Abstract
Methamphetamine (Meth) is a widely abused stimulant and its users are at increased risk for multiple infectious diseases. To determine the impact of meth on the immune system, we utilized a murine model that simulates the process of meth consumption in a typical addict. Our phenotypic analysis of leukocytes from this dose escalation model revealed that meth affected key immune subsets. Meth administration led to a decrease in abundance of natural killer (NK) cells and the remaining NK cells possessed a phenotype suggesting reduced responsiveness. Dendritic cells (DCs) and Gr-1high monocytes/macrophages were also decreased in abundance while Gr-1low monocytes/macrophages appear to show signs of perturbation. CD4 and CD8 T cell subsets were affected by methamphetamine, both showing a reduction in antigen-experienced subsets. CD4 T cells also exhibited signs of activation, with increased expression of CD150 on CD226-expressing cells and an expansion of KLRG1+, FoxP3− cells. These results exhibit that meth has the ability to disrupt immune homeostasis and impact key subsets of leukocytes which may leave users more vulnerable to pathogens.
Introduction
Methamphetamine (Meth) is a highly addictive psychostimulant and neurotoxicant of increasing popularity among drug-abusing populations worldwide [1]–[9]. This rising popularity has significant economic consequences, as in the US alone, the financial burden of meth use was calculated at >23 billion dollars annually [10]. Meth is typically administered nasally, intravenously or orally, and meth users experience feelings of euphoria, hyperactivity, reduced appetite, sleeplessness, and arousal after administration [11]. After injection, meth, a lipid-soluble monamine, has been shown to disseminate and accumulate widely throughout tissues in both humans and rats [12], [13], and there is an extensive body of data that describes the toxic effects of meth on the CNS and resulting neurologic damage and cognitive impairment [14], [15]. Meth users are prone to increased rates of several types of infections, including human immunodeficiency virus (HIV), hepatitis A, B, and C, and methillicin-resistant Staphylococcus aureus, due to their involvement in risky sexual practices and from the inherent dangers associated with intravenous drug use [16]–[23]. In addition to these behaviors putting users at increased risk of transmission of infectious agents, meth has been shown to promote HIV infection of human macrophages and increase hepatitis C replication within hepatocytes [24], [25].
Meth itself is an immunomodulatory substance with a wide range of effects that have been observed in diverse in vitro systems as well as murine and nonhuman primate models. Several lines of evidence support the notion that meth suppresses the immune system. Meth has been associated with reduced leukocyte proliferation [26]–[28], reduced IL-2 production [28]–[30], reduced immunoglobulin production [30], [31], and reduced macrophage and dendritic cell (DC) function [32]–[34]. Meth also promotes susceptibility to viral and fungal pathogens among hosts [27], [34], [35]. While meth appears to suppress the response of B and T cells, macrophages, and DCs, studies have shown that natural killer (NK) cells exhibit increased levels of activation after meth exposure [29], [30], [35], [36], although at least one study reported reduced NK cell function [37]. Meth has also been observed to alter immune function in the brain, with increased microglia activation and abundance after meth exposure [38]. Finally, meth has been reported to promote apoptosis in the thymus and spleen [39], as well as among cultured T cells [28].
Taken together, these studies reveal that meth has the ability to profoundly interfere with immunological networks, affecting diverse leukocyte subsets and thereby leaving the user vulnerable to pathogens. Although several studies on meth have employed flow cytometry to evaluate the immune response, this has not been performed in a comprehensive manner to elucidate specific cellular alterations induced by meth. Indeed, multiparameter flow cytometry can be used to generate a detailed analysis of the expression level of multiple proteins at the single cell level. This allows the investigator to generate a complex, yet more complete, picture of immunological networks in states of health, disease, and toxicant exposure, and ultimately produce a wealth of observations to guide further investigations. As previous studies suggested profound and detrimental impacts of meth on T cell, NK cell, and macrophage/DC responses, we hypothesized that these deficiencies would result in specific phenotypic alterations of these cells, thereby suggesting altered functionality in vivo. To explore this hypothesis, we treated C57BL/6 mice with levels of methamphetamine that simulate usage patterns observed in typical human addicts (following methods in [34]) and then examined multiple leukocyte subsets to observe how meth changes the phenotypic appearance of immune cells. Our results reveal that meth treatment reduces the overall abundance of activated/antigen-experienced CD4 and CD8 T cells while promoting activation and expansion of discrete CD4 T cell subsets. We also observed that meth impacts splenic myeloid cells, reducing the number of DCs and Gr-1high monocytes/macrophages, while promoting the perturbation of Gr-1low monocytes/macrophages. Finally, we observed that meth contributes to a reduction in number of splenic NK cells and leaves the majority of remaining NK cells with a phenotype suggesting reduced responsiveness and functional efficacy.
Methods
Mice and Meth Treatment
Male C57BL/6 mice 12–14 weeks of age were purchased from Jackson Labs, housed in specific pathogen free conditions, and given unlimited access to food and water. We purchased (+)-methamphetamine hydrochloride from Sigma-Aldrich and employed a treatment regimen similar to that described in [34] that simulates meth usage patterns of typical human addicts. Meth was administered via intraperitoneal injections in a ramped dosing schedule on days 1–4 with 2, 4, 6, and 8 mg/kg given per day. On days 5–14, we plateaued the dosage to 10 mg/kg/day. Control mice were given saline solution (vehicle) over this same time period. On day 14, within 3 hours of the final injection, mice were anesthetized with isofluorane and euthanized via cervical dislocation. The Institutional Animal Care and Use Committee of the University of Nebraska Medical Center approved all procedures.
Flow Cytometry
Spleens and mesenteric lymph nodes were harvested from freshly euthanized mice. These were then teased apart with fine forceps and ran through nylon mesh screens to produce single cell suspensions. Red blood cells (RBCs) were removed from splenocyte preparations by utilizing ammonium chloride RBC lysis buffer. Prior to labeling cells with antibodies, we stained cells with Live/Dead© Fixable.
Blue Dead Cell Stain (Life Technologies) and then blocked Fc receptors using unlabeled, irrelevant rat IgG. We used mouse monoclonal antibodies from multiple sources in our flow cytometry panels. From BD Pharmingen, we utilized B220(RA3-6B2), CD3e(500A2), CD11c(HL3), CD27(LG.3A10), CD44(IM7), CD80(16-10A1), and NKp46(29A1.4). From eBiosciences, we used CD4(GK1.5), CD8(53-6.7), CD11b(M1/70), CD25(PC61.5), CD62L(MEL-14), CD94(18d3), CD86(GL-1), Gr-1(RB6-8C5), NKG2D(CX5), FoxP3(FJK-16s), MHC II(M5/114.15.2), Ly-49H(3D10) and Ly-49G2(eBio4D11). Antibodies against CD3(17A2), CD27(LG.3A10), CD45RB(C363-16A), KLRG1(2F1/KLRG1), CD150(TC15-12F12.2), and CD226(10E5) were purchased from BioLegend. For surface staining, cells were suspended in staining buffer consisting of phosphate buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 0.05% sodium azide (NaN3). Surface-stained cells were fixed in 1.5% paraformaldehyde (PFA) for 1 hour, then washed and resuspended in staining buffer prior to analysis. For FoxP3 staining, we used FoxP3 Fixation/Permeabilization Concentrate and Diluent from eBiosciences according to the manufacturer’s recommendations. Cells were analyzed with an LSR II (BD Biosciences) within 24 hours of preparation.
Analysis and Statistics
We used FlowJo (v. 8.8.6, TreeStar, Inc.) to analyze flow cytometry data. Values from FlowJo were transferred into GraphPad Prism (v. 4.0, GraphPad Software) for statistical analysis. To test for significant differences between meth-treated and control animals, we utilized the Mann-Whitney U Test.
Results
Meth Causes a Reduction in Absolute Number and Proportion of Splenic Natural Killer Cells, Gr-1(Ly-6C)high Monocytes/macrophages, and DCs
To determine the impact of meth exposure on the proportion and number of splenic leukocyte subsets, we prepared splenocyte preparations for flow cytometric analysis with antibodies against CD3, B220, Gr-1, CD11b, CD11c, MHC II, and NKp46, allowing us to define and quantify T cells, B cells, natural killer (NK) cells, neutrophils, eosinophils, dendritic cells (DCs), and Gr-1high and Gr-1low monocytes/macrophages (see gating strategy Fig. 1 and Table 1). The Gr-1 antibody (clone RB6-8C5) binds to epitopes on Ly-6G and Ly-6C [40], and can be used to label neutrophils (SSCmed, Gr-1bright (co-expressing both Ly6G and Ly6C at high levels)) and divide splenic monocytes/macrophage into Gr-1high/low subsets, corresponding to high or low levels of Ly-6C expression [41], [42]. NKp46 is an activating receptor specifically expressed by the NK cell lineage and can be used to effectively label these cells [43].
Figure 1. Gating strategy for defining major subsets of leukocytes among splenocytes. A.
Dead cells were removed from the analysis using Live/Dead® fixable dead cell stain. B. Doublets were removed from living cells (Live/Dead−) using FSC-A and FSC-H. C. Gr-1bright splenocytes were gated and defined as neutrophils. D. NKp46+ events were gated from Gr-1low/− splenocytes and defined as natural killer (NK) cells. E. NKp46−, Gr-1low/− splenocytes were divided into MHC II+ and MHC II− populations. F. MHC II+ events were split by B220 and CD11c expression. MHC II+, B220+, CD11c− events were defined as B cells. MHC II+ B220−, CD11c+ events were defined as dendritic cells (DCs). G. We split MHC II− events by CD3 and CD11b expression. CD11b-, CD3+, MHC II- events were labeled as T cells. H. CD11b+, CD3−, MHC II- events were split according to SSC profile. Events with higher SSC values (suggesting greater internal granularity) were labeled as eosinophils. I. Events with lower SSC values were divided by Gr-1 expression, giving two populations: Gr-1(Ly-6C)high monocytes/macrophages and Gr-1(Ly-6C)low monocytes/macrophages (mono/MΦ).
Table 1. Phenotype of splenic leukocyte subsets analyzed after meth treatment.
| Leukocyte subset | Surface expression of relevant phenotypic markers |
| T cells | CD3+, MHC II−, Gr-1−, NKp46−, CD11b− |
| B cells | B220+, MHC II+, CD3−, Gr-1−, NKp46−, CD11c− |
| Natural killer (NK) cells | NKp46+, Gr-1−, MHCII−, CD3− |
| Dendritic cells (DCs) | CD11c+, MHC II+, CD11b+/−, CD3−, B220−, NKp46− |
| Neutrophils | CD11b+, Gr-1bright, SSCmed, CD3−, B220−, CD11c−, MHCII−, NKp46− |
| Eosinophils | CD11b+, Gr-1low, SSChigh, CD3−, B220−, CD11c−, MHCII−, NKp46− |
| Gr-1(Ly-6C)high mono/MΦ | CD11b+, Gr-1+, MHCII−, CD3−, B220−, CD11c−, NKp46− |
| Gr-1(Ly-6C)low mono/MΦ | CD11b+, Gr-1−, MHCII−, CD3−, B220−, CD11clow/−, NKp46− |
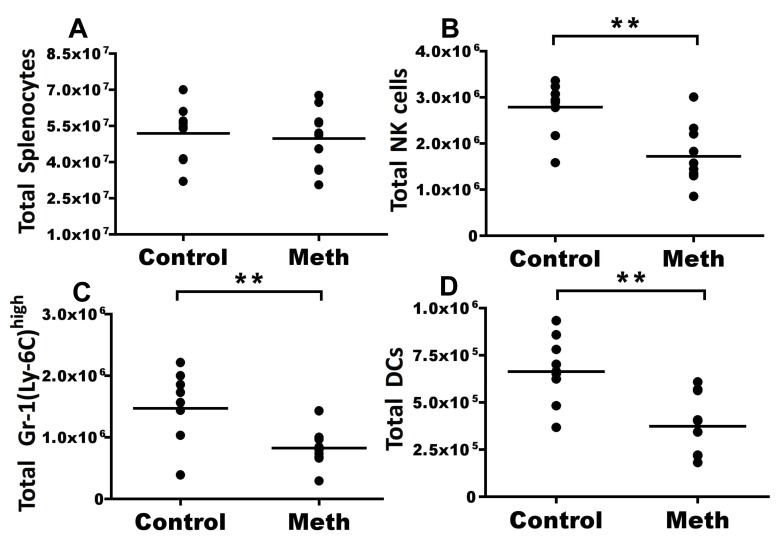
Our results indicated that although absolute counts of total splenocytes were not different between meth-treated and control groups (Fig. 2A), meth treatment caused significant decreases in proportion (data not shown) and number of NK cells (p = 0.003), Gr-1(Ly-6C)high monocytes/macrophages (p = 0.0041), and DCs (p = 0.0015) in meth-treated mice (Fig. 2B–D). T cells, B cells, neutrophils, eosinophils, and Gr-1(Ly-6C)low monocytes/macrophages exhibited no significant difference in proportion or absolute number after meth treatment (data not shown). These results show that meth has the ability to negatively impact the abundance of innate immune subsets.
Figure 2. Meth causes a reduction of splenic leukocyte subsets.
C57BL/6 mice were treated with vehicle (control) or ramped dosage of methamphetamine (meth – see methods) for 14 days. At day 14, mice were sacrificed and splenic leukocyte subsets were analyzed by flow cytometry. A. Meth treatment did not alter total splenocyte number. B. Meth causes a significant reduction in total natural killer (NK) cells (NKp46+, CD3−, B220−, Gr-1−, MHC II−). C. Gr-1high monocytes (CD11b+, Gr-1high, MHC II−, CD3−, B220−) were reduced after meth treatment. D. Meth treatment reduces dendritic cells (DCs) defined as CD11c+, MHC II+, B220−, Gr-1− events. Dead cells and doublets were removed prior to analysis. **P<0.01 calculated by Mann-Whitney U Test. Bars represent mean. Data are from 2 experiments of 5 animals per treatment group per experiment.
Meth Treatment Causes the Perturbation of Gr-1(Ly-6C)low Monocytes/macrophages
Meth treatment has been shown to upset antigen presentation pathways and promote apoptosis, thus suggesting an alteration of antigen presenting cell (APC) activation with meth treatment. To investigate the role of meth treatment on APC and APC-precursor activation levels, we labeled splenic DCs and monocytes/macrophages with antibodies against the costimulatory markers CD80 and CD86 [44], and then examined surface protein expression by flow cytometry. Interestingly, Gr-1low monocytes/macrophages showed statistically significant upregulation of CD80 (p = 0.0057; measured by mean fluorescence intensity (MFI)), but not CD86 (data not shown), after meth exposure (Fig. 3A & B). This increase in CD80 expression was coupled with a decrease in CD11b expression by Gr-1low monocytes/macrophages (p = 0.0004; Fig. 3C & D). There was no change in CD80 or CD86 expression levels by Gr-1high monocytes/macrophages and DCs (data not shown). An upregulation in CD80 in parallel with a downregulation of CD11b suggests that that Gr-1low monocytes/macrophages are perturbed after meth treatment, while Gr-1high monocytes/macrophages appear to be less sensitive to meth-induced activation.
Figure 3. Meth perturbs Gr-1low monocytes/macrophages.
Gr-1low monocytes/macrophages were analyzed for signs of activation after meth treatment using flow cytometry. A. Representative histogram showing CD80 (B7-1) expression by Gr-1ow monocytes/macrophages. Red represents a meth-treated animal, black represents a vehicle-treated animal, and grey is the isotype control. B. Meth causes increased expression of CD80 by Gr-1low, quantified by mean fluorescence intensity (MFI). C. Representative histogram showing CD11b (Mac-1) expression by Gr-1low monocytes/macrophages with colors as in A above. D. Meth causes decreased expression of CD11b by Gr-1low monocytes, quantified by MFI. Dead cells and doublets were removed prior to analysis. **p<0.01, ***p<0.001 calculated by Mann-Whitney U Test. Error bars represent SEM. Data are from 2 experiments of 5 animals per treatment group per experiment.
A Greater Proportion of NK Cells Possess a Phenotype Suggesting Terminal Differentiation After Meth Exposure
Meth treatment has been shown to both promote and inhibit NK cell activation. To address if NK cells exhibit an altered phenotype suggesting changes in maturation or development, we labeled NK cells (NK1.1+, CD4−, CD8−) with KLRG1, CD27, Ly-49H, Ly-49G2 and CD94. Among murine NK cells, CD27 expression is associated with naïve status of NK cells and KLRG1 expression is associated with terminal differentiation [45], [46]. We noticed a significant decrease in proportion of KLRG1−, CD27+ NK cells (p = 0.0011) concurrent with a significant increase in KLRG1+, CD27− NK cells (p = 0.0002; Fig. 4A–C). While a greater proportion of NK cells possessed a terminally differentiated phenotype, this did not correspond to an overall increase in number. We observed that total KLRG1+, CD27− NK cells were relatively similar between meth-treated and control animals (Fig. 4E). However, there was a significant decrease in total KLRG1−, CD27+ NK cells after meth treatment compared to control (p<0.0001; Fig. 4D). These results suggest that meth treatment may promote the terminal differentiation of NK cells and also reduce the abundance of non-terminally differentiated NK cells. No difference in Ly-49H, Ly-49G2, or CD94 expression was noted after treatment (data not shown).
Figure 4. Meth alters NK cell subsets.
We labeled splenic NK cells (NK1.1+, CD4−, CD8−) with antibodies against killer cell lectin-like receptor G1 (KLRG1) and CD27 to determine if meth treatment alters functionally distinct NK cell subsets. A. Representative gating showing KLRG1 and CD27 on gated NK cells. B and C. Meth treatment results in a decreased proportion of KLRG1−, CD27+ NK cells with an increase in proportion of KLRG1+, CD27− NKs. D and E. Although proportionally higher, KLRG1+, CD27− NK cells are not increased in absolute number. However, KLRG1−, CD27+ NK cells were significantly reduced in number. Dead cells and doublets were removed prior to analysis. ***p<0.001 calculated by Mann-Whitney U Test. Bars represent mean. Data are from 2 experiments of 5 animals per treatment group per experiment.
CD4 T Cells
Meth treatment results in decreased proportions of activated CD4 T cells in spleen and lymph node
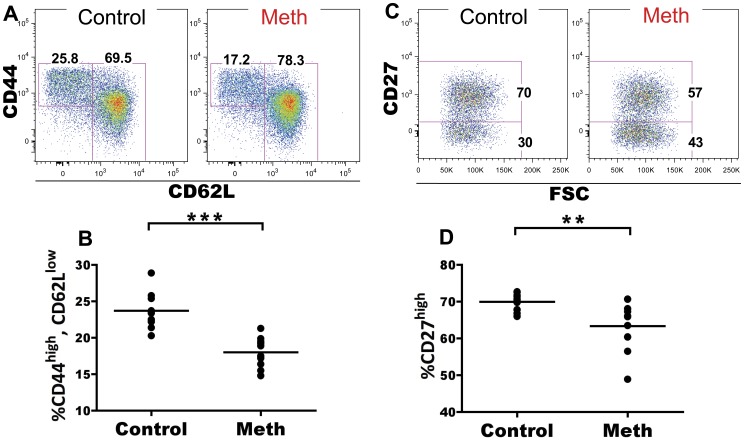
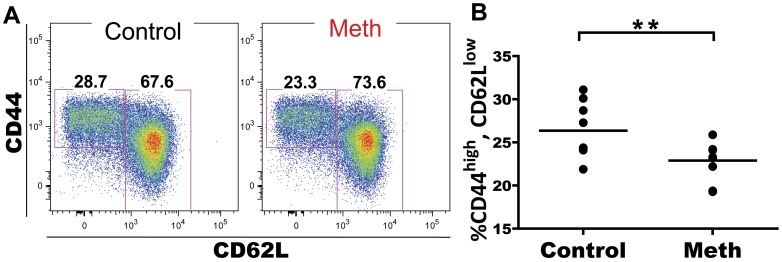
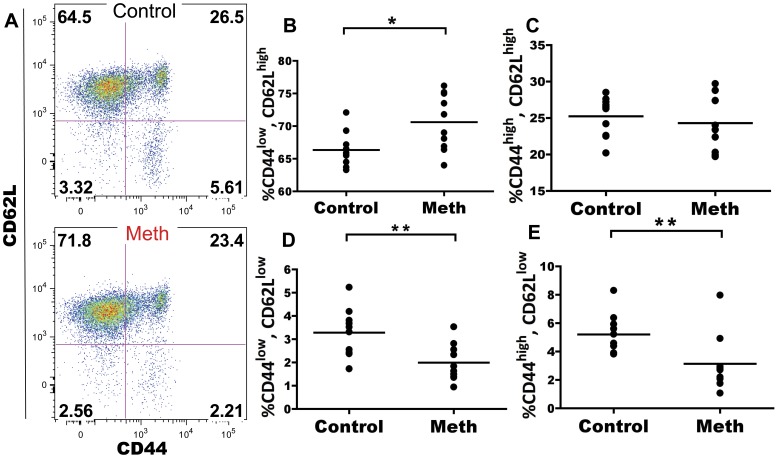
Meth has been associated with altered T cell activation and proliferation. We investigated the role of meth on CD4 and CD8 T cells in the spleen and mesenteric lymph node (MLN) to determine if meth influences antigen-experienced and naïve subsets of T cells in vivo. Within the splenic CD4 T cell compartment, we observed a significant decrease in proportion of CD62Llow, CD44high antigen-experienced cells after meth treatment (p<0.0001; Fig. 5A & B). Futhermore, within this CD62Llow, CD44high subset we observed a decreased proportion of cells expressing high levels of CD27 in meth-treated mice compared to controls (p = 0.0029; Fig. 5C & D). Among CD62L+ CD4 T cells, we observed a significant increase in proportion of naïve CD45RBbright, CD44low cells (p<0.0001), with significant decreases in proportion of CD45RBlow, CD44low (p = 0.0003) and CD45RBlow, CD44high cells (p = 0.0001; Fig. 6A–D). Combined, these results suggest a decrease in overall activation/antigen experience by CD4 T cells. While a similar decrease in CD62L−, CD44high proportion was noted in the MLN (p = 0.0052; Fig. 7A & B), we did not observe differences in CD27 expression within this subset in this organ (data not shown). We also observed no differences in the CD62L+ population of MLN CD4 T cells after meth treatment (data not shown). In total, meth appears to inhibit CD4 T cell activation in secondary lymphoid tissue.
Figure 5. Meth reduces proportions of CD62Llow, CD44high splenic CD4 T cells and these cells exhibit lower CD27 expression.
Splenic CD4 T cells were examined after meth treatment to determine if meth alters surface phenotypes suggesting activation/antigen experience and effector status. A. Representative gating showing CD62L and CD44 on CD4 T cells. B. Meth causes a reduction in proportion of CD44high, CD62Llow CD4 T cells. C. CD27 expression by CD44high, CD62Llow CD4 T cells. D. CD44high, CD62Llow CD4 T cells from meth treated animals exhibit a lower proportion of cells expressing CD27 at a high level. **p<0.01, ***p<0.001 calculated by Mann-Whitney U Test. Bars represent mean. Data are from 2 experiments of 5 animals per treatment group per experiment.
Figure 6. Meth causes reduced proportions of alternatively spliced CD4 T cells among the splenic CD62Lhigh compartment.
Meth and control mice were analyzed for surface expression of CD45RB and CD44 on the CD62Lhigh subset. CD45RB expression is downregulated upon antigen experience and can be used to gauge activation levels of CD4 T cells. A. CD45RB and CD44 on CD62Lhigh splenic CD4 T cells. B–D. Meth-treated animals had a higher proportion of CD45RBhigh, CD44low CD4 T cells compared to controls. Meth-treated animals also exhibited lower proportions of CD45RBlow, CD44low and CD45RBlow, CD44highsubsets compared to controls. ***p<0.001 calculated by Mann-Whitney U Test. Bars represent mean. Data are from 2 experiments of 5 animals per treatment group per experiment.
Figure 7. Meth treatment causes a reduction in proportion of CD62Llow, CD44high CD4 T cells in the mesenteric lymph node (MLN).
MLN CD4 T cells were labeled with CD62L and CD44 to determine proportions of antigen-experienced cells. A. CD62L and CD44 on MLN CD4 T cells from meth and vehicle treated animals. B. Meth causes a reduction in proportion of CD44high, CD62Llow CD4 T cells in the MLN. **p<0.01 calculated by Mann-Whitney U Test. Bars represent mean. Data are from 2 experiments of 5 animals per treatment group per experiment.
Meth causes upregulation of CD150 (SLAM) on CD226+ CD4 T cells
Members of the signaling lymphocytic activation (SLAM) family are associated with T cell activation, and signaling through CD150 (SLAM) results in IFN-γ production, differentiation, and proliferation by T cells [47]. CD226 expression has been associated with Th1 status in murine T cells [48]. We hypothesized that meth’s negative impact on T cell activation should correspond to a decrease in CD150 expression as well as a decreased proportion of CD226-expressing CD4 T cells. To test this hypothesis, we compared CD150 MFI and proportion of CD226+ CD4 T cells from meth-treated and control mice. Although we observed no differences in CD226 expression after meth treatment (data not shown), we did observe significantly increased expression of CD150 on CD226+ CD44+ CD4 T cells (p<0.0001; Fig. 8A & B). The upregulation of this costimulatory molecule suggests that meth promotes the activation of specific subsets of CD4 T cells.
Figure 8. Increased expression of CD150 on CD226+ CD4 T cells after meth treatment.
We examined the expression of the costimulatory markers CD150 and CD226 by splenic CD4 T cells after meth treatment. A. Representative gating and histogram showing selection of CD226+ CD4 T cells and increased expression of CD150 by meth treated animals compared to control. Red represents a meth-treated animal, black is vehicle-treated animal, and grey is the isotype control. B. CD226+ CD4 T cells from meth treated animals express higher levels of CD150 than those from control animals. Protein expression was calculated using mean fluorescence intensity (MFI). ***p<0.001 calculated by Mann-Whitney U Test. Error bars represent SEM. Data are from 2 experiments of 5 animals per treatment group per experiment.
Meth causes the expansion of a KLRG1+ CD4 T cell subset that is not FoxP3+
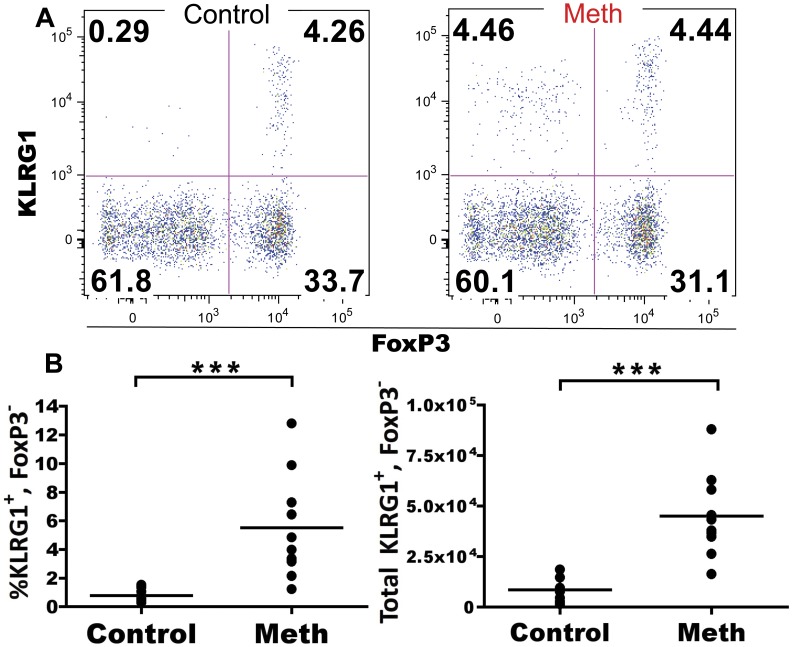
Regulatory T cells (TREGS) are fundamental in maintaining tolerance [49] and within the TREG population, a potent regulatory compartment expressing KLRG1 has been reported in healthy mice [50]. To determine the effect of meth on specific subsets of TREGs, we labeled splenic CD4 T cells with FoxP3, CD25 (IL-2Rα), and KLRG1, with additional markers of homing and differentiation. Although we observed no significant difference in KLRG1+/−, FoxP3+, CD25+ CD4 T cells after meth treatment (data not shown), we did observe a significant expansion of KLRG1+ FoxP3− CD4 T cells in proportion and absolute number after meth treatment (p<0.0001; Fig. 9A & B). These cells were CD44high, CD45RBlow, CD25low CD62Llow, and NKG2D− (data not shown), a profile of protein expression suggesting activation. A similar KLRG1+ expansion was observed in MLN, although FoxP3 staining was not performed in the MLN (data not shown). These data from spleen and MLN demonstrate that meth promotes the expansion of a CD4 T cell subset with a terminal effector phenotype, an unexpected find amidst signs of suppression.
Figure 9. Meth promotes the expansion of splenic KLRG1+, FoxP3− CD4 T cells.
Meth-treated animals possessed an expansion of KLRG1+ CD4 T cells within the spleen. These cells were CD44high and negative/low for FoxP3, CD25, CD62L, NKG2D, and CD45RB. A. Representative gating showing significant expansion of splenic KLRG1+, FoxP3− CD4 T cells among the CD44high, CD45RBlow compartment with relatively unchanged proportions of FoxP3-expressing subsets after meth treatment. B and C. Proportion and absolute number of KLRG1+, FoxP3− CD4 cells are increased with meth. ***p<0.001 calculated by Mann-Whitney U Test. Bars represent mean. Data are from 2 experiments of 5 animals per treatment group per experiment.
CD8 T Cells
Meth treatment results in decreased proportions of activated CD8 T cells in spleen and lymph node
Naïve CD8 T cells can differentiate into cytotoxic effectors with short-lived status or long-term memory potential. Since meth-induced deficiencies in these effector subsets may leave the user more vulnerable to pathogens, we investigated meth’s ability to alter CD8 T cell subpopulations. CD8 T cells can be divided into 4 subsets depending on levels of CD62L and CD44 expression: CD62Lhigh, CD44low (naïve CD8 T cell, TN), CD62Lhigh, CD44high, (central memory CD8 T cell, TCM), CD62Llow, CD44high (effector memory CD8 T cells, TEM), and CD62Llow, CD44low (acute/activated effector CD8 T cell, TAE) [51], [52]. Within the spleen, we observed a significant increase in proportion of CD8 TN cells (p = 0.0185) with a decrease in proportion of TEM (p = 0.0021) and TAE (p = 0.0021) subsets, but no change in TCM proportion (Fig. 10A–E). Similar alterations were observed in the MLN. Mesenteric lymph node CD8 T cells in meth-treated mice showed a significant increase in proportion of CD8 TN cells (p<0.0001) with a decrease in proportion of TEM (p<0.0001), TAE (0 = 0.0011), and TCM subsets (p = 0.0029; Fig. 11 A–E). From these results, it appears that meth also negatively impacts the accumulation of antigen-experienced CD8 T cells, similar to what was observed in CD4 T cells.
Figure 10. Meth-treated animals exhibit a greater proportion of naïve phenotype CD8 T cells and reduced proportions of antigen-experienced subsets within the spleen.
Splenic CD8 T cells were labeled with CD62L and CD44 and classified into 4 groups according the expression of these two proteins: CD62Lhigh, CD44low (Naïve CD8 T cell, TN), CD62Lhigh, CD44high, (central memory CD8 T cell, TCM), CD62Llow, CD44high (effector memory CD8 T cells, TEM), and CD62Llow, CD44low (acute effector CD8 T cell, TAE). A. Gating showing CD44 and CD62L expression by splenic CD8 T cells. B–E. After meth treatment, the TN compartment is increased in proportion, while the TAE and TEM showed a decrease in proportion and TCM appeared unchanged. *p<0.05, **p<0.01 calculated by Mann-Whitney U Test. Bars represent mean. Data are from 2 experiments of 5 animals per treatment group per experiment.
Figure 11. CD8 T cells from the mesenteric lymph node (MLN) also show reductions in proportion of antigen-experienced subsets with an increase in the naïve compartment.
Mesenteric lymph node CD8 T cells were labeled with CD62L and CD44 and classified as in Fig. 10. A. Gating showing CD44 and CD62L expression by MLN CD8 T cells. B–E. After meth treatment, the TN compartment is increased in proportion, while the TAE, TEM, and TCM compartments all revealed a decrease in proportion. **p<0.01, ***p<0.001 calculated by Mann-Whitney U Test. Bars represent mean. Data are from 2 experiments of 5 animals per treatment group per experiment.
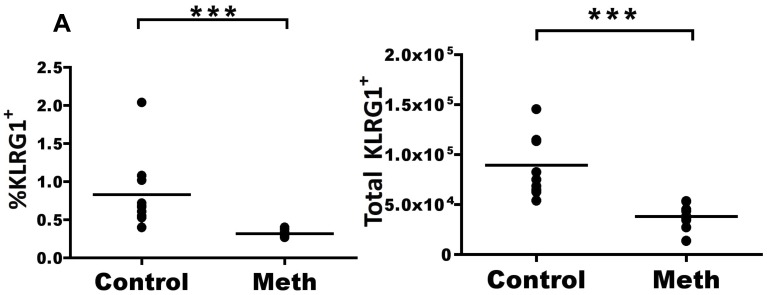
Meth treatment results in decreased numbers of KLRG1+ CD8 T cells in the MLN
KLRG1 is expressed by effector CD8 T cells [53], and we postulated that meth’s disruption of antigen presenting pathways and T cell activation would also effect the profile of this marker of CD8 T cell activation. Interestingly, we observed a significant decrease in KLRG1-expressing CD8 T cells (p<0.0001) in the MLN (Figure 12 A), yet we observed no difference in KLRG1 expression by CD8 T cells in the spleen (data not shown). This reduction in the MLN adds further evidence to support the notion that meth reduces specific effector T cells and may promote weakened immunity.
Figure 12. Meth causes a reduced proportion and number of KLGR1+ CD8 T cells in the MLN.
CD8 T cells were labeled with KLRG1 to investigate whether meth impacts subsets of antigen-experienced CD8 T cells. A. The proportion and number of CD8 T cells expressing KLRG1 in the MLN is reduced after meth treatment. *p<0.01, ***p<0.001 calculated by Mann-Whitney U Test. Bars represent mean. Data are from 2 experiments of 5 animals per treatment group per experiment.
Discussion
Meth Decreases the Abundance of Splenic Innate Leukocytes
Previous studies have suggested meth has the potential to disrupt immune homeostasis and leave the user more susceptible to pathogenic challenge. With meth impacting the function of NK cells and antigen-presenting cells (APCs), a basis of innate immunity becomes weakened, and consequent and appropriate adaptive responses by T and B cells are less likely. In this study, we observed an overall decrease in both proportion and number of splenic NK cells, DCs, and Gr-1high monocytes/macrophages. Meth-induced leukopenia had been previously reported by Saito et al. [37], who noted reduced numbers of leukocytes in the blood of male Slc:ddY mice at 1, 4, 24, and 48 hours after meth treatment, but did not determine which subsets were impacted by meth. Furthermore, our results showing no difference in splenic T and B cell proportion after meth treatment are in agreement with the findings of Wey et al. [31], who analyzed splenocytes from meth-treated BALB/c mice for proportion of B220, CD4 and CD8-expressing cells. While the effect of meth of promoting apoptosis [28], [39] may explain some of this reduction in cell number, it would also raise the question as to why certain subsets remain relatively unchanged after meth exposure.
The findings of Saito et al. showing leukocyte deficiencies in blood and our observed reductions of myeloid populations within the spleen may suggest a sensitivity to meth in the bone marrow, a lipid-rich environment that may store meth and have detrimental effects on bone marrow-derived leukocyte subsets. It is known that meth is lipophilic and can accumulate in solid tissue [12], [13], and it has been reported that meth can persist in bone marrow [54]–[56]. Additionally, In et al. [30] reported reduced granulocyte-macrophage colony formation in GM-CSF-treated cultures of bone marrow harvested from meth-treated CD-1 mice. For these reasons, it would be meaningful to perform additional investigations on the effects of meth on haematopoesis and the seeming imperviousness to meth by B cells, neutrophils, and Gr-1low monocytes/macrophages compared to other bone marrow-derived subsets.
The immunological roles of Gr-1(Ly-6C)low and Gr-1(Ly-6C)high monocytes/macrophages are not completely clear, but it has been shown that Ly-6Clow monocytes appear to be a more stable, tissue resident and endothelium-monitoring population capable of macrophage and DC differentiation, while Ly-6Chigh monocytes tend to home to areas of inflammation and mature to inflammatory DCs and macrophages [57]. Our observation of reduced splenic Gr-1(Ly-6C)high monocytes/macrophages fits well with the reduced number of peritoneal macrophages after meth exposure reported by In et al. [32]. In their study, they utilized the intraperitoneal thioglycolate injection model of inflammation to promote macrophage accumulation in the peritoneum. This model has been shown to cause the migration of Ly-6Chigh (but not Ly-6Clow) monocytes to the peritoneum [57], where they ultimately mature to macrophage. The noted deficiencies in peritoneal macrophages correlates with our observation of reduced numbers of splenic precursors to this population. Indeed, the spleen has been shown to be a significant reservoir of mature monocytes and upon inflammatory signaling, to exhibit substantial decreases in monocyte numbers as cells emigrate [42].
Perturbed Gr-1low Monocytes/macrophages may be Responding to Meth-induced Cytotoxicity and Exhibiting a Tolerogenic Function
In addition to alterations in splenic abundance, our results indicate that the Gr-1(Ly-6C)high and Gr-1(Ly-6C)low monocyte/macrophage populations are unequally affected by meth in terms of activation. While we observed no difference in CD86 and CD80 expression by Gr-1(Ly6C)high monocytes/macrophages, we did observe a significant increase in CD80 expression by Gr-1(Ly-6C)low monocytes/macrophages, with no change in their CD86 expression. In addition to their role in vascular repair and monitoring [58], Ly6Clow monocytes/macrophages have been reported to operate as phagocytes, capable of engulfing debris from dead cells and ultimately promoting T cell proliferation [59] or self tolerance [60]. If meth is capable of inducing increased rates of apoptosis as suggested by Potula et al. [28] and Iwasa et al. [39], perhaps this population of monocytes/macrophages is responding to an accumulation of dead material and maturing to promote tolerance. Indeed, CD80 has been shown to bind PDL-1 [61], [62] and this interaction results in reduced rates of proliferation among T cells [61], [63], suggesting a tolerogenic function of CD80 in addition to it’s known role in costimulation [64]. Although CD80-CTLA-4 interactions have also been proposed to terminate effector T cell responses in the periphery [44], our data do not lend themselves to this interpretation. The Ly-6Clow monocytes/macrophages in our study are MHC II−, thus they are not presenting antigen in this manner and therefore are unlikely to be involved in specific TCR-MHC II interactions. Furthermore, the only expansion of T cells we observed was within the CD4 compartment, suggesting MHC II dependency and providing little evidence for cross presentation.
Accumulation and removal of apoptotic cells may also explain our observed decrease in CD11b expression on Ly-6Clow monocytes/macrophages. A recent report by Schif-Zuck et al. [65] has described a decrease in CD11b expression on “satiated” macrophages resulting from iC3b directed internalization of apoptotic cells. Although work by Talloczy et al. [33] and Martinez et al. [34] has suggested reduced phagocytic efficacy due to the basic properties of meth impacting with the acidic endosome of macrophages, findings that suggest reduced antigen-presentation effectiveness and activation, their results did not suggest eliminated antigen processing and internalization capabilities, rather, attenuated, and therefore are not in disagreement with our findings.
Meth-induced Alterations in NK Cell Proportion and Number Suggest Functional Deficiencies
The fundamental roles performed by NK cells in viral immunity [66], tumor surveillance [67], and guiding the adaptive immune response have been revealed [68]. Consequently, a deficiency in NK cell number as well as NK cell function may lead to weakened immunity. Our observation of an increased proportion of terminally differentiated NK cells corresponds with previous reports suggesting altered activation levels of NK cells after meth exposure [30], [35]. KLRG1 expression by NK cells is almost exclusively restricted to the CD11bhigh, CD27low subset [45], [46], [69], a group that exhibits lower levels of homeostatic proliferation [45], [69] and possesses lower levels of cytotoxicity than its CD27high counterpart [45]. KLRG1-expressing NK cells have also been shown to produce lower levels of IFN-γ than KLRG1− NK cells [70]. Our data suggest that meth causes a shift in the NK cell compartment, with a marked reduction in CD27high, KLRG1− NK cells after meth treatment. This shift leaves the majority of NK cells in periphery with a phenotype suggesting lower responsiveness and effectiveness, a dangerous combination considering the importance of NK cells during viral infections and the increased susceptibility of meth users to several viruses.
T Cells Exhibit Signs of Suppression as well as Activation After Meth Treatment
We have observed that meth causes a reduction of activated/antigen-experienced CD4 and CD8 T cells. We noted substantial decreases in proportion of CD44high, CD62Llow CD4 T cells after meth treatment and within this population, we observed a decrease in proportion of cells expressing CD27. Combined, these results suggest a decrease in CD4 T cell activation perhaps due to diminished antigen presentation as well as a polarization to short-lived status, as CD27 expression has been associated with longevity in T cells [71].
Within the CD62L+ subset of CD4 T cells, we observed a decrease in proportion CD45RBlow/CD44low, and CD45RBlow/CD44high cells, with an increased proportion of CD45RBhigh/CD44low cells. While the majority of naïve splenic T cells express CD45RB, after antigen experience these cells will undergo alternate exon splicing and downregulate CD45RB [72]. The observed deficiencies in CD45RB downregulation may be induced by reduced APC effectiveness as well as the reduced number of DCs observed in our experiment. Alternatively, meth may directly obstruct CD45 exon splicing in some fashion. An additional possibility is that meth promotes increased thymic output or enhanced proliferation by naïve subsets within the spleen. However, our data do not reveal an increase in splenic T cell number, nor did we find an altered CD4:CD8 ratio after meth treatment. Although we did not investigate the thymus in our study, it is probable that meth also accumulates within thymic tissue and may impact thymic selection and output. Supporting this notion, Iwasa et al. [39] observed an increased proportion of apoptotic cells in the thymus after a single injection of meth, while In et al. [30] noted an increase in proportion of CD4 T cells in the thymus with decreases in proportion CD8 and double positive (DP) T cells accompanied with reduced thymic weight after meth use, findings which justify further investigations of the effects of meth on the thymus.
CD8 T cell activation patterns were similarly affected as those of CD4 T cells. We observed reduced proportions of effector memory (TEM) and acute effector (TAE) CD8 T cells subsets in the spleen and reduced proportions of TEM, central memory (TCM), and TAE subsets in the MLN, again suggesting either decreased antigen presentation and/or increased cell death among these reduced populations. These overall decreases in CD4 and CD8 T cell activation may be highly detrimental during the host’s response to pathogenic challenge. Considering that meth users are at increased risk for HIV and other viral infections, suppression of the immune adaptive response would be expected to lead to exacerbated infections. In agreement with this reasoning, meth use accompanying HIV has been associated with increased viral loads in human subjects [73].
Although our investigations revealed signs of suppression of the CD4 and CD8 T cell response, we did observe associations suggesting activation, namely, increased CD150 expression by CD226+ CD4 T cells and an expansion of KLRG1+ CD4 T cells. CD150 upregulation by T cells has been observed after concanavalin A [47] and anti-CD3 [74] stimulation, and CD150 costimulation with anti-CD3 antibodies induced increased IFN-γ prodution and proliferation compared to anti-CD3 antibody treatment alone [47]. In our analysis of the splenic CD4 T cell compartment after meth treatment, we noted an increase in CD150 expression among CD226+ CD4 T cells that are also CD44high and CD62Llow. This is an interesting finding considering that overall CD44high,CD62Llow numbers were decreased with meth use, thus suggesting a specific subset(s) of unknown TCR specificity is somehow activated by meth, while others are diminished.
Killer cell lectin-like receptor G1 (KLRG1) is an inhibitory C-type lectin receptor that binds to E, N, and R cadherins [75], [76] and we observed alterations in KLRG1 expression by NK cells and CD4 and CD8 T cells after meth treatment. Interestingly, we noted an expansion of KLRG1+ FoxP3− CD4 T cells that appears surprising in the midst of signs of several signs of immune suppression. Increased KLRG1+ CD4 T cells have been reported after Toxoplasma gondii infection [70] and Mycobacterium tuberculosis infection [77], while KLRG1+ CD4 T cells were found to be unresponsive to TCR-induced proliferation [50] and produced high levels of IFN-γ and TNF-α after peptide stimulation [77]. Beyersdorf et al. [50] also observed that a portion of FoxP3+ CD4 T cells coexpressed KLRG1 and CD152, were CD25+/−, and that KLRG1+, CD25+/− CD4 T cells were capable of reducing cellular proliferation of anti-CD3 stimulated naïve CD4 T cells in the presence of APC, as well as limiting H3 thymidine uptake by TCR-stimulated naïve CD4 T cells. From these studies, we can conclude that there are at least two main populations of KLRG1+ CD4 T cells in mice: one which represents a terminally differentiated effector, and another which is a potent TREG. While we did observe KLRG1+ expression amongst FoxP3+ CD4 T cells and these cells were CD25+/−, we did not find a difference in proportion or number of any FoxP3-expressing CD4 T cell subset after meth treatment. Phenotypically, our KLRG1+ CD4 T cell subset fits into an effector/memory phenotype, being CD62L−, CD45RBlow, CD25−, FoxP3− and CD44high. However, future studies must be conducted to determine the functional attributes of this population and to determine its Th status. 1558–1565.
Conclusions
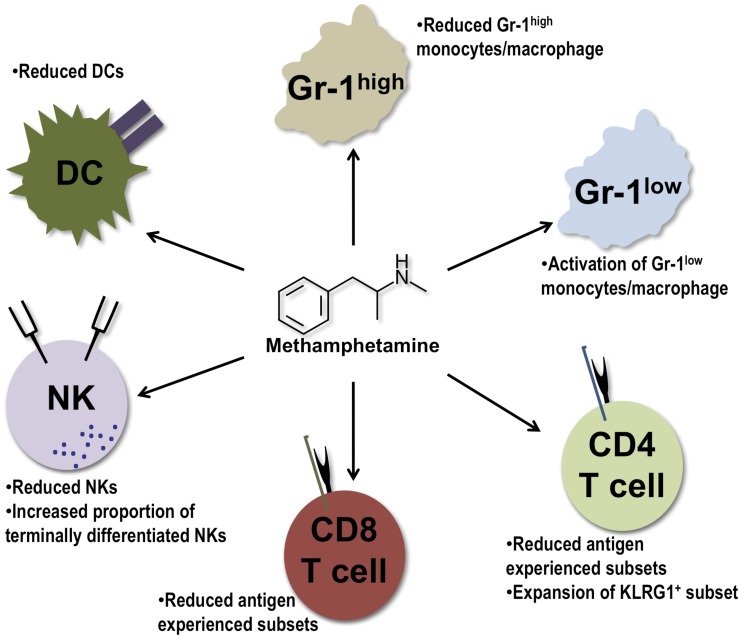
Our results demonstrate that meth impacts several leukocyte populations (Fig. 13). Indeed, meth’s role in inducing apoptosis and inhibiting APC function may have tremendous effects on T cell memory populations as well as on T cell activation, as our results and those of others have suggested. Furthermore, the combined effects of a decrease of Gr-1high monocytes/macrophages, DCs, and NK cell numbers, with a proportional increase of a less-responsive NK cell subset may leave the user less able to initiate a sufficient immune response and ultimately reduce the effectiveness of the adaptive response. Although our data reveal specific descriptive alterations induced by meth and suggest a reduced ability to respond efficiently to pathogens, additional investigations must focus on how the innate and adaptive arms are impacted in the face of viral and bacterial challenge concurrent with meth exposure. As meth addicts are at risk for increased rates of sexually transmitted diseases (STDs) and blood borne pathogens due to behavioral responses and drug administration routes, these investigations would be meaningful in revealing which mechanisms promote increased susceptibility to infections as suggested by this work and previous studies.
Figure 13. Methamphetamine impacts the innate and adaptive arms of immunity.
Summary of the effects of methamphetamine on immune cell subsets based upon the observations in this report.
Funding Statement
This work was funded by NIH RO1∶345160-2058101 from the National Institute on Drug Abuse. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brouwer KC, Case P, Ramos R, Magis-Rodriguez C, Bucardo J, et al. (2006) Trends in production, trafficking, and consumption of methamphetamine and cocaine in Mexico. Substance Use and Misuse 41: 707–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cronkwright Kirkos W, Carrique T, Griffen K, La Barge AP (2008) The York region methamphetamine strategy. Canadian Medical Association Journal 178: 1655–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Degenhardt L, Baker A, Maher L (2008) Methamphetamine: Geographic areas and populations at risk, and emerging evidence for effective interventions. Drug and Alcohol Review 27: 217–219. [DOI] [PubMed] [Google Scholar]
- 4. McKetin R, Kozel N, Douglas J, Ali R, Vicknasingam B, et al. (2008) The rise of methamphetamine in southeast and east Asia. Drug and Alcohol Review 27: 220–228. [DOI] [PubMed] [Google Scholar]
- 5. Mau MK, Asao K, Efird J, Saito E, Ratner R, et al. (2009) Risk factors associated with methamphetamine use and heart failure among native Hawaiians and other pacific island peoples. Journal of Vascular Health and Risk Management 5: 45–52. [PMC free article] [PubMed] [Google Scholar]
- 6. Sheridan J, Butler R, Wheeler A (2009) Initiation into methamphetamine use: qualitative findings from an exploration of first time use among a group of New Zealand users. Journal of Psychoactive Drugs 41: 11–17. [DOI] [PubMed] [Google Scholar]
- 7. Bonell CP, Hickson FC, Weatherburn P, Reid DS (2010) Methamphetamine use among gay men across the UK. The International Journal on Drug Policy 21: 244–246. [DOI] [PubMed] [Google Scholar]
- 8. Gonzales R, Mooney L, Rawson RA (2010) The methamphetamine problem in the United States. Annual Review of Public Health 31: 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peltzer K, Ramlagan S, Johnson BD, Phaswana-Mafuya N (2010) Illicit drug use and treatment in South Africa: a review. Substance Use and Misuse 45: 2221–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J (2009) The economic cost of methamphetamine use in the United States, 2005. Santa Monica, CA: RAND Corporation. Available: http://www.rand.org/pubs/monographs/MG829. Accessed 2010 June 22.
- 11. Cruickshank CC, Dyer KR (2009) A review of the clinical pharmacology of methamphetamine. Addiction 104: 1085–1099. [DOI] [PubMed] [Google Scholar]
- 12. Rivière GJ, Gentry WB, Owens SM (2000) Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. The Journal of Pharmacology and Experimental Therapeutics 292: 1042–1047. [PubMed] [Google Scholar]
- 13. Volkow ND, Fowler JS, Wang GJ, Shumay E, Telang F, et al. (2010) Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PLoS ONE 5: e15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nordahl TE, Salo R, Leamon M (2003) Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. Journal of Neuropsychiatry and Clinical Neuroscience 15: 317–325. [DOI] [PubMed] [Google Scholar]
- 15. Krasnova IN, Cadet JL (2009) Methamphetamine toxicity and messengers of death. Brain Research Reviews 60: 379–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hutin YJF, Sabin KM, Hutwagner LC, Schaben L, Shipp GM, et al. (2000) Multiple modes of hepatitis A virus transmission among methamphetamine users. American Journal of Epidemiology 152: 186–192. [DOI] [PubMed] [Google Scholar]
- 17. Gonzales RP, Marinelli-Casey P, Shoptaw S, Ang A, Rawson RA (2006) Hepatitis C virus infection among methamphetamine-dependent individuals in outpatient treatment. Journal of Substance Abuse Treatment 31: 195–202. [DOI] [PubMed] [Google Scholar]
- 18. Vogt TM, Perz JF, Van Houten Jr CK, Harrington R, Hansuld T, et al. (2006) An outbreak of hepatitis B virus among methamphetamine injectors: the role of sharing injection drug equipment. Addiction 101: 726–730. [DOI] [PubMed] [Google Scholar]
- 19. Cohen AL, Shuler C, McAllister S, Fosheim GE, Brown MG, et al. (2007) Methamphetamine use and methicillin-resistant Staphylococcus aureus skin infections. Emerging Infectious Diseases 13: 1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corsi KF, Booth RE (2008) HIV sex risk behaviors among heterosexual methamphetamine users: literature review from 2000 to present. Current Drug Abuse Reviews 1: 292–296. [DOI] [PubMed] [Google Scholar]
- 21. Forrest DW, Metsch LR, LaLota M, Cardenas G, Beck DW, et al. (2010) Crystal methamphetamine use and sexual risk behaviors among HIV-positive and HIV-negative men who have sex with men in south Florida. Journal of Urban Health: Bulleting of the New York Academy of Medicine 87: 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freeman P, Walker BC, Harris DR, Garofalo R, Willard N, et al. (2011) Methamphetamine use and risk for HIV among young men who have sex with men in 8 US cities. Archives of Pediatric and Adolescent Medicine 165: 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marshall BDL, Wood E, Shoveller JA, Patterson TL, Montaner JSG, et al. (2011) Pathways to HIV risk and vulnerability among lesbian, gay, bisexual, and transgendered methamphetamine users: A multi-cohort gender-based analysis. BMC Public Health 11: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang H, Wang X, Chen H, Song L, Ye L, et al. (2008) Methamphetamine enhances HIV infection of macrophages. American Journal of Pathology 172: 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye L, Peng JS, Wang X, Wang YJ, Luo GX, et al. (2008) Methamphetamine enhances hepatitis C virus replication in human hepatocytes. Journal of Viral Hepatology 15: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gagnon L, Lacroix F, Chan J, Buttar HS (1992) In vitro effects of ‘designer’ amphetamines on human peripheral blood mononuclear leukocytes proliferation and on natural killer cell activity. Toxicology Letters 63: 313–319. [DOI] [PubMed] [Google Scholar]
- 27. Yu Q, Zhang D, Walston M, Zhang J, Liu Y, et al. (2002) Chronic methamphetamine exposure alters immune function in normal and retrovirus-infected mice. International Immunopharmacology 2: 951–962. [DOI] [PubMed] [Google Scholar]
- 28. Potula R, Hawkins BJ, Cenna JM, Fan S, Dykstra H, et al. (2010) Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. Journal of Immunology 185: 2867–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. House RV, Thomas PT (1994) Comparison of immune functional parameters following in vitro exposure to natural and synthetic amphetamines. Immunopharmacology and Immunotoxicology 16: 1–21. [DOI] [PubMed] [Google Scholar]
- 30. In S, Son E, Rhee D, Pyo S (2005) Methamphetamine administration produces immunomodulation in mice. Journal of Toxicology and Environmental Health, Part A 68: 2133–2145. [DOI] [PubMed] [Google Scholar]
- 31. Wey S, Wu H, Chang F, Jan T (2008) Methamphetamine and diazepam suppress antigen-specific cytokine expression and antibody production in ovalbumin-sensitized BALB/c mice. Toxicology Letters 181: 157–162. [DOI] [PubMed] [Google Scholar]
- 32. In S, Son E, Rhee D, Pyo S (2004) Modulation of murine macrophage function by methamphetamine. Journal of Toxicology and Environmental Health, Part A 67: 1923–1937. [DOI] [PubMed] [Google Scholar]
- 33. Tallóczy Z, Martinez J, Joset D, Ray Y, Gácser A, et al. (2008) Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathogens 4: e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martinez LR, Mihu MR, Gácser A, Santambrogio L, Nosanchuk JD (2009) Methamphetamine enhances histoplasmosis by immunosupression of the host. The Journal of Infectious Diseases 200: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marcondes MCG, Flynn C, Watry DD, Zandonatti M, Fox HS (2010) Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. The American Journal of Pathology 177: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saito M, Yamaguchi T, Kawata T, Ito H, Kanai T, et al. (2005) Effects of methamphetamine on cortisone concentration, NK cell activity and mitogen response of T-lymphocytes in female cynomolgus monkeys. Experimental Animals 55: 477–481. [DOI] [PubMed] [Google Scholar]
- 37. Saito M, Terada M, Kawata T, Ito H, Shigematsu N, et al. (2008) Effects of single or repeated administrations of methamphetamine on immune response in mice. Experimental Animals 57: 35–43. [DOI] [PubMed] [Google Scholar]
- 38. Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM (2004) Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglia activation. The Journal of Pharmacology and Experimental Therapeutics 311: 1–7. [DOI] [PubMed] [Google Scholar]
- 39. Iwasa M, Maeno Y, Inoue H, Koyama H, Matoba R (1996) Induction of apoptotic cell death in rat thymus and spleen after bolus injection of methamphetamine. International Journal of Legal Medicine 109: 23–28. [DOI] [PubMed] [Google Scholar]
- 40. Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE (2008) Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of Leukocyte Biology 83: 64–70. [DOI] [PubMed] [Google Scholar]
- 41. Strauss-Ayali D, Conrad SM, Mosser DM (2007) Monocyte subpopulations and their differentiation patterns during infection. Journal of Leukocyte Biology 82: 244–252. [DOI] [PubMed] [Google Scholar]
- 42. Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, et al. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walzer T, Bléry M, Chaix J, Fuseri N, Chasson L, et al. (2007) Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proceedings of the National Academy of Sciences 104: 3384–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharpe AH, Freeman GJ (2002) The B7-CD28 superfamily. Nature Reviews Immunology 2: 116–126. [DOI] [PubMed] [Google Scholar]
- 45. Hayakawa Y, Smyth MJ (2006) CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. Journal of Immunology 176: 1517–1524. [DOI] [PubMed] [Google Scholar]
- 46. Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, et al. (2007) NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. Journal of Immunology 178: 4764–4770. [DOI] [PubMed] [Google Scholar]
- 47. Howie D, Okamoto S, Rietdijk S, Clarke K, Wang N, et al. (2002) The role of SAP in murine CD150 (SLAM) -mediated T-cell proliferation and interferon γ production. Blood 100: 2899–2907. [DOI] [PubMed] [Google Scholar]
- 48. Dardalhon V, Schubart AS, Reddy J, Meyers JH, Monney L, et al. (2005) CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions. Journal of Immunology 175: 1558–1565. [DOI] [PubMed] [Google Scholar]
- 49. Wing K, Sakaguchi S (2010) Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature Immunology 11: 7–13. [DOI] [PubMed] [Google Scholar]
- 50. Beyersdorf N, Ding X, Tietze JK, Hanke T (2007) Characterization of mouse CD4 T cell subsets defined by expression of KLRG1. European Journal of Immunology 37: 3445–3454. [DOI] [PubMed] [Google Scholar]
- 51. Tsujimura K, Obata Y, Matsudaira Y, Nishida K, Akatsuka Y, et al. (2006) Characterization of murine CD160+ CD8+ T lymphocytes. Immunology Letters 106: 48–56. [DOI] [PubMed] [Google Scholar]
- 52. Wiede F, Roomberg A, Cretney E, Lechner A, Fromm P, et al. (2009) Age-dependent, polyclonal hyperactivation of T cells is reduced in TNF-negative gld/gld mice. The Journal of Leukocyte Biology 85: 108–116. [DOI] [PubMed] [Google Scholar]
- 53. Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, et al. (2008) Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. The Journal of Experimental Medicine 205: 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kojima T, Okamoto I, Miyazaki T, Chikasue F, Yashiki M, et al. (1986) Detection of methamphetamine and amphetamine in a skeletonized body buried for 5 years. Forensic Science International 31: 93–102. [DOI] [PubMed] [Google Scholar]
- 55. Nagata T, Kimura K, Hara K, Kudo K (1990) Methamphetamine and amphetamine concentrations in postmortem rabbit tissues. Forensic Science International 48: 39–47. [DOI] [PubMed] [Google Scholar]
- 56. Sato Y, Kondo K, Takayasu T, Ohshima T (2000) Detection of methamphetamine in a severely burned cadaver – a case report. Nihon Hoigaku Zasshi 54: 420–424. [PubMed] [Google Scholar]
- 57. Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82. [DOI] [PubMed] [Google Scholar]
- 58. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, et al. (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of Experimental Medicine 204: 3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, et al. (2006) Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. The Journal of Experimental Medicine 203: 583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peng Y, Latchman Y, Elkon KB (2009) Ly6Clow monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. Journal of Immunology 182: 2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ (2007) Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity 27: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Butte MJ, Peña-Cruz V, Kim MJ, Freeman GJ, Sharpe AH (2008) Interaction of human PD-L1 and B7–1. Molecular Immunology 45: 3567–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, et al. (2010) B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 116: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bromley SK, Iaboni A, Davis SJ, Whitty A, Green JM, et al. (2001) The immunological synapse and CD28-CD80 interactions. Nature Immunology 2: 1159–1166. [DOI] [PubMed] [Google Scholar]
- 65. Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, et al. (2011) Saturated-efferocytosis generates pro-resolving CD11blow macrophages: modulation by resolvins and glucocorticoids. European Journal of Immunology 41: 366–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marcenaro E, Carlomagno S, Pesce S, Della Chiesa M, Parolini S, et al. (2011) NK cells and their receptors during viral infections. Immunotherapy 3: 1075–1086. [DOI] [PubMed] [Google Scholar]
- 67. Waldhauer I, Steinle A (2008) NK cells and cancer immunosurveillance. Oncogene 27: 2932–5943. [DOI] [PubMed] [Google Scholar]
- 68. Waggoner SN, Cornberg M, Selin LK, Welsh RM (2011) Natural killer cells act as rheostats modulating antiviral T cells. Nature 481: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, et al. (2009) Maturation of mouse NK cells is a 4-stage developmental program. Blood 113: 5488–5496. [DOI] [PubMed] [Google Scholar]
- 70. Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, et al. (2002) Cutting edge: Inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. Journal of Immunology 168: 2585–2589. [DOI] [PubMed] [Google Scholar]
- 71. Denoeud J, Moser M (2011) Role of CD27/CD70 pathway of activation in immunity and tolerance. Journal of Leukocyte Biology 89: 195–203. [DOI] [PubMed] [Google Scholar]
- 72. Hermiston ML, Xu Z, Weiss A (2003) CD45: A critical regulator of signaling thresholds in immune cells. Annual Review of Immunology 21: 107–137. [DOI] [PubMed] [Google Scholar]
- 73. Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, et al. (2003) Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. The Journal of Infectious Diseases 188: 1820–1826. [DOI] [PubMed] [Google Scholar]
- 74. Henning G, Kraft MS, Derfuss T, Pirzer R, de Saint-Basile G, at al (2001) Signaling lymphocyte activation molecule (SLAM) regulates T cellular cytotoxicity. European Journal of Immunology 31: 2741–2750. [DOI] [PubMed] [Google Scholar]
- 75. Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, et al. (2006) Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. The Journal of Experimental Medicine 203: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li Y, Hofmann M, Wang Q, Teng L, Chlewicki LK, et al. (2009) Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing self recognition. Immunity 31: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, et al. (2010) Distinct functions of antigen-specific CD4 T cells during Mycobacterium tuberculosis infection. Proceedings of the National Academy of Sciences 107: 19408–19413. [DOI] [PMC free article] [PubMed] [Google Scholar]