Abstract
The sorghum (Sorghum bicolor L. Moench) cultivar 58M, which contains the null mutant phytochrome B gene, shows reduced photoperiodic sensitivity and exhibits a shade-avoidance phenotype. Ethylene production by seedlings of wild-type and phytochrome B mutant cultivars was monitored every 3 h, and both cultivars were found to produce ethylene in a circadian rhythm, with peak production occurring during the day. The phytochrome B mutant produces rhythmic peaks of ethylene with approximately 10 times the amplitude of the wild-type counterpart with the same period and diurnal timing. The source of the mutant's additional ethylene is the shoot. The diurnal rhythm can be produced with either light or temperature cycles; however, both light and temperature cycles are required for circadian entrainment. The temperature signal overrides the light signal in the production of diurnal rhythms, because seedlings grown under thermoperiods reversed with the photoperiod produced ethylene peaks during the warm nights. To examine the effect of extreme shading on ethylene production, seedlings were grown under dim, far-red-enriched light. This treatment duplicated the phytochrome B mutant's shade-avoidance phenotype in the wild type and caused the wild type to produce ethylene peaks similar to those observed in the mutant. The results confirm that phytochrome B is not required for proper function of circadian timing, but it may be involved in modulating physiological rhythms driven by the biological clock oscillator.
Phytochrome is a light-sensing protein-chromophore complex present in higher plants. At least five species of phytochrome, from PhyA to PhyE, have been characterized in Arabidopsis thaliana (Sharrrock and Quail, 1989; Clack et al., 1994), and it has been proposed that at least some of these phytochromes have both overlapping and distinct functions (Reed et al., 1994; Smith, 1995). One of these functions is thought to be an involvement in setting the timing of the circadian clock (Lumsden, 1991). The circadian clock is believed to occur in all eukaryotic organisms, with a model consisting of environmental cues (generally light) entraining an oscillator, which in turn times rhythmic responses within a period of approximately 24 h (Bünning, 1967). Phytochrome is currently perceived to transduce the environmental light stimulus to time the oscillator; the majority of the work on the circadian clock in plants has focused on the role of light in entrainment.
The short-day, photoperiodic grass Sorghum bicolor is represented by a number of maturity cultivars that vary in their photoperiodic sensitivity (Quinby, 1967). One of these cultivars, 58M, has recently been shown to possess a frameshift mutation in the coding sequence of the PHYB gene, which results in a premature stop codon (Childs et al., 1997). The mutation in this gene results in a plant with immunologically undetectable levels of the light-stable phytochrome PhyB (Childs et al., 1992). The phyB mutant 58M exhibits classic symptoms of a PhyB-deficient plant; compared with its wild-type counterpart 100M, 58M appears to be etiolated even under high red-light irradiance, has less chlorophyll and anthocyanin, and shows reduced sensitivity to photoperiodic control of flowering by flowering early, even under long days (Childs et al., 1991).
As part of an ongoing investigation of the control of short-day photoperiodism, we have been examining rhythmic fluctuations in the levels of endogenous GAs. Levels of four of these GAs have been shown to cycle in a diurnal fashion in cv 90M (recessive phyB-2), cv 100M (dominant PHYB), and cv 58M (null mutant phyB-1). Furthermore, the timing of the peaks of bioactive GA1 and its precursor GA20 are shifted in the PhyB mutant, from afternoon in the wild type to early morning in the mutant (Foster and Morgan, 1995). The significance of these findings is enhanced by the observation that, when the mutant is grown under long, noninductive days, flowering is delayed and the GA1 and GA20 peaks shift to late in the day, similar to the timing observed for the wild type. Furthermore, under very short, inductive photoperiods, the wild type produces GA1 peaks early in the day and also flowers early (I.-J. Lee, K.R. Foster, and P.M. Morgan, unpublished data).
The plant hormone ethylene is involved in many plant growth and development responses and, with a few exceptions, is generally viewed as playing an inhibitory role in vegetative growth by reducing elongation of both roots and shoots (Abeles et al., 1992). Light is one of many environmental stimuli shown to be involved in modifying ethylene production both positively and negatively, with phytochrome implicated in the control of this response in several cases (Goeschl et al., 1967; Imaseki et al., 1971; Vangronsveld et al., 1988; Michalczuk and Rudnicki, 1993). Previous work has shown that ethylene production by cotton and bean follows a diurnal rhythm, with peaks occurring during the light cycle (Lipe and Morgan, 1973; Rikin et al., 1984; Morgan et al., 1990). Ethylene production rates of Stellaria were observed to fluctuate in a diurnal rhythm (Emery et al., 1994), and subsequently it was found that the level of ACC oxidase activity and ACC oxidase mRNA expression occur in a matching circadian rhythm, possibly influenced by phytochrome (Kathiresan et al., 1996). Recently, diurnal ethylene evolution was reported in Chenopodium rubrum; however, unlike the case for Stellaria, the rhythm was suggested to be a result of cyclic ACC synthase activity and was not shown to be circadian (Machácková et al., 1997). An endogenous ethylene rhythm in young cereal seedlings has also been demonstrated, but this rhythm occurred within an unusual period (12–16 h) and degraded upon leaf emergence (Ievenish and Kreic-bergs, 1992).
Because of the gross phenotypic differences observed between the PhyB mutant and its wild-type counterpart, and because of the history of rhythmic hormone production observed in this and other species, rhythmic ethylene production by the two sorghum cultivars 100M and 58M was examined. We sought to test the relative levels of ethylene produced by each cultivar during the day/night cycle and to examine how the entrainment of a possible ethylene rhythm was controlled.
MATERIALS AND METHODS
Two of the near-isogenic sorghum (Sorghum bicolor L. Moench) maturity cultivars, 100M (Ma1Ma1, Ma2Ma2, Ma3Ma3, and Ma4Ma4 for the maturity genes) and 58M (Ma1Ma1, Ma2Ma2, ma3Rma3R, and Ma4Ma4; Quinby, 1967) were used. Ma3 and ma3R were recently redesignated as PHYB and phyB-1, respectively, based on the determination that they encode the gene for phytochrome B and a null mutant version of the gene (Childs et al., 1997). Seeds were germinated vertically in the dark at 27°C on moistened germination paper. After 40 h three healthy, uniform seedlings were carefully transferred in upright orientation into 65-mL test tubes containing 20 mL of fritted clay previously saturated with one-quarter-strength Hoagland solution and drained. The bottoms of the tubes were covered with aluminum foil, and the tubes were placed in the dark for 24 h until the start of the first photoperiod. The plants received various photoperiods and temperature regimens as described in Results, with small fans ensuring constant air circulation. Lighting was provided by a combination of F48T12/CW/HO fluorescent lamps (Phillips, Mahwah, NJ) and 60-W incandescent lamps giving 225 μmol m−2 s−1 PAR with a red:far-red ratio of 1.42. For dim, far-red-enriched light treatments, light was provided by four 60-W incandescent lamps producing 29 μmol m−2 s−1 PAR with a red:far-red ratio of 0.75. Light measurements were made with a spectroradiometer (model no. 1800, Li-Cor, Lincoln, NE). Plant height was measured from substrate level at various times.
Ethylene Evolution
For analysis of ethylene evolution, the tubes were capped with rubber septa for 30 min and a 1-mL headspace sample was collected and analyzed using a 10S10 or 10SPlus gas chromatograph (Photovac, Markham, Ontario, Canada) equipped with a photoionization detector. Three identical tubes without plants were measured at each time to account for background ethylene. Ethylene production in this system was linear for at least 30 min.
To determine the relative contribution of the root and shoot to ethylene production, seedlings were removed from the tubes 6 d after sowing and the root and shoot were cut from the caryopsis. The organs were placed in 3-mL syringes equipped with a three-way valve attached to a 1-mL syringe. After 20 min of incubation a 1-mL headspace sample from the 3-mL syringe was transferred to the 1-mL syringe, and this was analyzed for ethylene as described above. Three syringes without plants were measured to account for background ethylene. Organ weight was determined immediately after sampling. Ethylene production from organs prepared in this way was linear for at least 20 min.
RESULTS
Ethylene Production by Wild Type and Null Phytochrome B Sorghum Mutant
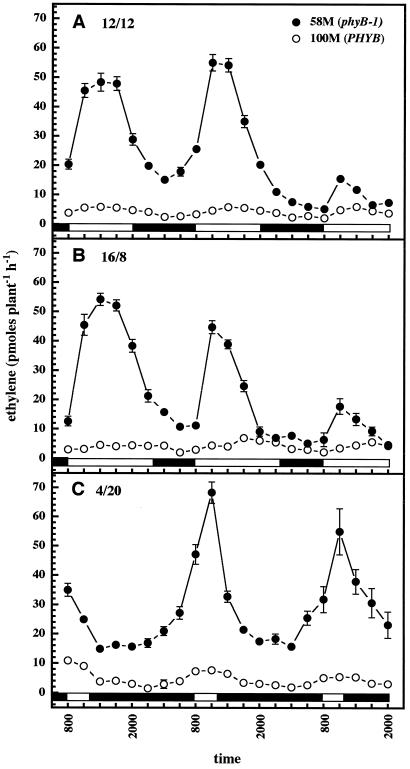
Figure 1A illustrates ethylene production by cv 58M (phyB-1) and cv 100M (PHYB) under a 12-h photo-/thermoperiod. Both cultivars showed rhythmic ethylene production with peaks near the middle of the light period and low ethylene, production at night. cv 58M grossly overproduced ethylene, with peak values about 10 times higher than cv 100M. The amplitude of peak ethylene production showed a tendency to decrease on the last day of sampling (d 7); however, plants maintained in unenclosed test tubes for longer periods also showed strong rhythmic ethylene production with the same daily pattern when assayed 14 and 15 d after sowing (data not shown).
Figure 1.
Diurnal ethylene production rates by sorghum under various photo-/thermoperiods. A and B, n = 7; C, n = 5; results are means ± se. White and black bars near the bottom indicate light and dark periods, respectively.
When roots and shoots of both cultivars grown under 12-h photo-/thermoperiods were cut and incubated separately, roots were found to produce ethylene at approximately the same rate. The shoots of cv 58M produced much more ethylene than shoots of cv 100M and much more ethylene than the roots (Table I).
Table I.
Ethylene production from dissected organs of sorghum cvs 58 M (phyB-1) and 100 M (PHYB)
| Organ | Ethylene Rate | Total Ethylene | Total Ethylene by Organ |
|---|---|---|---|
| pmol g−1 fresh wt−1 h | pmol h−1 | % | |
| cv 58 M root | 89.29 ± 21.1 | 2.89 ± 1.12 | 13.35 |
| cv 58 M shoot | 279.36 ± 14.40 | 18.81 ± 3.01 | 86.65 |
| cv 100 M root | 98.29 ± 25.41 | 3.03 ± 0.32 | 69.88 |
| cv 100 M shoot | 23.11 ± 6.56 | 1.30 ± 0.21 | 30.12 |
Values are means ± se, n = 3.
Plants grown under both shorter (10 h; data not shown) and longer (16 h; Fig. 1B) photo-/thermoperiods showed patterns of ethylene production similar to the 12-h case, with correspondingly narrower and broader peaks, respectively. Under the 16-h photo-/thermoperiod it was apparent that the peak in ethylene production by cv 58M actually occurred before the midday point in the photo-/thermoperiod (Fig. 1B). Plants grown under extremely short photo-/thermoperiods (4 h light:20 h dark) also showed a rhythmic production of ethylene, which anticipated the appearance of the light/warm period (Fig. 1C).
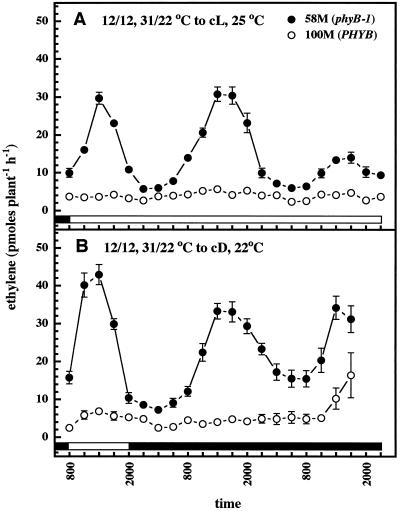
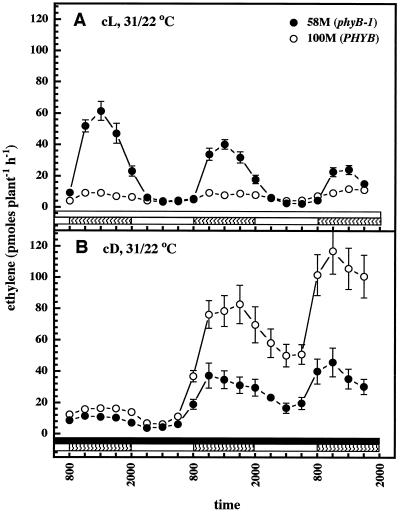
To test whether the rhythmic production of ethylene was circadian, plants were entrained for 2 d under 12-h photo-/thermoperiods and then switched to constant light and temperature. Figure 2A shows that the ethylene rhythm is free-running in both cultivars, with strong peaks produced by cv 58M occurring within an approximate 24-h period. Similarly entrained plants switched into constant dark and temperature also produced a free-running rhythm within a period of approximately 24 h (Fig. 2B). cv 100M did not exhibit a clear rhythm under these conditions. Both cultivars showed a gradual, general increase in ethylene production under the constant dark condition.
Figure 2.
Circadian ethylene production by sorghum under constant light (cL; A) or dark (cD; B) conditions. n = 5; results are means ± se. White and black bars near the bottom indicate light and dark periods, respectively.
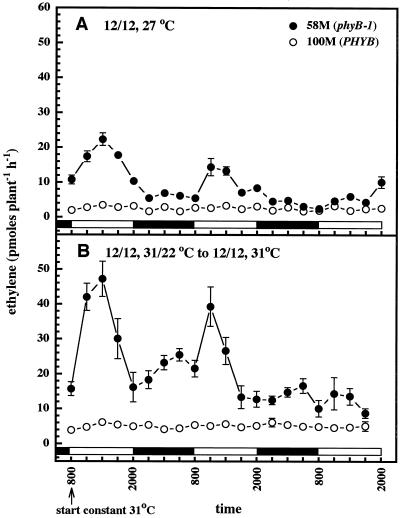
cv 58M seedlings grown with 12-h photoperiods under a regimen of constant temperature demonstrated erratic ethylene rhythms that decayed over three measured cycles, whereas cv 100M seedlings did not exhibit rhythmic ethylene production under these growth conditions (Fig. 3A). Mutant seedlings entrained with both 12-h photo- and thermoperiods for 2 d and then switched to constant temperature and 12-h photoperiods continued to cycle with a decaying rhythm. A second peak of lower amplitude was also observed, with a maximum late in the dark period. Again, cyclic ethylene production was not apparent in cv 100M (Fig. 3B).
Figure 3.
Rhythmic ethylene production by sorghum with cycling photoperiods and constant temperature from sowing (A) and cycling photo-/thermoperiods and then transferring to cycling photoperiods and constant temperature (B). n = 5; results are means ± se. White and black bars near the bottom indicate light and dark periods, respectively.
The remarkable effect of temperature on the rhythmic production of ethylene was studied in several ways. Seedlings were grown under constant light and given cycling thermoperiods beginning 3 d after sowing. Both cultivars showed rhythmic ethylene production with peaks during the warm period, and cv 58M overproduced ethylene (Fig. 4A). The pattern of ethylene production was strikingly similar to that observed under 12-h synchronous photo-/thermoperiods. Cycling temperature was also sufficient to cause plants grown in constant darkness to produce ethylene rhythms (Fig. 4B). Under a constant dark/cycling thermoperiod regimen the cv 100M seedlings actually produced more ethylene than did cv 58M. Growth of seedlings in the dark again resulted in a general increase in ethylene production by both cultivars.
Figure 4.
Rhythmic ethylene production by sorghum with cycling thermoperiods and constant light (cD, A) or dark (cD, B). n = 5; results are means ± se. Solid white and black bars near the bottom indicate light and dark periods, respectively; hatched bars indicate the warm period.
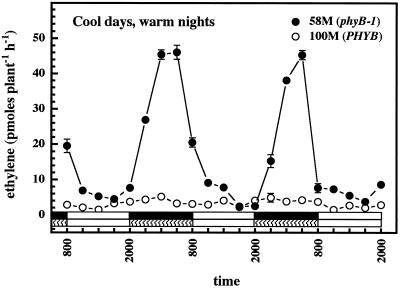
To examine the relative contributions of temperature and light, seedlings were grown under photoperiods with the temperature kept warm during the dark period and cool during the light. This regimen resulted in weak rhythmicity in cv 100M and a strong ethylene rhythm in the mutant, with the timing of peak ethylene production being reversed so that it occurred during the warm, dark period (Fig. 5).
Figure 5.
Rhythmic ethylene production by sorghum under a regimen of reversed photo- and thermoperiods (warm nights, cool days). n = 5; results are means ± se. Solid white and black bars near the bottom indicate light and dark periods, respectively; hatched bars indicate the warm period.
Seedlings entrained with either three 12-h photoperiods at 26°C constant temperature or with three 12-h 31/22°C thermoperiods under constant light did not continue to cycle when the timing stimulus (light or temperature) was made constant (data not shown).
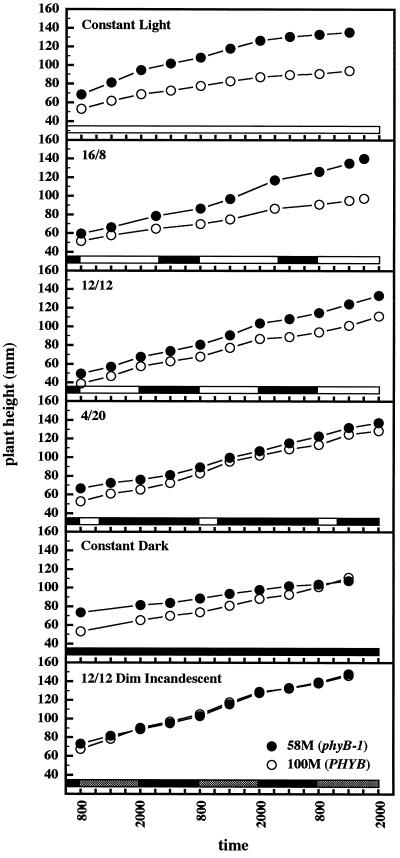
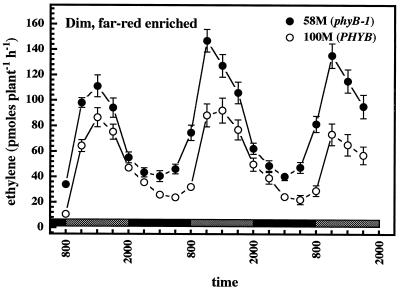
The shoot elongation rate of the seedlings was a function of both temperature and light. cv 58M elongated more rapidly than the wild type under illuminated conditions, and the differential in growth rate was enhanced under longer photoperiods (Fig. 6). In constant dark the elongation rates of the two cultivars were comparable. As noted earlier, ethylene production under prolonged dark conditions tended to increase after several days (Fig. 2B), and ethylene production by cv 100M in constant dark, cycling temperature was actually greater than that by the mutant (Fig. 4B). To examine the effect of shading on elongation and ethylene production, seedlings were grown under a regimen of dim, far-red-enriched light provided by incandescent lights only. Seedlings of the two cultivars grown in this way exhibited nearly identical elongation rates (Fig. 6, bottom) and appeared nearly identical to the eye. The cv 58M phenotype was changed little under these conditions, whereas the phenotype of cv 100M was a near phenocopy of cv 58M. Ethylene production by both cultivars was remarkably similar, echoing the pattern produced by cv 58M under bright, red-light-enriched conditions and yet with greater amplitude (Fig. 7).
Figure 6.
Elongation of sorghum seedlings grown under various light regimens. n = 15; results are means ± se. White and black bars near the bottom indicate light and dark periods, respectively.
Figure 7.
Rhythmic ethylene production by sorghum grown under dim, far-red-enriched photo-/thermoperiods. n = 5; results are means ± se. White and black bars near the bottom indicate light and dark periods, respectively.
Elongation rates of the two cultivars determined for the light and dark periods of d 6 are shown in Table II. Under a 12-h synchronized photo-/thermoperiod with far-red balanced light, cv 58M elongated about 1.2 times as fast as cv 100M during the light period, and the elongation rates of both cultivars were reduced during the dark period. When the plants were grown with 12-h synchronized photo-/thermoperiods with dim, far-red-enriched light, the light period elongation rate of cv 58M increased slightly over far-red balanced values (1.1 times), whereas the elongation rate for cv 100M increased by almost 1.3 times. The dark period elongation rate for cv 58M was unaffected by this light treatment, whereas the dark period elongation rate of cv 100M was increased by 1.31 times. A regimen of 12-h photo-/thermoperiods with cool days and warm nights resulted in both cultivars achieving maximal elongation rates during the warm night. The cool, light period elongation rates for both cultivars were similar to the dark period rates observed with 12-h synchronized far-red balanced conditions, whereas the warm, dark period rates were less than those observed for the 12-h synchronized far-red balanced light period (0.82 times for cv 58M and 0.51 times for cv 100M).
Table II.
Elongation rates of sorghum cvs 58 M (phyB-1) and 100 M (PHYB) grown under different photo/thermoperiod regimens
| Cultivar and Illumination | 12/12 Photo-Thermoperiod Elongation Rate | 12/12 Dim Incandescent Photo-Thermoperiod Elongation Rate | 12/12 Reversed Photo-Thermoperiod Elongation Rate |
|---|---|---|---|
| mm h−1 | |||
| 58 M-light period | 1.90 ± 0.07 (n = 21) | 2.09 ± 0.08 (n = 15) | 0.99 ± 0.04 (n = 13) |
| 100 M-light period | 1.58 ± 0.06 (n = 20) | 2.01 ± 0.05 (n = 15) | 0.61 ± 0.03 (n = 15) |
| 58 M-dark period | 0.94 ± 0.04 (n = 21) | 0.93 ± 0.04 (n = 15) | 1.56 ± 0.04 (n = 13) |
| 100 M-dark period | 0.58 ± 0.04 (n = 20) | 0.76 ± 0.03 (n = 15) | 0.81 ± 0.04 (n = 15) |
Rates were calculated over 12-h periods, beginning 6 d after sowing, from growth curve data presented in Figure 6. Values are means ± se.
DISCUSSION
The circadian ethylene biosynthesis rhythms we have described are significant in at least three interrelated ways. First, they are a convenient means of measuring an output of the circadian clock in sorghum. The assay of ethylene is relatively easy and yields readily quantifiable results. It will be possible to perform detailed studies of entrainment and perturbation of the clock under various environmental conditions using this system. The fact that the output signal is actually a plant hormone with multiple regulatory effects adds to the utility of the assay. Second, the discovery of these rhythms raises the question of how they are controlled by PhyB and other phytochromes. How PhyB effects overproduction of ethylene in sorghum is beginning to be discovered in our laboratory. The results of Kathiresan et al. (1996) suggest that a phytochrome may also be involved in timing the rhythm, although we cannot corroborate this in sorghum. Third, the possible role of ethylene in the control of the shade-avoidance syndrome, including vegetative growth and/or floral initiation, can be studied using these cultivars, which vary widely in their ethylene production, growth rates, and time to flowering under different light/temperature regimens.
We initially observed diurnal ethylene production by the two sorghum cultivars under cycling photo-/thermoperiods of different durations. Both cultivars produced ethylene in a cyclic rhythm, with peaks before or near the midpoint of the light period. The timing of the ethylene peaks was not discernibly different between the PhyB mutant (cv 58M) and the wild type (cv 100M); however, the amplitude of the ethylene biosynthesis peak was greatly enhanced in the case of the mutant. These results show that the plants are able to time the rhythm to daylengths of different durations. Even under a regimen of extremely short 4-h days, the ethylene rhythm was propagated in both cultivars, demonstrating the ability of the plant to track even abnormally short light signals, although the plants are not able to compress the ethylene peak to fit entirely into the light period. Under such short-day conditions, it is obvious from the predawn leadin to the ethylene peak that the plants anticipate the light period, and this effect can also be noted to a lesser degree in the 10-, 12-, and 16-h photo-/thermoperiods. The ethylene rhythm in wild-type plants was only weakly expressed in 12-h photo- and thermoperiods (Fig. 1), but in constant dark or dim, far-red-enriched light, existence of a rhythmic pattern of ethylene biosynthesis was clearly demonstrated (Figs. 4 and 7).
The ethylene biosynthesis rhythm was proven to be circadian by entraining the plants with two photo-/thermoperiods and then letting the system free-run in the light at a constant temperature. We observed that the rhythms persisted for at least three cycles without further timing stimuli. A subsequent experiment in which the plants were entrained and then allowed to free-run in constant dark with constant temperature produced similar results, indicating that light is not required for persistence of the rhythm once it has been entrained. The circadian ethylene rhythm appears to have the same period under either constant light or constant dark, and there is no difference in the period of the wild type compared with the mutant in constant light, although the exact timing of the wild-type peak was uncertain because of its low amplitude. In constant dark a rhythm could not be detected in the wild type. In contrast, the CAB gene expression rhythm in Arabidopsis has been shown to cycle with a longer period under constant dark as compared with constant light (Millar et al., 1995). A lengthening of the period of the same CAB expression cycle was also observed in seedlings possessing the hy1 mutation, which lack detectable phytochromes (Millar et al., 1995).
We next began examining the contributions of light and temperature signals to the rhythm. Mutant seedlings maintained with only a light/dark signal showed a weakened ethylene biosynthesis rhythm that decayed by the 3rd d of measurement, whereas no rhythm at all was detectable in the wild type (Fig. 3A). This entrainment did not prove to be circadian, since neither cultivar would cycle after being placed in constant light. Unexpectedly, plants entrained with both photo- and thermoperiods produced a weak, decaying rhythm after being switched to constant temperature, with the cycling photoperiod maintained (Fig. 3B). This treatment also resulted in the appearance of a second peak of ethylene release in the mutant. The second peak appeared late in the dark period, was of lower amplitude than the primary peak, and was not observed under any other treatments. The significance of the second peak and the decay of the rhythm after loss of the temperature signal has not been determined, but these results may indicate that the light- and temperature-entrained rhythms are independent manifestations of the clock, which produce clear, single-peak oscillations only when synchronized.
Under constant light a cycling thermoperiod regimen produced a strong ethylene biosynthesis rhythm in both cultivars; however, this rhythm did not prove to be circadian in nature. Similarly, constant dark coupled with cycling thermoperiods resulted in rhythmic ethylene production by both cultivars, with the wild type producing even more ethylene than the mutant (Fig. 4B). Under constant dark conditions, a general increase in ethylene production was often observed in both cultivars; however, this was the only treatment that increased ethylene production by the wild type over the mutant. The overriding importance of the temperature signal in eliciting the diurnal rhythm was confirmed when the plants were grown with cool days and warm nights. Seedlings of both cultivars grown in this way produced ethylene peaks during the warm night, once again with the mutant producing peaks with much greater amplitude than the wild type. Possibly, PhyB is not fundamentally involved with setting the oscillator but is involved in coupling the response between the oscillator and the rhythm. Other evidence supporting this hypothesis has been presented: although CAB expression levels were dissimilar between the wild type and the PHYB mutant, a circadian rhythm with similar timing persisted in both (Childs et al., 1995).
Earlier work showed that ethylene production is modified through the action of phytochrome. Pisum and Phaseolus seedlings have been shown to reduce ethylene biosynthesis in response to red-light treatments, an effect demonstrated to be a result of phytochrome action due to its far-red reversibility (Goeschl et al., 1967; Vangronsveld et al., 1988). The circadian rhythm of ACC oxidase activity and mRNA accumulation may also be controlled by phytochrome, since the rhythm was initiated by red- but not blue-light pulses (Kathiresan et al., 1996). On the other hand, the rhythms observed in sorghum are less sensitive to light. Single light pulses of different durations were not able to set the rhythm (S.A. Finlayson, I.-J. Lee, and P.W. Morgan, unpublished data), nor could a circadian rhythm be entrained by cycling photoperiods alone. It is possible that the weak diurnal rhythm observed under constant temperature and light/dark is actually due to cyclic radiant heating of the seedling rather than light per se.
Few rhythms are known to be entrained by temperature. Rhythmic accumulation of the Sinapis alba transcripts Sagrp and Saglp was observed under either cycling light or temperature, although only light was demonstrated to entrain accumulation under free-running conditions (Heintzen et al., 1994a, 1994b). It is interesting that, although the transcript levels fluctuated dramatically, the levels of protein encoded by these genes did not vary with appreciable amplitude. In tomato, polyamine levels fluctuate in a diurnal rhythm, with peaks occurring during cool nights (N′Doye et al., 1994). Diurnal polyamine levels cycled strongly with temperature under constant dark, and they also peaked during cool days in a regimen of cool days and warm nights, indicating the dominance of the temperature signal in the effect. However, temperature was not shown to entrain a circadian rhythm. Circadian entrainment of accumulation of CAB and Rubisco small subunit mRNAs can be induced by temperature alone, either as a short, cyclic heat shock or as alternating warm and cool thermoperiods under constant dark (Kloppstech et al., 1991). We report that temperature alone is not able to entrain free-running ethylene rhythms, but the ethylene rhythms in sorghum represent a novel system in higher plants in which entrainment of a circadian rhythm requires both light and temperature signals.
The shoot elongation rates of the two cultivars begin to converge under shorter photo-/thermoperiods; under constant dark the elongation rates are indistinguishable, and we also observed that the wild type can produce more ethylene than the mutant. Postulating that the effect observed under constant dark might reflect a response to the most extreme type of shading possible, we designed an experiment to simulate a high shade environment with dim far-red light. This treatment resulted in the wild-type seedlings assuming the elongation rate and phenotype of the mutant, which was unchanged. Additionally, total ethylene production by the two cultivars was very similar. These results can be interpreted as meaning that the production of ethylene with a high amplitude is a response to shading, which is constitutively expressed in the mutant as a result of the loss of PhyB function. Possibly, the large ethylene peaks are involved in generating the shade-avoidance phenotype; however, a causal relationship has not yet been established. It was noted that, when the wild type exhibited a strong rhythmic production of ethylene at rates similar to the mutant (Figs. 4 and 7), it also expressed aspects of the mutant phenotype, and elongation rates were very similar (Table II). Furthermore, we observed that maximal elongation rates occur concurrently with the expression of peak ethylene production, i.e. during the light period when plants are grown with 12-h synchronized photo-/thermoperiods and during the dark period when plants are grown with 12-h cool days and warm nights (Table II). Although peak ethylene production does correlate with maximum shoot elongation in the timing of the phenomena, it was also observed that the amplitude of the ethylene rhythm is much greater than the corresponding differences in growth rates presented in Table II.
In most instances ethylene is implicated as a inhibitor of vegetative growth, especially elongation (Abeles et al., 1992). The general exception to this paradigm is the case of plants adapted to partial submergence, such as deep water rice (Métraux and Kende, 1983) and Rumex spp. (Voesenek and Blom, 1989), in which ethylene actually stimulates elongation. Recently, two reports demonstrated that in both maize and sorghum ethylene can have a promotive effect on mesocotyl elongation under red light, whereas ethylene applied to seedlings in darkness inhibits elongation (Nishizawa and Suge, 1995; Suge and Nishizawa, 1995). It is difficult to reconcile these reports with our system in which both maximum elongation and ethylene production can occur in the dark when the warm temperature occurs concurrently. We are presently testing whether ethylene is involved in the growth responses of the sorghum cultivars, but we are also considering the possibility that the large ethylene peaks are a result of, rather than a cause of, rapid elongation. Few studies have addressed the role of endogenous ethylene in nonstressed vegetative growth, although it has been our experience that rapidly growing tissues produce more ethylene than less active tissues. Whether this ethylene is involved in eliciting the rapid growth or is produced as a consequence of this growth has yet to be answered. It is also possible that ethylene overproduction in cv 58M may reflect an inability of the seedlings to perceive ethylene and control its biosynthesis through a feedback inhibition loop.
A striking feature of the ethylene rhythms we have measured is the large amplitude overproduction by the mutant. How does the lesion result in ethylene overproduction? Previous evidence has suggested that ACC oxidase fluctuations are responsible for generating the ethylene rhythm observed in Stellaria longipes (Kathiresan et al., 1996), whereas a contrasting report of Chenopodium rubrum presents evidence that, although ACC oxidase activity does vary diurnally, the control of rhythmic ethylene production is actually a result of ACC synthase activity (Machácková et al., 1997). It is possible that both enzymes contribute to the rhythm. We are presently investigating the biosynthesis of ethylene in these cultivars by analyzing substrate levels, enzyme activity, and abundance of mRNAs encoding ACC synthase and ACC oxidase. At a more fundamental level we are concerned with the link between ethylene production and PhyB. When we have identified the enzyme(s) responsible for the generation of the ethylene rhythm in sorghum, we will have a starting point from which to trace the molecular events leading from PhyB to the resulting ethylene production.
ACKNOWLEDGMENTS
We acknowledge with thanks the seed supplied for this study by Dr. Bill Rooney (Department of Soil and Crop Sciences, Texas A&M, College Station) and the loan of a Photovac 10S10 gas chromatograph from David M. Reid and the University of Calgary (Alberta, Canada).
Footnotes
This work was supported by U.S. Department of Agriculture National Research Initiative Competitive Grants Program grant no. 91-37304-6582 and Texas Higher Education Board ATP grant no. 999902-87 to P.W.M., a predoctoral overseas Korean government scholarship to I.-J. L., and the Texas Agriculture Experiment Station.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology, Ed 2. Academic Press, New York
- Bünning E. The Physiological Clock, Ed 2. New York: Springer-Verlag; 1967. [Google Scholar]
- Childs KL, Cordonnier-Pratt M-M, Pratt LH, Morgan PW. Genetic regulation of development in Sorghum bicolor. VII. ma3R flowering mutant lacks a phytochrome that predominates in green tissue. Plant Physiol. 1992;99:765–770. doi: 10.1104/pp.99.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs KL, Lu J-L, Mullet JE, Morgan PW. Genetic regulation of development in Sorghum bicolor. X. Greatly attenuated photoperiod sensitivity in a phytochrome-deficient sorghum possessing a biological clock but lacking a red light-high irradiance response. Plant Physiol. 1995;108:345–351. doi: 10.1104/pp.108.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs KL, Miller FR, Cordonnier-Pratt M-M, Pratt LH, Morgan PW, Mullet JE. The sorghum photoperiod sensitivity gene, Ma3, encodes a phytochrome B. Plant Physiol. 1997;113:611–619. doi: 10.1104/pp.113.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs KL, Pratt LH, Morgan PW. Genetic regulation of development in Sorghum bicolor. VI. The ma3R allele results in abnormal phytochrome physiology. Plant Physiol. 1991;97:714–719. doi: 10.1104/pp.97.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RS. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Emery RJN, Reid DM, Chinnappa CC. Phenotypic plasticity of stem elongation in two ecotypes of Stellaria longipes: the role of ethylene and response to wind. Plant Cell Environ. 1994;17:691–700. [Google Scholar]
- Foster KR, Morgan PW. Genetic regulation of development in Sorghum bicolor. IX. The ma3R allele disrupts diurnal control of gibberellin biosynthesis. Plant Physiol. 1995;108:337–343. doi: 10.1104/pp.108.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl JD, Pratt HK, Bonner BA. An effect of light on the production of ethylene and the growth of the plumular portion of etiolated pea seedlings. Plant Physiol. 1967;42:1077–1080. doi: 10.1104/pp.42.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzen C, Fischer R, Melzer S, Kappeler S, Apel K, Staiger D. Circadian oscillations of a transcript encoding a germin-like protein that is associated with cell walls in young leaves of the long-day plant Sinapis alba L. Plant Physiol. 1994a;106:905–915. doi: 10.1104/pp.106.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzen C, Melzer S, Fischer R, Kappeler S, Apel K, Staiger D. A light- and temperature-entrained circadian clock controls expression of transcripts encoding nuclear proteins with homology to RNA-binding proteins in meristematic tissue. Plant J. 1994b;5:799–813. doi: 10.1046/j.1365-313x.1994.5060799.x. [DOI] [PubMed] [Google Scholar]
- Ievinsh G, Kreicbergs O. Endogenous rhythmicity of ethylene production in growing intact cereal seedlings. Plant Physiol. 1992;100:1389–1391. doi: 10.1104/pp.100.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaseki H, Pjon CJ, Furuya M. Phytochrome action in Oryza sativa L. IV. Red and far-red reversible effect on the production of ethylene in excised coleoptiles. Plant Physiol. 1971;48:241–244. doi: 10.1104/pp.48.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan A, Reid DM, Chinnappa CC. Light and temperature entrained circadian regulation of activity and mRNA accumulation of 1-aminocyclopropane-1-carboxylic acid oxidase in Stellaria longipes. Planta. 1996;199:329–335. doi: 10.1007/BF00195723. [DOI] [PubMed] [Google Scholar]
- Kloppstech K, Otto B, Sierralta W. Cyclic temperature treatments of dark-grown pea seedlings induce a rise in specific transcript levels of light-regulated genes related to photomorphogenesis. Mol Gen Genet. 1991;225:468–473. doi: 10.1007/BF00261689. [DOI] [PubMed] [Google Scholar]
- Lipe JA, Morgan PW. Ethylene, a regulator of young fruit abscission. Plant Physiol. 1973;51:949–953. doi: 10.1104/pp.51.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden PJ. Circadian rhythms and phytochrome. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:351–371. [Google Scholar]
- Machácková I, Chauvaux N, Dewitte W, Van Onckelen H. Diurnal fluctuations in ethylene formation in Chenopodium rubrum. Plant Physiol. 1997;113:981–985. doi: 10.1104/pp.113.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J-P, Kende H. The role of ethylene in the growth response of submerged deep water rice. Plant Physiol. 1983;72:441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk B, Rudnicki RM. The effect of monochromatic red light on ethylene production in leaves of Impatiens balsamina L. and other species. Plant Growth Regul. 1993;13:125–131. [Google Scholar]
- Millar AJ, Straume M, Chory J, Chua N-H, Kay SA. The regulation of circadian period by phototransduction pathways in Arabidopsis. Science. 1995;267:1163–1166. doi: 10.1126/science.7855596. [DOI] [PubMed] [Google Scholar]
- Morgan PW, He C-J, De Greef JA, De Proft MP. Does water deficit stress promote ethylene synthesis by intact plants? Plant Physiol. 1990;94:1616–1624. doi: 10.1104/pp.94.4.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N′Doye M, Millet B, Paynot M, Martin-Tanguy J. Diurnal modulation of polyamine content in seedlings of Lycopersicon esculentum Mill. cv. Ace 55 by temperature and light. Plant Sci. 1994;104:11–15. [Google Scholar]
- Nishizawa T, Suge H. The regulation of maize mesocotyl growth by ethylene and carbon dioxide. Jpn J Crop Sci. 1995;64:794–800. [Google Scholar]
- Quinby JR (1967) The maturity genes of sorghum. In AG Norman, ed, Advances in Agronomy, Vol 19. Academic Press, New York, pp 267–305
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikin A, Chalutz E, Anderson JD. Rhythmicity in ethylene production in cotton seedlings. Plant Physiol. 1984;75:493–495. doi: 10.1104/pp.75.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Smith H. Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- Suge H, Nishizawa T. Opposite effects of ethylene on the growth of sorghum mesocotyl under red light and in darkness. Jpn J Crop Sci. 1995;64:139–143. [Google Scholar]
- Vangronsveld J, Clijsters H, Van Poucke M. Phytochrome-controlled ethylene biosynthesis of intact etiolated bean seedlings. Planta. 1988;174:19–24. doi: 10.1007/BF00394868. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Blom CWPM. Growth responses of Rumex species in relation to submergence and ethylene. Plant Cell Environ. 1989;12:433–439. [Google Scholar]