Abstract
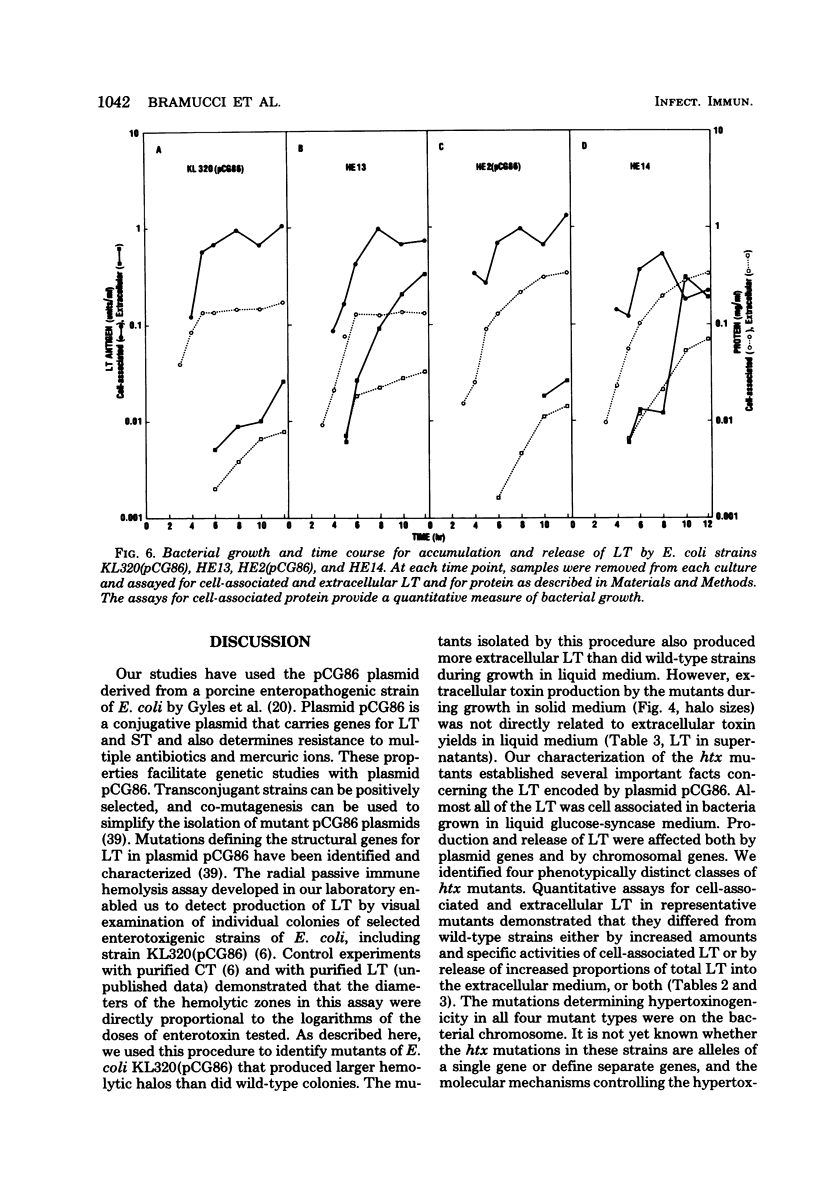
The structural genes for heat-labile enterotoxin (LT) are present on plasmid pCG86. Escherichia coli KL320(pCG86), LT was found to be cell associated. LT was present as a soluble protein in sonic lysates of KL320(pCG86). Thirty-one mutants of KL320(pCG86) that produced increased amounts of extracellular LT were isolated. These hypertoxinogenic (htx) mutants were assigned to four phenotypically distinct classes based on the amounts of cell-associated and extracellular LT in early-stationary-phase cultures. Type 1 and type 2 htx mutants produced significantly increased amounts of cell-associated LT. Type 3 and type 4 htx mutants produced normal or decreased amounts of cell-associated LT was similar to that of the wild type. In the mutants of types 1, 3, and 4, the ratios of extracellular to cell-associated LT were higher than that of the wild type and were characteristic for each strain. Cell lysis or leakage of macromolecular cytoplasmic constituents appeared to be significant for release of LT by mutants of types 1, 3, and 4, because supernatants from cultures of these mutants also contained increased amounts of protein and of the cytoplasmic enzyme glucose 6-phosphate dehydrogenase. In all four representative htx mutants, the hypertoxinogenic phenotypes were dependent on chromosomal mutations. The resident pCG86 plasmids were eliminated from the htx mutants of types 2 and 3. After wild-type plasmid pCG86 was introduced into the cured strains by conjugation, their hypertoxinogenic phenotypes were restored. We conclude that chromosomal loci in E. coli KL320 are important in regulating expression of the LT structural genes of plasmid pCG86.
Full text
PDF


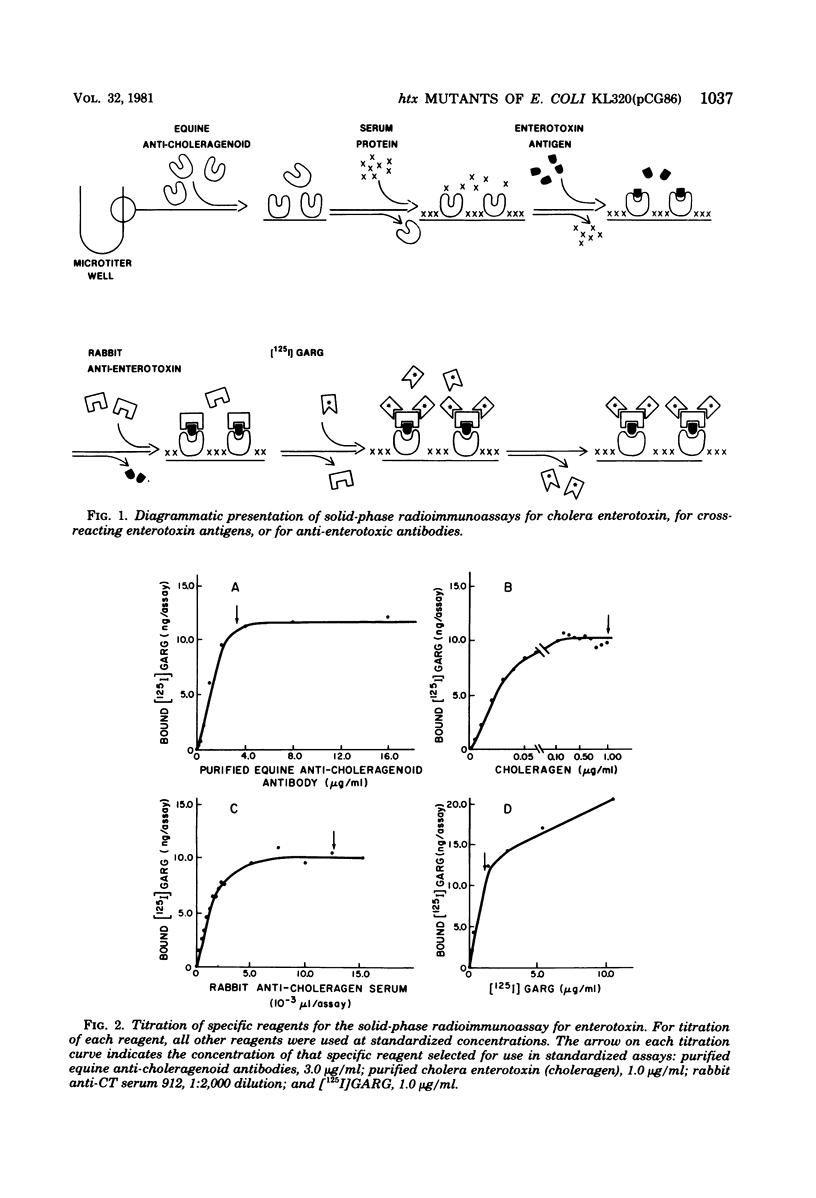
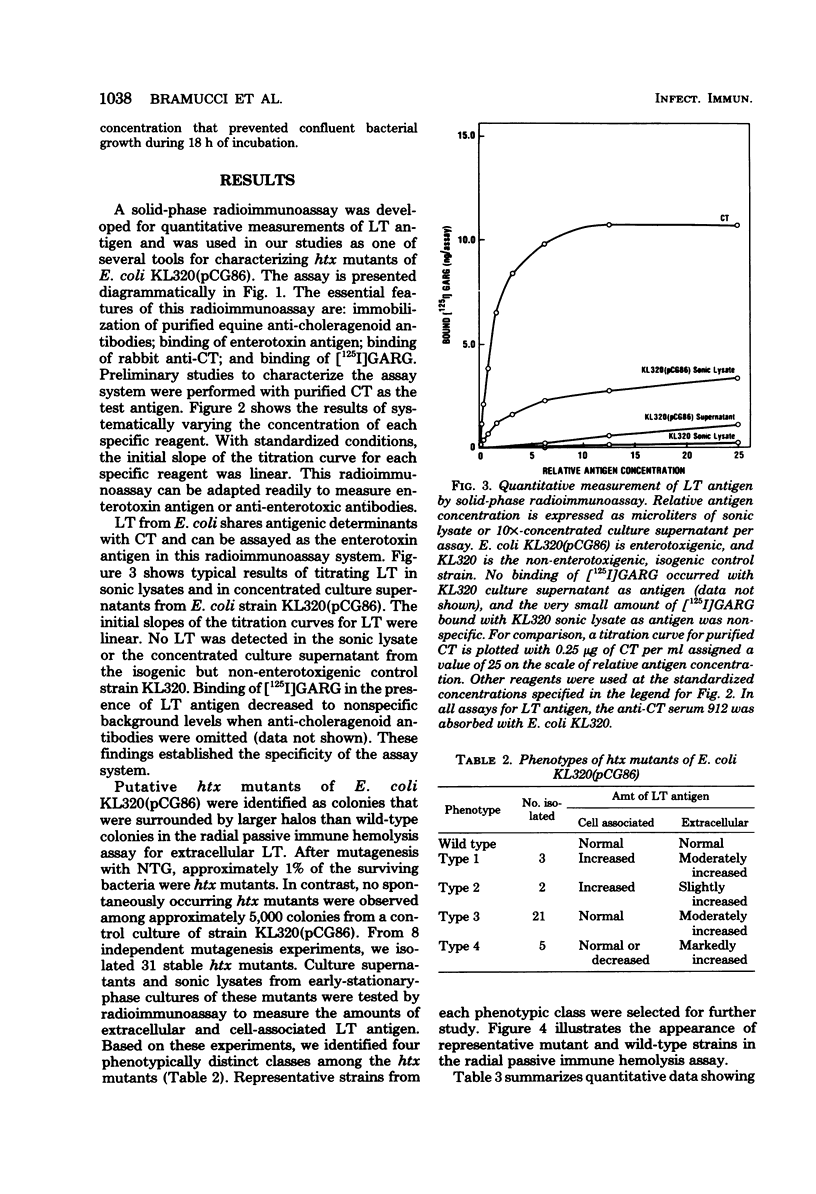
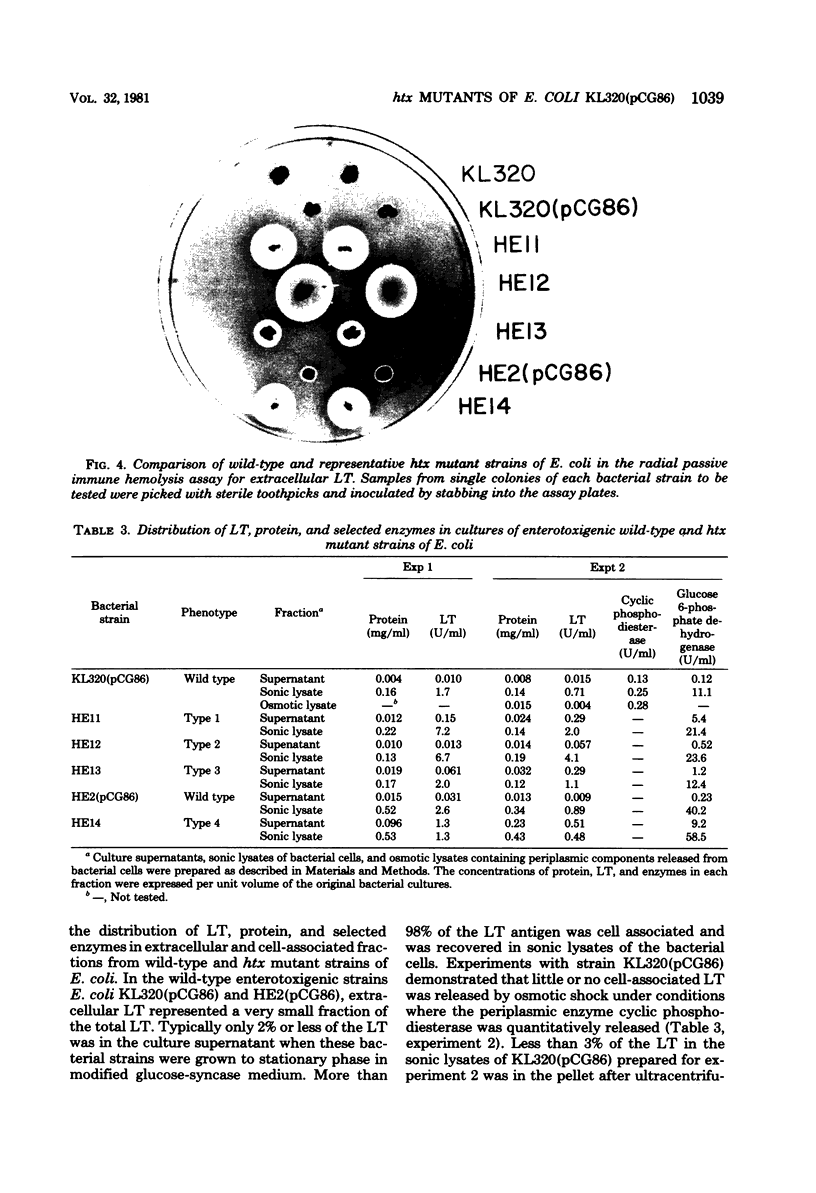

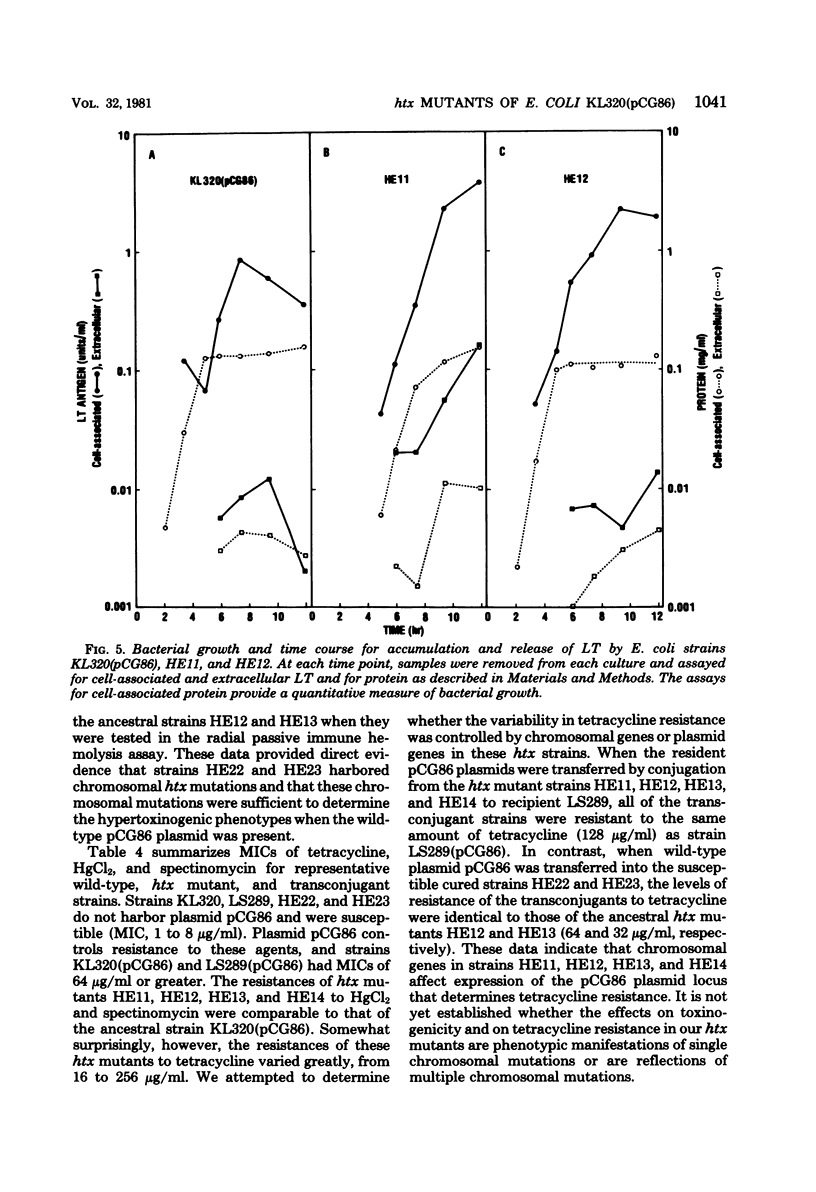


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Robertson D. C. Purification and chemical characterization of the heat-stable enterotoxin produced by porcine strains of enterotoxigenic Escherichia coli. Infect Immun. 1978 Mar;19(3):1021–1030. doi: 10.1128/iai.19.3.1021-1030.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Bramucci M. G., Holmes R. K. Radial passive immune hemolysis assay for detection of heat-labile enterotoxin produced by individual colonies of Escherichia coli or Vibrio cholerae. J Clin Microbiol. 1978 Aug;8(2):252–255. doi: 10.1128/jcm.8.2.252-255.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Demonstration of shared and unique immunological determinants in enterotoxins from Vibrio cholerae and Escherichia coli. Infect Immun. 1978 Dec;22(3):709–713. doi: 10.1128/iai.22.3.709-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Welkos S. L., Holmes R. K. Immunochemical studies of diphtherial toxin and related nontoxic mutant proteins. Infect Immun. 1980 Dec;30(3):835–846. doi: 10.1128/iai.30.3.835-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Richardson S. H., Gorbach S. L. Purification of the polymyxin-released, heat-labile enterotoxin of Escherichia coli. J Infect Dis. 1976 Mar;133 (Suppl):97–102. doi: 10.1093/infdis/133.supplement_1.s97. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C. L., Palchaudhuri S., Maas W. K. Naturally occurring plasmid carrying genes for enterotoxin production and drug resistance. Science. 1977 Oct 14;198(4313):198–199. doi: 10.1126/science.333581. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Relationships among heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. J Infect Dis. 1974 Mar;129(3):277–283. doi: 10.1093/infdis/129.3.277. [DOI] [PubMed] [Google Scholar]
- Gyles C., So M., Falkow S. The enterotoxin plasmids of Escherichia coli. J Infect Dis. 1974 Jul;130(1):40–49. doi: 10.1093/infdis/130.1.40. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Baine W. B., Vasil M. L. Quantitative measurements of cholera enterotoxin in cultures of toxinogenic wild-type and nontoxinogenic mutant strains of Vibrio cholerae by using a sensitive and specific reversed passive hemagglutination assay for cholera enerotoxin. Infect Immun. 1978 Jan;19(1):101–106. doi: 10.1128/iai.19.1.101-106.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Johnson W. M., Lior H., Johnson K. G. Heat-stable enterotoxin from Escherichia coli: factors involved in growth and toxin production. Infect Immun. 1978 May;20(2):352–359. doi: 10.1128/iai.20.2.352-359.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Robertson D. C. Purification and chemical characterization of the heat-labile enterotoxin produced by enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):586–596. doi: 10.1128/iai.25.2.586-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Moss J., Richardson S. H. Activation of adenylate cyclase by heat-labile Escherichia coli enterotoxin. Evidence for ADP-ribosyltransferase activity similar to that of choleragen. J Clin Invest. 1978 Aug;62(2):281–285. doi: 10.1172/JCI109127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Newsome P. M., Burgess M. N., Mullan N. A. Effect of Escherichia coli heat-stable enterotoxin on cyclic GMP levels in mouse intestine. Infect Immun. 1978 Oct;22(1):290–291. doi: 10.1128/iai.22.1.290-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Parker C., Romig W. R. Self-transfer and genetic recombination mediated by P, the sex factor of Vibrio cholerae. J Bacteriol. 1972 Nov;112(2):707–714. doi: 10.1128/jb.112.2.707-714.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Silva M. L., Maas W. K., Gyles C. L. Isolation and characterization of enterotoxin-deficient mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1384–1388. doi: 10.1073/pnas.75.3.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]



