Abstract
Cigarette smoking is a common addiction that increases the risk for many diseases, including lung cancer and chronic obstructive pulmonary disease. Genome-wide association studies (GWAS) have successfully identified and validated several susceptibility loci for nicotine consumption and dependence. However, the trait variance explained by these genes is only a small fraction of the estimated genetic risk. Pathway analysis complements single marker methods by including biological knowledge into the evaluation of GWAS, under the assumption that causal variants lie in functionally related genes, enabling the evaluation of a broad range of signals. Our approach to the identification of pathways enriched for multiple genes associated with smoking quantity includes the analysis of two studies and the replication of common findings in a third dataset. This study identified pathways for the cholinergic receptors, which included SNPs known to be genome-wide significant; as well as novel pathways, such as genes involved in the sensory perception of smell, that do not contain any single SNP that achieves that stringent threshold.
Introduction
Cigarette smoking is a common habit that has detrimental effects on physical health including an increased risk of heart disease, cancer, stroke, and chronic lung disease. In the United States, cigarette smoking is the leading cause of morbidity and mortality; accounting for 30% of all cancer deaths and 80% of deaths from chronic obstructive pulmonary disease [1]. Although tobacco smoking is a complex multidimensional behavior, research has highlighted smoking quantity, usually evaluated by cigarettes per day (CPD), as a predictor of nicotine dependence [2].
Epidemiological studies have demonstrated that both environmental and genetic factors are associated with different dimensions of smoking behavior [3]. The heritability of smoking quantity is estimated to be between 0.49 and 0.56 [4], and different GWAS have identified and replicated signals within the nicotinic acetylcholine receptor genes on chromosome 15q25 (CHRNA5-A3-B4) [5]–[10] and chromosome 8p11 (CHRNB3-A6) [8], [9], as well as nicotine metabolizing genes on chromosome 19q13 (CYP2A6-B6) [9], [10];. Each of these variants explains 0.5∼1.9% of the phenotypic variance for subjects of European ancestry (EA) [10], [11]. Additional meta-analysis efforts [7] have been carried out to identify more loci, presumably with smaller effect and only detectable by the power gained by a larger number of samples. The most encouraging signals found in a discovery phase, that included more than 15,000 subjects with reported values for smoking quantity, were followed up in a replication phase which encompassed two studies that gathered even larger pools of subjects (ENGAGE [9] n = 46,481; and TAG [10] n = 74,035). However, no additional locus achieved genome-wide significance (p-value<5.0E−8 for 1 million SNPs tested), raising the possibility that structural or rare variants with strong effects explain the missing heritability [12].
That only a small fraction of the heritability of smoking quantity is detected by GWAS is a characteristic common to many other complex traits [13]. Preliminary estimates of the total amount of phenotypic variance explained by common SNPs (minor allele frequency >1%) [13], suggests that similar to other complex traits [13], [14], the percentage of genetic variance captured by GWAS chips is higher than that explained by the loci identified so far; this suggests that many of the signals that fail to reach genome-wide significance in current datasets are true signals.
Pathway analysis offers a complementary perspective to interpret GWAS, incorporating repositories of expert knowledge, represented in biological pathway databases and gene ontologies. This approach evaluates whether the signals detected by a GWAS are overrepresented for families of biologically related genes. By shifting from the evaluation of individual SNPs to pathways of genes –under the commonly accepted hypothesis that causal variants are not randomly distributed across the genome, but instead lie in functionally related genes– we can prioritize the variants that do not reach genome-wide significance level [15]. This concept was extensively employed in the identification of expression profiles of microarrays, and since then has been adapted to mine GWAS datasets [15]–[17]. Employing different statistical methods and implementations, pathway analysis has been applied to a variety of neurological and psychiatric diseases [17]; implicating axon guidance for Parkinson disease [18], neuronal cell adhesion and membrane scaffolding for schizophrenia and Bipolar disorder [19], and immune system and cholesterol metabolism for Alzheimer’s disease [20].
We applied pathway analysis to two substance dependence GWAS datasets, the “Nicotine addiction Genetics” (OZALC-NAG) [21], and the “Study of Addiction: Genetics and Environment” (SAGE) [22], analyzing a commonly used measure of smoking behavior [7], [9], [10], cigarettes per day (CPD )(Table 1), to identify enriched candidate sets of genes defined as Gene Ontology (GO) terms [23] and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways [24]. We carried out the statistical analysis by executing the algorithm ALIGATOR [15] an overrepresentation method that analyzes genes exhibiting significance below a specified threshold. We analyzed these two studies independently, and selected those terms and pathways that were statistically significant (p-value<0.05) in both datasets, and further verified their role by analyzing the EA subjects from the Atherosclerosis Risk in Communities study (ARIC) [25], [26]. We applied this same approach by executing the algorithm MAGENTA [27] an implementation of the method Gene Set Enrichment Analysis (GSEA).
Table 1. Characteristics of the studies and subjects analyzed.
| OZALC-NAG | SAGE | ARIC | ||
| # Subjects | 4038 | 2014 | 5198 | |
| % Men/%Women | 49%/51% | 44%/56% | 56%/44% | |
| Age, mean ± S.D. | 45.16±10.43 | 37.97±9.09 | 54.30±5.7 | |
| Cigarettes per day | ||||
| 0 to 10 | 1159 | 1001 | 1047 | |
| 11 to 20 | 876 | 518 | 2155 | |
| 21 to 30 | 958 | 223 | 941 | |
| 30 or more | 1045 | 272 | 1052 | |
CPD: Cigarettes per day.
Materials and Methods
Samples and Study Design
We analyzed the subjects with European ancestry with reported cigarettes per day (CPD) values included in the Nicotine Addiction Genetics (OZALC-NAG) study [28]; the Study of Addiction: Genetics and Environment (SAGE) [22]; and the Atherosclerosis Risk in Communities study (ARIC) [25], [26] (Table 1). Both the OZALC-NAG, which is an Australian family based study, and the SAGE study, which includes unrelated North American subjects, are GWAS ascertained based on substance dependence. In contrast ARIC is a population-based study designed to investigate the etiology of atherosclerosis in middle-aged adults [25]. Additional details for each of these studies is provided in Text S1 and elsewhere [28], [22] and [26].
Genotypes
We implemented a unifying strategy that employed both genotyped and imputed SNPs to analyze the same set of SNPs in each of the three studies (Text S2). We analyzed all of the SNPs included in the Illumina Human 1 M beadchip to maximize the signals ascertained in the two exploratory studies.
Pathway Analysis
ALIGATOR [15]
This method performs an overrepresentation analysis, evaluating the significance for each category of genes empirically. This method can be applied to both unrelated and family based datasets. It selects the set of genes, of size n, which are tagged by SNPs located within gene sequences or in the 20 kb up/downstream flanking these gene regions, which are more significant than a specific threshold (i.e., <0.001; 0.005; 0.01; and 0.05). The association p-value is estimated using standard GWAS methods, and is detailed in Text S3. A pruning process that eliminates SNPs in linkage disequilibrium is performed by considering only the most significant SNP among all of the SNPs that have r2>0.2 and are within 1 Mb. If one SNP tags more than one gene, all of these genes are included as significant. Although more than one SNP in linkage equilibrium (r2<0.2) might tag a gene, each gene is counted only once. The statistical significance of the overrepresentation of each set of genes (category-specific p-value) is calculated by comparing the number of significant genes to the number of genes expected by chance. For this purpose, the algorithm generates 50,000 sets of genes, by randomly selecting SNPs until a list of n tagged genes is formed.
Random sets of genes are also employed to estimate the study wide p-value by applying a bootstrap method: one of the 50,000 sets of genes is selected as a reference and compared to a subsample of 5,000 random lists (selection with replacement). This is repeated 1,000 times, comparing the most significant p-value in each iteration to the p-value of each category of genes, estimating how often the significance level is obtained by random chance. In addition, the number of sets of genes that achieved different threshold levels (i.e., <0.005; 0.01 and 0.05) is compared to the values obtained by random reference studies to calculate the excess of significantly overrepresented sets of genes [15]. The method can correct for any bias due to genes that are physically located within the same region of the chromosome (genes which are less than 1 Mb apart) associated with the same signal and assigned to the same category of genes. This is done by grouping these genes as a single entity, which is tagged by a set of SNPs mapping within the genes.
MAGENTA [27]
This method implements a gene set enrichment analysis [29] (GSEA) without requiring the empirical phenotype-based test procedure to estimate the significance of the categories of genes, enabling its application to family based studies as well as population-based genome-wide association study meta-analyses. In our work. each gene is scored by the most significant p-value among all of the SNPs located within the gene or up to 20 kb from the 5′ and 3′ ends of the genic sequences (Text S2). This value is corrected for confounding effects: the gene size, number of SNPs per kb, number of independent SNPs per kb, number of recombination hotspots per kb, linkage disequilibrium units per kb, and genetic distance measured in centiMorgans per kb. This is done by applying a step-wise multiple linear regression analysis to the normalized (Z-score) p-value of each gene [27]. MAGENTA also corrects for physical proximity along the chromosome retaining only one gene per cluster of genes in a category of genes. The nominal GSEA p-value is calculated by comparing the extent of the “leading edge fraction” (i.e., the subset of genes whose corrected p-values are among the 95th percentile) of each category of genes (of size n), to the one observed in 10,000 random samples of n genes. Finally, the method uses Bonferroni multiple test correction (p-value = 0.05), and also computes the false discovery rate, a less stringent approach to correct for the burden of testing multiple hypotheses.
Enrichment of Categories of Genes Common to OZALC-NAG and SAGE Studies
The statistical significance of the number of categories of genes enriched in both the OZALC-NAG and SAGE studies was empirically evaluated by executing ALIGATOR to evaluate the OZALC-NAG study on the subset of categories of genes significantly enriched in the SAGE study.
Biological Repositories of Expert Knowledge Analyzed
For the current analysis we included Gene Ontology terms [23] (April 2010) and Kyoto Encyclopedia of Genes and Genomes pathways [24] (October 2010) that contain between 3 and 500 genes interrogated by the Illumina 1 M platform. This resulted in a total of 7962 GO terms and 217 KEGG pathways that we refer to as categories of genes.
Results
Approach to Identify GO Terms and KEGG Pathways Affecting Cigarette Consumption
We applied the ALIGATOR method independently to the OZALC-NAG study and the EA subjects from the SAGE study; analyzing the subset of SNPs that reached different significance levels (i.e., SNP p-value<0.001; 0.005; 0.01; and 0.05), to circumvent any bias introduced by this parameter [15]. We observed that for every combination of SNP p-value and category of gene enrichment p-value thresholds, there were a significant number of categories of genes enriched in common in the OZALC-NAG and SAGE studies (p-value<0.01) (Table 2). We selected the thresholds for the SNP and category of gene p-values (Table 2) that identified a significant excess of enriched categories of genes (p-value<0.05) for both studies. (See Table S1 panels A and B to see specific Gene Ontology and KEGG results respectively). We discarded those values for the SNP and category of gene threshold that were significant for only a single study: for example, although the OZALC-NAG study show a significant excess of pathways (p-value = 0.0346) with p-values lower than 0.005 when we analyzed the SNPs with p-values lower than 0.005, the SAGE study was not significant (p-value = 0.141) for these same cutoff values (Table 2). Because of this lack of consistency, we did not consider the categories of genes identified by these specific values for the thresholds. In contrast, there was a replicated significant excess of categories of genes for both studies when the SNPs with p-values <0.001 were analyzed. This occurred independently of the threshold for categories considered (i.e., 0.005, 0.01 and 0.05) (Table 2); thus we considered all of the terms and pathways with a category-specific p-value that satisfied the most relaxed constraint for category cutoff (i.e., = 0.05), which includes all of the pathways satisfying the more stringent constraints. When we analyzed the SNPs with p-values <0.05 we observed a significant excess of enriched categories of genes with p-values <0.005 and 0.01 consistently in both OZALC-NAG and SAGE studies (Table 2). Applying the same criteria described above, we analyzed the categories with category-specific p-values <0.01. Our replication strategy was to evaluate the subset of categories of genes that were significant for both the OZALC-NAG and the SAGE studies in the ARIC study (p-value<0.05) (See Table 2 for the number of common categories of genes between the two studies and its statistical significance).
Table 2. Number of categories of genes identified by ALIGATOR for OZALC-NAG and SAGE studies for smoking quantity.
| Category of Gene enrichment threshold | |||||||||
| 0.005 | 0.01 | 0.05 | |||||||
| SNP Threshold | Study | # SNPs | # Genes | #cat. | p-value | #cat. | p-value | #cat. | p-value |
| 0.001 | OZALC-NAG | 270 | 264 | 24 | 1.40E−03 | 36 | 3.80E−03 | 102 | 1.32E−02 |
| SAGE | 206 | 225 | 15 | 7.60E−03 | 35 | 2.40E−03 | 122 | 1.60E−03 | |
| Common | 4 | <2.00E−4 | 5 | <2.00E−4 | 7 | 8.20E−03 | |||
| 0.005 | OZALC-NAG | 1158 | 1078 | 18 | 3.46E−02 | 27 | 1.04E−01 | 147 | 7.94E−02 |
| SAGE | 1094 | 1127 | 11 | 1.14E−01 | 35 | 5.40E−02 | 181 | 2.92E−02 | |
| Common | 2 | <2.00E−4 | 2 | <2.00E−4 | 14 | 1.20E−03 | |||
| 0.01 | OZALC-NAG | 2242 | 1962 | 19 | 6.18E−02 | 37 | 1.06E−01 | 175 | 1.41E−01 |
| SAGE | 2150 | 2021 | 21 | 4.42E−02 | 41 | 7.72E−02 | 197 | 8.16E−02 | |
| Common | 2 | <2.00E−4 | 4 | <2.00E−4 | 12 | 1.60E−03 | |||
| 0.05 | OZALC-NAG | 10374 | 6832 | 39 | 2.62E−02 | 72 | 4.32E−02 | 281 | 1.70E−01 |
| SAGE | 9906 | 6901 | 46 | 1.06E−02 | 85 | 1.88E−02 | 326 | 6.70E−02 | |
| Common | 9 | <2.00E−4 | 12 | <2.00E−4 | 55 | <2.00E−4 | |||
SNPs: number of SNPs that achieved a significant for each threshold for OZALC-NAG and SAGE studies;
Genes: number of genes mapped;
cat and p-value reflect the excess of categories of genes (GO terms or KEGG pathways) for the thresholds applied to the category-specific p-values in the OZALC-NAG and the SAGE studies. “Common” rows show the number of overlapping categories of genes significantly enriched for both studies and the corresponding statistical significance.
For the most stringent threshold for SNPs (p-value<0.001) ALIGATOR identified 102 and 122 categories of genes in OZALC-NAG and SAGE respectively (category-specific p-value<0.05)(Table 2). Seven GO terms were significant for both studies (Table S2) but no significant KEGG pathways were common to both studies. Moreover, all seven GO terms were also significant for the ARIC study (p-value<0.05) (Table S2). These terms group genes in the cluster of cholinergic nicotinic receptor genes on chromosomes 15 and 8, and closely related genes.
We also inspected the categories of genes identified by the most relaxed threshold for SNPs (i.e., <0.05). A total of 72 and 85 categories were significant for OZALC-NAG and SAGE respectively (category-specific p-value <0.01) (Table 2). Eleven GO terms and one KEGG pathway were common to both studies. These terms and pathways showed an overlap among the constituent genes; thus we grouped these into 4 clusters of categories of genes that reflected the similarity of the genes included (Table 3; Figure S1). These clusters were labeled i) cholinergic nicotinic receptors, ii) sensory perception of chemical stimulus/smell related genes, iii) ribosome genes, and iv) retinoid binding genes. Eight of these categories of genes were significant in ARIC (category-specific p-value<0.05). Neither of the two retinoid binding GO terms showed statistical significance for the ARIC study. In contrast, each of the remaining 3 clusters included at least one GO term with category-specific p-values lower than 0.01 for the ARIC study.
Table 3. ALIGATOR identified common OZALC-NAG and SAGE GO terms and KEGG pathways for the analysis of SNPs p-value<0.05 and ARIC replication results.
| OZALC-NAG | SAGE | ARIC | Combined | ||||||||||
| acc | name | # Genes Cat. | # Genes | p-value | Expected | # Genes | p-value | Expected | # Genes | p-value | Expected | p-value * | |
| Cholinergic nicotinic receptors | |||||||||||||
| GO:0042166 | acetylcholine binding | 22 | 11 | 5.80E−04 | 3.30 | 10 | 2.32E−03 | 3.3348 | 8 | 7.10E−03 | 2.69 | 2.57E−06 | |
| GO:0015464 | acetylcholine receptor activity | 18 | 10 | 9.00E−04 | 2.86 | 8 | 9.98E−03 | 2.8908 | 7 | 1.12E−02 | 2.34 | 1.17E−05 | |
| GO:0004889 | nicotinic acetylcholine-activated cation-selective channel activity | 17 | 9 | 4.40E−03 | 3.05 | 10 | 1.20E−03 | 3.0838 | 7 | 1.50E−02 | 2.49 | 2.40E−05 | |
| GO:0005892 | nicotinic acetylcholine-gatedreceptor-channel complex | 16 | 9 | 4.40E−03 | 3.05 | 10 | 1.20E−03 | 3.0838 | 7 | 1.50E−02 | 2.49 | 1.24E−04 | |
| Sensory perception | |||||||||||||
| GO:0004984 | olfactory receptor activity | 369 | 82 | 4.00E−05 | 48.48 | 78 | 1.00E−04 | 48.9594 | 61 | 1.08E−03 | 39.61 | 8.11E−09 | |
| GO:0007608 | sensory perception of smell | 386 | 91 | 9.40E−04 | 64.19 | 84 | 9.16E−03 | 64.8288 | 66 | 3.71E−02 | 52.42 | 5.44E−05 | |
| GO:0007606 | sensory perception of chemical stimulus | 430 | 99 | 8.00E−04 | 70.76 | 91 | 9.06E−03 | 71.4626 | 70 | 5.20E−02 | 57.77 | 7.70E−05 | |
| Ribosome | |||||||||||||
| hsa03010 | Ribosome | 88 | 15 | 3.40E−04 | 5.36 | 19 | 2.00E−04 | 5.4116 | 14 | 1.60E−04 | 4.37 | 1.13E−08 | |
| GO:0005840 | ribosome | 192 | 34 | 5.00E−04 | 17.97 | 40 | 2.00E−04 | 18.1458 | 32 | 8.00E−04 | 14.67 | 5.69E−08 | |
| GO:0022625 | cytosolic large ribosomal subunit | 36 | 8 | 4.82E−03 | 2.53 | 9 | 1.52E−03 | 2.557 | 5 | 6.17E−02 | 2.05 | 1.73E−04 | |
| Retinoid | |||||||||||||
| GO:0005501 | retinoid binding | 26 | 9 | 2.36E−03 | 5.54 | 9 | 2.60E−03 | 4.0218 | 6 | 1.71E−01 | 4.84 | 5.53E−04 | |
| GO:0001972 | retinoic acid binding | 11 | 3 | 1.82E−03 | 0.68 | 4 | 6.40E−04 | 1.0174 | 1 | 5.82E−01 | 3.00 | 2.80E−03 | |
acc: Category of gene (prefix GO indicates Gene ontology and hsa KEGG pathways) and corresponding name;
Genes Cat.: the total number of genes grouped by the category. For each study:
genes significant; corresponding category-specific p-value; and the Expected number of genes.
Combined p-values were calculated employing the weighted Z-score method.
GO Terms Enriched for Cholinergic Receptor Genes
We identified terms for cholinergic nicotinic receptor genes (CHRN) that were significant independent of the threshold used to select the SNPs. Three molecular function and one cellular component terms were significant for both OZALC-NAG and SAGE, regardless of the threshold for SNPs considered (Table 3 and Table S2). These four terms were replicated in ARIC, again for both p-value thresholds applied to SNP selection. Despite specificity differences in the genes grouped by each term, all of them contain a majority of the nicotinic (CHRN) and muscarinic (CHRM) cholinergic receptor subunit genes, and other closely related genes (Figure 1).
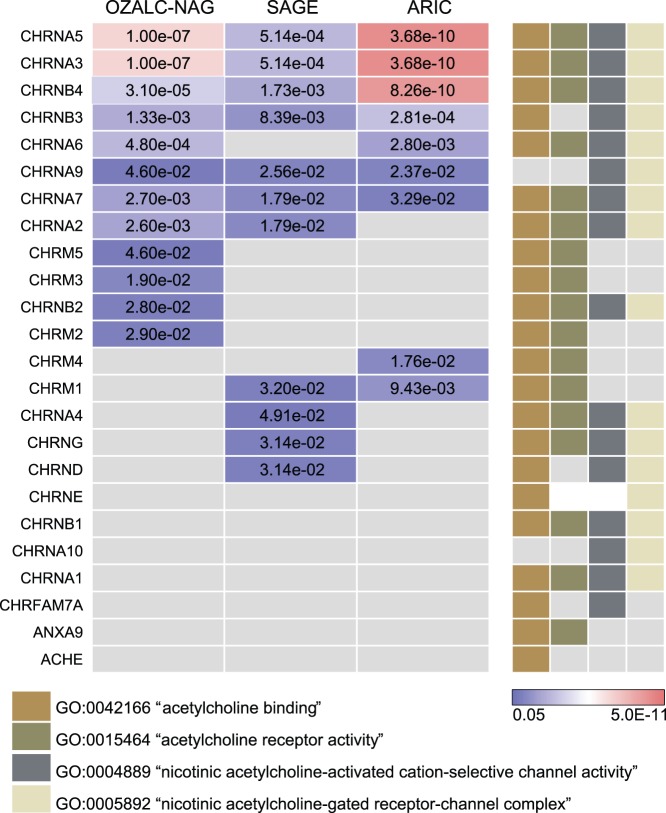
Figure 1. Go terms for cholinergic receptors and significant genes.
The p-value of each gene was assigned based on the most significant SNP in gene sequences and flanking regions (Left panel). SNPs in linkage disequilibrium (r2>0.2) and in a local proximity (1 Mb) were removed. Colored boxes in the right panel reflect the assignment of each gene to the different GO terms.
All of these terms included the clusters of cholinergic receptors on chromosome 15 (CHRNA5-A3-B4) [5]–[10] and chromosome 8 (CHRNB3-A6) [8]–[10]. By virtue of the broad range of SNP significance analyzed, these genes were tagged in each of the 3 studies, whether or not the significant SNPs achieved genome-wide level (Figure 1; See Table S3 for the rs numbers of the SNPs tagging the genes in cholinergic receptor genes). In addition, several other cholinergic receptors were tagged by SNPs with more moderate p-values (<0.05) (Figure 1). CHRNA7 (chr. 15q14) [11] and CHRNA9 (chr. 4p14) were tagged in each of the three studies (Figure 1), suggesting that variants in these genes, are also implicated in the genetic susceptibility to smoking quantity. To determine whether the nicotinic receptor genes on chromosomes 15 and 8 drove the statistical significance of these terms we analyzed a reduced dataset that did not include these genes (i.e., we removed CHRNA5-A3-B4 and CHRNB3-A6 genes). Although there was a considerable difference in the significance, the terms GO:0005892, GO:0042166 and GO:0004889 showed p-values <0.05 for SAGE (i.e., 0.014, 0.02 and 0.014 respectively) and a marginal p-value of 0.056 for the term GO:0015464. In the OZALC-NAG study, the term GO:0015464 showed a p-value of 0.049, and the term GO:0042166 a marginal p-value of 0.06. None of these terms was significant in ARIC, which reported the fewest significant genes for these terms (Table 3; and Figure 1), and only included 4 genes (CHRNA7/9 and CHRM4/1) after dropping the genome-wide significant genes.
The MAGENTA algorithm only identified GO terms related to cholinergic nicotinic genes (See Table 4 for significance results, and Table S4 for false discovery rates). Seven of the terms identified for OZALC-NAG and SAGE were also significant in ARIC (nominal p-value<0.05). Moreover, three of these terms were previously detected by ALIGATOR (i.e., GO:0015464, GO:0005892 and GO:0004889). In contrast to the other terms detected by MAGENTA, the term GO:0005230, ancestor of GO:0005230, includes genes other than the cholinergic receptors. However, none of these seven terms remained significant in any study when we performed the analysis dropping the clusters of cholinergic genes located in chromosomes 15 and 8, indicating that this core of cholinergic nicotinic receptors drives the results observed using MAGENTA.
Table 4. MAGENTA Significant categories of genes with nominal p-value<0.05 in the OZALC-NAG and the SAGE studies and ARIC corresponding results.
| OZALC-NAG | SAGE | ARIC | Combined | |||
| Threshold | Acc | Name | p-value | p-value | p-value | p-value * |
| 0.005 | GO:0035095 | behavioral response to nicotine | 2.20E−03 | 2.20E−03 | 2.00E−03 | 8.29E−06 |
| GO:0060084 | synaptic transmission involved in micturition | 8.50E−03 | 7.20E−03 | 7.90E−03 | 1.91E−04 | |
| 0.01 | GO:0006942 | regulation of striated muscle contraction | 6.50E−03 | 6.30E−03 | 1.00E+00 | 2.94E−01 |
| 0.05 | GO:0004889 | nicotinic acetylcholine-activated cation-selective channel activity | 8.50E−05 | 4.06E−02 | 6.00E−04 | 7.83E−07 |
| GO:0005892 | nicotinic acetylcholine-gated receptor-channel complex | 8.20E−05 | 4.36E−02 | 1.10E−03 | 1.60E−06 | |
| GO:0015464 | acetylcholine receptor activity | 2.68E−04 | 2.50E−02 | 2.20E−03 | 5.90E−06 | |
| GO:0005230 | extracellular ligand-gated ion channel activity | 3.50E−03 | 1.65E−02 | 1.76E−02 | 3.43E−04 | |
| GO:0007271 | synaptic transmission, cholinergic | 3.30E−03 | 2.63E−02 | 2.60E−02 | 6.41E−04 | |
| GO:0042060 | wound healing | 3.00E−04 | 1.69E−02 | 3.22E−01 | 6.05E−03 | |
| GO:0006940 | regulation of smooth muscle contraction | 2.50E−02 | 2.61E−02 | 1.33E−01 | 1.68E−02 | |
| GO:0005216 | ion channel activity | 2.52E−02 | 1.53E−02 | 1.88E−01 | 2.23E−02 | |
| GO:0042552 | myelination | 1.51E−02 | 1.64E−02 | 4.35E−01 | 5.94E−02 | |
| GO:0007257 | activation of JUN kinase activity | 3.42E−02 | 3.61E−02 | 3.52E−01 | 7.47E−02 | |
| GO:0006548 | histidine catabolic process | 4.71E−02 | 4.27E−02 | 3.04E−01 | 7.52E−02 | |
| GO:0034185 | apolipoprotein binding | 4.20E−02 | 4.12E−02 | 1.00E+00 | 1.45E−01 | |
| GO:0007265 | Ras protein signal transduction | 4.17E−02 | 4.99E−02 | 8.33E−01 | 3.14E−01 |
Threshold of significance satisfied by both NAF and SAGE; acc: identifier for the category of genes and name. For each study, the nominal GSEA p-value is shown (see Table S4 for false discovery rate).
Combined p-values were calculated employing the weighted Z-score method.
Sensory Perception of Chemical Stimulus and Smell
The most abundant categories of genes, with regard to the number of genes included, that ALIGATOR identified in the OZALC-NAG and SAGE studies grouped highly similar sets of genes. The biological processes GO:0007608, its ancestor the term GO:0007606 and the molecular function term GO:0004984 shared 368 genes in common (Table 3). From the pool of 431 different genes grouped by these terms, 56 were significant for at least two studies (Figure S2), and 7 genes were common to all three studies. This list includes the glutamate receptors, metabotropic 7 (GRM7– chr. 3p26.1-p25.1) and 8 (GRM8– chr. 7q31.3-q32.1); the olfactory receptors OR10P1 (chr. 12q13.2); OR52E2 (chr. 11p15.4), OR52J3 (chr. 11p15.4), and OR8D4 (chr. 11q24.1); and the bitter taste receptor TAS2R1 (chr. 5p15).
Both GRM7 and GRM8 are part of the glutamate signaling pathway and were previously reported to be associated with nicotine dependence [30], [31] and smoking initiation [32] respectively. In addition, a genetic linkage peak near GRM7 and nominal association was reported for GRM7 and major depression in the OZALC-NAG heavy smoking families [33]. rs963843 (MAF = 0.14) in GRM8 was the most significant SNP for SAGE (p-value = 5.0E−3), with an increased number of CPD. Although not significant, we observed the same direction of effect for this SNP in both OZALC-NAG (p-value = 0.074) and ARIC (p-value = 0.10), with the combined p-value = 1.16E−3. Two additional SNPs, the protective rs1557644 (p-value = 3.0E−3; MAF = 0.35) and the risk rs1018854 (p-value = 3.27E−4; MAF = 0.44) were significant in OZALC-NAG and ARIC respectively. Moreover, the haplotype derived from the risk alleles showed a p-value = 5.40E−3 (effect = 0.21) for SAGE.
Retinoid Binding Genes
Two molecular function GO terms significant for both the OZALC-NAG and SAGE studies group genes related to retinoid binding (Table 3), and exhibit a hierarchical relationship among them, GO:0001972 being the most specific and the term GO:0005501 the most general. Both studies share 4 significant genes (Figure S3) encoding the cellular retinoic acid binding proteins 1 (CRABP1 - chr 15q24), and 2 (CRABP2 - chr. 1q21.3); insulin-like growth (IGF2R - chr 6q26); and the complex of genes UDP glucuronosyltransferase 1 family, polypeptide A (UGT1A1/3/4/6/7/8/9/10), which are associated with the metabolism of nicotine [34]. Due to the physical overlap on the chromosome of this complex of genes, we employed UGT1A4, as a representative of the entire complex, eliminating the other members from the database of terms.
None of these terms was significant for ARIC, though the term GO:0016918 “retinal binding”, which represents a subset of the term GO:0005501 was significant (p-value = 0.045). This term also includes the gene CRABP1 (Figure S3), which was significant for ARIC. Among the other genes in the term GO:0016918 in ARIC we found 3 common to OZALC-NAG (Figure S3). A posterior inspection of the ARIC study showed that the gene IGF2R, also had significant SNPs (i.e., rs8191772 with a p-value = 0.01). In contrast, no significant SNP tagged either CRABP2 nor UGT1A4.
Discussion
Our approach to analyzing susceptibility pathways for smoking quantity was based on the identification of GO terms and KEGG pathways common to the OZALC-NAG and SAGE datasets that showed replication in the ARIC study. Our decision to unify the set of SNPs analyzed in each of the two exploratory studies, genotyped by different Illumina chips, proved to be a valid option that identifies common categories of genes while maximizing the chances of observing the same enriching genes. We implemented this strategy by extending the set of SNPs originally genotyped in the OZALC-NAG study, incorporating imputed data to encompass the ones ascertained in SAGE. We applied this same approach to analyze the ARIC dataset, which was genotyped using the Affymetrix platform. Moreover, apart from the differences in the enriching genes, all of the replicated GO terms and KEGG pathways were also significant when we restricted analysis of ARIC to the genotyped SNPs in the Affymetrix Human SNP Array 6.0 (data not shown).
We found GO terms for the cholinergic receptors, that included genes tagged by SNPs previously reported to achieve genome-wide significant levels (i.e. 5.0E−08), although these SNPs did not necessarily achieve this level in the datasets we evaluated. In addition, other cholinergic receptor genes with more modest p-values were also enriched in these GO terms. Each study identified CHRNA7 but the significant SNPs were different and in low r2 (<0.20) but high D’ (>0.80) values, suggesting the existence of a shared risk allele. However, we could not identify a same SNP that was significant for the three studies in the region (p-value<0.05). This might indicate the presence of an untyped SNP, possibly with a minor allele frequency too low to be accurately imputed, or might be a synthetic association representing the effects of multiple rare variants. In contrast, for the CHRNA9 gene, we could neither identify a common significant SNP nor a common allele tagged by SNPs in linkage disequilibrium (r2>0.5 or D’>0.5). Despite this, the pathway analysis was robust enough to highlight the associations of these two genes to smoking quantity. Indeed, the presence of these moderate signals in CHRNA7 and CHRNA9, as well as the ones in cholinergic muscarinic receptors, sustained the significance of the terms GO:0005892, GO:0042166, GO:0004889 and GO:0015464 for the analysis of the least stringent threshold for SNPs (Table 3). In contrast, the absence of these moderate signals resulted in the terms GO:0035095, GO:0035094 and GO:0007274 only being significant for the analysis of the SNPs that satisfied the most stringent threshold (Table S2).
We did not restrict our analysis to the ALIGATOR method, but also applied the MAGENTA method, as each of these methods can provide complementary findings [35]. Only the GO terms for cholinergic receptors were consistently significant in the OZALC-NAG, SAGE and ARIC studies using the MAGENTA method, increasing the levels of certainty of the original ALIGATOR predictions. In contrast, the ALIGATOR method identified other GO terms and KEGG pathways common to the three datasets. We verified if the assumption of one causal gene per signal made by the MAGENTA method caused the different results. However, correcting or not for physically proximal genes did not change substantially the MAGENTA results for these categories of genes. It could be argued that ALIGATOR results are a product of the specificity of the method, and are not susceptibility factors for smoking. However, some of the genes included in these significant GO terms were previously reported to influence smoking behaviors, which increases the confidence in these findings (e.g., GRM8 [32] and GRM7 [31], [30]). Our analysis detected other genes common to all datasets, including the bitter taste receptor TAS2R1, suspected to be able to sense the nicotine in cigarette smoke [36]. It has been shown that nicotine activates taste receptor pathways both specific for nicotine and also common to other bitter substances [37], [38]. This provides support for the finding that variants in some of the taste receptors can modulate cigarette consumption. Similarly, retinoic acid genes were also specific to the ALIGATOR analysis; but again it has been suggested that the activation of nicotinic receptors affects cellular signaling associated with retinoic acid target genes [39].
One caveat to consider when performing pathway analysis is that the results obtained are biased, or at least restricted, to the biological knowledge that is incorporated into the GWAS, as well as its representation and modeling. This may explain the absence of any replicated GO term or KEGG pathway for the metabolism of nicotine. CYP2A6 encodes the enzyme that metabolizes approximately 70 to 80% of nicotine to cotinine [10], [40], [41]. A single SNP in this chromosomal region is typed by the Illumina Human 1 M chip.: rs3733829, which is in the first intron of EGLN2 located 40 kb downstream from CYP2A6 [10]. This SNP is not in high linkage disequilibrium with any other SNP included in the chip in the extended genetic region considered for the CYP2A6. Moreover, CYP2A6 is a complex locus involving structural and rare functional variants that are not well tagged by the SNPs included in the genotyping platforms. The UGT complex of genes, which catalyze nicotine and cotinine glucuronidation [34], were significant in both the OZALC-NAG and SAGE studies, and were identified among the other retinoid binding genes. Furthermore, the flavin monooxygenase 1 gene (FMO1) is also associated with nicotine metabolism [42] and was tagged in the three studies. There is one KEGG pathway (hsa00982 “Drug metabolism – cytochrome P450”) and two GO terms (GO:0005792 “microsome” and GO:0042598 “vesicular fraction”) with less than 500 genes that include FMO1 and UGT1A4. However, each of these terms and pathways has a very large number of genes (i.e., 73, 241 and 248 genes respectively); and thus are not specific enough to formally represent nicotine metabolism genes. In contrast, the identification of ribosome genes was an unexpected result of our analysis. The relationship, if any, of this gene family to nicotine consumption is not currently understood.
Both ALIGATOR and MAGENTA provide methods to correct for the multiple pathways tested. ALIGATOR applies a bootstrap approach [15] whereas MAGENTA implements both Bonferroni multiple test correction and false discovery rate method [27]. To analyze the combined evidence of the multiple studies evaluated independently (Table 3) we chose the most stringent method, the Bonferroni multiple test correction, although it is most likely too conservative for the nested organization of GO terms. The corrected p-value is 6.11E−6 for the nominal p-value = 0.05 and 8179 pathways tested. Using this significance level the terms GO:0042166 and GO:0004984 were significant for the three combined studies (weighted Z-method [43]) (Table 3). Similarly, both methods provide mechanisms to correct for linkage disequilibrium; and thus avoid the situation that a same signal, which spans across multiple genes, inflates the number of significant genes in a same pathway. Because this inflation can be a source of false positive results for terms including clusters of genes, we re-executed the ALIGATOR method collapsing all physically proximal genes in the same category into a single entity. The GO terms representing the cholinergic receptor genes remained significant (category-specific p-value <0.05) after this correction (Table S5) for the OZALC-NAG, the SAGE and the ARIC studies. Similarly the GO term GO:0004984 “olfactory receptor activity” remained significant in the three studies, but the other two sensory perception GO terms were not consistently significant (Table S5).
Our systematic analysis of smoking quantity, conditioning GWAS to extrinsic information, has identified both expected and novel GO terms and KEGG pathways. This new information should lead to a further prioritization of genes that do not include genome-wide significance SNPs. Many genetic variants, each one with small effects, are expected to be associated to complex traits [44], Pathway analysis can be considered as a signal-to-noise filter for the true signals that are not strong enough to clearly stand out from the statistical background in a traditional GWAS.
Supporting Information
Hierarchical clustering of identified GO terms and KEGG pathway. We calculated the similarity matrix among the genes included in the 11 GO terms and KEGG pathway; and created a dendrogram by employing the single linkage method (i.e., nearest-neighbor).
(TIFF)
GO terms for the sensory perception of chemical stimulus and smell and significant genes. The p-value of each gene was assigned based on the most significant SNP in gene sequences and flanking regions (Left panel). SNPs in linkage disequilibrium (r2>0.2) and in a local proximity (1 Mb) were removed. Colored boxes in the right panel reflect the assignment of each gene to the different GO terms. Only genes significant in at least two studies are reported.
(TIFF)
Significant genes for significant GO terms related to Retinoid binding terms. Similar to Figure S2, the p-value of genes significant in any of the three studies (OZALC-NAG, SAGE or ARIC) is reported.
(TIFF)
Excess of enriched categories of genes identified by ALIGATOR for OZALC-NAG and SAGE studies for smoking quantity for Gene Ontoloty terms (A) and KEGG pathways (B).
(PDF)
ALIGATOR identified common OZALC-NAG and SAGE GO terms for the analysis of SNPS p-value <0.001 and ARIC replication results. acc: Category of gene and corresponding name; # Genes Cat.: the total number of genes grouped by the category; # genes significant; and category specific p-value; and Expected number of genes for SAGE, OZALC-NAG and ARIC studies. *Combined p-values were calculated employing the weighted Z-score method {Stouffer:1949ua}
(PDF)
RefSNP (rs) numbers for the SNPs tagging the GO terms for the cholinergic receptor genes.
(PDF)
MAGENTA False discovery rate for categories of genes with nominal p-value<0.05 in the OZALC-NAG and SAGE studies and ARIC corresponding results.
(PDF)
ALIGATOR enrichment analysis of the collapsed genes (physical distance <1 Mb) for GO terms representing the cholinergic receptor and the sensory perception genes.
(PDF)
Samples and study design.
(DOCX)
Genotypes.
(DOCX)
Statistical analysis.
(DOCX)
Funding Statement
O.H is supported by the National Institute of Drug Abuse (DA027995) and the Barnes-Jewish Hospital Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Centers for Disease Control and Prevention (CDC) (2008) Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004. MMWR Morb Mortal Wkly Rep 57: 1226–1228. [PubMed] [Google Scholar]
- 2. Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, et al. (2008) Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev 17: 3517–3525 doi:10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vink JM, Willemsen G, Boomsma DI (2005) Heritability of smoking initiation and nicotine dependence. Behav Genet 35: 397–406 doi:10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- 4. Broms U, Silventoinen K, Kaprio J, 5 (2006) Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res Hum Genet 9: 64–72 doi:10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- 5. Berrettini W, Yuan X, Tozzi F, Song K, Francks C, et al. (2008) Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry 13: 368–373 doi:10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, et al. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165: 1163–1171 doi:10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, et al. (2010) Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet 42: 436–440 doi:10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, et al. (2007) Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet 16: 24–35 doi:10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, et al. (2010) Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet 42: 448–453 doi:10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tobacco and Genetics Consortium (2010) Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 42: 441–447 doi:10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, et al.. (2010) Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. doi:10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed]
- 12.Haller G, Druley T, Vallania FL, Mitra RD, Li P, et al.. (2011) Rare missense variants in CHRNB4 are associated with reduced risk of nicotine dependence. Human Molecular Genetics. doi:10.1093/hmg/ddr498. [DOI] [PMC free article] [PubMed]
- 13. Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, et al. (2010) Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42: 565–569 doi:10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SH, Wray NR, Goddard ME, Visscher PM (2011) Estimating Missing Heritability for Disease from Genome-wide Association Studies. Am J Hum Genet 88: 294–305 doi:10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holmans P, Green EK, Pahwa JS, Ferreira MAR, Purcell SM, et al. (2009) Gene Ontology Analysis of GWA Study Data Sets Provides Insights into the Biology of Bipolar Disorder. The American Journal of Human Genetics 85: 13–24 doi:10.1016/j.ajhg.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang K, Li M, Bucan M (2007) Pathway-Based Approaches for Analysis of Genomewide Association Studies. The American Journal of Human Genetics 81: 1278–1283 doi:10.1086/522374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang K, Li M, Hakonarson H (2010) Analysing biological pathways in genome-wide association studies. Nature Publishing Group 11: 843–854 doi:10.1038/nrg2884. [DOI] [PubMed] [Google Scholar]
- 18. Lesnick TG, Papapetropoulos S, Mash DC, Ffrench-Mullen J, Shehadeh L, et al. (2007) A genomic pathway approach to a complex disease: axon guidance and Parkinson disease. PLoS Genet 3: e98 doi:10.1371/journal.pgen.0030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Dushlaine C, Kenny E, Heron E, Donohoe G, Gill M, et al. (2011) Molecular pathways involved in neuronal cell adhesion and membrane scaffolding contribute to schizophrenia and bipolar disorder susceptibility. Mol Psychiatry 16: 286–292 doi:10.1038/mp.2010.7. [DOI] [PubMed] [Google Scholar]
- 20. Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, et al. (2010) Genetic Evidence Implicates the Immune System and Cholesterol Metabolism in the Aetiology of Alzheimer’s Disease. PLoS ONE 5: e13950 doi:10.1371/journal.pone.0013950.t006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saccone S (2007) Genetic Linkage to Chromosome 22q12 for a Heavy-Smoking Quantitative Trait in Two Independent Samples. The American Journal of Human Genetics 80: 856–866 doi:10.1086/513703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, et al. (2010) A genome-wide association study of alcohol dependence. Proc Natl Acad Sci USA 107: 5082–5087 doi:10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, et al. (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29 doi:10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M (2010) KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res 38: D355–D360 doi:10.1093/nar/gkp896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharrett AR (1992) The Atherosclerosis Risk in Communities (ARIC) Study. Introduction and objectives of the hemostasis component. Ann Epidemiol 2: 467–469. [DOI] [PubMed] [Google Scholar]
- 26. Rasmussen-Torvik LJ, Alonso A, Li M, Kao W, Köttgen A, et al. (2010) Impact of repeated measures and sample selection on genome-wide association studies of fasting glucose. Genet Epidemiol 34: 665–673 doi:10.1002/gepi.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Segrè AV, DIAGRAM Consortium, MAGIC investigators, Groop L, Mootha VK, et al. (2010) Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits. PLoS Genet 6: e1001058 doi:10.1371/journal.pgen.1001058.t004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, et al.. (2011) A Quantitative-Trait Genome-Wide Association Study of Alcoholism Risk in the Community: Findings and Implications. Biol Psychiatry. doi:10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed]
- 29. Subramanian AA, Tamayo PP, Mesirov JPJ, 11 (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550 doi:10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Uhl GR, Liu Q-R, Drgon T, Johnson C, Walther D, et al. (2007) Molecular genetics of nicotine dependence and abstinence: whole genome association using 520,000 SNPs. BMC Genet 8: 10 doi:10.1186/1471-2156-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Q-R, Drgon T, Johnson C, Walther D, Hess J, et al. (2006) Addiction molecular genetics: 639,401 SNP whole genome association identifies many “cell adhesion” genes. Am J Med Genet B Neuropsychiatr Genet 141B: 918–925 doi:10.1002/ajmg.b.30436. [DOI] [PubMed] [Google Scholar]
- 32. Vink JM, Smit AB, de Geus EJC, Sullivan P, Willemsen G, et al. (2009) Genome-wide association study of smoking initiation and current smoking. Am J Hum Genet 84: 367–379 doi:10.1016/j.ajhg.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pergadia ML, Glowinski AL, Wray NR, Agrawal A, Saccone SF, et al. (2011) A 3p26–3p25 Genetic Linkage Finding for DSM-IV Major Depression in Heavy Smoking Families. Am J Psychiatry 168: 848–852 doi:10.1176/appi.ajp.2011.10091319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuehl GE, Murphy SE (2003) N-glucuronidation of nicotine and cotinine by human liver microsomes and heterologously expressed UDP-glucuronosyltransferases. Drug Metab Dispos 31: 1361–1368 doi:10.1124/dmd.31.11.1361. [DOI] [PubMed] [Google Scholar]
- 35. Gui H, Li M, Sham PC, Cherny SS (2011) Comparisons of seven algorithms for pathway analysis using the WTCCC Crohn’s Disease Dataset. BMC Res Notes 4: 386 doi:10.1186/1756-0500-4-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ (2009) Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134 doi:10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan T-HT, Mummalaneni S, et al. (2009) Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci USA 106: 1596–1601 doi:10.1073/pnas.0810184106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reed DR, Zhu G, Breslin PAS, Duke FF, Henders AK, et al. (2010) The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Human Molecular Genetics 19: 4278–4285 doi:10.1093/hmg/ddq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Osanai M, Lee G-H (2011) Nicotine-mediated suppression of the retinoic acid metabolizing enzyme CYP26A1 limits the oncogenic potential of breast cancer. Cancer Sci 102: 1158–1163 doi:10.1111/j.1349-7006.2011.01920.x. [DOI] [PubMed] [Google Scholar]
- 40. Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, et al. (2006) Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry 11: 400–409 doi:10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- 41. Bloom J, Hinrichs AL, Wang JC, Weymarn von LB, Kharasch ED, et al. (2011) The contribution of common CYP2A6 alleles to variation in nicotine metabolism among European-Americans. Pharmacogenet Genomics 21: 403–416 doi:10.1097/FPC.0b013e328346e8c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hinrichs AL, Murphy SE, Wang JC, Saccone S, Saccone N, et al. (2011) Common polymorphisms in FMO1 are associated with nicotine dependence. Pharmacogenet Genomics 21: 397–402 doi:10.1097/FPC.0b013e328346886f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whitlock MC (2005) Combining probability from independent tests: the weighted Z-method is superior to Fisher’s approach. J Evol Biol 18: 1368–1373 doi:10.1111/j.1420-9101.2005.00917.x. [DOI] [PubMed] [Google Scholar]
- 44.Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, et al.. (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. doi:10.1038/nature08185. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hierarchical clustering of identified GO terms and KEGG pathway. We calculated the similarity matrix among the genes included in the 11 GO terms and KEGG pathway; and created a dendrogram by employing the single linkage method (i.e., nearest-neighbor).
(TIFF)
GO terms for the sensory perception of chemical stimulus and smell and significant genes. The p-value of each gene was assigned based on the most significant SNP in gene sequences and flanking regions (Left panel). SNPs in linkage disequilibrium (r2>0.2) and in a local proximity (1 Mb) were removed. Colored boxes in the right panel reflect the assignment of each gene to the different GO terms. Only genes significant in at least two studies are reported.
(TIFF)
Significant genes for significant GO terms related to Retinoid binding terms. Similar to Figure S2, the p-value of genes significant in any of the three studies (OZALC-NAG, SAGE or ARIC) is reported.
(TIFF)
Excess of enriched categories of genes identified by ALIGATOR for OZALC-NAG and SAGE studies for smoking quantity for Gene Ontoloty terms (A) and KEGG pathways (B).
(PDF)
ALIGATOR identified common OZALC-NAG and SAGE GO terms for the analysis of SNPS p-value <0.001 and ARIC replication results. acc: Category of gene and corresponding name; # Genes Cat.: the total number of genes grouped by the category; # genes significant; and category specific p-value; and Expected number of genes for SAGE, OZALC-NAG and ARIC studies. *Combined p-values were calculated employing the weighted Z-score method {Stouffer:1949ua}
(PDF)
RefSNP (rs) numbers for the SNPs tagging the GO terms for the cholinergic receptor genes.
(PDF)
MAGENTA False discovery rate for categories of genes with nominal p-value<0.05 in the OZALC-NAG and SAGE studies and ARIC corresponding results.
(PDF)
ALIGATOR enrichment analysis of the collapsed genes (physical distance <1 Mb) for GO terms representing the cholinergic receptor and the sensory perception genes.
(PDF)
Samples and study design.
(DOCX)
Genotypes.
(DOCX)
Statistical analysis.
(DOCX)