with enzyme function in vitro and can have stabilizing or protective effects (Gröne and Kirst, 1991; Nishiguchi and Somero, 1992). Like betaines, DMSP relieves osmotic inhibition of growth in bacteria and accumulates to concentrations greater than 1 m in the stressed bacterial cells (Mason and Blunden, 1989; Paquet et al., 1994). DMSP is also known to accumulate to osmotically significant levels (≥100 mm) in the cytoplasm of algal cells (Dickson et al., 1980; Dickson and Kirst, 1986). These observations make it likely that DMSP acts as a compatible cytoplasmic osmolyte in DMSP-rich flowering plants (Larher et al., 1977; Storey et al., 1993), but there is no evidence that it is actually localized in cytoplasmic compartments rather than the vacuole.
Although the subcellular compartmentation of DMSP is unknown, in W. biflora the compartmentation of the enzymes that make it has been established (Trossat et al., 1996). Two compartments are involved: in the cytosol, a methyltransferase converts Met to SMM; the SMM is then imported into the chloroplast, where deamination and decarboxylation yield 3-dimethylsulfoniopropionaldehyde, which is oxidized by a dehydrogenase to DMSP. This arrangement implies that chloroplasts must contain a pool of the nonprotein amino acid SMM. It also raises the possibility that SMM transport into the chloroplast has a role in regulating DMSP synthesis.
Gly betaine has been localized in the cytoplasm and in chloroplasts by using aqueous procedures to fractionate protoplasts (Matoh et al., 1987) and to prepare chloroplasts (Robinson and Jones, 1986; Schröppel-Meier and Kaiser, 1988). Aqueous isolation procedures have also been used to study chloroplastic pools of amino acids (Mills and Joy, 1980). We therefore used such procedures to isolate chloroplasts from mesophyll protoplasts from unsalinized and salinized W. biflora, and determined levels of DMSP and SMM. A sensitive and specific MALDI-MS assay was developed to quantify the small amounts of SMM.
MATERIALS AND METHODS
Wollastonia biflora (L.) DC. genotype H was grown (one plant per 2.5-L pot) in Metro-Mix (Grace Sierra, Milpitas, CA) in a growth chamber (12-h day, 25°C, PPFD 200–300 μE m−2 s−1, 22°C night) and propagated by cuttings. Irrigation was with one-half-strength Hoagland nutrient solution. Salinization began 5 weeks after propagation by adding artificial seawater (Flowers et al., 1990) to the nutrient solution in 10% (v/v) steps every 3 d to a final level of 30%, which was maintained for at least 10 d before experiments. Pots received 1.5 L of irrigation solution daily. Insect control (Trossat et al., 1996) and osmotic-pressure measurements on leaf discs (Grumet and Hanson, 1986) were as described. Osmotic-pressure values were not corrected to 100% relative water content because the leaves of interest were expanding, precluding accurate estimation of this parameter. Leaf discs were lyophilized to determine dry weight. Spinach (Spinacia oleracea L. cv Savoy Hybrid 612) and pea (Pisum sativum L. cv Laxton's Progress 9) plants were grown as previously described (Cline, 1986; Weretilnyk et al., 1995).
Preparation of W. biflora Protoplasts and Chloroplasts
The procedures of Trossat et al. (1996) were used to prepare mesophyll protoplasts and chloroplasts, with the following modifications for salinized plants. (a) Macerase, Pectinase (5 mg mL−1, Calbiochem) was added to the digestion medium. (b) Protoplasts were purified by layering 1-mL portions of the crude preparation onto 2 mL of 15% (v/v) Percoll and centrifuging in a swing-out rotor (250g, 3 min); protoplasts were harvested from the top of the Percoll layer. (c) The step gradient used to purify chloroplasts contained 2 mL each of 30 and 35% (v/v) Percoll. (d) The sorbitol concentrations of all media were raised by 0.35 m, increasing osmotic pressure by 0.9 MPa. This increase was greater than the osmotic adjustment measured in salinized plants (see text), but was found to be needed for optimum yields. For washing experiments, purified chloroplasts (350–500 μg of Chl) were resuspended three times in 2 mL of lysis medium and pelleted by centrifugation (630g, 1 min). Chloroplast intactness was evaluated by phase-contrast microscopy (Lilley et al., 1975) and by the GAPDH:Chl ratio; GAPDH was assayed as previously described (Rathinasabapathi et al., 1994) with minor modifications (Trossat et al., 1996). Chl was determined according to Arnon (1949). [35S]DMSP and [35S]SMM used as internal standards were synthesized as previously described (Hanson et al., 1994; Gage et al., 1997).
[35S]Met-Labeling Experiments
[35S]Met (44 GBq μmol−1, NEN-DuPont) was mixed with Met to give a specific activity of 56 kBq nmol−1. W. biflora protoplasts from unsalinized leaves (50 μg of Chl) were incubated with 1 nmol of [35S]Met for 1 h at 25°C in an illuminated water bath (Burnet et al., 1995) in 100 μL of buffer containing 10 mm Mes-KOH, 1 mm CaCl2, 0.5 m sorbitol, and 10 mm KHCO3, final pH 6.4. The samples were agitated gently during incubation, which was stopped by freezing with liquid N2 after adding 1 mL of water containing SMM and DMSP carriers (0.25 μmol each). [35S]SMM and [35S]DMSP were analyzed using the ion-exchange chromatography and electrophoresis procedures described previously (Hanson et al., 1994).
Preparation of Chloroplasts from Plants Lacking DMSP
Spinach leaves were shown previously to lack detectable DMSP (<0.01 μmol g−1 fresh weight) (Paquet et al., 1995); pea leaves were shown to have undetectable levels using the assay cited below. Spinach and pea chloroplasts were isolated directly from leaves by mechanical grinding followed by purification using Percoll gradients, as previously described (Cline, 1986; Weretilnyk et al., 1995).
Amino Acid and DMSP Determination
Amino acids were assayed by a microscale version of Rosen's method (1957). Protoplast or chloroplast samples (2.5 or 5 μg of Chl, respectively) were ruptured by freezing at −80°C and thawing, brought to 220 μL with water, and centrifuged at 16,000g for 5 min. A 200-μL portion of the supernatant was mixed with 100 μL each of 0.2 mm NaCN in acetate buffer (2.65 m sodium acetate plus 6.7% [v/v] acetic acid) and 3% (w/v) ninhydrin in 2-methoxyethanol and heated at 100°C for 15 min. After adding 1 mL of isopropanol/water (1:1, v/v), samples were shaken vigorously and cooled to 22°C before reading A570. Gly was used as a standard. DMSP was determined by the dimethylsulfide-release assay described by Paquet et al. (1994), using protoplast or chloroplast samples containing 25 to 50 μg of Chl, or about 50 mg fresh weight of leaf tissue. Leaf tissue samples were frozen in liquid N2 and thawed in the assay vials just before starting assays.
SMM Isolation and Determination by MALDI-MS
W. biflora protoplast and chloroplast samples (200 μg of Chl) were frozen at −80°C, thawed, and diluted with 1 mL of water. Representative samples were spiked with [35S]SMM (30 pmol, 7.8 kBq). Membranes were removed by centrifugation (16,000g, 2 min), and the supernatant was applied to 1-mL columns (Dowex-1 [OH−] and BioRex-70 [H+]) arranged in a series. After each column was washed with 10 mL of water, SMM was eluted from the BioRex-70 column with 5 mL of 1 n HCl. The eluate was lyophilized; [35S]SMM recovery in the eluate averaged 82.5%. For MALDI-MS analysis, protoplast and chloroplast samples were taken up in 300 or 80 μL of water, respectively, containing 30 or 12 nmol of the [methyl-2H6]SMM internal standard (Hanson et al., 1994), and further diluted 20-fold. To prepare the MALDI-MS targets, 1 μL of diluted sample solution was applied to a sample stage well (10 × 10 well array on the sample plate) with 0.5 μL of a 0.1% aqueous solution of fluorosilicic acid (Aldrich) and 0.6 μL of a matrix solution consisting of 4 mg of 2,5-dihydroxybenzoic acid and 1 mg of sinapinic aldehyde oxime in 500 μL of tetrahydrofuran. The oxime was prepared from sinapinic aldehyde by standard procedures (Furniss et al., 1989) and recrystallized from aqueous ethanol before use (yield, 65%; fast-atom bombardment-MS in glycerol matrix, [MH]+ at m/z 224).
The sample matrix solution was mixed well and air-dried for 5 to 10 min. The sample stage was then introduced via a vacuum lock into the source chamber of a Voyager Elite time-of-flight MALDI mass spectrometer (PerSeptive Biosystems, Framingham, MA) equipped with an N2 laser emitting at 337 nm (3-ns pulse). Spectra were acquired in linear delayed-extraction mode (50- to 100-ns delay); the acceleration voltage was 22 kV. Each spectrum was produced by averaging 128 laser shots; at least three spectra were acquired from different regions of each target. Peak areas for m/z 164 (unlabeled SMM) and m/z 170 ([methyl-2H6]SMM) were integrated to quantify endogenous SMM. Corrections were made for matrix background signals using blanks without sample, the peak areas of which at m/z 164 and 170 were measured relative to that of the major matrix signal at m/z 123, and used to calculate appropriate proportional subtractions for the protoplast and chloroplast samples. These values were corrected for the 82.5% recovery of SMM before addition of the [methyl-2H6]SMM internal standard. Spinach and pea chloroplast samples were analyzed in the same way except that the internal standard was added before the ion-exchange step. In the post-source decay experiment, precursor ion selection was accomplished by means of an electrically switched ion gate. Post-source decay spectra were acquired by an incremental reduction in the reflectron voltage.
RESULTS
Leaves Used to Prepare Protoplasts
Tests showed that expanding W. biflora leaves that had reached about 70% of their final size were best for isolating protoplasts from control and salinized plants; the respective mean protoplast yields were 31 and 12% on a Chl basis. Some relevant characteristics of 70% expanded leaves are summarized in Table I. Their DMSP contents per unit fresh weight or plant water were comparable to those reported for mature leaves (Storey et al., 1993; Hanson et al., 1994). Table I gives DMSP levels on a Chl basis, for a comparison with the protoplast and chloroplast data below. Because salinized leaves had less Chl, the salinity-induced increase in DMSP was greater when expressed per unit of Chl (70%) than per unit fresh weight or plant water (40%).
Table I.
Characteristics of W. biflora leaves used for protoplast isolation
| Characteristic | Control | Salinized |
|---|---|---|
| Osmotic pressure (MPa) | 1.20 (0.07) | 1.48 (0.04) |
| Fresh wt:dry wt ratio | 8.91 (0.08) | 8.25 (0.51) |
| Chl content (mg g−1 fresh wt) | 2.16 (0.12) | 1.81 (0.07) |
| DMSP content (μmol g−1 fresh wt) | 23.2 (1.1) | 32.9 (0.7) |
| DMSP content (mmol L−1 plant water) | 26.2 (1.2) | 37.5 (0.8) |
| DMSP content (μmol mg−1 Chl) | 10.7 (0.5) | 18.2 (0.4) |
Leaves that had reached 70% of their final size (judged from that of adjacent mature leaves) were harvested 4 to 5 h after the start of the light period. Data are means and se (in parentheses) of three replicates.
SMM and DMSP Synthesis in W. biflora Protoplasts
Because the conditions used to isolate protoplasts may perturb metabolism, we tested protoplasts for their capacity to convert tracer [35S]Met to SMM and DMSP (Table II). The amounts of radiolabeled SMM and DMSP produced in 1 h were similar to those reported for leaf discs when expressed per unit of Chl (Table II). This result indicates that the protoplasts remained metabolically functional, and that endogenous pools of the intermediate SMM are unlikely to have changed much during protoplast preparation. Measurements of SMM pools in protoplasts and protoplast-derived chloroplasts should, therefore, be physiologically meaningful.
Table II.
Metabolism of radiolabeled Met by W. biflora protoplasts and leaf discs
| Tissue | Met Dose | Labeled Metabolites
|
|
|---|---|---|---|
| SMM | DMSP | ||
| pmol | |||
| Protoplasts | 1000 | 63 | 5.5 |
| Leaf discs | 640 | 32 | 3.8 |
Protoplasts and discs came from unsalinized plants. Protoplasts received 1 nmol (56 kBq) of [35S]Met per 50 μg of Chl, and leaf discs received 7.7 nmol (73 kBq) of [U-14C]Met per nine discs (equivalent to 600 μg of Chl). Incubation was for 1 h in the light. The data for leaf discs are calculated from figure 4 of Hanson et al. (1994). The protoplast experiment was repeated, with similar results. Values are expressed per 50 μg of Chl.
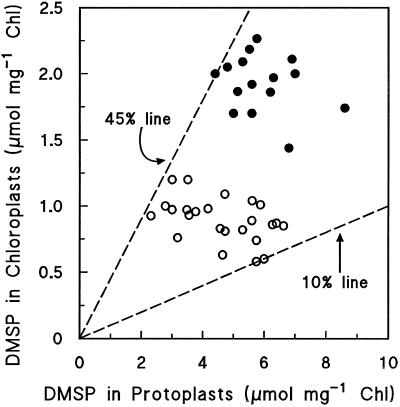
DMSP in W. biflora Protoplasts and Chloroplasts
Figure 1 is a scatter plot showing the DMSP levels in 38 independent mesophyll protoplast preparations and in the corresponding chloroplasts. The chloroplasts were isolated by a procedure that included a wash step followed by centrifugation through a Percoll gradient; they were ≥90% intact, as judged by phase-contrast microscopy and by the activity of the stromal marker GAPDH relative to that in protoplasts. W. biflora chloroplasts prepared in this way are negligibly contaminated (≤5%) with microbodies, mitochondria, and cytosol (Trossat et al., 1996). The chloroplasts were shown not to bind or absorb any DMSP released from other compartments during the isolation process by adding tracer [35S]DMSP to protoplasts just before they were lysed; essentially no label (<0.2%) was recovered in the purified chloroplasts.
Figure 1.
Scatter plot showing the levels of DMSP in W. biflora mesophyll protoplasts and in the chloroplasts derived from them. Each data point is for a separate experiment. The dashed lines correspond to chloroplast DMSP contents of 10 and 45% of the total in protoplasts, as indicated. ○, Control; •, salinization.
Looking first at protoplasts, Figure 1 shows that although there was variation among control and salinized populations, their mean DMSP levels were quite distinct (4.6 versus 6.0 μmol mg−1 Chl, significantly different at P = 0.05). These mean values are lower than those for the corresponding leaves (Table I), suggesting that the mesophyll cells that gave rise to protoplasts were not those richest in DMSP, or that some DMSP was lost during protoplast isolation. The implication in either case is that our DMSP data should probably be taken as minimum values.
Turning to the chloroplasts, Figure 1 indicates that DMSP levels were always substantial, accounting for 10 to 45% of the total DMSP in protoplasts, and that the mean DMSP level in salinized chloroplasts was twice that in the controls (1.9 versus 0.9 μmol mg−1 Chl, significantly different at P = 0.05). This doubling was attributable to an increase in the proportion of DMSP in the chloroplasts (34 versus 22%), as well as to the higher DMSP level in salinized protoplasts.
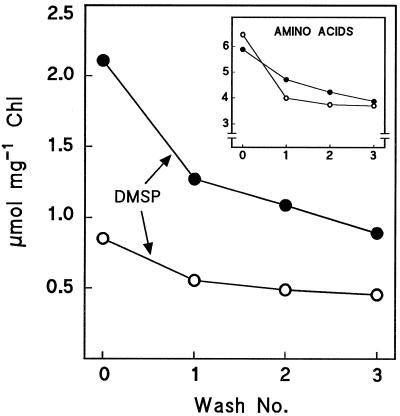
DMSP Leakage from W. biflora Chloroplasts during Washing
Spinach chloroplasts lose Gly betaine during washing (Robinson and Jones, 1986). Therefore, we tested the effects of washing on the DMSP levels in chloroplasts from control and salinized plants. As benchmarks, we also monitored GAPDH and total amino acids. GAPDH activity (6.4 ± 0.4 μmol min−1 mg−1 Chl, mean ± se, n = 3) was not lowered by three washes, showing that little outright chloroplast breakage occurred. In contrast, DMSP levels decreased progressively and to a similar extent in control and salinized chloroplasts (Fig. 2). Amino acid levels also decreased during washing, although less sharply than DMSP in the case of salinized chloroplasts (Fig. 2, inset). For six experiments with control and salinized chloroplasts, the mean (± se) decline in DMSP after two washes was 52 ± 2%. If it is assumed that the wash and Percoll-gradient steps in the isolation procedure would together have given losses similar to two washes, then the DMSP levels of chloroplasts in vivo would be approximately twice those found after isolation.
Figure 2.
Effect of washing on levels of DMSP and free amino acids (inset) in W. biflora chloroplasts. Chloroplasts from control plants (○) or salinized plants (•) were analyzed immediately upon isolation or after one to three additional wash steps. The experiment was repeated, with similar results. Chloroplasts broken by a freeze-thaw treatment lost >90% of their DMSP, showing that it was not adsorbed to chloroplast membranes.
Development of a MALDI-MS Assay for SMM
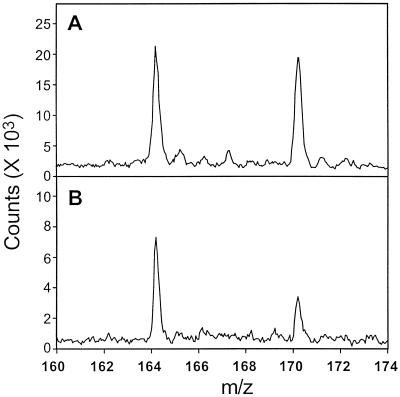
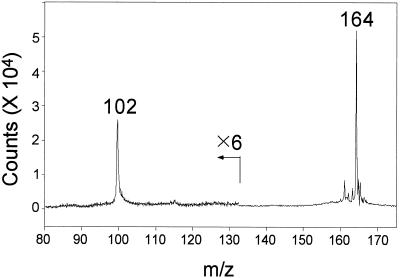
In W. biflora and most other plants leaf SMM levels are <0.5 μmol g−1 fresh weight (Bezzubov and Gessler, 1992; Hanson et al., 1994), so that determining SMM in small samples of protoplasts or chloroplasts requires a sensitive method. Because published assays for SMM lack sensitivity or specificity (Bezzubov and Gessler, 1992; Rhodes and Hanson, 1993), we developed a MALDI-MS assay in which a base fraction prepared by ion-exchange chromatography was analyzed without derivatization. Because the low-mass region in MALDI-MS spectra is typically cluttered with matrix-related peaks, a novel matrix was prepared to minimize the background in the region of interest (m/z 160–173). The low concentrations of SMM relative to those of inorganic ions in some samples, particularly from chloroplasts, gave poor signal:noise ratios in the MALDI spectra. We found that the addition of a dilute solution of fluorosilicic acid to the matrix greatly enhanced the response of SMM. Representative spectra from protoplast and chloroplast samples are shown in Figure 3. A standard curve prepared with unlabeled SMM and [methyl-2H6]SMM (not shown) indicated that they gave the same response on a molar basis. Endogenous SMM was therefore quantified from the ratio of peak areas at m/z 164 (unlabeled SMM) and m/z 170 ([methyl-2H6]SMM). The detection limit for SMM was 0.2 pmol applied to the target. Confirmation that the peak at m/z 164 represented SMM was provided by a MALDI-post-source decay experiment (Fig. 4). This showed that the expected fragment at m/z 102 was formed from the m/z 164 precursor ion by the elimination of dimethylsulfide (164-C2H6S).
Figure 3.
MALDI-MS analysis of the base fractions from protoplasts (A) and chloroplasts (B) isolated from salinized W. biflora leaves. The signals at m/z 164 and 170 correspond to the endogenous SMM and the [methyl-2H6]SMM internal standard, respectively. The peaks in both spectra are unsmoothed.
Figure 4.
MALDI-post-source decay spectrum of the m/z 164 peak from an unsalinized W. biflora chloroplast sample. The ion at m/z 102 represents a fragment formed by a neutral loss of dimethylsulfide, and is consistent with the identification of the m/z 164 peak as SMM.
SMM in W. biflora Protoplasts and Chloroplasts
As for DMSP, tests with the tracer [35S]SMM showed that chloroplasts did not acquire SMM that was released from other compartments during the isolation process. The SMM levels in protoplasts (Table III) were quite comparable to those reported for leaves (Hanson et al., 1994), supporting the evidence from radiolabeling that protoplast isolation does not greatly perturb SMM pools. The SMM levels in protoplasts from salinized leaves were significantly lower than in unsalinized controls (about 190 versus 290 nmol mg−1 Chl), but levels in salinized and control chloroplasts were both about 120 nmol mg−1 Chl (Table III), so that chloroplastic SMM accounted for about 80% of the total in salinized plants but accounted for only one-half of this in the controls. The data for salinized treatments in Table III indicate that SMM loss during chloroplast isolation was small; consistent with this, levels decreased by as little as 8% when isolated chloroplasts were given three additional washes.
Table III.
SMM levels in W. biflora mesophyll protoplasts and protoplast-derived chloroplasts
| Treatment | SMM Level
|
Chloroplastic SMM | |
|---|---|---|---|
| Protoplasts | Chloroplasts | ||
| nmol mg−1 Chl | % of total | ||
| Control | 289a | 114c | 39 (4) |
| Salinized | 192b | 130c | 76 (15) |
Data are means from five and six experiments for control and salinized plants, respectively, in each of which SMM was determined in a protoplast preparation and in chloroplasts derived therefrom. Values for SMM levels followed by different letters are significantly different (P ≤ 0.05) by analysis of variance. The values for chloroplastic SMM (SMM in chloroplasts/SMM in protoplasts × 100) are means and se (in parentheses).
SMM in Spinach and Pea Chloroplasts
Whereas few higher plants accumulate DMSP, most if not all contain SMM (Giovanelli et al., 1980; Bezzubov and Gessler, 1992), and it has been proposed that interconversion of SMM and Met (the SMM cycle) plays an important metabolic role in helping sustain the pool of free Met (Mudd and Datko, 1990). We therefore analyzed spinach and pea, both of which have SMM but not DMSP, to determine whether they resemble W. biflora in having chloroplastic pools of SMM (Table IV). Pea had leaf SMM levels close to those in W. biflora and a chloroplast SMM pool that was detectable but represented only 1% of the total. Spinach had a far lower leaf SMM level, and a small chloroplastic SMM pool equivalent to 15% of the total. The chloroplastic SMM pool in both species was at least 25-fold smaller than that in W. biflora.
Table IV.
SMM levels in spinach and pea leaves and chloroplasts
| Species | SMM Level
|
Chloroplastic SMM | |
|---|---|---|---|
| Leaves | Chloroplasts | ||
| nmol mg−1 Chl | % of total | ||
| Pea | 238 | 2.7 | 1 |
| Spinach | 30 | 4.5 | 15 |
Chloroplasts were isolated from leaves of unsalinized plants by mechanical grinding and purified on Percoll gradients. Data are means of duplicate determinations.
DISCUSSION
DMSP as a Chloroplast Osmolyte
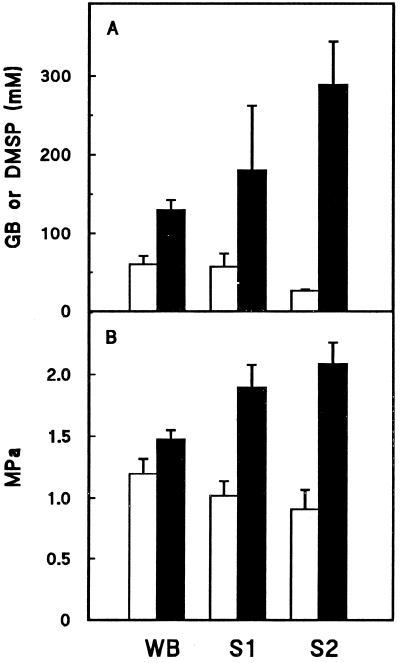
Our data show that much of the DMSP in W. biflora mesophyll cells is located in the chloroplasts. The proportion cannot be measured precisely because DMSP is lost from chloroplasts during isolation. This loss can be estimated as approximately 50% by extrapolation from the results of washing experiments. If a 50% loss is assumed, it may be calculated from the data of Figure 1 that 44 ± 4% of the DMSP is chloroplastic in control cells, and 69 ± 4% is chloroplastic in salinized cells (means ± se). Assuming that the volume of the stromal compartment in unsalinized or salinized W. biflora is in the middle of the range (25–35 μL mg−1 Chl) reported for other plants (Robinson and Jones, 1986; Winter et al., 1993), it can be further calculated that mean stromal DMSP concentrations are 60 mm in control and 130 mm in salinized chloroplasts (Fig. 5A). These values are comparable to those reported for Gly betaine in control and salinized spinach chloroplasts (Fig. 5A), particularly when the smaller overall osmotic adjustment in W. biflora is taken into account (Fig. 5B). The salinization-induced increase in the proportion of DMSP located in chloroplasts suggests that DMSP might be actively redistributed between cellular compartments in response to osmotic stress, as proposed for Gly betaine (Leigh et al., 1981).
Figure 5.
A, Estimated stromal DMSP concentrations in W. biflora chloroplasts compared with published values for Gly betaine (GB) in spinach chloroplasts. B, Leaf osmotic pressures. Open and solid bars are mean values for unsalinized and salinized plants, respectively. Error bars indicate sd values. WB, Data for W. biflora; S1, data for spinach from Schröppel-Meier and Kaiser (1988); and S2, data for spinach calculated from Robinson and Jones (1986).
A salinization-induced increase in DMSP concentration of 70 mm would contribute 0.18 MPa to stromal osmotic pressure, suggesting that about two-thirds of the overall 0.28-MPa osmotic adjustment (Table I) is achieved in the chloroplasts by DMSP accumulation. The role of DMSP in chloroplasts, however, may go beyond that of a nontoxic osmolyte in view of the evidence that its analog, Gly betaine, can protect the photosynthetic machinery against the effects of elevated salt concentrations at concentrations as low as 50 to 100 mm (Deshnium et al., 1995; Nomura et al., 1995). In this context, it is interesting to note that amino acids were important osmolytes in control and salinized chloroplasts but did not accumulate in response to salinization (Fig. 2, inset). Amino acid levels were also found not to increase in salinized spinach chloroplasts (Schröppel-Meier and Kaiser, 1988).
Chloroplastic and Extrachloroplastic Pools of SMM
This study of SMM is the first, to our knowledge, to address its subcellular compartmentation. We found that W. biflora has both chloroplastic and extrachloroplastic pools of SMM, which fits well with the finding that SMM is synthesized in the cytosol but converted to DMSP in the chloroplast (Trossat et al., 1996). Assuming a stromal volume of 30 μL mg−1 Chl (see above) and negligible SMM loss during chloroplast isolation, in vivo stromal concentrations of SMM would be approximately 4 mm in both control and salinized plants. This is a high value and helps explain or strengthen two previous findings concerning the conversion of SMM to DMSP. First, intact W. biflora chloroplasts convert tracer [35S]SMM to DMSP at rates no agreater than 70 pmol mg−1 Chl h−1 (Trossat et al., 1996), which is 2 to 3 orders of magnitude below the in vivo rate of DMSP synthesis. That these rates are so low can be accounted for by a massive (probably >100-fold) isotope dilution by endogenous chloroplastic SMM. Second, in vivo 15N-labeling evidence indicates that the conversion of SMM to 3-dimethylsulfoniopropionaldehyde in W. biflora is mediated by a transaminase (Rhodes et al., 1997). Because transaminases typically have Km values of several millimolar for their amino acid substrates (Christen and Metzler, 1985), a high chloroplastic SMM concentration might be expected.
Whereas salinization had little effect on the chloroplastic SMM pool, it reduced the extrachloroplastic SMM pool(s) by around 100 nmol mg−1 Chl, or 60% (Table III). If we assume for simplicity that there is only one such pool and that it is in the cytosol (Trossat et al., 1996), it follows that a markedly lower cytosolic SMM concentration in salinized plants drives a somewhat higher rate of SMM transport into the chloroplast (and then conversion to DMSP). This would suggest that salinization increases the capacity or affinity of an SMM transporter in the chloroplast envelope. Such a transporter could be a key component in the control architecture of the DMSP pathway. That spinach and pea have small chloroplastic SMM pools further suggests that the putative transporter is not specific to the DMSP pathway but a general feature of flowering plants that is amplified in W. biflora, leading to a greatly increased transmembrane flux of SMM and a much-expanded chloroplastic pool of SMM.
ACKNOWLEDGMENT
We thank Dr. Kurt D. Nolte for help in growing plants and analyzing DMSP.
Abbreviations:
- Chl
chlorophyll
- DMSP
3-dimethylsulfoniopropionate
- GAPDH
NADP-linked glyceraldehyde-3-phosphate dehydrogenase
- MALDI
matrix-assisted laser desorption-ionization
- SMM
S-methyl-l-Met
Footnotes
This work was supported in part by National Science Foundation grant nos. IBN-9514336 (to A.D.H.) and IBN-9628750 (to D.A.G.) and by an endowment from the C.V. Griffin, Sr., Foundation. Mass-spectral data were acquired at the Michigan State University-National Institutes of Health (NIH) Mass Spectrometry Facility, which is supported in part by grant RR 00484 from NIH, National Center for Research Resources. This is University of Florida Agricultural Experiment Station journal series no. R-06059.
LITERATURE CITED
- Arnon DI. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzubov AA, Gessler NN. Plant sources of S-methylmethionine. Prikl Biokhim Mikrobiol. 1992;28:423–429. [PubMed] [Google Scholar]
- Blunden G, Gordon SM. Betaines and their sulphonio analogues in marine algae. Prog Phycol Res. 1986;4:39–80. [Google Scholar]
- Burnet M, Lafontaine PJ, Hanson AD. Assay, purification, and partial characterization of choline monooxygenase from spinach. Plant Physiol. 1995;108:581–588. doi: 10.1104/pp.108.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen P, Metzler DE (1985) Transaminases, Vol 2. Wiley, New York
- Cline K. Import of proteins into chloroplasts: membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem. 1986;261:14804–14810. [PubMed] [Google Scholar]
- Colmer TD, Fan TW-M, Läuchli A, Higashi RM. Interactive effects of salinity, nitrogen and sulphur on the organic solutes in Spartina alterniflora leaf blades. J Exp Bot. 1996;47:369–375. [Google Scholar]
- Deshnium P, Los DA, Hyashi H, Mustardy L, Murata N. Transformation of Synechococcus with a gene for choline oxidase enhances tolerance to salt stress. Plant Mol Biol. 1995;29:897–907. doi: 10.1007/BF00014964. [DOI] [PubMed] [Google Scholar]
- Dickson DMJ, Kirst GO. The role of β-dimethylsulphonio-propionate, glycine betaine and homarine in the osmoacclimation of Platymonas subcordiformis. Planta. 1986;167:536–543. doi: 10.1007/BF00391230. [DOI] [PubMed] [Google Scholar]
- Dickson DMJ, Wyn Jones RG, Davenport J. Steady state osmotic adaptation in Ulva lactuca. Planta. 1980;150:158–165. doi: 10.1007/BF00582360. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Flowers SJ, Hajibagheri MA, Yeo AR. Salt tolerance in the halophytic wild rice, Porteresia coarctata Tateoka. New Phytol. 1990;114:675–684. [Google Scholar]
- Furniss BS, Hannaford AJ, Smith PWG, Thatchell AR (1989) Vogel's Textbook of Practical Organic Chemistry, Ed 5. London Scientific and Technical Press, London, p 1259
- Gage DA, Rhodes D, Nolte KD, Hicks WA, Leustek T, Cooper AJL, Hanson AD. A new route for synthesis of dimethylsulphoniopropionate in marine algae. Nature. 1997;387:891–894. doi: 10.1038/43160. [DOI] [PubMed] [Google Scholar]
- Giovanelli J, Mudd SH, Datko A (1980) Sulfur amino acids in plants. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 5: Amino Acids and Derivatives. Academic Press, New York, pp 453–505
- Gröne T, Kirst GO. Aspects of dimethylsulfoniopropionate effects on enzymes isolated from the marine phytoplankter Tetraselmis subcordiformis (Stein) J Plant Physiol. 1991;138:85–91. [Google Scholar]
- Grumet R, Hanson AD. Genetic evidence for an osmoregulatory function of glycinebetaine accumulation in barley. Aust J Plant Physiol. 1986;13:353–364. [Google Scholar]
- Hanson AD, Gage DA (1996) 3-Dimethylsulfoniopropionate biosynthesis and use by flowering plants. In RP Kiene, PT Visscher, MD Keller, GO Kirst, eds, Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Plenum Press, New York, pp 75–86
- Hanson AD, Rivoal J, Paquet L, Gage DA. Biosynthesis of 3-dimethylsulfoniopropionate in Wollastonia biflora (L.) DC. Evidence that S-methylmethionine is an intermediate. Plant Physiol. 1994;105:103–110. doi: 10.1104/pp.105.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MD, Bellows WK, Guillard RRL. Dimethyl sulfide production in marine phytoplankton. In: Saltzman ES, Cooper WJ, editors. Biogenic Sulfur in the Environment. Washington, DC: American Chemical Society; 1989. pp. 167–182. [Google Scholar]
- Larher F, Hamelin J, Stewart GR. L'acide diméthylsulfo-nium-5 propanoïque de Spartina anglica. Phytochemistry. 1977;16:2019–2020. [Google Scholar]
- Leigh RA, Ahmad N, Wyn Jones RG. Assessment of glycinebetaine and proline compartmentation by analysis of isolated beet vacuoles. Planta. 1981;153:34–41. doi: 10.1007/BF00385315. [DOI] [PubMed] [Google Scholar]
- Lilley RM, Fitzgerald MP, Rienits KG, Walker DA. Criteria of intactness and the photosynthetic activity of spinach chloroplast preparations. New Phytol. 1975;75:1–10. [Google Scholar]
- Malin G (1996) The role of DMSP and DMS in the global sulfur cycle and climate regulation. In RP Kiene, PT Visscher, MD Keller, GO Kirst, eds, Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Plenum Press, New York, pp 177–189
- Mason TG, Blunden G. Quaternary ammonium and tertiary sulfonium compounds of algal origin as alleviators of osmotic stress. Bot Mar. 1989;32:313–316. [Google Scholar]
- Matoh T, Watanabe J, Takahashi E. Sodium, potassium, chloride and betaine concentrations in isolated vacuoles from salt-grown Atriplex gmelini leaves. Plant Physiol. 1987;84:173–177. doi: 10.1104/pp.84.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills WR, Joy KW. A rapid method for isolation of purified, physiologically active chloroplasts, used to study the intracellular distribution of amino acids in pea leaves. Planta. 1980;148:75–83. doi: 10.1007/BF00385445. [DOI] [PubMed] [Google Scholar]
- Mudd SH, Datko AH. The S-methylmethionine cycle in Lemna paucicostata. Plant Physiol. 1990;93:623–630. doi: 10.1104/pp.93.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi MK, Somero GN. Temperature- and concentra-tion-dependence of compatibility of the organic osmolyte β-dimethylsulfoniopropionate. Cryobiology. 1992;29:118–124. doi: 10.1016/0011-2240(92)90011-p. [DOI] [PubMed] [Google Scholar]
- Nomura M, Ishitani M, Takabe T, Rai AK, Takabe T. Synechococcus sp. PCC7942 transformed with Escherichia coli bet genes produces glycine betaine from choline and acquires resistance to salt stress. Plant Physiol. 1995;107:703–708. doi: 10.1104/pp.107.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet L, Lafontaine PJ, Saini HS, James F, Hanson AD. Évidence en faveur de la présence du 3-diméthylsulfonio-propionate chez une large gamme d'angiospermes. Can J Bot. 1995;73:1889–1896. [Google Scholar]
- Paquet L, Rathinasabapathi B, Saini H, Zamir L, Gage DA, Huang Z-H, Hanson AD. Accumulation of the compatible solute 3-dimethylsulfoniopropionate in sugarcane and its relatives but not other gramineous crops. Aust J Plant Physiol. 1994;21:37–48. [Google Scholar]
- Rathinasabapathi B, McKue KF, Gage DA, Hanson AD. Metabolic engineering of glycine betaine biosynthesis: plant betaine aldehyde dehydrogenases lacking typical transit peptides are targeted to tobacco chloroplasts where they confer betaine aldehyde resistance. Planta. 1994;193:155–162. doi: 10.1007/BF00192524. [DOI] [PubMed] [Google Scholar]
- Rhodes D, Gage DA, Cooper AJL, Hanson AD. S-Methylmethionine conversion to dimethylsulfoniopropionate: evidence for an unusual transamination reaction. Plant Physiol. 1997;115:1541–1548. doi: 10.1104/pp.115.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D, Hanson AD. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:357–384. [Google Scholar]
- Robinson SP, Jones GP. Accumulation of glycinebetaine in chloroplasts provides osmotic adjustment during salt stress. Aust J Plant Physiol. 1986;13:659–668. [Google Scholar]
- Rosen H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957;67:10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Schröppel-Meier G, Kaiser WM. Ion homeostasis in chloroplasts under salinity and mineral deficiency. I. Solute concentrations in leaves and chloroplasts from spinach plants under NaCl or NaNO3 salinity. Plant Physiol. 1988;87:822–827. doi: 10.1104/pp.87.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey R, Gorham J, Pitman MG, Hanson AD, Gage DA. Response of Melanthera biflora to salinity and water stress. J Exp Bot. 1993;44:1551–1560. [Google Scholar]
- Trossat C, Nolte KD, Hanson AD. Evidence that the pathway of dimethylsulfoniopropionate biosynthesis begins in the cytosol and ends in the chloroplast. Plant Physiol. 1996;111:965–973. doi: 10.1104/pp.111.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weretilnyk EA, Smith DD, Wilch GA, Summers PS. Enzymes of choline synthesis in spinach. Response of phospho-base N-methyltransferase activities to light and salinity. Plant Physiol. 1995;109:1085–1091. doi: 10.1104/pp.109.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in barley leaves. Planta. 1993;191:180–190. [Google Scholar]