Abstract
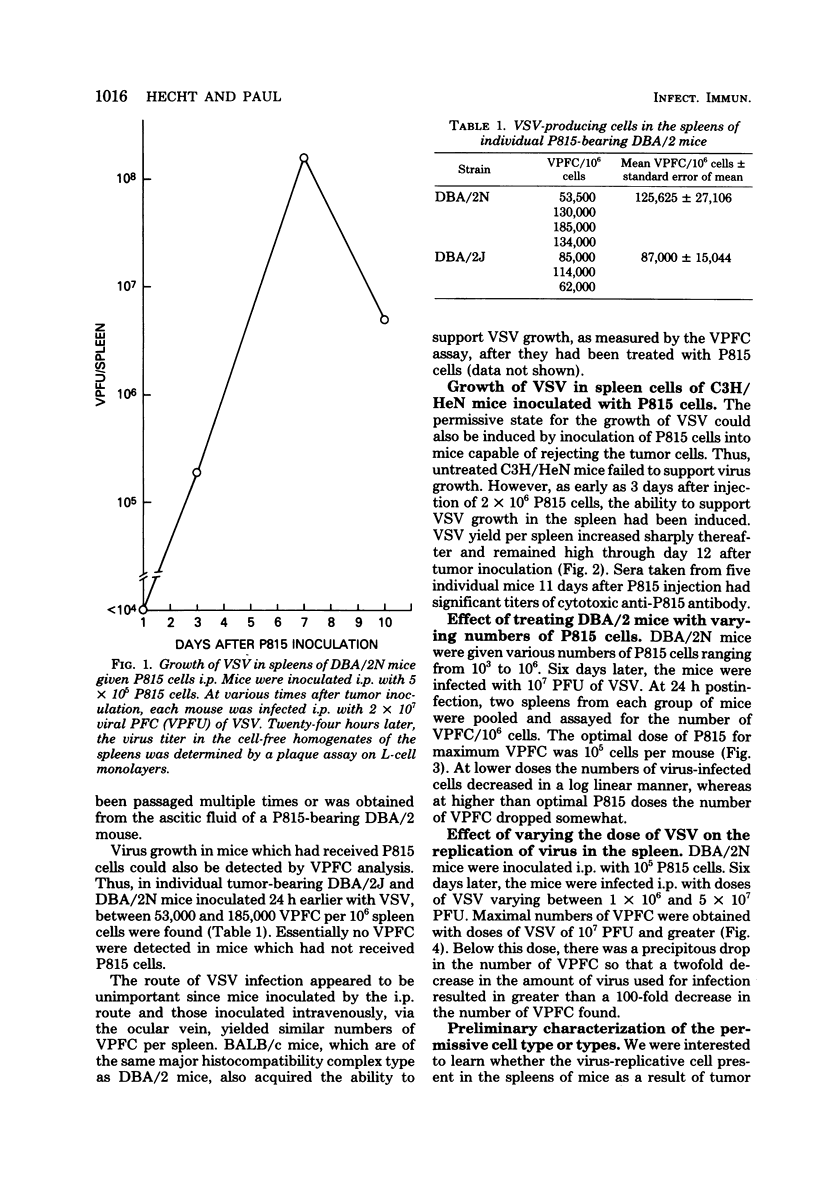
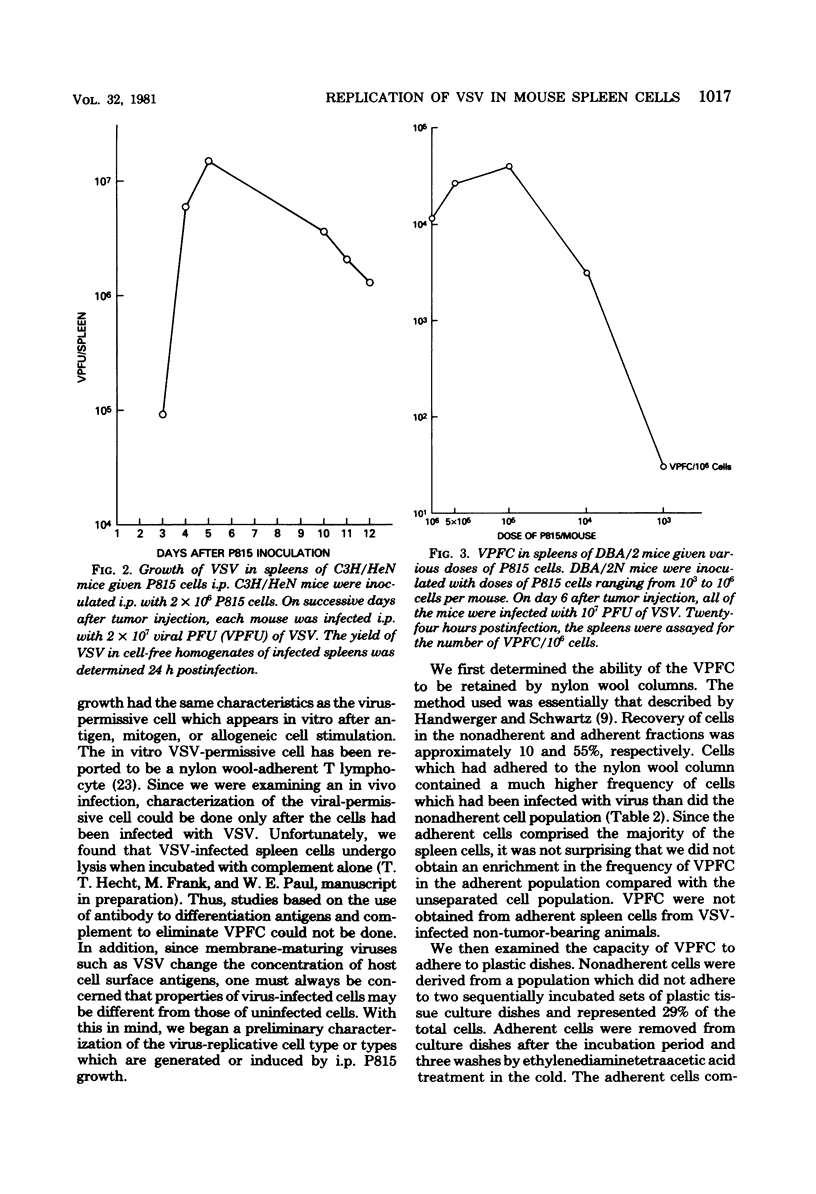
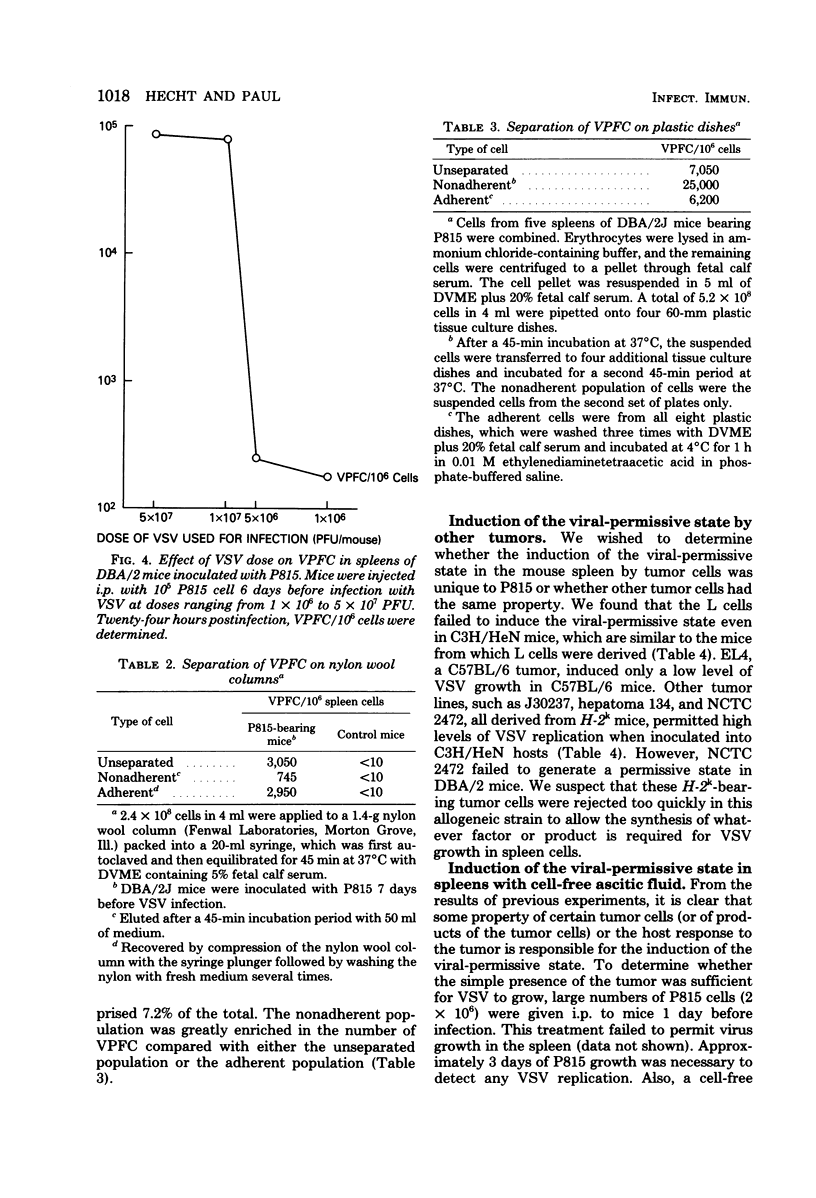
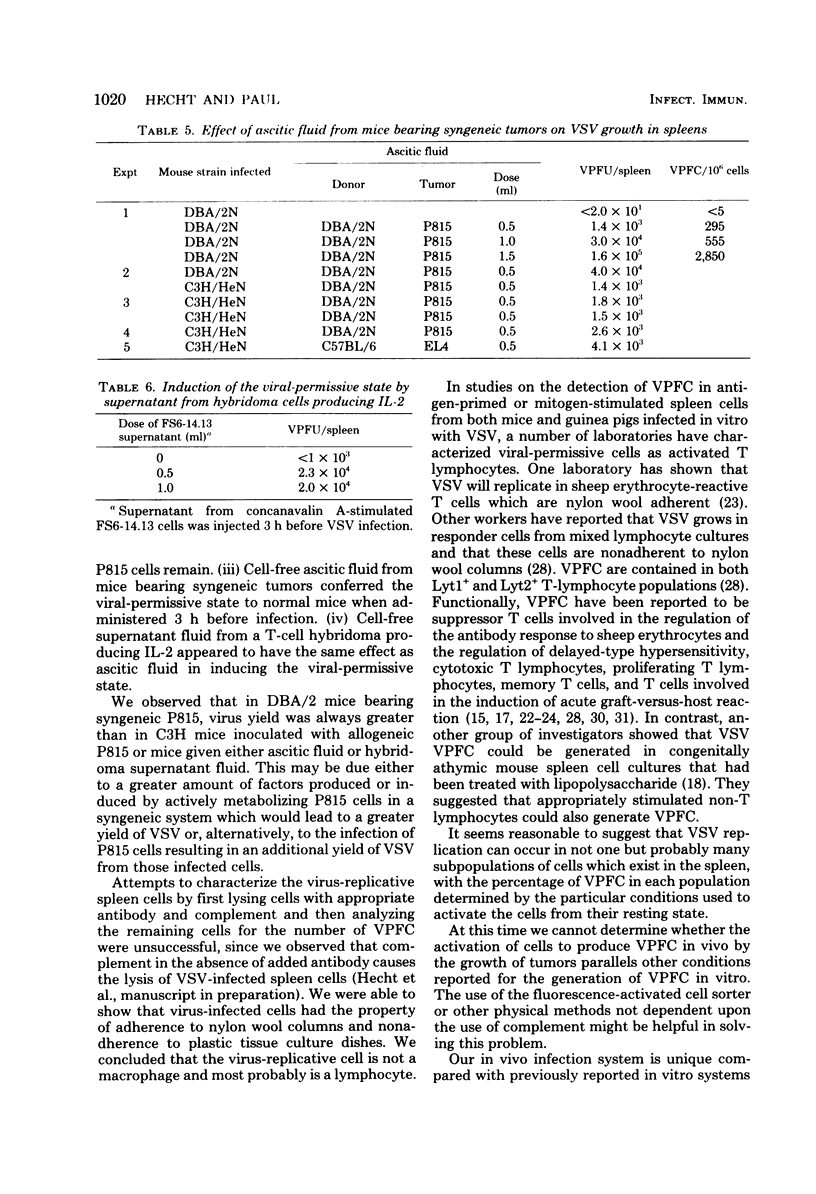
Mouse spleen cells which normally cannot support the in vivo replication of vesicular stomatitis virus (VSV) became susceptible to VSV infection after the intraperitoneal growth of certain syngeneic and allogeneic tumors. After 3 days' growth of P815 tumor cells in syngeneic DBA/2 mice, the viral-permissive state for VSV replication had been established. By 7 days after tumor in inoculation, up to 18% of the spleen cells were producing virus yielding greater than 10(8) plaque-forming units per spleen. Similarly, P815 cells induced the viral-permissive state in allogeneic C3H/HeN mice. Tumors other than P815 were also effective in permitting VSV growth in the spleen. The presence of tumor cells themselves was not sufficient for VSV growth, yet cell-free ascitic fluid from mice bearing syngeneic tumors inoculated 3 h before infection allowed for VSV replication. Cell-free supernatant from a T-cell hybridoma synthesizing interleukin-2 was also effective in permitting virus growth when inoculated 3 h before infection. The virus-permissive cell has been characterized as a nylon wool-adherent and plastic dish-nonadherent spleen cell.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandlow G., Kühne J. Abnormal lysozyme production of peritoneal macrophages from mastocytoma P-815 bearing C3D2F1-mice. Med Microbiol Immunol. 1980 Feb;168(1):55–62. doi: 10.1007/BF02121652. [DOI] [PubMed] [Google Scholar]
- Emerson S. U. Vesicular stomatitis virus: structure and function of virion components. Curr Top Microbiol Immunol. 1976;73:1–34. doi: 10.1007/978-3-642-66306-2_1. [DOI] [PubMed] [Google Scholar]
- Friedman H., Specter S., Watanabe M., Pan S. H. Tumor-induced immunosuppression. Am J Pathol. 1978 Nov;93(2):499–514. [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regualtion of the immune response to tumor antigens. I. Immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):791–799. [PubMed] [Google Scholar]
- HANSON R. P. The natural history of vesicular stomatitis. Bacteriol Rev. 1952 Sep;16(3):179–204. doi: 10.1128/br.16.3.179-204.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger B. S., Schwartz R. H. Separation of murine lymphoid cells using nylon wool columns. Recovery of the B cell-enriched population. Transplantation. 1974 Dec;18(6):544–548. doi: 10.1097/00007890-197412000-00013. [DOI] [PubMed] [Google Scholar]
- Harwell L., Skidmore B., Marrack P., Kappler J. Concanavalin A-inducible, interleukin-2-producing T cell hybridoma. J Exp Med. 1980 Oct 1;152(4):893–904. doi: 10.1084/jem.152.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Effect of vesicular stomatitis virus infection on the histocompatibility antigen of L cells. J Virol. 1972 Oct;10(4):578–585. doi: 10.1128/jvi.10.4.578-585.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Interactions of vesicular stomatitis virus with murine cell surface antigens. J Virol. 1976 Sep;19(3):833–845. doi: 10.1128/jvi.19.3.833-845.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez L., Bloom B. R., Blume M. R., Oettgen H. F. On the number and nature of antigen-sensitive lymphocytes in the blood of delayed-hypersensitive human donors. J Exp Med. 1971 Apr 1;133(4):740–751. doi: 10.1084/jem.133.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano S., Bloom B. R., Howe M. L. Enumeration of activated thymus-derived lymphocytes by the virus plaque assay. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2299–2303. doi: 10.1073/pnas.70.8.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T., Shioiri-Nakano K., Sugiura A. Detection of mitogen-activated T and non-T lymphocytes by virus plaque assay. Virus plaque assay on the cells fractionated by unit gravity sedimentation. Immunology. 1977 Jun;32(6):875–883. [PMC free article] [PubMed] [Google Scholar]
- Lafferty K. J., Andrus L., Prowse S. J. Role of lymphokine and antigen in the control of specific T cell responses. Immunol Rev. 1980;51:279–314. doi: 10.1111/j.1600-065x.1980.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Mills B. J., Beebe D. P., Cooper N. R. Antibody-independent neutralization of vesicular stomatitis virus by human complement. II. Formation of VSV-lipoprotein complexes in human serum and complement-dependent viral lysis. J Immunol. 1979 Dec;123(6):2518–2524. [PubMed] [Google Scholar]
- Minato N., Katsura Y. Virus-replicating T cells in the immune response of mice. I. Virus plaque assay of the lymphocytes reactive to sheep erythrocytes. J Exp Med. 1977 Feb 1;145(2):390–404. doi: 10.1084/jem.145.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Katsura Y. Virus-replicating T cells in the immune response of mice. II. Characterization of T cells capable of replicating vesicular stomatitis virus. J Exp Med. 1978 Oct 1;148(4):837–849. doi: 10.1084/jem.148.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Katsura Y. Virus-replicating T cells in the immune response of mice. III. Role of vesicular stomatitis virus-replicating T cells in the antibody response. J Exp Med. 1978 Oct 1;148(4):850–861. doi: 10.1084/jem.148.4.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Kirstein D. P., Tuttle R. L. Subversion of host defense mechanisms by murine tumors. I. A circulating factor that suppresses macrophage-mediated resistance to infection. J Exp Med. 1976 Mar 1;143(3):559–573. doi: 10.1084/jem.143.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Schnitzlein W. M. Defective interfering particles of rhabdoviruses. Curr Top Microbiol Immunol. 1979;86:123–168. doi: 10.1007/978-3-642-67341-2_4. [DOI] [PubMed] [Google Scholar]
- Senik A., Bloom B. R. Differentiation of memory T cells to virus plaque-forming cells and cytotoxic T lymphocytes. J Exp Med. 1977 Jul 1;146(1):11–21. doi: 10.1084/jem.146.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu D. T., Romano T. J., Bloom B. R., Thorbecke G. J. Diminution by vesicular stomatitis virus of acute graft vs host mortality in mice. Cell Immunol. 1979 Sep 1;46(2):416–421. doi: 10.1016/0008-8749(79)90429-5. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Stabilization of enveloped viruses by dimethyl sulfoxide. J Virol. 1968 Sep;2(9):953–954. doi: 10.1128/jvi.2.9.953-954.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


