Abstract
Thiostrepton, a powerful antibiotic belonging to the thiopeptide class, was synthesized in the laboratory for the first time in 2004 through an arduous campaign involving novel strategies and tactics, scenic detours and unexpected roadblocks. In this article the author narrates the long journey to success not so dissimilar to Odysseus’ return voyage to Ithaca, full of adventure, knowledge and wisdom.
Inspired by the writing form of Mahaffy,[1] the author has taken liberties with the style in which this article is written.
Introduction: Thiostrepton and Penicillin
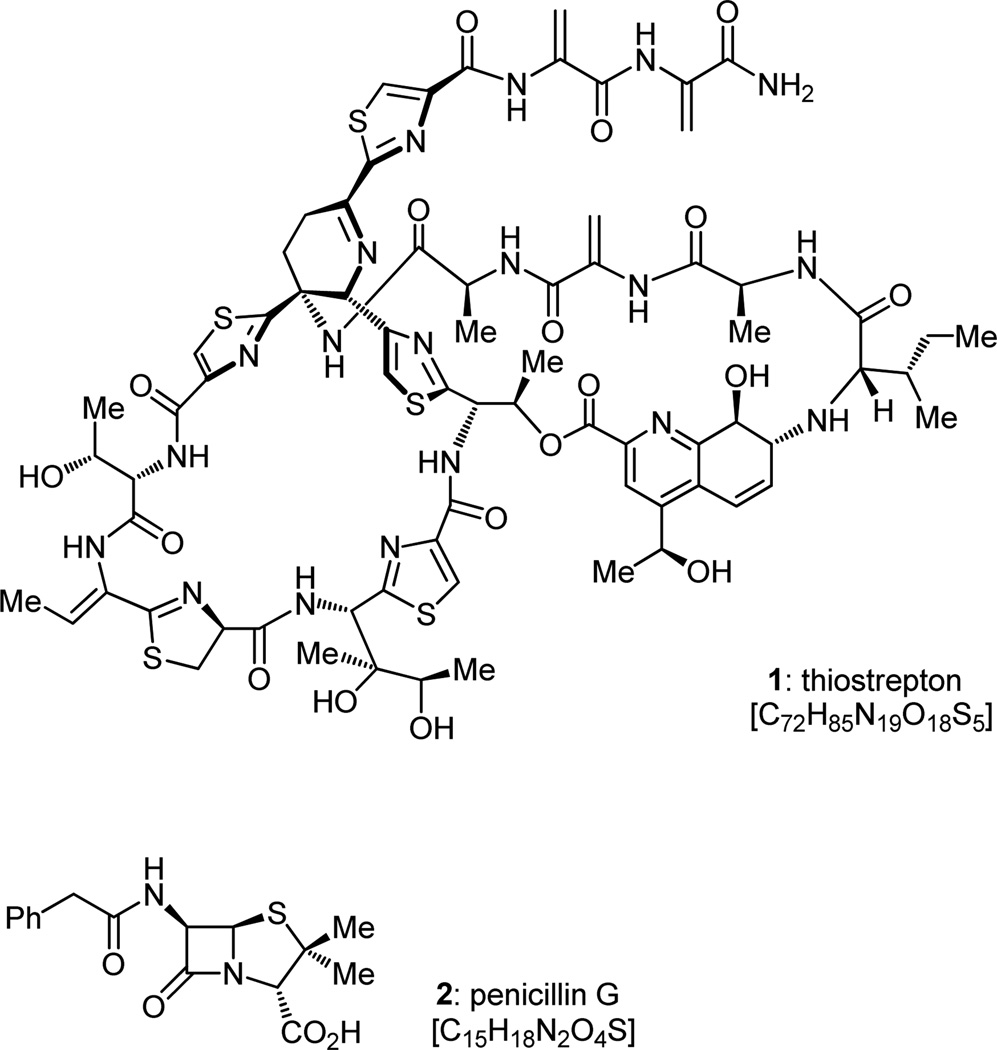
Thiostrepton (1, Figure 1), a substance first isolated from the three Streptomyces species azureus ATCC 14921, hawaiiensis ATCC 12236, and laurentii ATCC 31255 in 1954[2] and structurally elucidated in subsequent years by chemical,[3] spectroscopic,[4] and X-ray crystallographic techniques,[5] is of considerable interest from a number of perspectives. Belonging to the growing family of thiopeptide antibiotics,[6] this natural product boasts a number of unique features. Its magnificent structure places it at the top of its class (the thiopeptide antibiotics, of which there are many), a royal status that is also reflected in its impressive biological properties. These include (beside antibacterial)[7] antimalarial,[8] immunosuppressive,[9] and cytotoxic[10] actions. The longevity of its use in veterinary medicine bodes well for its potential employment in human applications if suitable modifications can be made to its structure and formulation so as to improve its pharmacology and method of delivery to the patient. Both thiostrepton’s biosynthesis and mode of action are fascinating, and their contemplation at the molecular level is worth a moment or two. We will briefly comment on the mode of action of this most intriguing molecule; its biosynthesis will have to await later commentary, when we come to consider its laboratory synthesis, for it was inspirational to the conception of the adopted synthetic strategy. In a painstaking, but illuminating investigation reported in 1996, Xing and Draper concluded that thiostrepton exerts its action against Gram-positive bacteria by grabbing onto a specific site of the ribosome composed of the 23S ribosomal t-RNA and protein L11, an unfortunate event for the unaware bacteria, for this binding leads to their death through inhibition of protein biosynthesis.[11]
Figure 1.
Structures of thiostrepton (1) and penicillin G (2).
The molecular architecture of thiostrepton (1, Figure 1), so precisely laid naked by X-ray crystallographic analysis, must not be underestimated for its structural complexity and chemical sensitivity, just like that of the penicillins[12] [exemplified by penicillin G (2, Figure 1)] in a previous era. In that instance, the deceptively simple structure of penicillin eluded armies of organic chemists for decades until it yielded to chemical synthesis in the late 1950s.[13] A comparison between the two antibiotics, thiostrepton (1) and penicillin G (2), would be instructive not only in placing the two structures in perspective, but also in aiding those less initiated in such matters to appreciate the strides made by the artisans of chemical synthesis in the intervening half a century between the two campaigns toward their total synthesis. The two molecules strike one with amazing commonalities, not the least of which is the fact that their first X-ray crystallographic structures were reported by one and the same Dorothy Crowfoot Hodgkin of Oxford University, that of penicillin in 1945[5c] and thiostrepton in 1970[5a] (albeit the latter was missing its terminal dehydroalanine tail end residue). Thiostrepton and penicillin contain within their unique structures atoms of the same elements: carbon, hydrogen, oxygen, nitrogen and sulfur, but the former contains many more than the latter, as revealed by their respective formulas (thiostrepton: C72H85N19O18S5; penicillin G: C15H18N2O4S). Both contain amide bonds: thiostrepton has eleven, penicillin has two. Both possess impressive ring frameworks: thiostrepton is made out of ten rings, penicillin of only two (in addition to the phenyl ring attached to the end of the sidechain). Both contain a sole sulfur atom in each of the 5-membered rings within their structures (five in thiostrepton, one in penicillin G). A little imagination also allows the recognition of a subtle, but interesting equivalence between the β-lactam ring of penicillin and each of the dehydroalanine groupings of thiostrepton. Imagine joining the nitrogen atom of the dehydroalanine unit with the terminal carbon of its olefinic bond while concurrently transferring the nitrogen-bound hydrogen atom to the other carbon of the same double bond: a β-lactam is born! The intrigue does not end there, for both the β-lactam ring and the dehydroalanine moieties are reactive species, and both vulnerable to the aggressive action of nucleophiles.
The intriguing ring collection of thiostrepton includes a substituted pyridine system fused onto a cyclohexene ring in the form of a quinaldic acid moiety. In turn, the quinaldic acid moiety is part of a 27-membered ring along the periphery of which are situated (in addition to four peptide bonds) an ester linkage, a secondary amine functionality, and a thiazole ring. The same thiazole ring is part of a second tetrapeptide macrocycle, a 26- membered ring containing two additional thiazoles and one thiazoline moiety. Both macrocycles are tied onto a dehydropiperidine core in a fascinating knot that, at this point, may look so diabolical as if only Alexander’s sword can possibly cut or undo! Finally, the dehydropiperidine ring system carries a sidechain directly attached onto its imine functionality, and which extends, through a thiazole ring, into the bis-dehydroalanine chain alluded to above. Penicillin G by comparison is characterized by a mere thiazolidine ring fused onto a 4-membered ring (β-lactam). The β-lactam ring is, of course, not to be underestimated, either for its obstinacy to chemical synthesis or its enormous contributions made to humanity through its enchanting chemistry. It was John Sheehan who succeeded in developing, in 1957, the first total synthesis of penicillin V,[13] another prominent member of the β- lactam family of antibiotics. What were the prospects of synthesizing thiostrepton several decades later, at the dawn of the twenty-first century? As we pondered this question in 2000, and despite its imposing structure, the odds looked good. But, first, courage was needed to sail toward “Ithaca” into the seas of the unknown, and then perseverance to overcome, with body and mind, the obstacles that surely were awaiting us along the way. Here is how this “odyssey” was planned and traveled, not without frustration, to its final destination, with much learning, satisfaction, and joy.[14,15]
Total Synthesis of Thiostrepton: Retrosynthetic Analysis and Execution of the Synthesis
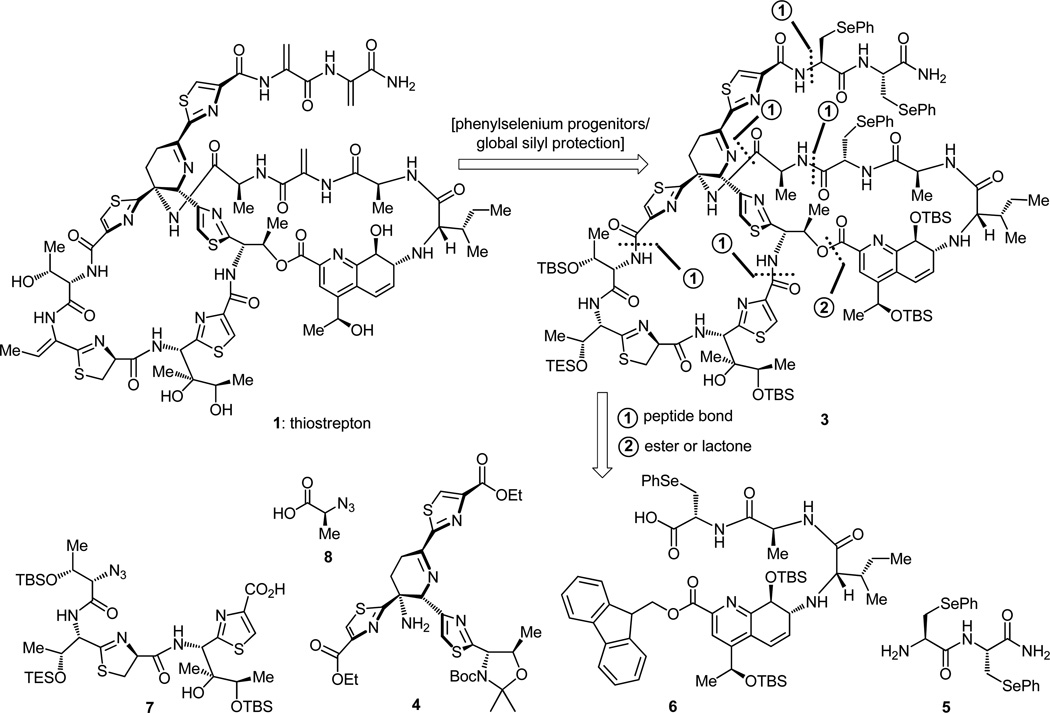
In contemplating a total synthesis of a complex molecular structure as that of thiostrepton (1), the prudent warrior would concern himself or herself not only with the first steps that would define the starting materials, but also with the last that would ensure the emergence and survival of the final target and the advanced intermediates leading up to it. It was with regard to the latter consideration that we decided, in our retrosynthetic analysis, to transform certain reactive functionalities of thiostrepton into more robust groups in order to safely sail them through the arduous journey that would lie ahead and, in a timely fashion, gently release them so as to produce the highly sensitive structure of the targeted molecule. Thus, the two dehydroalanine units constituting the longest side-chain of the molecule and the one embedded within its quinaldic acid-containing macrocycle were retrosynthetically converted to phenylselenyl moieties. It was to these moieties that we entrusted the eventual generation of the reactive dehydroalanine groupings, primarily because of the high propensity of selenium to oxidation as compared to that of sulfur and nitrogen, many atoms of which are present in thiostrepton. Such a daring hypothesis needed, of course, verification, for it would be costly to arrive a breath away from the target to find out that it was wrong. The verification came from model studies which are so often called upon in total synthesis to gather early intelligence, not always reliable but, nevertheless, often useful. The second potential source of trouble along the way was the Z-double bond of thiostrepton conjugated to the thiazoline ring, which confers enhanced reactivity to its vicinal functionalities. In a maneuver that had the same objective as that employed to protect the reactive dehydroalanine groups, this double bond was disguised behind a progenitor functionality, this time a TES-protected secondary alcohol. Taken together, these four retrosynthetic transformations allowed us to generate compound 3 (Figure 2) as a potential precursor to thiostrepton. All that was needed to reach 1 from 3 in the final stages of the projected synthesis was the selective oxidation of the selenium groups, an event that would set the stage for syn-eliminations of the anticipated selenoxides to cast all three dehydroalanine units in their place, and remove all silicon-based protecting groups. The challenge of this latter operation was to encourage the planted hydroxy group to depart in a geometrically correct fashion, that is to say anti, so as to generate the desired Z-olefin adjacent to the thiazoline ring. This hypothesis too had to be tested, and it was, with success, employing suitable model systems.
Figure 2.
Retrosynthetic analysis of thiostrepton (1).
Having settled on advanced intermediate 3 as a trusted progenitor of our target molecule (1), we were in the rather luxurious position to choose between several series of disconnections that would disassemble it into simpler building blocks for further consideration. From the many strategic bonds, and for reasons beyond the scope of this article, we focused on those marked by dotted lines in structure 3. These dissections generated, upon suitable protecting group manipulations, key building blocks 4 (representing the dehydropiperidine core region of the molecule), 5 (representing the bis-dehydroalanine tail), 6 (representing the quinaldic acid domain), 7 (representing the thiazoline-thiazole region), and 8 (representing the alanine residue attached to the hindered amino group of the dehydropiperidine ring). This latter “tiny building block” was to play a major role in overcoming a thorny problem that arose early on in the synthesis and one that we will shortly discuss. One should note at this point that parts of this final retrosynthetic analysis are based on hindsight; the original did not differ by much. While considerable simplification of thiostrepton (1) from a formidable target to an array of manageable fragments has been achieved by this “imaginary degradation”, two problems remained. First and foremost was the two-pronged question of how to join them together once secured and in what order, for these are the most pressing issues faced in endeavors of this sort. There are five peptide bonds to be constructed and one ester linkage to be formed for which there are 720 sequences from which to choose, and these are only the options offered by the one set of disconnections applied (there were many more of those to consider as alluded to above). The reasons for the specific sequence used for the final assembly are beyond the space intended for this occasion, leaving only a brief outline of the sequence itself as the only option, a commentary to which we shall return shortly. The second challenge, albeit lesser in magnitude than the one mentioned above, is the construction of the defined key building blocks (4–8), of which only 4, 6, and 7 are serious enough to warrant our special attention. And of these three, we will only discuss here in some detail the dehydropiperidine fragment 4, for its construction exemplifies many of the aspects of the art and science of total synthesis. Indeed, within its architecture one can find many of the secrets of beauty and logic of synthesis: how the architecture of a molecule fascinates and lures the practitioner into falling in love with it; how the practitioner goes about designing a strategy for the molecule’s conquest; how the molecule tantalizes the practitioner and leads him or her into dead-ends and nightmarish pitfalls from which an escape has to be planned with rationale and patience; how the practitioner faces the drama of defeat and how he or she must regroup with renewed vigor and stamina to think and design a new strategy for the next assault; and how, in the end, he or she prevails and shares the joy and exhilaration with others… only to start all over again with a new challenge, often greater than the one bygone.
The dehydropiperidine moiety within subtarget 4 is clearly the heart of the structure of thiostrepton. You conquer that, and the molecule is yours, or at least so it seems. It is also its façade, where most of its architectural beauty resides. It is highly sensitive, for the imine functionality is well known for its propensity to hydrolyze; and yet, nature provided for its protection and survival. The three thiazole substituents are its royal guards against external forces, while its primary amino group is its potent enemy from within – unless quickly neutralized as (perhaps) an amide, an eventuality that has to materialize sooner or later if we hope to make thiostrepton from it. These descriptions will soon become apparent, but first let us review the literature of the prior art with relevance to the problem at hand, a prerequisite for planning a strategy toward any complex synthetic target.
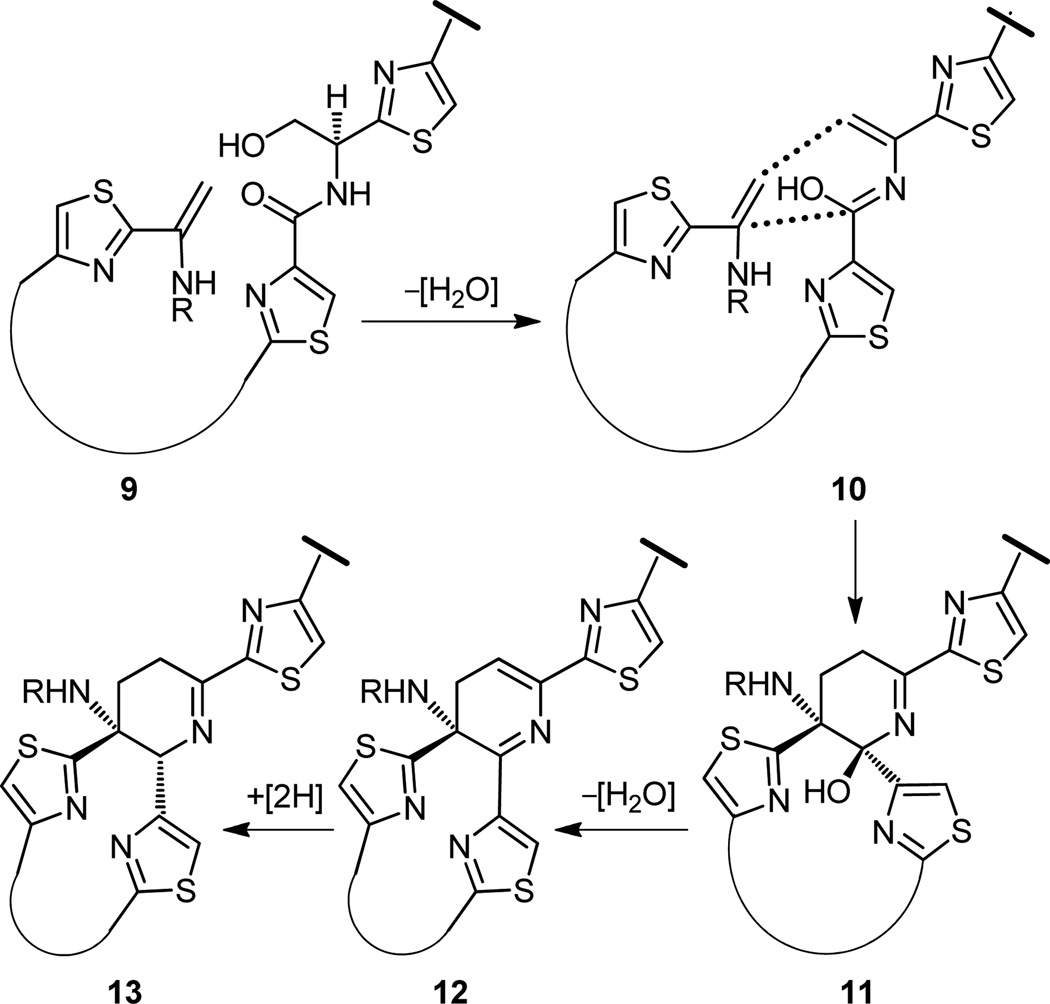
Within the realm of natural products chemistry, biosynthesis is a branch that so elegantly provides glimpses of nature’s magical ways of constructing its own molecules. Luckily, in this instance, studies carried out by Floss and his collaborators[16] shined considerable light, some by experiment, some by insightful speculation, that would be of considerable interest to us as synthetic chemists. If we could mimic nature in the laboratory to synthesize thiostrepton’s dehydropiperidine core 4, we would. Mimicry of a superior artisan—and nature is the supreme master of the art of synthesis—is not to be shamed, especially if we recognize the importance of developing reagents and strategies that may exhibit comparable powers to those of enzymes, a long standing dream of practitioners of chemical synthesis. Floss and coworkers,[16] and Bycroft and Gowland before them,[17] proposed the chemistry shown in Figure 3 as nature’s way to the dehydropiperidine system of thiostrepton. According to this mechanism, and within the enzymatic machinery of the producing organism, a highly unsaturated intermediate (i.e. 10, Figure 3) is swiftly formed from an assembled peptide (9) that cyclizes to a hydroxydehydropiperidine intermediate (11) (through an intramolecular Diels–Alder reaction) from which a molecule of water is lost through a 1,4-elimination reaction. The resulting piperidine ring system (12) then undergoes a stereoselective 1,4- reduction in which two hydrogen atoms are delivered from opposite sides of the acceptor diene, resulting in the generation of the dehydropiperidine core (13) with the two thiazoles oriented in space trans with respect to each other. That these reactions exhibit the exquisite stereoselectivity that they do, or indeed the fact that they proceed at all, is a tribute to the awesome power of enzyme chemistry.
Figure 3.
Proposal for the biosynthesis of the dehydropiperidine domain of thiostrepton (1).[16,17]
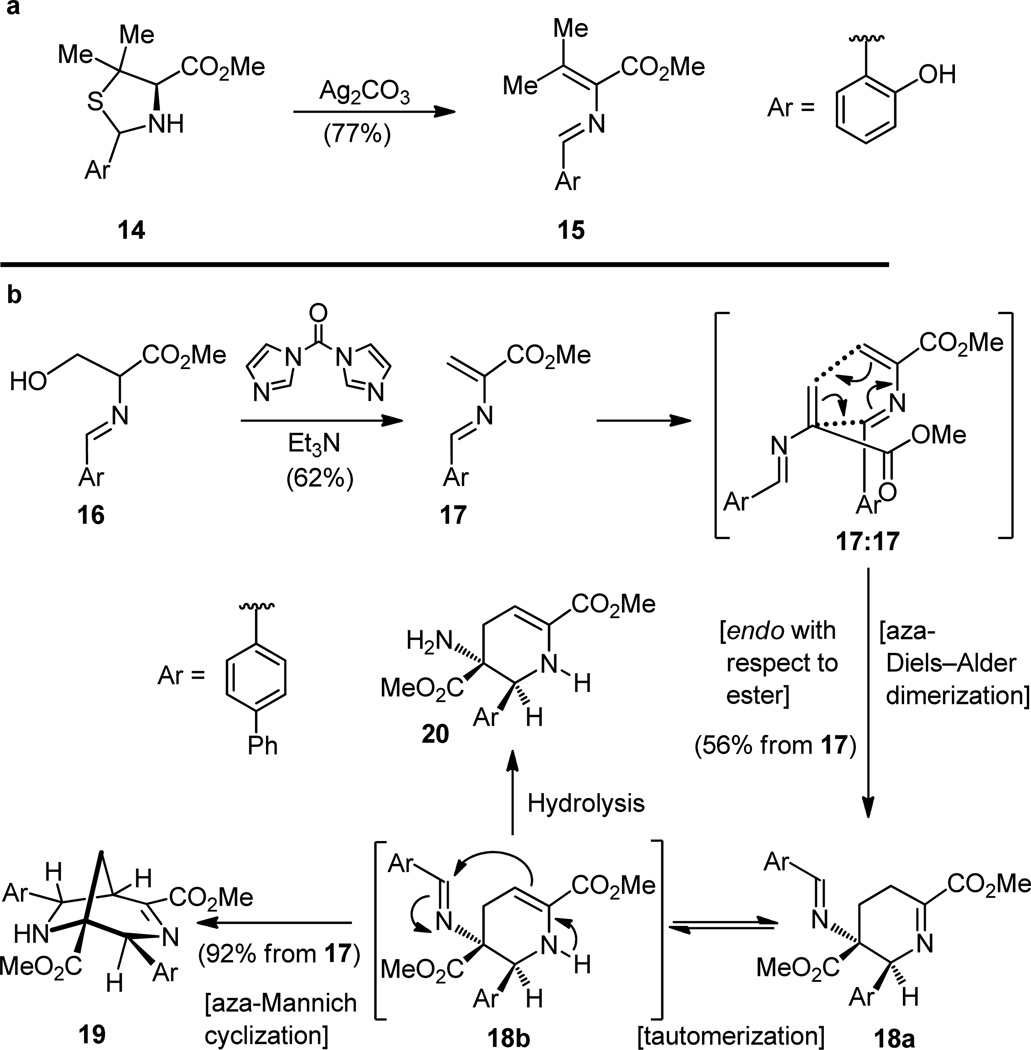
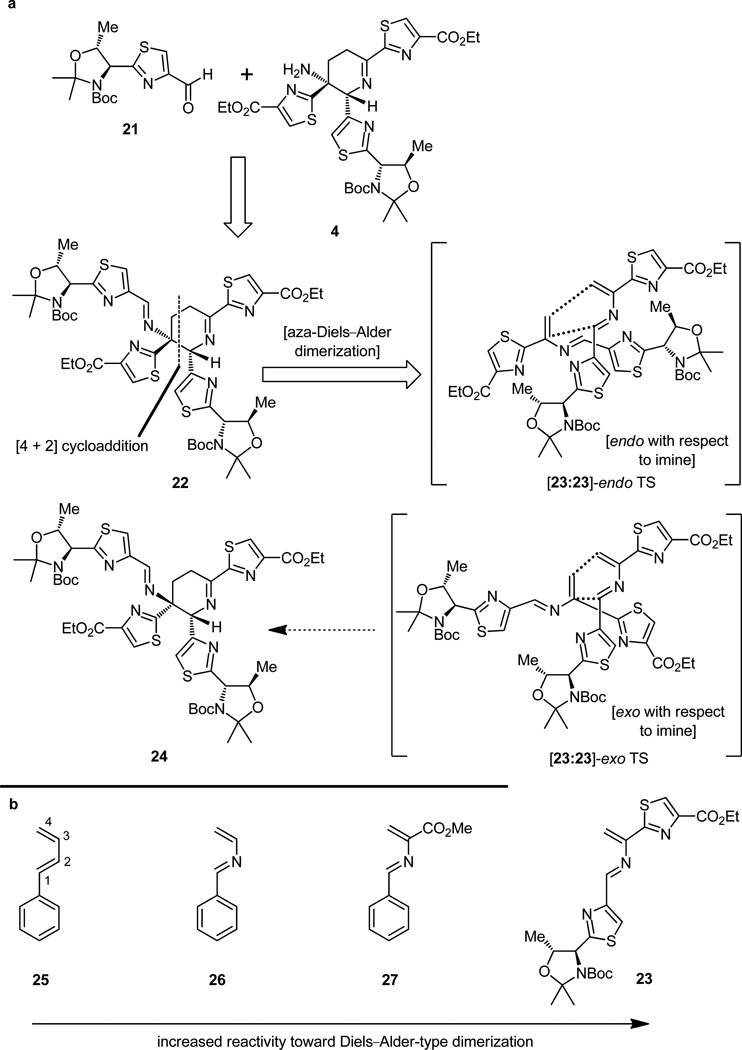
Whether the same outcome can be accomplished in the laboratory with man-made reagents and conceived designs is another matter. A dim glimmer of hope for such a process existed in the chemical literature. Schmidt and associates[18] had shown, in the 1970s, that azadienes can be generated from thiazolidines by formally removing the elements of hydrogen sulfide with silver carbonate (14 → 15, Figure 4a), and Wulff and coworkers[19] subsequently demonstrated that these transient species react with themselves to form cyclic compounds reminiscent of our dehydropiperidine system. The case shown in Figure 4b is illustrative. But whereas the bicyclic Mannich-type product 19 was formed starting from iminoalcohol 16 in excellent yield (through endo transition state 17:17 and transient intermediates 18a and 18b), the monocyclic dehydropiperidine product (20) was observed only in very low yield (through 18 and 19). There were other problems too. The double bond was in the enamine (see structure 20), and not the desired imine position, and the two substituents on the formed ring (corresponding to the thiazole rings of the natural dehydropiperidine system) were cis to each other (ester/phenyl groups, see structure 20), rather than trans as desired for our purposes. In other words, the state of affairs in this area was a long way from the ideal situation for our comfort. Nevertheless, this observation together with the biosynthetic considerations alluded to above were of great inspirational value and, upon taking note of them, we committed ourselves to the biomimetic strategy idea toward the dehydropiperidine core of thiostrepton as depicted in Figure 5a. This one idea would shape the character and set the style of our total synthesis, as is often the case in such campaigns. The idea may seem ingenious but it is not truly original in the absolute sense. It was simply the assimilation of what others have thought and done before, now recast in a new light to address the problem before us. Risky as it was, however, it needed to be adapted, improved, and modified. This is, in fact, how most of the progress in science is made–with only occasional true genius and original ideas like those associated with Archimedes, Newton, and Einstein, among a selected few. Our recast idea is shown in Figure 5a. In a maneuver that appears to proceed counter to the concept of retrosynthetic analysis, we temporarily borrowed a molecule of aldehyde 21 and combined it with the desired amine 4 to form, on paper and without need for experimental verification, now or ever, the more complex bis-imine 22. In contrast to the above retrosynthetic transformation, the conversion of 22 to 4 had to be a viable laboratory procedure and, therefore, should be of concern to us, for we will have to actually carry it out at some point later on in the synthetic sequence. Considering the more exposed nature of the exocyclic imine as opposed to the shielded endocyclic imine, we reasoned that the functionality we wished to degrade would preferentially collapse under appropriately mild conditions to afford the desired product (i.e. 4). This is the essence of our retrosynthetic analysis. Provided that the crucial cyclization would proceed in the required endo mode (i.e. 23:23-endo TS, and not the exo mode 23:23-exo TS that would lead to the undesired cis diastereoisomer 24), if assumed to be a concerted [4 + 2] cycloaddition reaction, then it would give the desired product [if only it could be liberated from its suicidal form (22), the external imine]. And that rescue mission had to be accomplished before the latter compound (22) had the chance to transform itself into the Mannich-type bicyclic system, an anathema for us, concerned as we were, with the total synthesis of thiostrepton. It was with these hopes that we embarked on our journey to explore the reactivity of these systems. Theoretical studies were encouraging, for they had shown a favorable reactivity trend toward Diels–Alder type dimerizations within the diene series 25–27 (Figure 5b, vide infra for further discussion on this issue).
Figure 4.
Literature precedent for the generation of azadienes from thiazolidines (a)[18] and dimerization of azadienes (b).[19]
Figure 5.
Retrosynthetic analysis of dehydropiperidine system 4 (a) and reactivity trends of dienes 25–27, 23 toward Diels–Alder type dimerization (b).
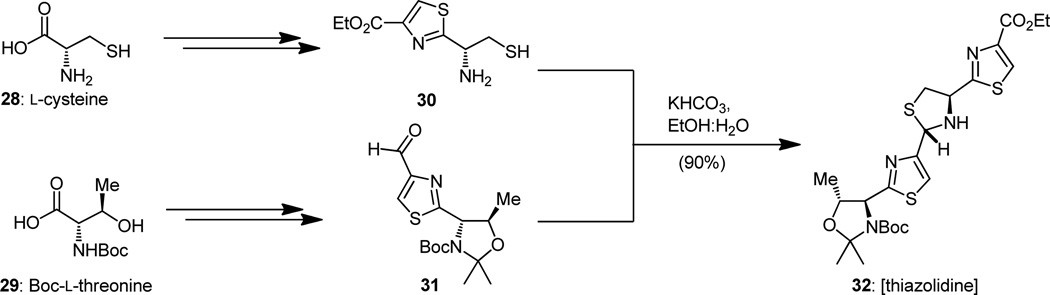
Returning now to the dehydropiperidine core of thiostrepton and employing our newly sharpened retrosynthetic sword, we can apply an imaginary scission at the indicated bonds of the molecule, effectively slicing it into two identical halves (see structures 22 and 23:23, Figure 5a). The so-obtained unit (23) is nothing more than a sophisticated version of Wulff’s azadiene system (i.e 17, Figure 4b),[19] but no less than an appropriate substrate for the potential generation of our subtarget molecule. In fact, it is more suited to become a progenitor to the dehydropiperidine ring system than the postulated biosynthetic intermediate (i.e. 10, Figure 3), for it lacks the apparently superfluous hydroxyl group. But, will it react, if indeed it can be prepared, in the required manner to deliver the correct trans bis-thiazole imine product (i.e. 22, Figure 5a); and will the latter behave in a manner conducive to its isolation and further manipulation, thereby violating the precedent set forth by its earlier sibling cited above? These were the issues to be resolved as we entered into the laboratory from the drawing board excited about the prospects of success, but aware of the dangers and surprises that may lie ahead, seen only under the dim light of speculation and fog of hope. There were some good reasons to be encouraged. First we must explain why 2-azadienes tend to dimerize in the way they do as exemplified by the cited case in Figure 4.[19] In a typical diene such as 25 (Figure 5b) there exists a relatively large LUMO-HOMO energy gap which is responsible for its inertness toward itself. Upon placement of a nitrogen atom within the diene system at the 2-position (diene 26, Figure 5b), both the HOMO and LUMO energy levels are lowered, a downfall that is further exacerbated by the placement of an electron-withdrawing group such as an ester at the 3-position (system 27).[20] However, placement of an aromatic moiety at the 1-position, as in both 27 and 23 (Figure 5b), raises the HOMO energy level, producing a uniquely small HOMO-LUMO energy gap, thereby making the diene capable of reacting with itself in a formal Diels–Alder reaction. That the reaction of 23 will proceed in the desired direction as depicted in the endo (with respect to the imino group) transition state (23:23-endo TS) in Figure 5a, a requirement for the formation of the desired 1,2-trans (thiazole/thiazole) adduct 22 rather than follow the opposite mechanism (as it did in the case of 17 ,Figure 4b), was a point of contention. The prevailing argument, whose origin admittedly was based perhaps more on wishful thinking than on mathematics, was that in the case of 23 the imino group of the dienophile would take precedent over the aromatic moiety in directing the system toward the normal endo arrangement, as opposed to the case of 17 where the ester group apparently wins over the aromatic moiety in the battle for stereochemical control. Should our azadiene (23) opt to take the opposite course (i.e. 23:23-exo TS → 24, Figure 5a) in its dimerization to that wished, our proposition would turn into a painful disappointment. There were also good reasons to choose Lcysteine (28) and Boc-L-threonine (29) as the starting points for our guest of the amino thiol 30 and aldehyde 31, materials that were needed for the construction of compound 32 (Figure 6), the designed precursor of azadiene 23 (see Figure 5a). Following a swift combination of known reactions and new procedures we soon arrived at our first destinations with large and expediently renewable supplies of intermediates 30 and 31.[14a,c, 15b] Coaxing them to combine into thiazolidine 32 (Figure 6, ca. 1:1 mixture of two diastereomers) was no major challenge, as this reaction was easily effected under the influence of potassium hydrogen carbonate in aqueous ethanol in high yield.[14a,c,15b]
Figure 6.
Synthesis of azadiene precursor thiazolidine 32.
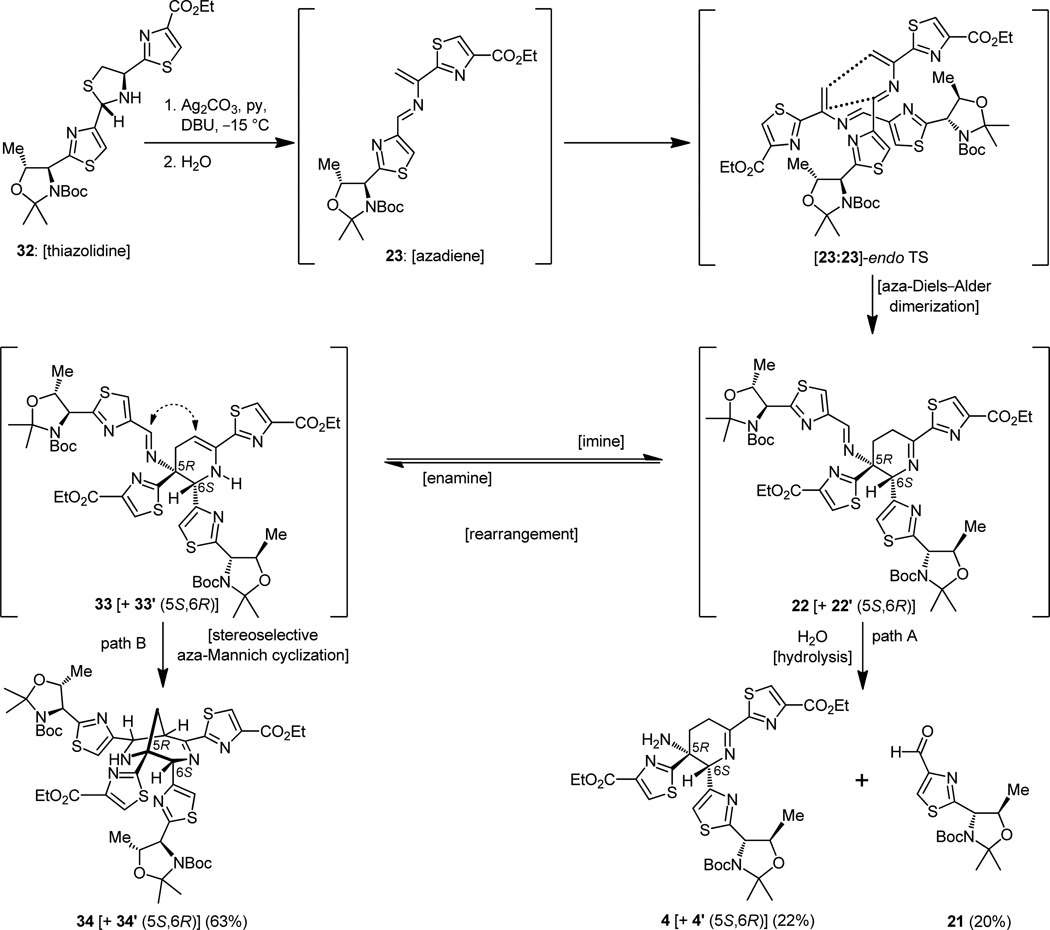
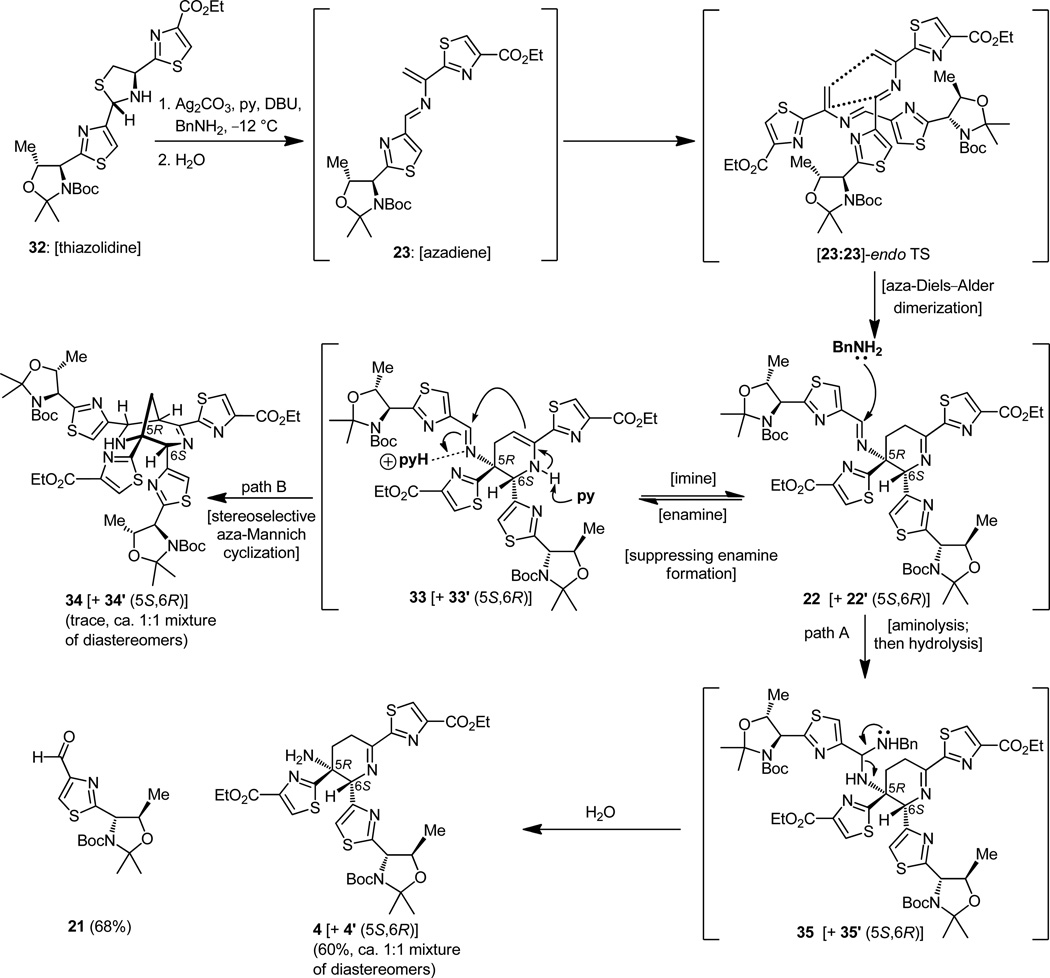
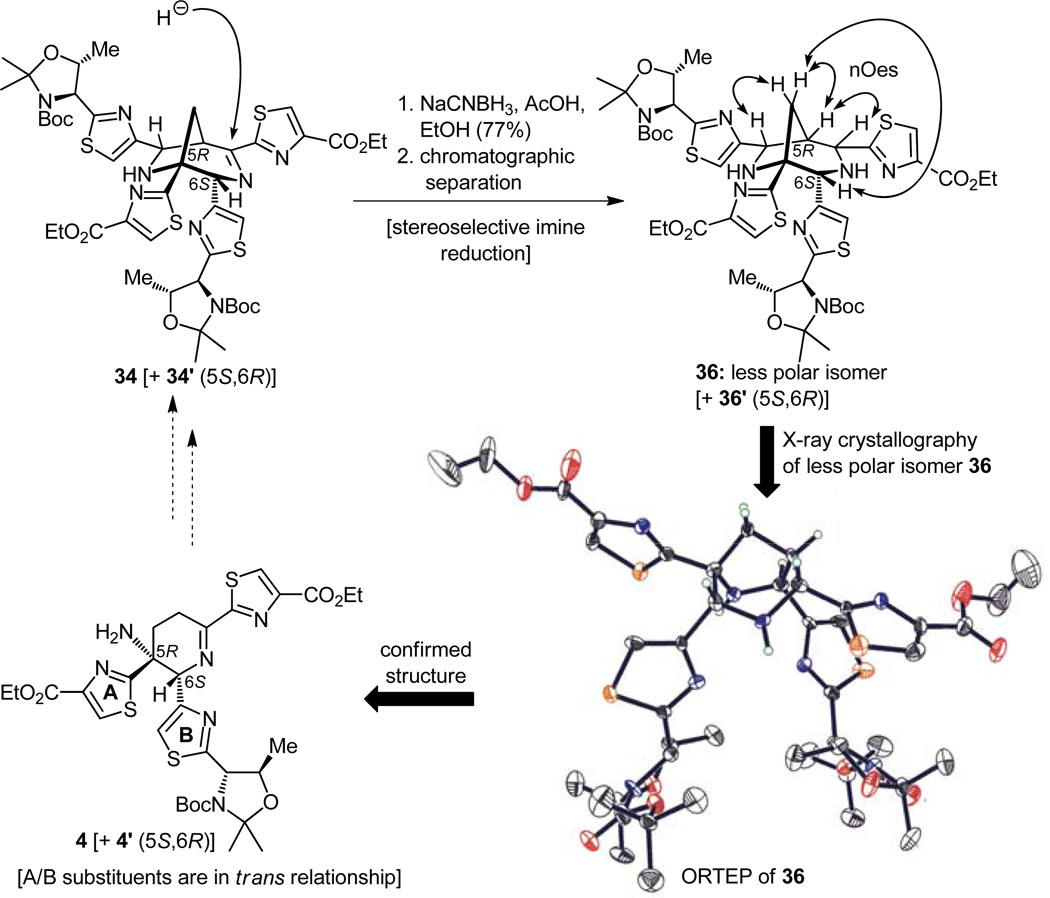
Recognizing the significance of the moment and with considerable trepidation, we then proceeded to treat thiazolidine 32 with silver carbonate and DBU in pyridine, conditions that were expected to decompose it to the much coveted azadiene (23) and hopefully allow isolation of dimeric materials arising from this reactive intermediate (Figure 7). Indeed, under the prescribed conditions, three compounds were isolated from the reaction mixture. Spectroscopic analysis revealed that they were, in order of preponderance, the bridged Mannich-type polycycle 34 [+ 34′ (5S,6R diastereomer ca. 1:1 dr), 63% yield], the expected imine product 4 [+ 4′ (5S,6R diastereomer ca. 1:1 dr), 22% yield], and thiazolyl aldehyde 21 (20% yield). The stereochemical identities of the two diastereomers remained unknown at the time. This result was encouraging but by no means perfect or completely understood. To be sure, it was exhilarating that we did observe some of the desired product with the imine functionality within the central ring, although the tentatively assigned stereochemical relationship of the two adjacent thiazoles was not apparent at this stage due to the absence of clear spectroscopic evidence. This ambiguity had to be resolved and, furthermore, the formation of the useless by-product had to be suppressed. With an eye on resolving the latter issue first, and borrowing from the work of Wulff,[19] we carried out the same experiment, but now in the presence of excess benzylamine, an agent that was meant to rescue the imine product before it had the chance to rearrange, opening the path to its destruction through the incipient enamine (see 33 → 34, Figure 8). The outcome was delightful, for not only was the formation of the Mannich-type products (34 + 34′) suppressed to a minimum, but more importantly the desired dehydropiperidine compounds (4 + 4′) were isolated in 60% yield.[14a,c] The mechanistic underpinnings of these transformations are shown in Figure 8 (see structures 33 and 35).
Figure 7.
Generation of azadiene 23 and initial results of its Diels–Alder dimerization.
Figure 8.
Optimization of the Diels–Alder dimerization of azadiene 23 toward the desired dehydropiperidine core and mechanistic considerations.
Having secured a viable route to 4 + 4′ (5S,6R), we then turned our attention to the determination of their stereochemistry. The postulated Diels–Alder cycloaddition-dimerization of the azadiene monomeric unit (23) could, in principle, lead to four diastereomers, two arising from the endo/exo modes of reaction, and two deriving from the alternative facial, top/bottom orientation of one azadiene over the other; this, of course, was assuming only one regiochemical arrangement in the transition state. As we have seen, in the event only two isomers were formed, most likely the two corresponding to the two top/bottom orientation options since there was no compelling reason for the two reactive molecules to behave otherwise (their stereocenters are too far away from the action site to exert any influence). Fortunate as this specific pathway was, the literature precedent cited above[19] of the exclusive exo mode of reaction leading to the dreaded cis disubstituted arrangement (see Figure 4b) painted a gloomy picture, for if that was to be the case here the project would have come to an abrupt halt, a devastating dead-end but not uncommon in endeavors of total synthesis. Returning now to the issue of stereochemistry, the structure of dehydropiperidine 4 [+ 4′ (5S,6R)] was far from ideal as an object of NMR spectroscopic investigation for a number of reasons, the least of which was the relative scarcity of protons directly attached to the ring of interest. In view of this predicament and in the absence of crystallinity, we resorted to a more classical approach of converting the compound in question, or one related to it, to a derivative whose properties, we hoped, might improve the chances of absolute structural proof. To this end, compound 34 [and its diastereomer 34′ (5S,6R)] was treated with sodium cyanoborohydride in ethanol in the presence of acetic acid with the intention of reducing its imino group, an expectation that was not only reasonable but also one that had close precedent in the work of Hashimoto and his group.[21] Thus, the chromatographically inseparable mixture of imine 34 and its diastereomer 34′ (5S,6R) were converted to their corresponding amines under the aforementioned conditions. Pleasantly, these derivatives proved separable by chromatography, an event that led to pure samples of the two diastereomers. With the addition of two extra protons and the conformational change that accompanied this reduction, the less polar isomer so-obtained (i.e. amine 36, Figure 9) yielded to NMR spectroscopic analysis (see nOe’s indicated on structure 36, Figure 9), revealing its stereochemical identity. Even more pleasing was the successful crystallization of compound 36 in aesthetically pleasing crystals, whose X-ray crystallographic analysis confirmed beyond doubt its structure (see ORTEP in Figure 9). Tracing back the origin of this intermediate (36) and considering its genealogical relationship to our coveted subtarget 4 (see Figures 8 and 9), the seemingly logical conclusion that the latter compound possessed the desired stereochemistry was drawn. The X-ray structure of 36 had also established, in addition to the trans relationship of the thiazole rings within 4 and 4′, their relative stereochemistry with respect to the remote asymmetric centers appended onto the chain extending beyond one of them; therefore, we now knew that the desired dehydropiperidine system was in the mixture of the two diastereoisomers we had obtained through the Diels–Alder reaction. The twins (i.e. 4 + 4′), however, would not separate until further elaboration.
Figure 9.
Confirmation of relative stereochemistry of the dehydropiperidine core 4 by NMR spectroscopy and X-ray crystallographic analysis of bicyclic derivative 36.
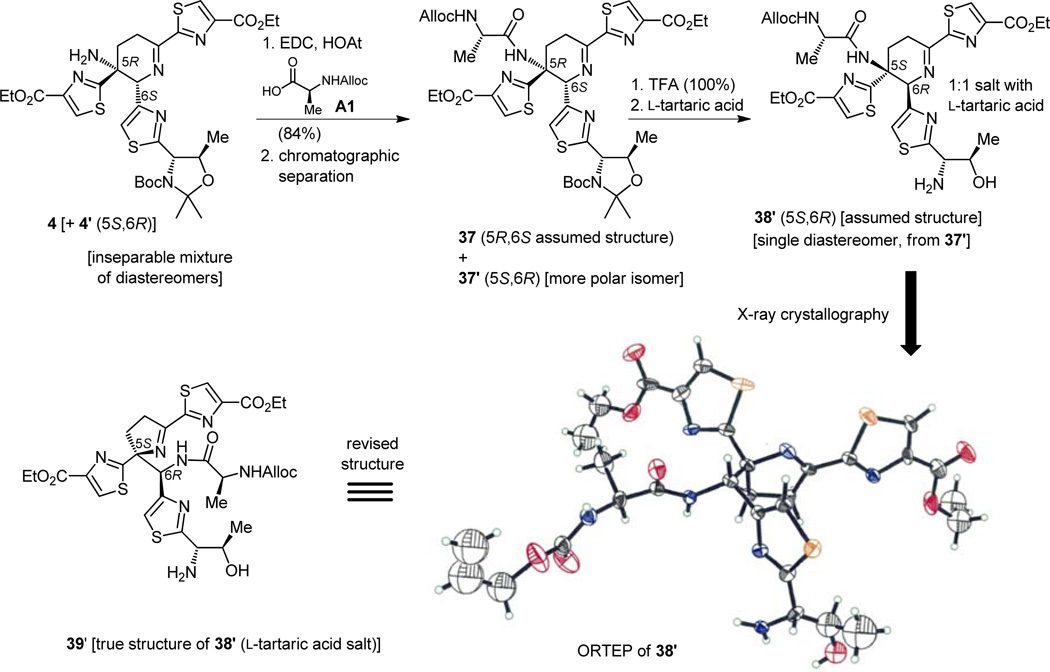
With this stereochemical knowledge boosting our confidence, we surged ahead with the diastereomeric mixture represented by structures 4 + 4′. Our plan called for a peptide coupling that would engage the amino group with an incoming amino acid derivative as a means of branching out from the crowded dehydropiperidine ring system and onto the thiostrepton chain extending from one of its corners. Despite its hindered nature, the amino group in the spotlight reacted with the N-Alloc alanine derivative A1 (Figure 10) in the presence of EDC and HOAt—two superb reagents whose combination proved to be a much superior peptide coupling reagent than that developed and used by Sheehan in his original penicillin synthesis [N,N′-dicyclohexylcarbodiimide (DCC)][13]—to afford dipeptide 37. At this stage we were rewarded with the extra bonus of being able to separate, chromatographically and for the first time, the two diastereomeric forms of our mainstream dehydropiperidine intermediates. A second reward would come in the form of crystals of a subsequent intermediate derived from one of them, namely 1:1 L-tartaric acid salt of the amino alcohol obtained by exposure of the more polar isomer 37′ (5S,6R) to trifluoroacidic acid (TFA). The latter fell out of an ethyl acetate solution containing L-tartaric acid after standing for one month! There was no delay in obtaining the X-ray structure of one of these crystals, an exercise that was destined to reveal a major surprise, one that would bring our excitement and momentum to a sudden halt and send us into a mental spin. Lying bare in front of our eyes was the structure of, not a dehydropiperidine molecule as we assumed all along, but that of a dehydropyrrolidine (see ORTEP and structure 38′, Figure 10)![14a,c] Such unexpected perils are not uncommon in total synthesis, and they are not always unwelcome, for sometimes they are blessings in disguise and can turn into major discoveries. This time, however, the pitfall was not welcome, and a way out had to be found. For that to occur we needed to understand the causes of the nuisance, having sensed its crippling consequences.
Figure 10.
Preparation and X-ray crystallographic analysis of presumed dihydropiperidine core structure 38′ and revision of its structure to 39′.
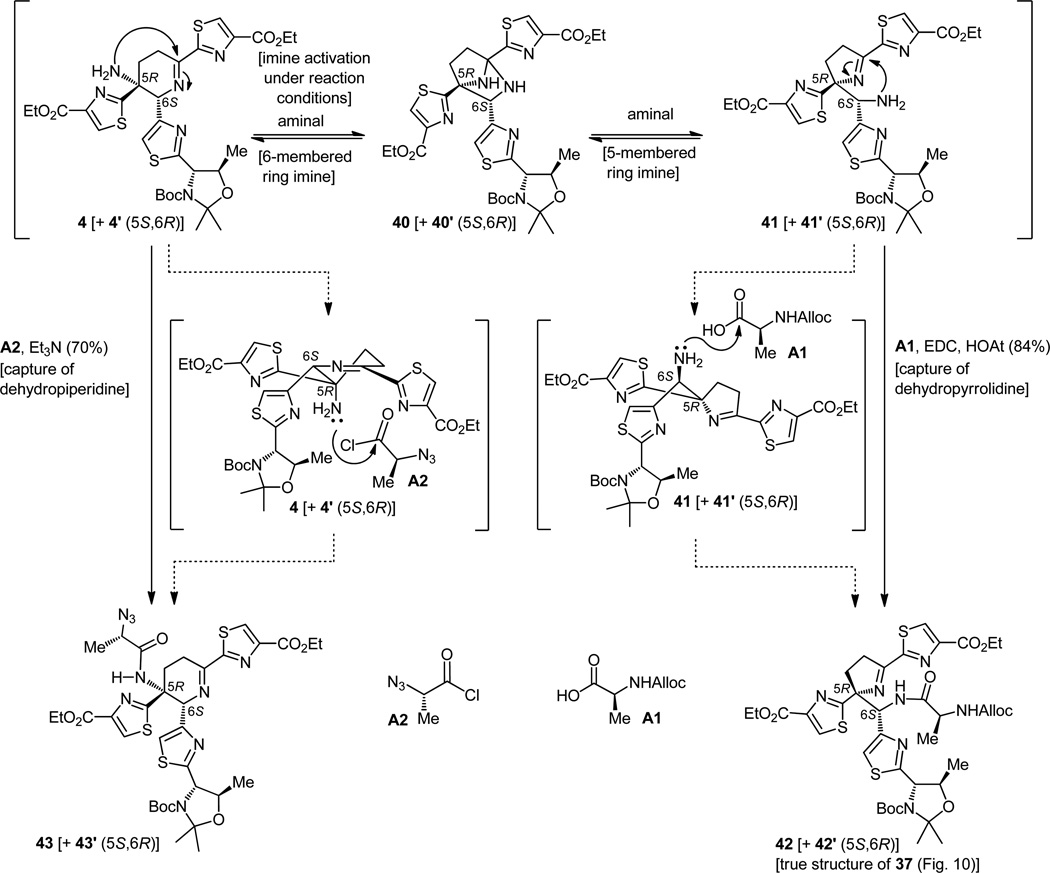
Our postulated explanation of what happened is depicted graphically in Figure 11. Apparently, once liberated from its initial imine partner, the primary amino group within the dehydropiperidine structure 4 (+ 4′) finds another partner, this time within its own hostel, the imino group with which, upon appropriate activation, it forms an internal aminal [40 (+ 40′)]. This rearrangement of connectivity may continue, always in a reversible manner, to the dehydropyrrolidine structure 41 (+ 41′) which now exposes a different primary amino group, one that, being less hindered than the original, reacts preferentially with the alanine derivative A1 in the peptide coupling reaction to afford the observed dipeptide 42 (+ 42′) containing the 5-membered imine ring. The equilibrium, which apparently lies to the side of the dehydropiperidine structure as revealed by 1H NMR spectroscopy, is driven to the side of the dehydropyrrolidine by the siphoning of the latter into the observed and only viable product [i.e. 42 (+ 42′)]. How did we, you may ask, miss this rearrangement in the earlier stages of our investigation? In retrospect, two assumptions, both based on incomplete information, led us to the acceptance of the dehydropiperidine structure that we so much desired. The first was the coupling constant observed for the 1H NMR signal corresponding to H-6 (J = 9.6 Hz) in compound 42 (Figure 11) that we mistakenly attributed to its long range W-type interaction with the N-H proton, four bonds away (see structure 43, Figure 11). It was, in reality, a coupling of this proton (H-6) with the N-H proton three bonds away within structure 42 (this would explain its rather large value of 9.6 Hz). The second unfortunate mistake we committed was hastening to relate the X-ray structure of compound 36 (Figure 9) derived from the Mannich-type product 34 with that of the alanine-coupled system 42 (Figure 11) without further questioning and experimentation.
Figure 11.
Mechanistic explanation for the dehydropiperidine ring contraction (4 → 41 → 42) and solution to the problem through the use of azido alanine equivalent A2 to afford 43.
Leaving behind the realm of explanations, speculative as some were, we needed to move beyond what happened and to the domain of such new thinking that would lead us out of the predicament we found ourselves. Given the equilibrium that we postulated between the two imines via their common relative [aminal 40 (+ 40′), Figure 11] and our contention that it was the bulky nature of the coupling partner [the N-Alloc derivative of L-alanine (A1)] that was responsible for shifting the equilibrium toward the direction of the dehydropyrrolidine by its preference for it, we reasoned that a smaller alanine equivalent might lose this propensity, especially if it was quick to capture the initially abundant dehydropiperidine form 4 (+ 4′) of our substrate. The azido acyl chloride alanine precursor A2 (Figure 11) seemed to fulfill these requirements. More importantly, it performed admirably well in its intended role, combining with dehydropiperidine 4 (+ 4′) in the presence of triethylamine to form dipeptide azide 43 (+ 43′) in good yield. There might be an alternate and more sophisticated explanation for the observed difference between the azide chloride (A2) and the N-Alloc alanine (A1) equivalents in their reaction with the dehydropiperidine 4 that could be advanced; the one just noted above and used in our working hypothesis to break the dead lock is perhaps the most simplistic, and sufficed for our purposes (see transition states in brackets, Figure 11).
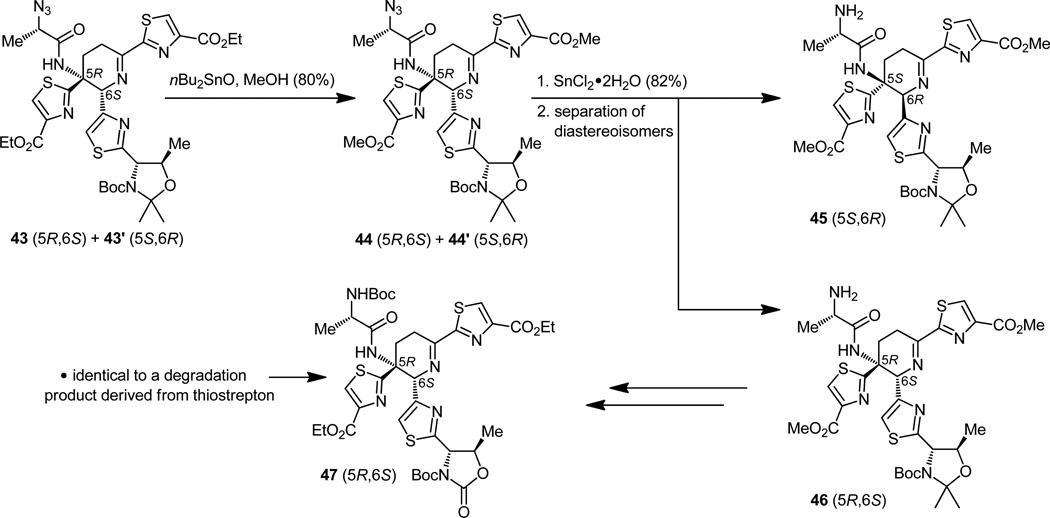
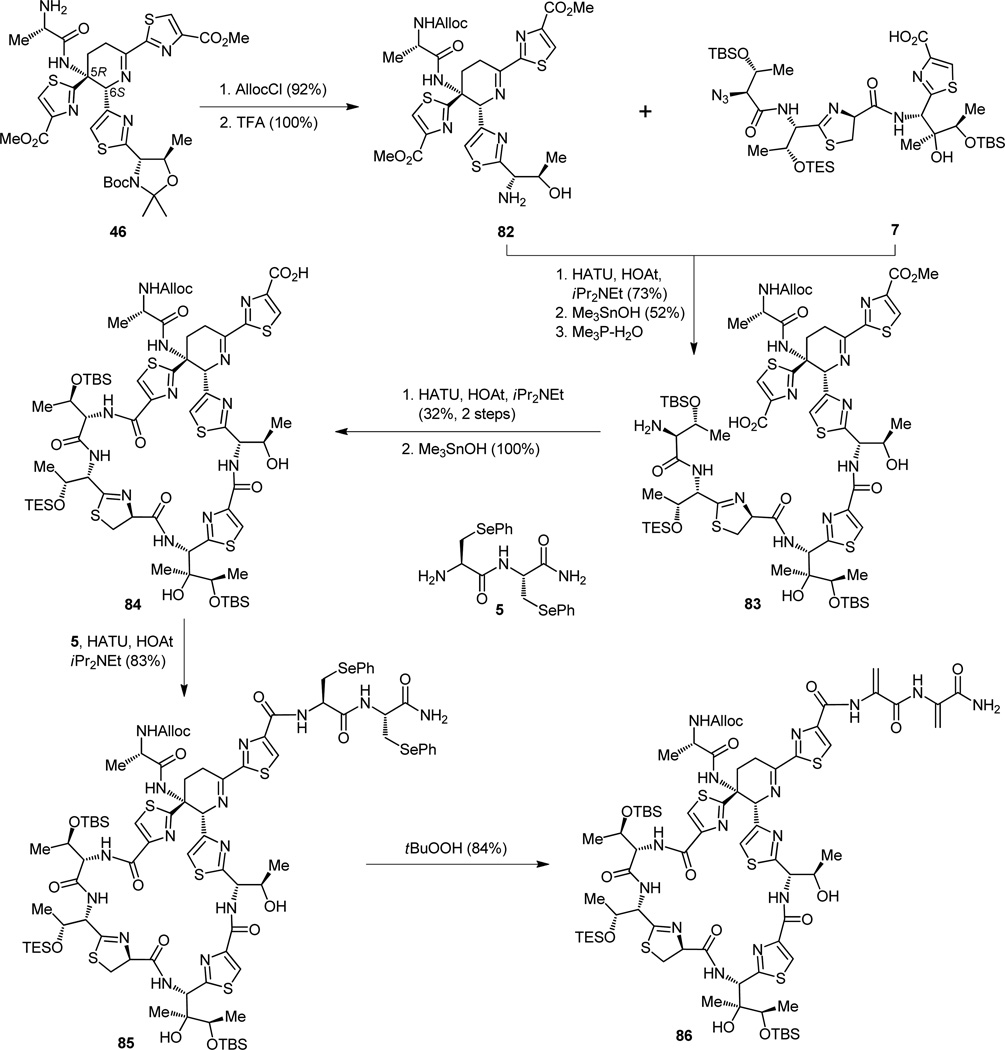
The next step in the synthesis was dictated by a need for a mild procedure that would be needed later to liberate the carboxylic acid groups within the growing thiostrepton-bound molecule under the mildest of conditions such that no epimerization of any of its stereocenters or destruction of its structure would occur. Surely the methyl group would be a more suitable candidate to guard the carboxylate moieties for the time being, for although robust enough its expulsion at the right moment would be easier than that of the more resistant ethyl group. It was, therefore, both prudent and timely to replace the ethyl groups of the two ester functionalities inherited from its simple ancestors, a choice solely made on the basis of their commercial availability. To this end, the diester 43 (+43′) was refluxed in methanol in the presence of di-n-butyltin oxide[22] to afford the desired dimethyl ester (44, Figure 12) through a simple exchange reaction, at this stage still a binary mixture with its diastereomer 44′, for it was not possible, up to now, to separate the two diastereomers in a convenient manner. This accomplishment would have to wait until the next step, when employment of tin dichloride dihydrate reduced the azido group to a primary amine. The resulting compounds (45 and 46), just like their five-membered ring relatives before them (Figure 10), exhibited sufficiently different chromatographic properties to allow their separation. In order to establish the stereochemical identities of the newly separated diastereomeric imines, compound 46 (less polar isomer) was then advanced to an intermediate identical to a previously reported[21] degradation product of thiostrepton (i.e. 47), thereby confirming its 5R,6S configuration. Thus, the structural platform containing the “heart” of thiostrepton was constructed. What remained was to attach the rest of the appendages and frameworks demanded by the entire architecture of the target molecule, a challenge not to be dismissed as trivial by any means. For the sake of expedience our description of the construction of the other fragments will be brief, focusing only on some selected biomimetic and asymmetric aspects of the synthetic chemistry involved, both of which are demonstrative of the impressive strides made in these two areas in the last few decades.
Figure 12.
Separation of the dehydropiperidine diastereomers and identification of the desired 5R,6S isomer (46) through comparison of a derivative to a thiostrepton degradation product (47).
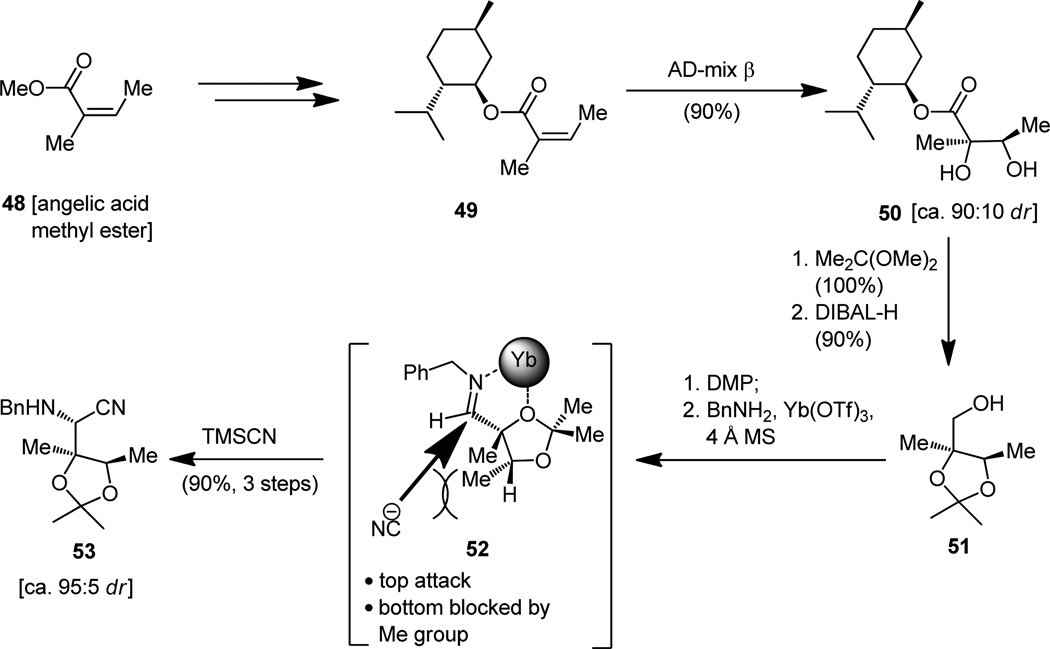
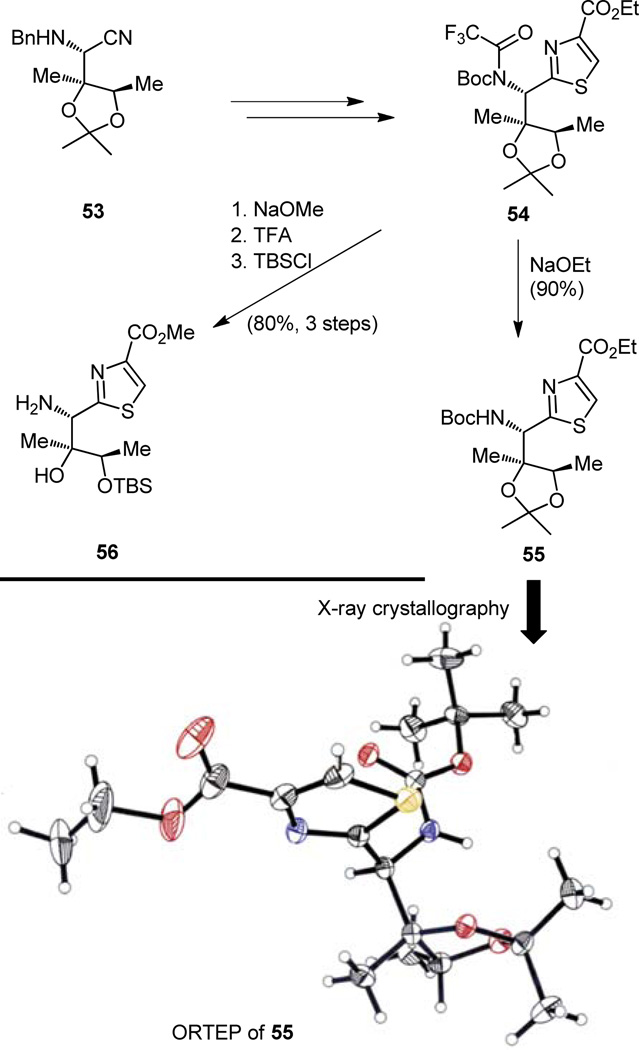
The enantioselective synthesis of building block 53[14a,c] (destined to become part of the required thiazoline-thiazole fragment 7, Figure 2) depicted in Figure 13 is a vivid demonstration of the power of the sharp tools of contemporary synthetic chemical technology. While the three-step conversion of the prochiral and commercially available angelic acid methyl ester (48) to its menthol counterpart (49) was conventional, the reaction of the latter with AD-mix β was impressive both for its high chemical yield and its exceptional diastereoselectivity (ca. 90:10),[23] affording diol 50 in ample quantities for further elaboration. This pleasing result is, no doubt, a consequence of the cooperative matching of the two chiral groups (the menthol moiety and the chiral ligand contained within the ADmix-β) that must be communicating during this asymmetric dihydroxylation process. The two steps that followed, and which produced the hydroxy acetonide 51 from dihydroxy ester 50, were also of a well-known variety, as if a pause was needed before another breathtaking sequence would follow. It was the three-step, one-pot procedure that converted primary alcohol 51 to the benzylamine nitrile 53 in which one more asymmetric center was introduced with exquisite stereochemical control that caused the next excitement. In this sequence, the primary alcohol is oxidized to the corresponding aldehyde and thence converted to the benzyl imine, a reactive species onto which the cyanide moiety was planted, more or less, exclusively and in high overall yield by attack from the top less-hindered face of the molecule (see 52, Figure 13, ca. 95:5 ratio of diastereomers), the delivery system being none other than the distinguished trimethylsilyl derivative of hydrogen cyanide (TMSCN). That the stereochemistry of 53 was of the desired disposition as depicted, and in order to prove that its usefulness extended to the preparation of the more advanced desired intermediate 56, the transformations depicted in Figure 14 (53 → 54 → 56) were experimentally executed as originally planned, spare for the X-ray structural analysis (see ORTEP of 55, Figure 14) that was performed when it was merely because the opportunity for a crystalline derivative arose at that juncture. Such pleasant occasions cannot but rarely be planned, in contrast to downstream synthetic transformations which can, these days, be predicted even in impressively multistep synthetic sequences, a tribute to the astonishing advances in strategy design that have been made in recent years.
Figure 13.
Construction of benzylamine nitrile 53.
Figure 14.
Construction of amino alcohol 56 and ORTEP of compound 55 confirming the stereochemistry of 56.
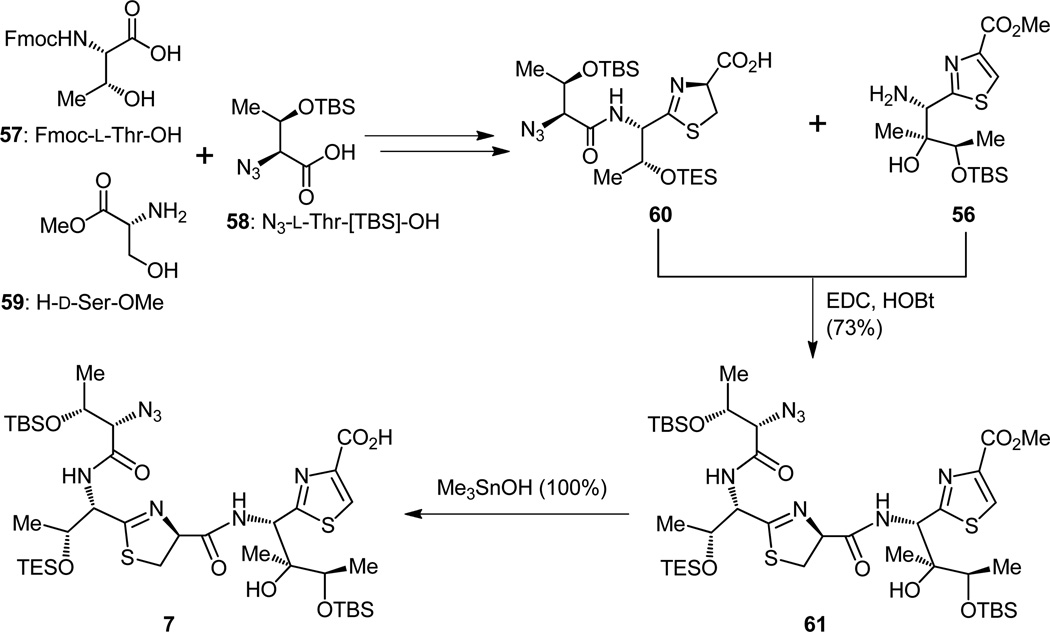
It was during the final drive toward the thiazoline-thiazole fragment 7 when another noteworthy discovery was made. That enlightenment came when we were faced with the task of gently hydrolyzing the methyl ester residing on the thiazole residue of the masked tripeptide 61 that was obtained by coupling the two building blocks shown in Figure 15, namely carboxylic acid 60, which was generated from two L-Thr equivalents (57 and 58), D-Ser-OMe (59), and amine 56 (EDC, HOBt), whose synthesis has already been presented (Figure 14). The difficulty, not completely unanticipated, was encountered as a consequence of the sensitivity of the compound under investigation (i.e. 61) to basic conditions, even those associated with the employment of the often used and much respected lithium hydroxide. Indeed, it was due to this difficulty that we found ourselves in hot pursuit of a new method to accomplish this hydrolysis without causing collateral damage to the neighboring sites of the molecule. The relatively obscure and rarely used Me3SnOH[24] provided the solution to this thorny problem, for it was through its magical action that we were able to obtain the desired carboxylic acid (7, Figure 15) without altering any of the other sensitive sites of the molecule, including the stereogenic center bearing the thiazoline moiety.[25] There is no room here to expand on the scope of this seemingly trivial, but useful observation. It suffices to say, however, so useful and unique this reagent proved time and again in the total synthesis under discussion that we were tempted to pronounce it the “reagent of the total synthesis of thiostrepton”. To be sure, we were to call upon it on several other occasions during this campaign to carry out surgical operations on strategic sites of molecules yet to be encountered, but which will soon be in sight.[14,15]
Figure 15.
Construction of thiazoline-thiazole fragment 7.
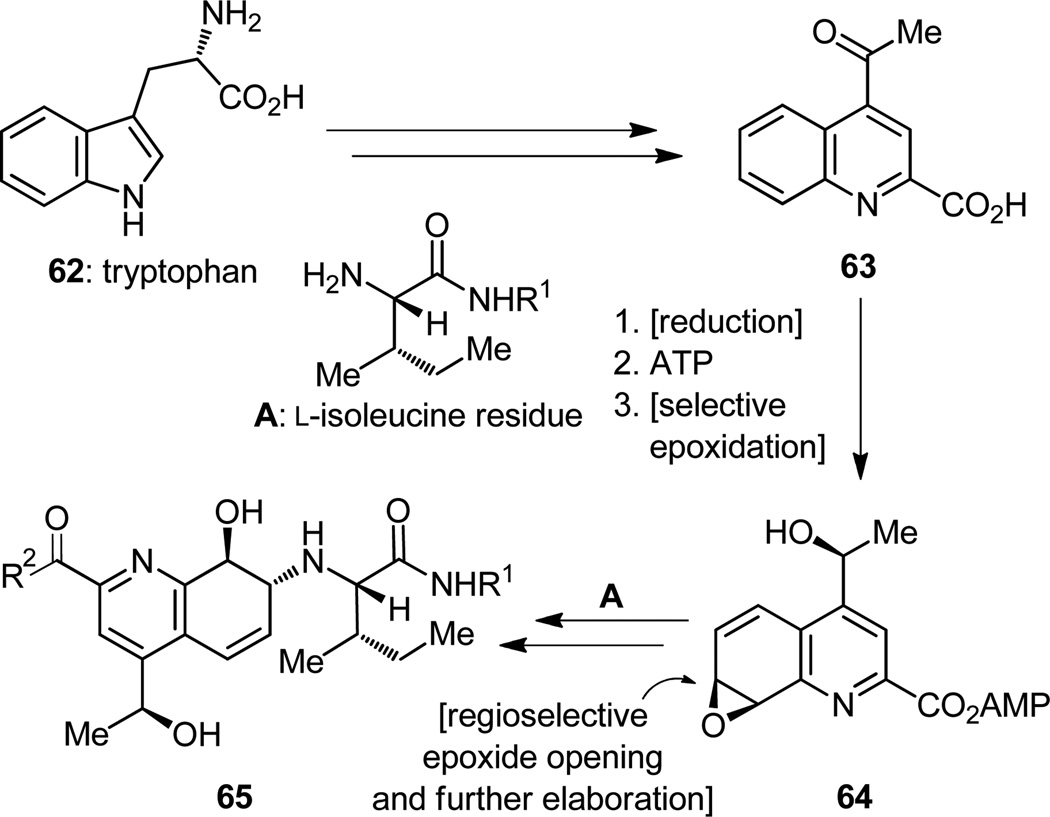
We shall now turn our attention to the third, major key intermediate, specifically the quinaldic acid domain (i.e. 65, Figure 16), whose biosynthesis had already been investigated and proposals made as to how nature may be fashioning its delicate structure from tryptophan (62, Figure 16). These insights, it was hoped, would provide the inspiration for our total synthesis design and the opportunity to compare the power of the available chemical tools with that of the enzymes so deftly utilized by nature to manufacture thiostrepton.
Figure 16.
Proposed biosynthetic scheme for the quinaldic acid domain 65 of thiostrepton.[16b]
Highlights of the proposed biosynthetic scheme[16b] are shown in Figure 16, and they include asymmetric reduction of the ketone functionality of 63 followed by another impressive reaction, this time an epoxidation of an aromatic ring that, in addition to being highly regioselective, is also distinguished for its exquisite diastereoselectivity (63 → 64). The incorporation of L-isoleucine into the quinaldic acid epoxide moiety to afford amino alcohol 65 in nature’s scheme is also admirable, particularly with regard to regioselectivity, given the fact that the incoming nucleophile is confronted with an epoxide whose ends appear equally activated. With all our modern advantages, how do we fare in facing such challenging synthetic tasks? A comparison of our accumen with that of enzymes in regard to these steps may be instructive, we thought, if not useful, and so we entered into the gamble.
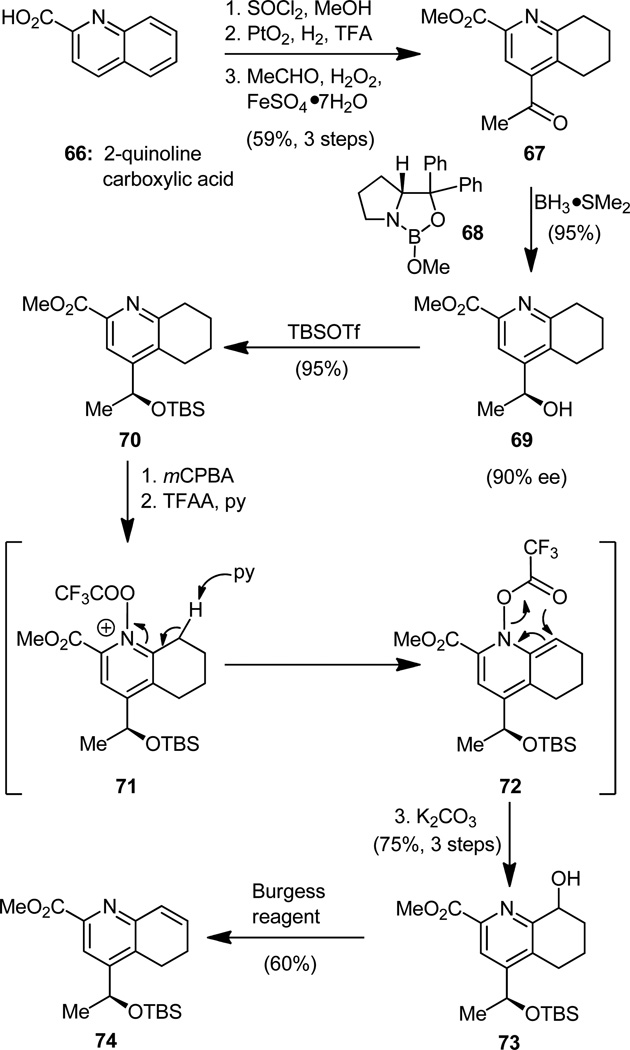
Our journey to the required quinaldic acid fragment (6, Figure 2)[14a,c] began with the commercially available 2-quinoline carboxylic acid (66), which was swiftly converted to the prochiral methyl ketone 67 by three conventional steps (Figure 17). We then called upon the Corey–Bakshi–Shibata (CBS) catalyst (68)[26] to do what it took an enzyme to accomplish in the thiostrepton-producing bacteria, namely to carry out the asymmetric reduction of the ketonic carbonyl group of 67, a task that the catalyst performed admirably well using borane-dimethyl sulfide complex as the reducing agent, to furnish alcohol 69 in high yield and excellent stereoselectivity (90% ee). Impressive as this last accomplishment may have been in unfolding the next phase of our strategy, we then exposed our weaknesses as artisans of synthesis, for we found ourselves in the rather embarrassing situation to have to introduce, one at a time, the two double bonds we had just erased at the outset of our synthetic route! Be that as it may, the installation of the first double bond—the one that we will have to epoxidize next— proceeded well and not without elegance (see Figure 17). Thus, protection of the hydroxyl group of 69, followed by oxidation of the nitrogen residing within the pyridine ring of 70, gave the expected N-oxide intermediate. Through a procedure known as the Boekelheide rearrangement[27]—not so dissimilar to the better known Polonovski reaction[28]—that involves treatment with trifluoroacetic anhydride in the presence of pyridine, this N-oxide intermediate was then converted, upon final exposure to aqueous potassium carbonate, to the corresponding secondary alcohol 73 via 71 and 72. The latter intermediate was obtained as a mixture of diastereomers, an inconsequential occurrence, for the instigating stereocenter would soon be erased. Remaining before arrival at the desired olefin (74) was the removal of a molecule of water from the latter compound, an objective that was easily accomplished by the Burgess reagent,[29] a sulfur-containing compound possessing a remarkable thirst for water.
Figure 17.
Construction of quinaldic acid derivative 74.
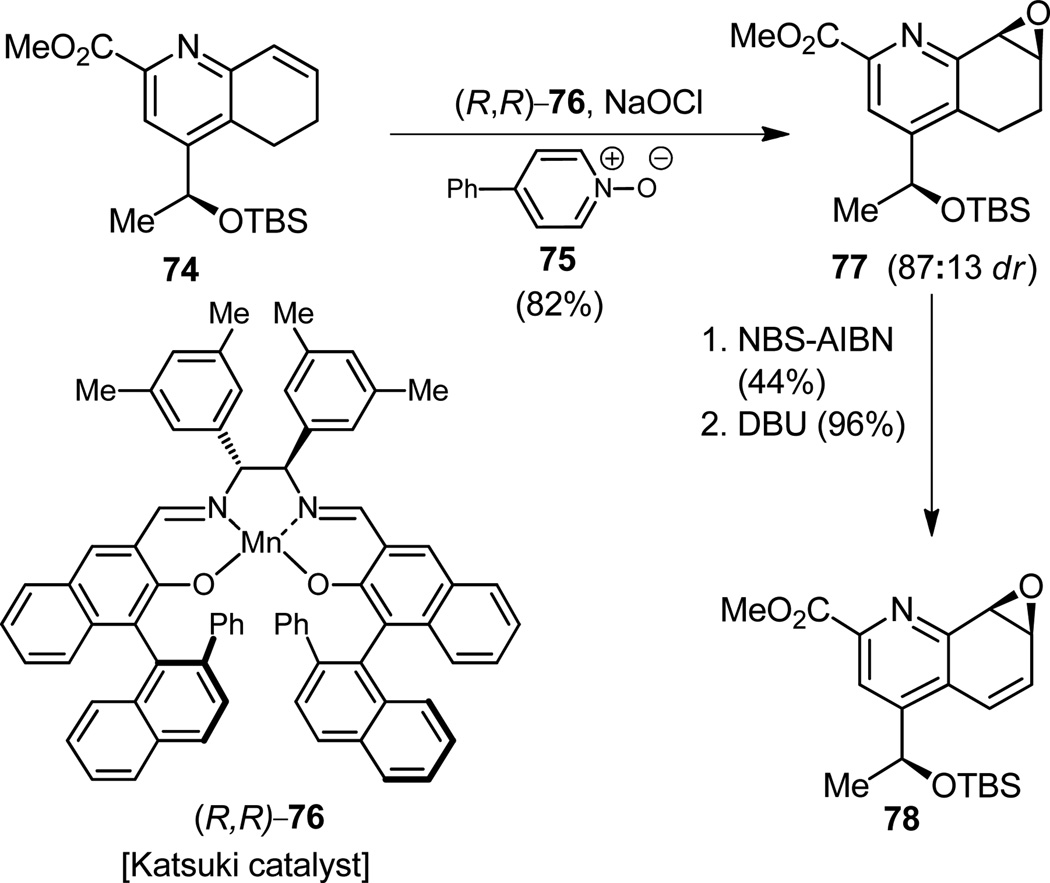
With access to olefinic substrate 74, it was now time for us to attempt to show off again our ability to match nature in the art of asymmetric catalysis. The accomplishment of the asymmetric synthesis of epoxide 77 (Figure 18) from olefin 74 by oxidation with 4-phenylpyridine-N-oxide (75) assisted by Katsuki’s catalyst (R,R- 76)[30] with 87:13 dr, while pleasantly received, did not match nature’s result, which must be close to 100:0 dr, if not exactly that! The second double bond was then engraved into the molecule by classical means (77 → 78, Figure 18), and soon we were ready for the next challenging operation of the sequence, that of the regioselective opening of the epoxide moiety within the delicate substrate just arrived at (78) with the appropriate amine nucleophile.
Figure 18.
Construction of quinaldic acid epoxide derivative 78.
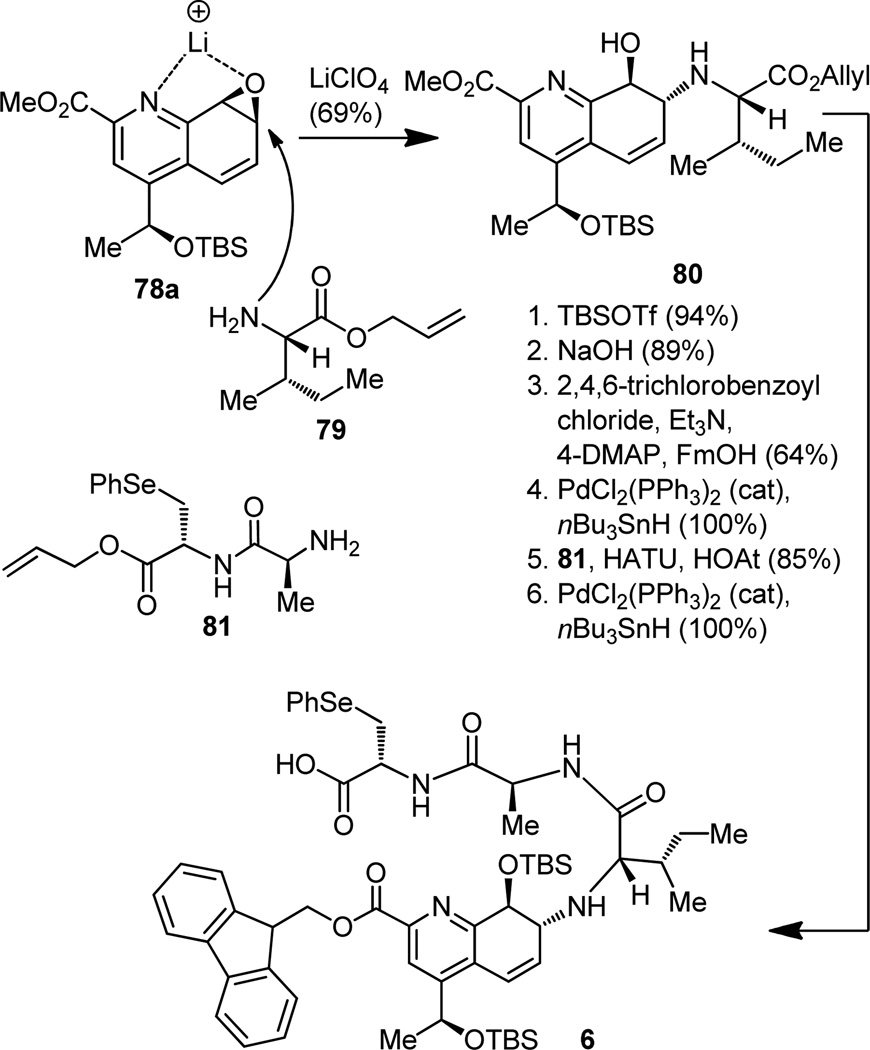
A cursory inspection of quinaldic acid epoxide 78 revealed that its two vulnerable sites are equally activated, more or less, by their surroundings. Further inspection, however, led to a supposition that a reagent as simple as lithium perchlorate may provide the necessary differentiation between the two sites by lithium complexation with the heteroatoms favorably disposed on the same side of the molecule as demonstrated in structure 78a (Figure 19). The incoming nucleophile would then attack the epoxide at the indicated and desired site, be it by virtue of steric or electronic reasons or a combination of the two. In the event, mixing epoxide 78 with amino acid derivative 79 in acetonitrile and in the presence of lithium perchlorate indeed gave the anticipated hydroxy amine (80) as the sole regioisomer, which was readily converted to the well-fortified carboxylic acid 6 by a series of standard synthetic operations that included incorporation of fragment 81. By then the quinaldic acid domain was ready for incorporation into the final structure, whose assembly became our next challenge. But before we leave this topic a comment regarding the reactivity of the secondary amine residing within the structure of intermediate 6 is in order. Those well versed in these matters will know that we, synthetic chemists, still need to guard or restrain reactive functional groups within our growing molecules, especially those that are likely to misbehave or are vulnerable to attack under the circumstances that may befall upon them. And so it was prudent for us to attempt such a protection of what we reasonably perceived as the most reactive site within the structure of 6, namely the basic nitrogen moiety. Somewhat surprisingly, however, this secondary amine resisted all our attempts to engage it with a protecting group cleavable at the end of the synthesis (e.g. trifluoroacetate, Troc). We were puzzled, and for a brief period of time agonized over the issue, not realizing that the answer—or rather speculative hypothesis—to our predicament was right in front of our eyes. Could the neighboring bulky substituents surrounding this amino group be responsible for its obstinacy, and could this safety net allow the group to pass through the perilous territory that lies ahead and arrive at its rendezvous with natural thiostrepton intact? It took courage to invest in this postulate; however, it turned into a fortunate prophecy. It was both remarkable and joyful to watch this secondary amine, bare as it was, march through the sequence surviving reaction after reaction, including several peptide couplings, a lactonization, and an oxidation! Applying such tactics of no functional group protection in synthesis in a general sense would certainly be a welcome development and one of which chemists are currently dreaming. To be sure, nature does it, and so we can do it, too… one day, but not quite yet, although admirable progress has been made in recent years.[31]
Figure 19.
Synthesis of quinaldic acid fragment 6.
Having constructed all the required fragments, including the bisphenylselenyl equivalent 5 (Figure 2)[14,15,22] corresponding to the tail of thiostrepton through a route that will look trivial to the expert, it was time to deal with their union and elaboration into their final destination. Our description of this part of the story will be even more brief than the tale just told, for the chemistry involved in accomplishing this goal was distinguished not so much for its methodological novelty, but rather for its strategy and logistics. Indeed, there are scores of chemical tools available for accomplishing, at least in principle, the remaining tasks of bond construction and functional group manipulation required for the completion of the total synthesis at hand. The specific reagents and conditions necessary were defined during the careful experimentation in the investigation that followed, and upon which the foundation was laid from where the final assault was to be launched for the conquest of thiostrepton.
It should be noted that there were many abortive attempts toward thiostrepton before the final one succeeded, for it was not possible a priori to know which of the myriad possible combinations of steps would be successful. To exemplify the drama that the practitioner of total synthesis is accustomed to experiencing, we will mention two incidents that led to the downfall of two of the early strategies toward 1, in both of which the bis-dehydroalanine tail was the defending hero who, in the end, preferred to self-destruct rather than surrender to our advances.
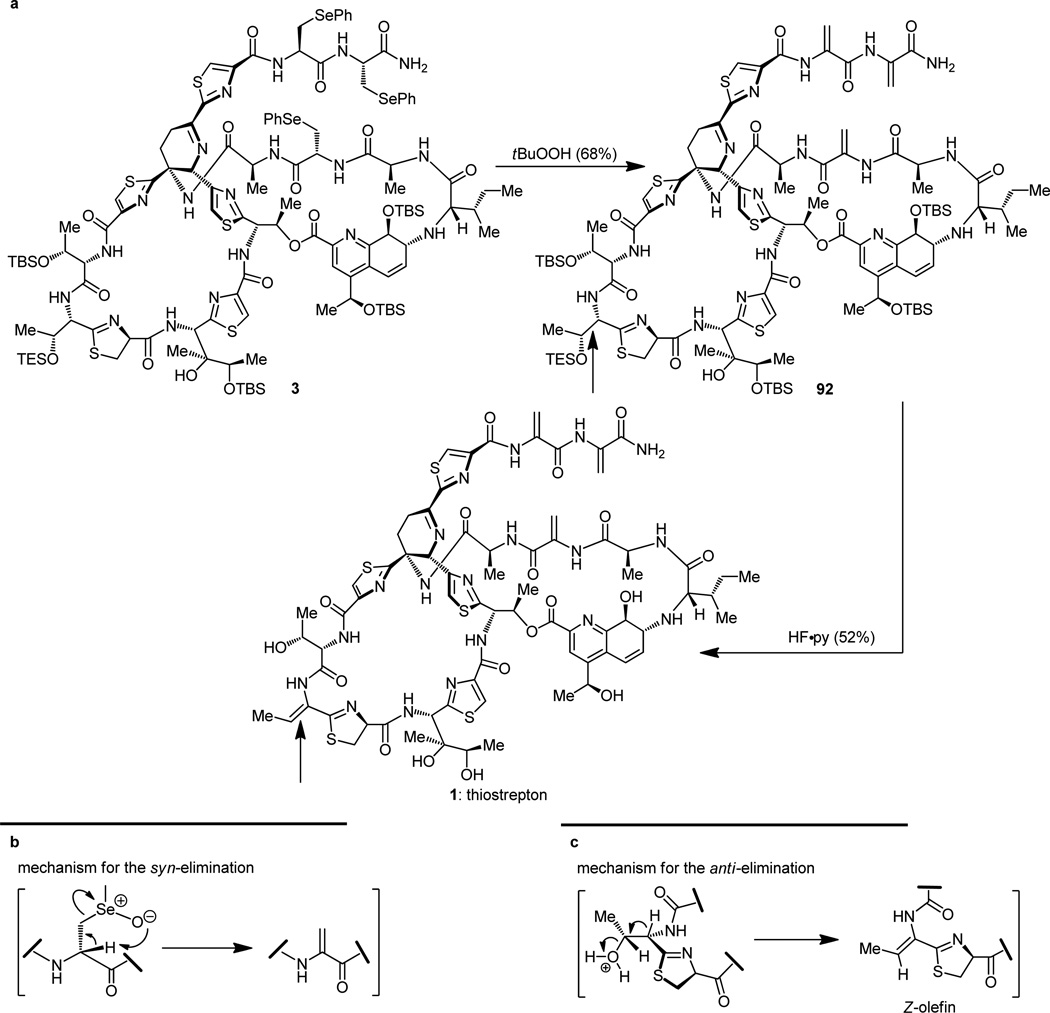
The initial steps toward the final assembly of the described building blocks to the targeted thiostrepton are summarized in Figure 20. Thus, the dehydropiperidine core 46 was taken to its N-Alloc hydroxy amine descendant 82 by first protecting its primary amine and then disassembling its acetonide-based fortification. The newly generated hydroxy amine (82) was then coupled with the thiazoline-containing carboxylic acid fragment 7 through a peptide coupling process. Next, the amino acid 83, obtained by suitable manipulation of the resulting product (selective ester hydrolysis and azide reduction), was cyclized into a macrolactam whose remaining carboxylic acid moiety was liberated from its methyl ester through the action of Me3SnOH[25] to afford advanced intermediate 84 as shown. This functionality within 84 was then used as a handle to embrace covalently the masked side-chain 5 through another peptide coupling reaction to furnish advanced intermediate 85. Oxidation/elimination of the resident phenylselenenium groups by the action of tBuOOH then produced the long-sought bisdehydroalanine containing compound 86.
Figure 20.
Construction of advanced intermediate 86 containing the 27-membered thiazoline macrocycle and the bis-dehydroalanine tail.
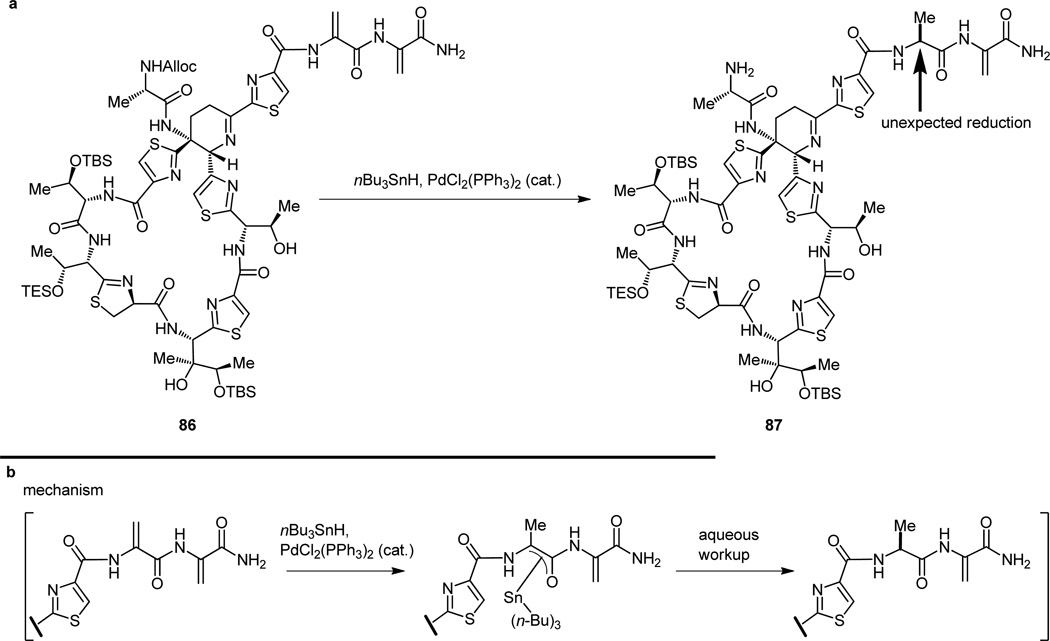
It then became necessary to free the primary amino group of the advanced intermediate 86 from its Alloc derivative, an operation that required the use of tri-n-butyltin hydride [nBu3SnH] and bis(triphenylphosphine) palladium dichloride [PdCl2(PPh3)2] as a catalyst.[32] In the first instance of interference from the bisdehydroalanine tail, and despite the repeated and highly successful use of this combination of reagents to achieve similar objectives in this and other research programs, the catalyst chose to misbehave (or more precisely, we did not anticipate its reactivity), leading to irreparable damage to the tail end of our substrate (86 → 87, Figure 21a). The damage detected was reduction of one of the olefinic bonds of the bis-dehydroalanine tail, presumably as a consequence of a postulated palladium-catalyzed hydrostannylation followed by protonolysis of the resulting enol stannane, as illustrated in Figure 21b.
Figure 21.
Unexpected reduction of the bis-dehydroalanine tail (a) and mechanistic rationale for its occurrence (b).
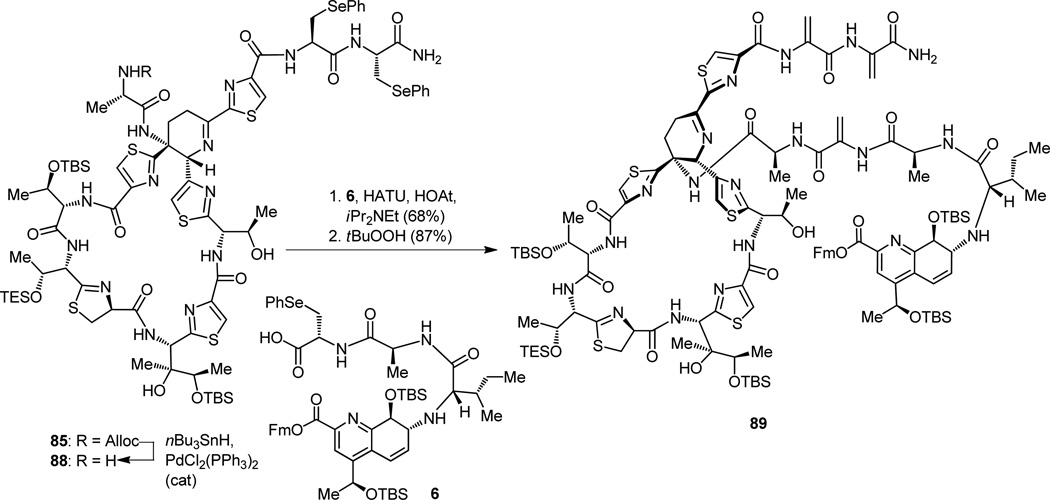
In order to circumvent this unwelcome side reaction, we retreated to bis-phenylselenenyl-containing macrocycle 85 (Figure 20), and attempted the Alloc removal at that stage. In this instance, the combination of nBu3SnH–PdCl2(PPh3)2 (cat.) succeeded admirably in removing the Alloc protecting group and liberating, at the same time, the corresponding amine (88, Figure 22), thus allowing us to proceed forward. The final fragment (6) was then incorporated into the growing structure by yet another peptide coupling, facilitated by HATU, HOAt, and iPr2NEt, and the resulting product was treated with tBuOOH to oxidatively remove the phenylselenenyl groups, furnishing the tris-dehydroalanine containing compound 89 as shown in Figure 22.
Figure 22.
Construction of advanced tris-dehydroalanine seco compound 89 containing all carbon atoms of thiostrepton.
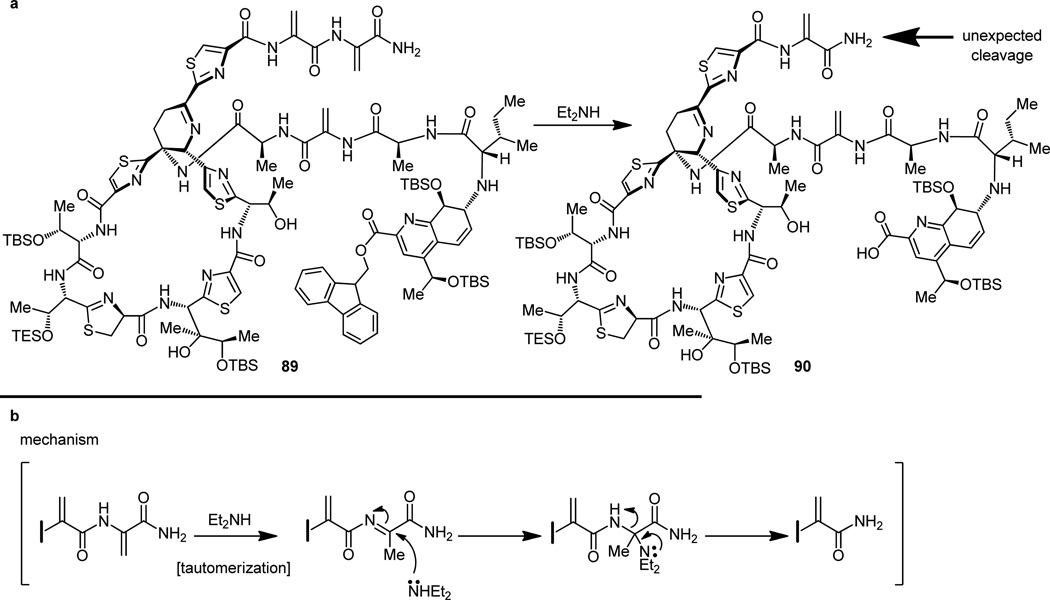
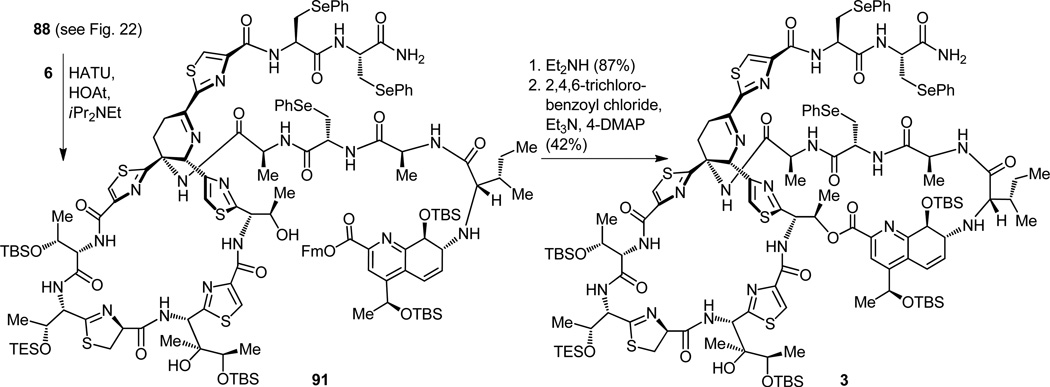
The second frustrating episode of bis-dehydroalanine tail interference took place at this point as an attempt was made to remove the fluorenylmethyl (Fm) protecting group from the carboxylic acid moiety of intermediate 89 (Figure 23a).[14b,d] This seemingly routine dismantling of an Fm ester under the mild conditions of diethylamine caused, in addition to bringing about the desired change, cleavage of a dehydroalanine unit from the tail of the molecule. Since we could not imagine milder circumstances that would perhaps lead to the desired outcome, we decided to halt operations along these lines and offered the mechanistic explanation for the unexpected collapse of the side-chain shown in Figure 23b as our final act of this particular expedition. Despite the setbacks, however, and out of the maze of synthetic avenues to the final target traveled, came the final road that did lead to thiostrepton, our long sought and coveted prize.[14] Thus, removal of the Fm group from the tris-phenylselenenyl-containing progenitor 91 (obtained from 88 and 6 after the HATU coupling) proceeded without incident to unravel the corresponding seco hydroxy acid (Figure 24). This newly generated carboxylic acid was then tied up with the, so far idle, hydroxy group under the influence of the highly effective reagent 2,4,6-trichlorobenzoyl chloride in the Yamaguchi macrolactonization reaction[33] that ensued. With this operation successfully completed, the entire polycyclic framework of thiostrepton was in place as structure 3 (Figure 24). This substance is nothing less than a delicately protected form of the natural product (1), for it has the potential to bear thiostrepton, if only the mildest of conditions could be employed to recover our sensitive and unforgiving synthetic target. The selective oxidation of the three selenenium groups with tert- butyl hydroperoxide within structure 3 (Figure 25a), where several potentially oxidizable sulfur and nitrogen groups reside, is remarkable. Also remarkable is the spontaneous syn-elimination of all three selenoxide moieties (see mechanism, Figure 25b) formed during the reaction, and which led to the penultimate precursor to thiostrepton, compound 92 (Figure 25a), a molecule that contained all functional groups required (albeit some in protected forms), except for the double bond conjugated to the thiazoline ring (see arrows in Figure 25a). At this stage the plan called for the concurrent removal of all five silicon protecting groups from the latest intermediate and the subsequent antielimination (see mechanism, Figure 25c) of the hydroxyl moiety closest to the thiazoline ring, hopefully under the same conditions. That is precisely what happened on exposure of compound 92 to hydrogen fluoride-pyridine complex. Synthetic thiostrepton (1), emerging from the reaction mixture as a laboratory-born equal to the natural substance, registered its arrival with identical chromatographic and spectroscopic signatures to those of a natural sample. And so it was that thiostrepton was made in the laboratory on June 29, 2004, a little over four years after it was targeted for total synthesis.[34]
Figure 23.
Unexpected dehydroalanine tail cleavage during removal of the fluorenylmethyl ester (a) and mechanistic rationale for its occurence (b).
Figure 24.
Construction of precursor 3 containing the complete ring framework of thiostrepton.
Figure 25.
Final stages of the total synthesis of thiostrepton (a) and mechanistic rationale for the formation of the olefinic bonds (b, c).
Other Total Syntheses of Thiopeptide Antibiotics
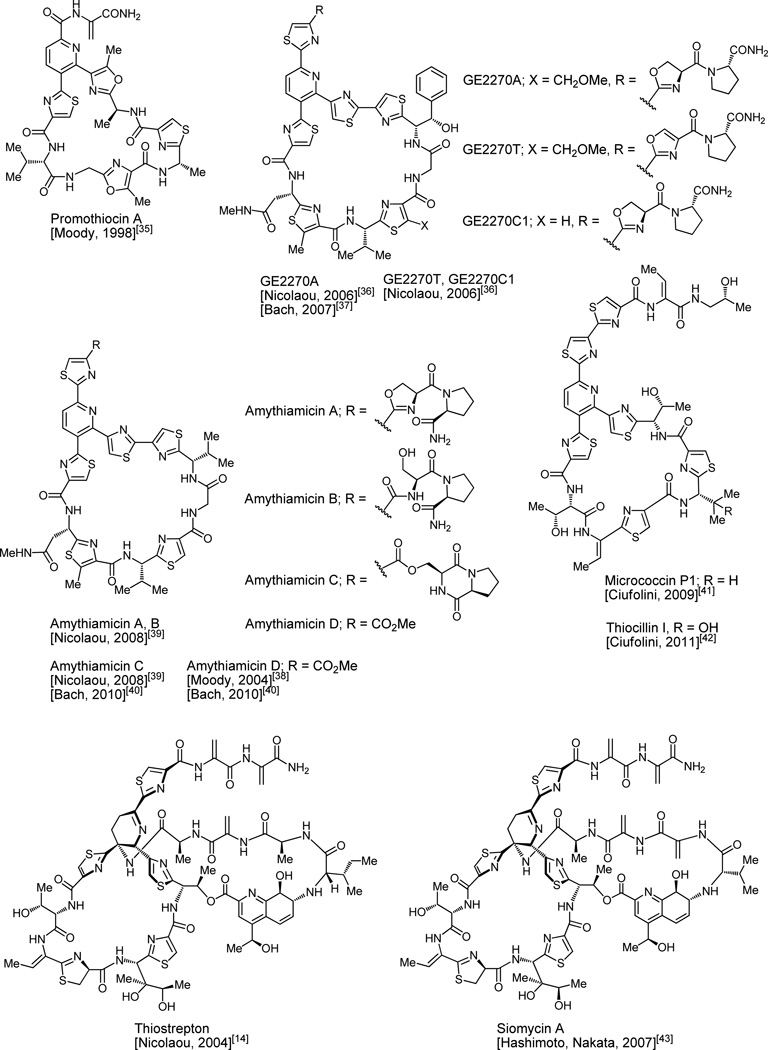
In addition to thiostrepton, a number of other members of the thiopeptide antibiotics family have been synthesized in the laboratory (see Figure 26). Thus, promothiocin A was the first thiopeptide antibiotic to be reached by total synthesis (Moody, 1998).[35] The total syntheses of thiopeptide antibiotics GE2270A, GE2270T, GE2270C1 were reported in 2006 (Nicolaou, Chen).[36] The Bach group added their elegant total synthesis of GE2270A to the literature in 2007.[37] The first total synthesis of amythiamicin D was reported by Moody et al. in 2004,[38] while those of amythiamicins A, B and C were published in 2008 (Nicolaou, Chen).[39] Bach and Ammer reported the total synthesis amythiamicins C and D in 2010.[40] Ciufolini and Kefranc’s total synthesis of micrococcin P1 (2009)[41] was accompanied by revision of its structure. Ciufolini and Aulakh reported the total synthesis of the related thiopeptide antibiotic thiocillin I in 2011.[42] The total synthesis of siomycin A, whose structure is reminiscent of that of thiostrepton, was reported by the Hashimoto–Nakata group in 2007.[43] No doubt, further advances in the field will be forthcoming, most likely incorporating new synthetic methodologies and biological aspects along the conduits of the total synthesis or semisynthesis endeavors.
Figure 26.
Synthesized thiopeptide antibiotics.
Epilogue
Described within are, in their full extent, the details of the campaign to construct in the laboratory through total synthesis thiostrepton, the flagship member of the thiopeptide class of antibiotics. While exciting and full of adventure and suspense, the unexpected ups and downs of this description reveal imperfections of the art of strategy design as much as they provide a demonsration of its power to reach complex bioactive molecules. Future goals of practitioners of total synthesis should, therefore, include ways to improve predictability in strategy design in order to avoid costly pitfalls, and develop and incorporate within such strategies more effective and reliable reactions, as well as streamline and shorten synthetic sequences. Both theoretical and experimental advances are required for such improvements to be realized.
In celebrating the high level of the curent state of the art of total synthesis one should recall the instrument revolution that occurred in the 1940s and 1950s and the dramatic support that it provided to synthesis in terms of structural analysis.[44] The impetus so acquired by the field, coupled with enormous advances in new synthetic reactions and strategy design, brought us to the present state of the art.[45] Interestingly, our times also reveal new trends that were not present in the early days of this revolution in total synthesis, namely the incorporation of method development and chemical biology aspects within total synthesis research programs. The latter, in particular, is an integral part of the movement toward collaborative and translational research. This movement had already begun during the latter decades of the twentieth century.
Acknowledgements
It is with great pleasure that I acknowledge the extraordinary contributions of my co-workers, whose names are found in the cited papers, without whom this article would not have been possible. I also wish to express my love and appreciation for my wife, Georgette and our children Colette, Alex, Chris and PJ for their unconditional love, patience and support. My deep gratitude and appreciation also go to my office staff, Vicky Nielsen Armstrong, Janise Petrey, Natasha Zepeda and Mary Mulshine, for their loyalty and assistance over the years and their contributions to the preparation of this article. I also wish to thank David J. Edmonds, James J. Crawford, Christopher R.H. Hale and Christian Nilewski for their assistance and proofreading of the manuscript. Last but not least, I am grateful to the National Institutes of Health (USA), The Skaggs Institute for Chemical Biology, A*STAR Singapore, and my many colleagues and friends from the pharmaceutical and biotechnology sector for their generous financial support that made the work described herein possible.
Biography

K. C. Nicolaou, born in Cyprus and educated in UK and USA, is Chairman of Chemistry, The Scripps Research Institute, where he holds the Darlene Shiley Chair in Chemistry and Aline W. & L. S. Skaggs Professorship in Chemical Biology, and is Distinguished Professor of Chemistry, University of California, San Diego. The impact of his work in chemistry, biology and medicine flows from his contributions to chemical synthesis as described in over 740 publications. His dedication to chemical education is reflected in his Classics in Total Synthesis book series and Molecules That Changed the World, and his training of hundreds of graduate students and postdoctoral fellows. He is a Member of the National Academy of Sciences (USA), the German Academy of Sciences Leopoldina, and the American Philosophical Society, and Corresponding Member of the Academy of Athens.
References
- 1.Mahaffy JP. What Have the Greeks Done for Modern Civilisation. New York: G.P. Putnam’s Sons; 1910. p. 263. [Google Scholar]
- 2.a) Pagano JF, Weinstein MJ, Stout HA, Donovick R. Antibiot. Ann. 1955–1956:554–559. [PubMed] [Google Scholar]; b) Vandeputte J, Dutcher JD. Antibiot. Ann. 1955–1956:560–561. [PubMed] [Google Scholar]; c) Steinberg BA, Jambor WP, Suydam LO. Antibiot. Ann. 1955–1956:562–565. [PubMed] [Google Scholar]
- 3.a) Bodanszky M, Sheehan JT, Fried J, Williams NJ, Birkhimer CA. J. Am. Chem. Soc. 1960;82:4747–4748. [Google Scholar]; b) Kenner GW, Sheppard RC, Stehr CE. Tetrahedron Lett. 1960;1:23–26. [Google Scholar]; c) Drey CNC, Kenner GW, Law HD, Bodanszky M, Fried J, Williams NJ, Sheehan JT. J. Am. Chem. Soc. 1961;83:3906–3908. [Google Scholar]; d) cross DFW, Kenner GW, Sheppard RC, Stehr CE. J. Chem. Soc. 1963:2143–2150. [Google Scholar]; e) Bodanszky M, Fried J, Sheehan JT, Williams NJ, Alicino J, Cohen AI, Keeler BT, Birkhimer CA. J. Am. Chem. Soc. 1964;86:2478–2490. [Google Scholar]; f) Bodanszky M, Scozzie JA, Muramatsu I. J. Am. Chem. Soc. 1969;91:4934–4936. doi: 10.1021/ja01037a028. [DOI] [PubMed] [Google Scholar]; g) Bodanszky M, Scozzie JA, Muramatsu I. J. Antibiot. 1970;23:9–12. doi: 10.7164/antibiotics.23.9. [DOI] [PubMed] [Google Scholar]
- 4.a) Hensens OD, Albers-SchÖnberg G. J. Antibiot. 1983;36:799–813. doi: 10.7164/antibiotics.36.799. [DOI] [PubMed] [Google Scholar]; b) Hensens OD, Albers-SchÖnberg G. J. Antibiot. 1983;36:814–831. doi: 10.7164/antibiotics.36.814. [DOI] [PubMed] [Google Scholar]; c) Hensens OD, Albers-SchÖnberg G. J. Antibiot. 1983;36:732–845. doi: 10.7164/antibiotics.36.832. [DOI] [PubMed] [Google Scholar]
- 5.a) Anderson B, Hodgkin DC, Viswamitra MA. Nature. 1970;225:233–235. doi: 10.1038/225233a0. [DOI] [PubMed] [Google Scholar]; b) Bond CS, Shaw MP, Alphey MS, Hunter WN. Acta Cryst. 2001;D57:755–758. doi: 10.1107/s0907444901003134. [DOI] [PubMed] [Google Scholar]; c) Crowfoot D, Bunn CW, Rogers-Low BW, Turner-Jones A. Ch. 23. In: Clarke HT, Johnson JR, Robinson R, editors. The Chemistry of Penicillin. Princeton: Princeton University Press; 1949. pp. 893–894. [Google Scholar]
- 6.For selected reviews on thopeptide antibiotics, see: Bagley MC, Dale JW, Merritt EA, Xiong X. Chem. Rev. 2005;105:685–714. doi: 10.1021/cr0300441. Hughes RA, Moody CJ. Angew. Chem. 2007;119:8076–8101. Angew. Chem. Int. Ed. 2007;46:7930–7954. doi: 10.1002/anie.200700728..
- 7.Dennis SM, Nagaraja TG, Dayton AD. Res. Vet. Sci. 1986;41:251–256. [PubMed] [Google Scholar]
- 8.a) McConkey GA, Rogers MJ, McCutchan TF. J. Biol. Chem. 1997;272:2046–2049. doi: 10.1074/jbc.272.4.2046. [DOI] [PubMed] [Google Scholar]; b) Hardman JG, Limbird LE, Molinoff PB, Ruddon RWA, Gilman G. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 9th Ed. New York: The McGraw-Hill Companies; 1996. pp. 965–985. [Google Scholar]
- 9.Ueno M, Furukawa S, Abe F, Ushioda M, Fujine K, Johki S, Hatori H, Ueda J. J. Antibiot. 2004;57:590–596. doi: 10.7164/antibiotics.57.590. [DOI] [PubMed] [Google Scholar]
- 10.Jonghee K. PCT Int. Appl. 2002 WO 2002066046. [Google Scholar]
- 11.Xing Y, Draper DE. Biochemistry. 1996;35:1581–1588. doi: 10.1021/bi952132o. [DOI] [PubMed] [Google Scholar]
- 12.a) Fleming A. Brit. J. Exp. Path. 1929;10:226–236. [Google Scholar]; b) Chain E, Florey HW, Gardner AD, Heatley NG, Jennings MA, Orr-Ewing J, Sanders AG. Lancet. 1940;239:226–228. [Google Scholar]; c) Sheehan JC. The Enchanted Ring: The Untold Story of Penicillin. Cambridge, Massachussets: The MIT Press; 1982. p. 224. [Google Scholar]
- 13.a) Sheehan JC, Henery-Logan KR. J. Am. Chem. Soc. 1957;79:1262–1263. [Google Scholar]; b) Sheehan JC, Henery-Logan KR. J. Am. Chem. Soc. 1959;81:3089–3094. [Google Scholar]
- 14.a) Nicolaou KC, Safina BS, Zak M, Estrada AA, Lee SH. Angew. Chem. 2004;116:5197–5202. [Google Scholar]; Angew. Chem. Int. Ed. 2004;43:5087–5092. doi: 10.1002/anie.200461340. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Zak M, Safina BS, Lee SH, Estrada AA. Angew. Chem. 2004;116:5202–5207. [Google Scholar]; Angew. Chem. Int. Ed. 2004;43:5092–5097. doi: 10.1002/anie.200461341. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Safina BS, Zak M, Lee SH, Nevalainen M, Bella M, Estrada AA, Funke C, Zécri FJ, Bulat S. J. Am. Chem. Soc. 2005;127:11159–11175. doi: 10.1021/ja0529337. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Zak M, Safina BS, Estrada AA, Lee SH, Nevalainen M. J. Am. Chem. Soc. 2005;127:11176–11183. doi: 10.1021/ja052934z. [DOI] [PubMed] [Google Scholar]
- 15.a) Nicolaou KC, Safina BS, Funke C, Zak M, Zécri FJ. Angew. Chem. 2002;114:2017–2020. [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2002;41:1937–1940. [PubMed] [Google Scholar]; b) Nicolaou KC, Nevalainen M, Safina BS, Zak M, Bulat S. Angew. Chem. 2002;114:2021–2025. [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2002;41:1941–1945. [PubMed] [Google Scholar]; c) Nicolaou KC, Nevalainen M, Zak M, Bulat S, Bella M, Safina BS. Angew. Chem. 2003;115:3540–3546. doi: 10.1002/anie.200351745. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2003;42:3418–3424. doi: 10.1002/anie.200351745. [DOI] [PubMed] [Google Scholar]
- 16.a) Mocek U, Zeng Z, O’Hagan D, Zhou P, Fan L-DG, Beale JM, Floss HG. J. Am. Chem. Soc. 1993;115:7992–8001. [Google Scholar]; b) Priestley ND, Smith TM, Shipley PR, Floss HG. Bioorg. Med. Chem. 1996;4:1135–1147. doi: 10.1016/0968-0896(96)00126-5. [DOI] [PubMed] [Google Scholar]
- 17.Bycroft BW, Gowland MS. J. Chem. Soc. Chem. Commun. 1978:256–258. [Google Scholar]
- 18.Öhler E, Schmidt U. Chem. Ber. 1979;112:107–115. [Google Scholar]
- 19.a) Wulff G, Klinken HT. Tetrahedron. 1992;48:5985–5990. [Google Scholar]; b) Wulff G, Lindner HG, BÖhnke H, Steigel A, Klinken HT. Liebigs Ann. Chem. 1989:527–531. [Google Scholar]; c) Wulff G, BÖhnke H. Angew. Chem., Int. Ed. Engl. 1986;25:90–92. [Google Scholar]
- 20.Pinho e Melo TMVD, Fausto R, d’A. Rocha Gonsalves AM, Gilchrist TL. J. Org. Chem. 1998;63:5350–5355. [Google Scholar]
- 21.Higashibayashi S, Hashimoto K, Nakata M. Tetrahedron Lett. 2002;43:105–110. [Google Scholar]
- 22.Baumhof P, Mazitschek R, Giannis A. Angew. Chem. 2001;113:3784–3786. doi: 10.1002/1521-3773(20011001)40:19<3672::aid-anie3672>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2001;40:3672–3674. doi: 10.1002/1521-3773(20011001)40:19<3672::aid-anie3672>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23.Torres-Valencia JM, Cerda-García-Rojas CM, Joseph-Nathan P. Tetrahedron: Asymm. 1998;9:757–764. [Google Scholar]
- 24.a) Furlan RLE, Mata EG, Mascaretti OA. J. Chem. Soc., Perkin Trans. 1998;1:355–358. [Google Scholar]; b) Furlan RLE, Mata EG, Mascaretti OA, Pena C, Coba MP. Tetrahedron. 1998;54:13023–13034. [Google Scholar]; c) Furlan RLE, Mascaretti OA. Aldrichim. Acta. 1997;30:55–69. [Google Scholar]; d) Furlan RLE, Mata EG, Mascaretti OA. Tetrahedron Lett. 1996;37:5229–5232. [Google Scholar]
- 25.Nicolaou KC, Estrada AA, Zak M, Lee SH, Safina BS. Angew. Chem. 2005;117:1402–1406. doi: 10.1002/anie.200462207. [DOI] [PubMed] [Google Scholar]; Nicolaou KC, Estrada AA, Zak M, Lee SH, Safina BS. Angew. Chem. Int. Ed. 2005;44:1378–1382. doi: 10.1002/anie.200462207. [DOI] [PubMed] [Google Scholar]
- 26.a) For a review on CBS reduction, see: Corey EJ, Helal CJ. Angew. Chem. 1998;110:2092–2118. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. Angew. Chem. Int. Ed. 1998;37:1986–2012. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. Masui M, Shioiri T. Synlett. 1997:273–274..
- 27.Fontenas C, Bejan E, Haddou HA, Galavoine GA. Synth. Commun. 1995;25:629–633. [Google Scholar]; Boekelheide V, Linn WJ. J. Am. Chem. Soc. 1954;76:1286–1294. [Google Scholar]
- 28.Grierson D. Org. React. 1990;39:85–295. [Google Scholar]
- 29.Burgess EM, Penton HR, Taylor EA. J. Org. Chem. 1973;38:26–31. [Google Scholar]
- 30. Ito K, Yoshitake M, Katsuki T. Tetrahedron. 1996;52:3905–3920. Sasaki H, Irie R, Hamada T, Suzuki K, Katsuki T. Tetrahedron. 1994;50:11827–11838. c) For a review in this area, see: Katsuki T. Current Organic Chemistry. 2001;5:663–678..
- 31.a) Gaich T, Baran PS. J. Org. Chem. 2010;75:4657–4673. doi: 10.1021/jo1006812. [DOI] [PubMed] [Google Scholar]; b) Baran PS, Maimone TJ, Richter JM. Nature. 2007;446:404–408. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- 32. de la Torre BG, Torres JL, Bardají E, Clapés P, Xaus N, Jorba X, Calvet S, Albericio F, Valencia G. J. Chem. Soc. Chem. Comm. 1990:965–967. b) For a review on the use and removal of allylic protecting groups, see: Guibé F. Tetrahedron. 1998;54:2967–3042..
- 33.a) Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989–1993. [Google Scholar]; b) Nicolaou KC, Patron AP, Ajito K, Richter PK, Khatuya H, Bertinato P, Miller RA, Tomaszewski MJ. Chem. Eur. J. 1996;2:847–868. [Google Scholar]
- 34.For a recent review on the synthesis of thiostrepton and other antibiotics, see Nicolaou KC, Chen JS, Edmonds DJ, Estrada AA. Angew. Chem. 2009;121:670–732. doi: 10.1002/anie.200801695. Angew. Chem. Int. Ed. 2009;48:660–719. doi: 10.1002/anie.200801695..
- 35.Moody CJ, Bagley MC. Chem. Commun. 1998:2049–2050. [Google Scholar]
- 36.a) Nicolaou KC, Zou B, Dethe D-H, Li DB, Chen DY-K. Angew. Chem. 2006;118:7950–7956. doi: 10.1002/anie.200602798. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:7786–7792. doi: 10.1002/anie.200602798. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Dethe DH, Leung GYC, Zou B, Chen DY-K. Chem. Asian J. 2008;3:413–429. doi: 10.1002/asia.200700361. [DOI] [PubMed] [Google Scholar]
- 37.Müller HM, Delgado O, Bach T. Angew. Chem. 2007;119:4855–4858. doi: 10.1002/anie.200700684. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:4771–4774. doi: 10.1002/anie.200700684. [DOI] [PubMed] [Google Scholar]
- 38.Hughes RA, Thompson SP, Alcaraz L, Moody CJ. Chem. Commun. 2004:946–948. doi: 10.1039/b401580k. [DOI] [PubMed] [Google Scholar]
- 39.Nicolaou KC, Dethe DH, Chen DY-K. Chem. Commun. 2008:2632–2634. doi: 10.1039/b805069b. [DOI] [PubMed] [Google Scholar]
- 40.Ammer C, Bach T. Chem. Eur. J. 2010;16:14083–14093. doi: 10.1002/chem.201002144. [DOI] [PubMed] [Google Scholar]
- 41.Lefranc D, Ciufolini MA. Angew. Chem. 2009;121:4262–4265. doi: 10.1002/anie.200900621. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48:4198–4201. doi: 10.1002/anie.200900621. [DOI] [PubMed] [Google Scholar]
- 42.Aulakh VS, Ciufolini MA. J. Am. Chem. Soc. 2011;133:5900–5904. doi: 10.1021/ja110166x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori T, Higashibayashi S, Goto T, Kohno M, Satouchi Y, Shinko K, Suzuki K, Suzuki S, Tohmiya H, Hashimoto K, Nakata M. Tetrahedron Lett. 2007;48:1331–1335. [Google Scholar]
- 44.Slater LB. Stud. Hist. Phil. Sci. 2002;33:1–33. [Google Scholar]
- 45.For recent reviews on other total syntheses from the author’s laboratories, see: Nicolaou KC, Hale CRH, Nilewski C. Chem. Rec. 2012 doi: 10.1002/tcr.201200005. Nicolaou KC, Hale CRH, Nilewski C, Ioannidou HA. Chem. Soc. Rev. 2012;41:5185–5138. doi: 10.1039/c2cs35116a..