Abstract
Objective
To examine the mechanisms involved in hypotension induced by transient receptor potential vanilloid 4 (TRPV4) activation.
Methods
Wistar rats were given 50 mg/kg capsaicin subcutaneously 1-2 days postnatally to cause degeneration of capsaicin-sensitive sensory nerves. Vehicle was given to the corresponding newborn rats as the control group. After being weaned, male rats were picked for further investigation. At the age of 8 weeks, mean arterial pressure (MAP) and its response to 4α-phorbol 12,13-didecanoate (4α-PDD, a selective TRPV4 activator, 2.5 mg/kg, i.v.) with/without CGRP8-37 (1 mg/kg/min, i.v.), an antagonist of calcitonin gene-related peptide (CGRP, a potent vasodilator released from sensory nerves), in vehicle-/capsaicin-pretreated rats anesthetized with sodium pentobarbital (50 mg/kg, i.p.) were monitored to observe the contributions of neuropeptides released from sensory nerves to the 4α-PDD-induced hypotension. To detect the roles of various vasodilating factors released by vascular endothelium in the hypotensive effect induced by TRPV4 activation, the corresponding inhibitors/blockers, including indomethacin (a cyclooxygenase inhibitor, 10 mg/kg, i.v.), Nω-Nitro-L-arginine (L-NA, a NO synthase inhibitor, 20 mg/kg, i.v.), apamin (a blocker of small-conductance Ca2+-activated K+ (MaxiК) channels, 50 μg/kg, i.v.) combined with charybdotoxin (a blocker of intermediate- and large-conductance MaxiК channels, 50 μg/kg, i.v.), were used at various time before 4α-PDD injection. Plasma CGRP and substance P levels of rats before/after administration were measured using the corresponding Radioimmunoassays. At last, immunohistochemistry stainings were performed to observe expression of TRPV4/CGRP/MaxiК in mesenteric resistant arteries and sensory neurons/nerve fibers.
Results
Intravenous administration of 4α-PDD produced remarkable hypotension in vehicle-pretreated rats. The depressor effect was attenuated by degeneration of capsaicin-sensitive sensory nerves (P<0.05) or administration of CGRP8-37 (P<0.05). In both vehicle- and capsaicin-pretreated rats, the combined administration of apamin and charybdotoxin markedly reduced the 4α-PDD-induced hypotensive effect (P<0.05), but i.v. administration of indomethacin and L-NA didn’t produce the similar effect. Intravenous administration of 4α-PDD increased plasma CGRP but not substance P levels in vehicle-pretreated rats only (P<0.05), which was not affected by indomethacin, L-NA, or apamin plus charybdotoxin. Immunohistochemistry staining showed that TRPV4 colocalized with MaxiК channels in endothelium of mesenteric resistant arteries and with CGRP in sensory neurons/nerve fibers.
Conclusion
Our data show that the hypotensive effect induced by TRPV4 activation attributes to, at least in part, activation of MaxiК channels and CGRP receptors upon CGRP release from sensory nerves.
Keywords: blood pressure, calcitonin gene-related peptide, sensory nerves, Ca2+-activated K+ channels
Introduction
TRPV4, a member of the vanilloid subfamily of the transient receptor potential (TRP) channels, is a non-selective cation channel with a moderate Ca2+ selectivity (PCa/PNa=6, in which P is permeability) [1]. Similarly as other TRP channels, TRPV4 has six transmembrane domains flanked by cytoplasmic N- and C-termini [2]. TRPV4 displays a broad expression in various cells and tissues and has been implicated in diverse physiological processes. TRPV4 can be activated by a variety of stimuli, including heat, cell swelling, exogenous phorbol ester compounds, or endogenous, physiologically active lipids such as 5′,6′-epoxyeicosatrienoic acid (5′,6′-EET) [3]. These TRPV4-activating stimuli promote the channel opening by two distinct pathways: the PLA2-cytochrome P450 epoxygenase-dependent pathway or direct activation mediated by an aromatic residue at the N terminus of the third transmembrane domain [4].
TRPV4 is constitutively active at body temperature [5]. Recent findings reveal that TRPV4 plays an important role in systemic osmoregulation, mechanoregulation and thermoregulation [6-10]. Moreover, TRPV4 is also involved in the regulation of vascular tone, and activation of TRPV4 produces vasodilation [3]. We have previously shown that activation of TRPV4 by 4α-phorbol 12, 13-didecanoate (4α-PDD), a selective TRPV4 activator, leads to dose-dependent decreases in blood pressure in rats [11]. However, the mechanisms responsible for TRPV4-induced depressor effects are largely unknown.
Our previous studies also showed that release of calcitonin gene-related peptide (CGRP) played an important role in hypotension induced by activation of TRPV1, another member of the vanilloid subfamily. As well known, CGRP is one of the most potent vasodilatory neuropeptides and mainly released from sensory nerves expressing TRPV1 [12, 13]. Similar to TRPV1, TRPV4 has been reported to express in sensory nerves and colocalize with CGRP and substance P, which is also a vasodilatory neuropeptide [14, 15]. Based on the information stated above, we hypothesize that the depressor effect of 4α-PDD is mediated, at least partly, through stimulus of sensory nerves and the subsequent release of vasodilatory neuropeptides. It is well established that capsaicin administered at 50 mg/kg subcutaneously 1-2 days postnatally when sensory nerves are not fully developed leads to permanent impairment and degeneration of capsaicin-sensitive sensory nerves [16, 17]. Using this model combined with the use of antagonists to block neuropeptide receptors as well as the measurement of plasma CGRP and substance P levels, we aimed at examining the role of neuropeptides released from sensory nerves in TRPV4-mediated depressor effects.
Vascular endothelium plays an important role in controlling the tone of the underlying vascular smooth muscle by releasing vasodilating factors including prostacyclin, nitric oxide (NO), and endothelium-derived hyperpolarizing factor (EDHF) [18, 19]. It has been shown that activation of TRPV4 in endothelial cells causes endothelium-dependent vascular relaxation in rat coronary artery [20]. Furthermore, the loss-of-function of TRPV4 results in a loss of shear stress-induced vasodilation, a response pattern critically dependent on endothelial TRPV4 expression [21]. These findings suggest that vasodilation induced by TRPV4 activation is, at least in part, endothelium-dependent. To identify which endothelial pathway is involved in the TRPV4-mediated hypotensive effect, we used various inhibitors/blockers in combination with 4α-PDD to achieve this goal. These inhibitors/blockers include indomethacin (a cyclooxygenase inhibitor), Nω-Nitro-L-arginine (L-NA, a NO synthase inhibitor), and apamin [an inhibitor of small-conductance Ca2+-activated K+ (MaxiК) channels, SKCa] plus charybdotoxin [an inhibitor of intermediate- and large-conductance MaxiК channels, IKCa and BKCa].
Overall, the present study was designed to inquire the mechanisms involved in the hypotensive effects induced by TRPV4 activation. First, we determined the 4α-PDD-induced hypotensive effect in vehicle- or capsaicin-pretreated rats to test if neuropeptides released from sensory nerves play a role in the depressor effect, and then, the specific endothelium-dependent pathway involved was identified by use of various inhibitors/blockers.
Methods
Animal preparation
All of the experiments were approved by the Institutional Animals Care and Use Committee. Pregnant Wistar rats (Charles River Laboratories, Wilmington, Massachusetts, USA) were housed in the animal facility for at least 1 week before parturition. Neonatal rats received capsaicin 50 mg/kg s.c. in a volume of 10 mL/kg 1-2 days postnatally as described [16, 17]. Control neonatal rats were treated with equal volumes of vehicle solution (5% ethanol, 5% Tween 80 in saline). After 3 weeks of weaning, male and female rats were separated, and only male rats were used at the age of 8 weeks in the present study.
Surgical preparation
Under anesthesia with sodium pentobarbital at 50 mg/kg i.p., the left carotid artery was cannulated with a polyethylene tube containing isotonic heparin saline solution (5 U/ml) and connected with a Statham 231 D pressure transducer coupled to a Gould 2400s recorder for continuous monitoring of mean arterial pressure (MAP). The right jugular vein was cannulated for intravenous administration of drugs.
Preparation of samples
When the depressor phase approached the lowest point in response to bolus administration of 4α-PDD, i.e. 4-6 min after injection, the rats were killed by decapitation. Arterial and venous blood was collected and centrifuged for plasma CGRP and substance P assays. To determine colocalization of TRPV4 with MaxiК in mesenteric resistant arteries or with CGRP in dorsal root ganglia (DRG) neurons/perivascular sensory nerve fibers, vehicle-pretreated rats were killed by decapitation without subjecting to acute experiments, and mesenteric resistant arteries and DRG were collected for immunohistochemistry staining.
Experimental protocols Mean arterial pressure and drug administration
The effectiveness of degeneration of capsaicin-sensitive sensory nerves was verified by monitoring MAP responses to bolus administration of capsaicin at 30 μg/kg i.v. to vehicle- or capsaicin-pretreated rats. Vehicle- and capsaicin-pretreated rats were further divided into five groups for i.v. administration of vehicle or 4α-PDD in the presence or absence of indomethacin, L-NA, or a combined administration of apamin and charybdotoxin. Indomethacin at 10 mg/kg, L-NA at 20 mg/kg, or combination of apamin at 50 μg/kg and charybdotoxin at 50 μg/kg were given i.v. 15 min, 30 min or 20 min, respectively, before i.v. administration of 4α-PDD at 2.5 mg/kg. The doses of indomethacin, L-NA, apamin and charybdotoxin were chosen based on previous studies [22-27]. The i.v. injection volume for each drug was 400 μl. At the above specified doses, these drugs nearly completely inhibited/blocked activities of the corresponding endothelial pathways.
To examine the effect of CGRP8-37, an antagonist of CGRP receptor, on 4α-PDD-induced hypotension, CGRP8-37 was intravenously administered at the rate of 1 mg/kg per min for 2 min, followed by administration at the rate of 0.4 mg/kg per min for 15 min in vehicle-treated rats. 4α-PDD at 2.5 mg/kg was given i.v. bolus 1 min after CGRP8-37 administration. The dose and time frame for CGRP8-37 were chosen based on the previous study [28].
Radioimmunoassay
To determine immunoactive CGRP and substance P in plasma, commercially available rabbit anti-rat CGRP radioimmunoassay kit and rabbit anti-rat substance P radioimmunoassay kit (Bachem Americas Inc., Torrance, CA) were used. The assays were performed as recommended by the manufacturer.
Immunohistochemistry
Colocalization of TRPV4 and CGRP in perivascular sensory nerves innervating the mesenteric resistance arteries
Mesenteric resistance arteries were fixed with Zamboni’s fixative solution containing 2% formaldehyde, 15% picric acid and 0.1 M phosphate buffer on ice for 2 hours, washed with dimethyl sulfoxide (1×10 min) and phosphate-buffered saline (PBS) (3×10 min) at room temperature, and incubated in 0.4% Triton-X 100 for permeabilization and 5% fetal bovine serum in PBS for 30 minutes at room temperature to block nonspecific binding. The arteries were subsequently incubated with goat anti-rat CGRP (1:500, Santa Cruz Biotechnology, Santa Cruz, CA) and/or rabbit anti-rat TRPV4 (1:300, Alomone labs, Jerusalem, Israel), followed by a secondary antibody cocktail of donkey anti-goat CY3 (1:500, Jackson ImmunoResearch, West Grove, PA) and donkey anti-rabbit FITC (1:300, Jackson ImmunoResearch, West Grove, PA) for 30 minutes. After washing with PBS, the vessels were mounted to slides and optical sections of the vessels were viewed under Zeiss Pascal confocal laser scanning microscope using 488-nm and 543-nm laser. Negative control was performed by omission of primary antibodies, which showed no immunoreactivity.
Colocalization of TRPV4 and CGRP in DRG neurons
DRG was embedded in OCT compound and placed in liquid nitrogen. Samples of freshly frozen DRG were sectioned at 5 μm on a Cryotome FSE (Thermo Electron, Pittsburgh, PA), placed on 3-aminoalkyethoxysilane coated slides, fixed in 75% Acetone/Ethanol (v/v) for 5 minutes and incubated in normal donkey serum (1:28, Jackson ImmunoResearch labs, West Grove, PA) for 30 minutes. DRG sections were examined for CGRP and TRPV4 expression as described above with the use of goat anti-rat CGRP (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-rat TRPV4 (1:50, Alomone labs, Jerusalem, Israel), followed by a secondary antibody cocktail of donkey anti-goat CY3 (1:136, Jackson ImmunoResearch labs, West Grove, PA) and donkey anti-rabbit FITC (1:136, Jackson ImmunoResearch labs, West Grove, PA) for 30 minutes. The slides were viewed under Zeiss Pascal confocal laser scanning microscope using 488-nm and 543-nm laser. Negative control was performed by omission of primary antibodies, which showed no immunoreactivity.
Colocalization of TRPV4 and MaxiК in the endothelium of mesenteric resistant arteries
Mesenteric resistant arteries were embedded in OCT compound and placed in liquid nitrogen. Samples of freshly frozen mesenteric resistant arteries were sectioned at 5 μm on a Cryotome FSE (Thermo Electron, Pittsburgh, PA), placed on 3-aminoalkyethoxysilane coated slides, fixed in 75% Acetone/Ethanol (v/v) for 5 minutes and incubated in normal donkey serum (1:28, Jackson ImmunoResearch labs, West Grove, PA) for 30 minutes. Mesenteric resistant artery sections were incubated in avidin (Vector) and d-biotin (Sigma Chemical, St. Louis, MO) for 15 minutes. After washing with PBS, the arteries were incubated at 4 °C overnight in a primary antibody cocktail of goat anti-rat MaxiКβ (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-rat TRPV4 (1:100, Alomone labs, Jerusalem, Israel), followed by a secondary antibody cocktail of donkey anti-goat CY3 (1:136, Jackson ImmunoResearch labs, West Grove, PA) and donkey anti-rabbit FITC (1:136, Jackson ImmunoResearch labs, West Grove, PA) for 30 minutes. The slides were viewed under Zeiss Pascal confocal laser scanning microscope using 488-nm and 543-nm laser. Negative control was performed by omission of primary antibodies, which showed no immunoreactivity.
Drugs
Capsaicin and 4α-PDD (LC Laboratories, Woburn, MA) were dissolved in ethanol (5% v/v), Tween-80 (5% v/v) and saline to be used freshly. Indomethacin was dissolved in 0.24% Na2CO3 solution. Apamin, charybdotoxin, L-NA, or CGRP8-37 was dissolved in saline.
Statistical analysis
All values were expressed as means ± SE. Differences between 2 groups were analyzed by the use of the unpaired Student t test. Differences among groups were analyzed using one-way ANOVA followed by a Bonferroni’s adjustment for multiple comparisons. Differences were considered statistically significant at P<0.05.
Results
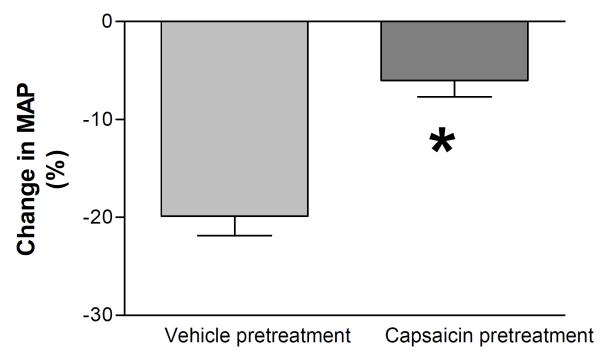
To examine the effectiveness of degeneration of capsaicin-sensitive sensory nerves, MAP responses to bolus i.v. injection of capsaicin, a selective TRPV1 channel activator, were examined in vehicle- or capsaicin-pretreated rats. Baseline MAP measured 1 hour after surgery was slightly but significantly lower in capsaicin pretreated compared to vehicle-pretreated rats (100 ± 4 mmHg vs 116 ± 3 mmHg, P<0.05). Bolus i.v. injection of capsaicin caused a greater decrease in MAP in vehicle-pretreated rats (-20 ± 1%) than in capsaicin-pretreated rats (-6 ± 1%, P<0.05) (Fig. 1), indicating that capsaicin-sensitive sensory nerves were effectively denervated by capsaicin neonatal pretreatment [17, 28, 29].
Figure 1.
MAP responses to intravenous injection of capsaicin (CAP, 30 μg/kg) in anesthetized vehicle- or capsaicin-pretreated rats. Values are mean ± SE (n=5 to 6). *P<0.05 compared with the corresponding vehicle-pretreated group.
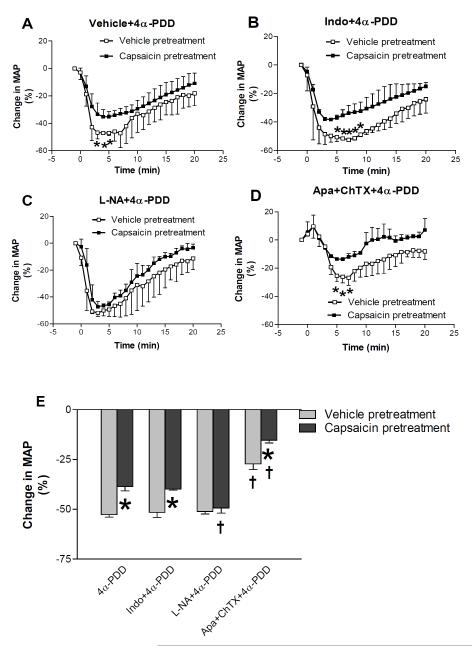
Fig. 2 shows that i.v. administration of 4α-PDD produced remarkable hypotension in vehicle-treated rats (-53 ± 1%). MAP decreased promptly, reached the lowest point around 4-6 minutes and returned to baseline gradually over the course of 20 minute-recording. To identify which endothelial pathway is involved in the TRPV4-mediated hypotensive effect, various inhibitors/blockers, indomethacin, L-NA, and apamin plus charybdotoxin, were used in the present study. Intravenous administration of indomethacin or apamin plus charybdotoxin induced a very transient increase of MAP while L-NA caused a stable increase of MAP 20 min after injection (vehicle pretreatment, 115 ± 3 mmHg vs 143 ± 4 mmHg, P<0.05; capsaicin pretreatment, 99 ± 5 vs 124 ± 4 mmHg, P<0.05). 4α-PDD was injected 10-15 minutes after MAP returned to baselines in the cases of indomethacin or apamin plus charybdotoxin administration and 30 minutes after L-NA administration. In vehicle-pretreated rats, the hypotensive effect of 4α-PDD was attenuated by combined administration of apamin and charybdotoxin (-27 ± 3%, P<0.05), but not by indomethacin (-52 ± 3%) or L-NA (-51 ± 2%), indicating that MaxiК channels play a key role in 4α-PDD-induced hypotension.
Figure 2.
MAP responses to intravenous injection of 4α-PDD (2.5 mg/kg) with or without indomethacin (10 mg/kg, i.v.), L-NA (20 mg/kg, i.v.) and apamin (50 μg/kg, i.v.) plus charybdotoxin (50 μg/kg, iv.) in anesthetized vehicle- or capsaicin-pretreated rats. A: Time-course responses of mean arterial pressure (MAP) to bolus injection of 4α-PDD in rats. B-D: Time-course responses of mean arterial pressure (MAP) to bolus injection of 4α-PDD with indomethacin, L-NA or apamin plus charybdotoxin, respectively. E: maximal changes of MAP in response to intravenous administration of drugs. Values are mean ± SE (n=5 to 6). *P<0.05 compared with the corresponding vehicle-pretreated group, †P<0.05 compared with the corresponding group administered 4α-PDD.
Compared to vehicle-treated rats, denervation of capsaicin-sensitive sensory nerves significantly attenuated 4α-PDD-induced hypotensive effects (-39 ± 2%, P<0.05), implicating that sensory nerves and/or their neuropeptides regulate 4α-PDD action. Furthermore, the depressor effect of 4α-PDD was further attenuated by combined administration of apamin and charybdotoxin (-15 ± 1%, P<0.05), but not by indomethacin (-40 ± 1%). Interestingly, administration of L-NA slightly enhanced the depressor effect of 4α-PDD in capsaicin-pretreated rats (-49 ± 2%, P<0.05). Taken together, these findings indicate involvement of MaxiК channels and sensory nerves in 4α-PDD-induced hypotension and absence of contributions of NO and prostacyclin to the depressor effect.
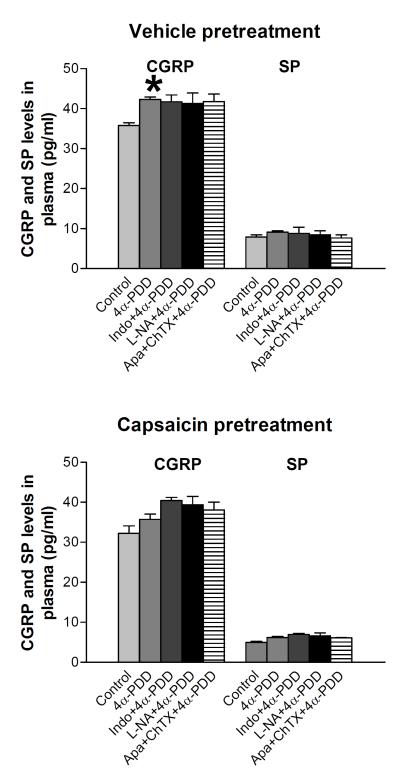
Plasma CGRP and substance P levels in each of the five groups are shown in Fig. 3. Intravenous administration of 4α-PDD significantly increased plasma CGRP levels in vehicle-pretreated rats (35.8 ± 1.0 pg/ml vs 42.3 ± 1.1 pg/ml, P<0.05), but not in capsaicin-pretreated group (32.3 ± 2.1 pg/ml vs 36.3 ± 1.5 pg/ml). Indomethacin, L-NA, or combined administration of apamin and charybdotoxin had no effect on 4α-PDD-inudced increases in plasma CGRP levels in vehicle-pretreated rats, but in capsaicin-pretreated rats, administration of these inhibitors/blockers slightly elevated 4α-PDD-induced release of CGRP though no significant changes were observed. In contrast, i.v. administration of 4α-PDD with or without indomethacin, L-NA, or combined administration of apamin and charybdotoxin didn’t affect plasma substance P levels in either vehicle-pretreated (7.9 ± 0.6 pg/ml vs 9.1 ± 0.4 pg/ml) or capsaicin-pretreated rats (5.0 ± 0.5 pg/ml vs 6.2 ± 0.4 pg/ml). These results indicate that activation of TRPV4 causes CGRP but not substance P release from sensory nerves only when sensory nerves are intact.
Figure 3.
/B> Plasma CGRP and substance P (SP) levels in response to bolus injection 4α-PDD (2.5 mg/kg) with or without indomethacin (10 mg/kg, i.v.), L-NA (20 mg/kg) and apamin (50 μg/kg, i.v.) plus charybdotoxin (50 μg/kg, i.v.). Values are mean ± SE (n=4-7). *P<0.05 compared with the corresponding control group.
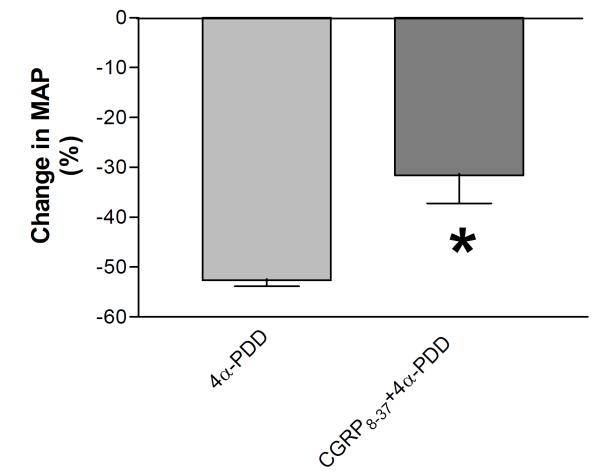
To assess the role of increased CGRP release in 4α-PDD-induced hypotension in vehicle-pretreated rats, MAP responses to 4α-PDD with or without CGRP8-37 were examined. Blockade of CGRP receptors with CGRP8-37 significantly attenuated the depressor effect of 4α-PDD in vehicle-pretreated rats (P<0.05), indicating that activation of CGRP receptors upon CGRP release induced by 4α-PDD contributes to TRPV4-mediated hypotension (Fig. 4). Given that the magnitude of MAP reduction in response to CGRP8-37 plus 4α-PDD treatment in vehicle-pretreated rats (-32 ± 4%) was very similar to that induced by 4α-PDD treatment in capsaicin-pretreated rats (-39 ± 2%), these results indicate that inability of 4α-PDD in inducing CGRP release in capsaicin-pretreated rats may account for attenuated hypotensive effects of 4α-PDD in these rats bearing sensory nerve impairment.
Figure 4.
Mean arterial pressure responses to bolus injection of 4α-PDD (2.5 mg/kg) with or without CGRP8-37 in vehicle-pretreated rats. Values are mean ± SE (n=5 to 6). *P<0.05 compared with the corresponding 4α-PDD-treated group.
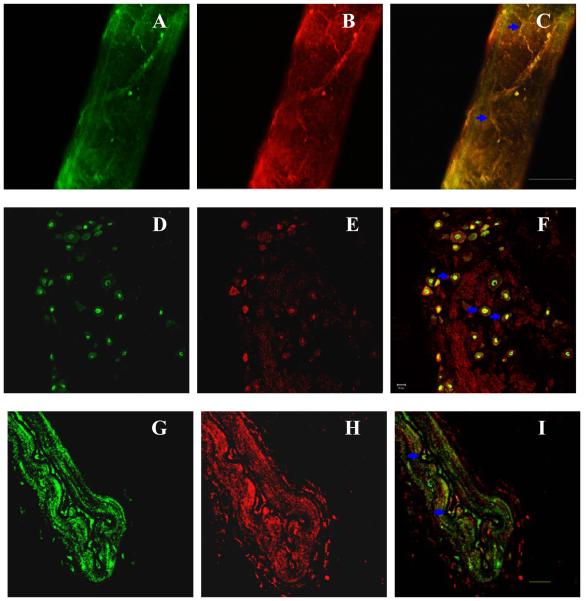
Double staining revealed that TRPV4, expressed in sensory neurons in DRG and sensory nerve fibers innervating mesenteric resistant arteries, colocalized with CGRP (Fig. 5). Moreover, immunohistochemistry staining of mesenteric resistant artery sections showed the colocalization of TRPV4 and MaxiК channels in vascular endothelium (Fig. 5). Colocalization is indicated by blue arrows. These results provide anatomical support for the notion that TRPV4 activation leads to CGRP release or opening of MaxiК channels.
Figure 5.
Confocal microscope images of double-immunofluorescence staining of mesenteric resistant arteries (A, B, C, G, H, I) and DRG neurons (D, E, F). A, D and G: FITC-labeled TRPV4 receptor staining (green). B and E: Cy3-labeled CGRP staining (red). C and F: colocalization of TRPV4 and CGRP (yellow). H: Cy3-labeled MaxiК channels staining (red). I: colocalization of TRPV4 and MaxiК channels (yellow). Blue arrows: the colocalized areas. Negative control not shown. Scale bars, 20 μm.
Discussion
The present study aimed at defining the mechanisms underlying the hypotensive effects induced by TRPV4 activation. We found that 1) 4α-PDD is less effective in decreasing blood pressure when sensory nerves are impaired by neonatal capsaicin pretreatment, indicating that sensory nerves and/or their neuropeptides contribute to 4α-PDD-induced hypotension; 2) 4α-PDD increases plasma CGRP but not substance P levels when sensory nerves are intact, and blockade of the CGRP receptors attenuates 4α-PDD-induced hypotension. These results support the notion that CGRP receptor activation upon CGRP release triggered by TRPV4 activation contributes to 4α-PDD-induced hypotension; 3) blockade of MaxiК channels but not inhibition of cyclooxygenase or NO synthase markedly attenuates 4α-PDD-induced hypotension, indicating contribution of MaxiК channels in 4α-PDD-induced hypotension and the lack of a significant role for NO and prostacyclin in the depressor effect induced by 4α-PDD; and 4) TRPV4 colocalizes with MaxiК channels in resistant vascular endothelium and with CGRP in sensory neurons/nerve fibers, providing anatomical evidence for interaction between TRPV4 and MaxiК channels and TRPV4-mediated CGRP release. Taken together, these data indicate that the hypotensive effect of TRPV4 attributes to opening of MaxiК channels and activation of CGRP receptors following CGRP release induced by activation of TRPV4.
A dense perivascular CGRP-containing sensory nerve network is found around blood vessels in virtually all vascular beds and counterbalances the prohypertensive effects of several neurohormonal systems to maintain normal blood pressure. In the present study, we were expecting to see an elevated baseline blood pressure in capsaicin-pretreated rats. However, we saw an opposite result in anesthetized rats. A possible explanation is that degeneration of capsaicin-sensitive sensory nerves leads to relatively increased sympathetic nerve activity and suppression of which by anesthesia lowers blood pressure.
An endogenous cannabinoid lipid, anandamide, may activate TRPV1 leading to vasodilation and subsequent hypotension that depends on CGRP release from perivascular sensory nerves [30-32]. Similarly, TRPV4 is expressed in sensory neurons that contain CGRP [33], a finding consistent with the results obtained in the present study showing that TRPV4 colocalizes with CGRP in DRG and perivascular nerve fibers. Moreover, the data in the present study showed that TRPV4 activation increased plasma CGRP levels and that blockade of the CGRP receptors inhibited 4α-PDD-induced depressor effects in a manner similarly as that of sensory nerve impairment. These findings indicate a key role for CGRP in mediating TRPV4-mediated hypotension.
Sensory nerve fibers may be classified as capsaicin-sensitive and capsaicin-insensitive populations. Capsaicin administered subcutaneously at a dose of 50 mg/kg to neonatal rats leads to an irreversible loss of 70-80% of small-diameter sensory neurons in DRG and depletion of neuronal stores of neuropeptides, whereas plasma levels of these neuropeptides may not be altered significantly due to the supplement from other tissues such as the thyroid gland [17, 28, 31, 32]. As shown in Fig. 3, plasma CGRP levels were at the similar level albeit tended to be lower in capsaicin-pretreated compared to vehicle-pretreated rats, a result consistent with previous findings.
CGRP may cause hypotension via its direct effect as a potent vasodilator or by inhibiting sympathetic nerve activity [13]. To date, CGRP is the most potent vasodilator discovered. It has been shown that plasma immunoreactive CGRP levels elevate in the animal models of acquired hypertension and that CGRP plays a compensatory role to buffer the increase of blood pressure [34]. In the present study, i.v. administration of indomethacin/apamin plus charybdotoxin induced a transient increase of blood pressure, and administration of L-NA led to a prolonged hypertensive effect. While these inhibitors/blockers had no effect on 4α-PDD-induced CGRP release in vehicle-pretreated rats, these drugs tended to potentiate 4α-PDD-induced CGRP release in capsaicin-pretreated rats albeit the enhancement was not statistically significant. Moreover, it has been shown that L-NAME (a derivative of L-NA)-induced hypertension increases sensitivity of vascular responses to CGRP [34]. Taken together, enhanced CGRP release as well as vascular responsiveness to CGRP may underlie the enhanced hypotension in capsaicin-pretreated rats given L-NA plus 4α-PDD. Moreover, previous studies showed that cyclooxygenase-mediated increases in prostaglandin production participated in bradykinin-mediated CGRP synthesis and release [34, 35], which may explain the delayed hypotensive effects observed in rats treated with indomethacin plus 4α-PDD.
Vascular endothelium controls the tone of the underlying vascular smooth muscle by releasing mainly three kinds of vasodilating factors including prostacyclin, NO, and EDHF [18, 19]. It has been shown that 4α-PDD activates TRPV4 expressed in endothelial cells leading to endothelium-dependent vascular relaxation in rat coronary and cerebral arteries [20]. Opening of TRPV4 channels, mimicking that of TRPV1 activation, leads to increases in Ca2+ influx [36, 37] and possibly Ca2+-induced release of a vasodilator, NO [20]. An inhibitor of NO synthase was therefore used to examine the role of NO in TRPV4 action. We found however that inhibition of NO synthase had no effect on 4α-PDD-induced decreases in blood pressure. Furthermore, inhibition of the cyclooxygenase pathway failed to attenuate 4α-PDD-induced hypotension. These results indicate that neither NO nor prostacyclin may be a key player in TRPV4-induced hypotensive effects.
EDHF is synthesized and released by the endothelium, and the most likely candidates for EDHF include epoxyeicosatrienoic acids (EETs), endothelium-derived potassium ions (K+), hydrogen peroxide (H2O2), and C-type natriuretic peptide [38]. EDHF is as powerful as NO or prostacyclin (PGI2) in terms of regulating blood pressure, and impairment in the EDHF signaling contributes to several cardiovascular pathologies including hypertension, chronic renal failure, and diabetes [39]. EDHF may increase intracellular calcium concentrations that lead to the opening of MaxiК (SKCa, IKCa, and BKCa) channels and hyperpolarization of endothelial cells [40]. Accumulating evidence shows possible involvement of EDHF in arterial dilation induced by activation of TRPV4 [41-43]. The data in the present study showed that blockade of SKCa, IKCa, and BKCa by combined administration of apamin and charybdotoxin attenuated 4α-PDD-induced hypotension possibly via preventing the opening of MaxiК channels, indicating that TRPV4-mediated depressor effects are, to a great extent, endothelial- and MaxiК channel-dependent.
In conclusion, our data demonstrate that TRPV4, co-expressed with MaxiК channels in resistant vessels and with CGRP in sensory neurons/nerve fibers, mediates profound hypotensive effects. The hypotensive effect of TRPV4 attributes to activation of MaxiК channels and CGRP receptors triggered by TRPV4-dependent CGRP release from sensory nerves. These findings may have important implications for future development of antihypertensive therapy.
Acknowledgments
Sources of Funding This work was supported in part by National Institutes of Health (grants HL-57853, HL-73287, and DK67620) and a grant from Michigan Economic Development Corporation.
Footnotes
Disclosures None.
References
- (1).Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278:26541–9. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- (2).Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- (3).Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–8. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- (4).Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286:C195–205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- (6).Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–35. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci U S A. 2003;100:13698–703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100:14531–6. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–46. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- (10).Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–14. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Gao F, Sui D, Garavito RM, Worden RM, Wang DH. Salt intake augments hypotensive effects of transient receptor potential vanilloid 4: functional significance and implication. Hypertension. 2009;53(2):228–35. doi: 10.1161/HYPERTENSIONAHA.108.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–7. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- (13).Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, Imai Y, Kayaba Y, Nishimatsu H, Suematsu Y, Hirata Y, Yazaki Y, Nagai R, Kuwaki T, Kurihara H. Elevated sympathetic nervous activity in mice deficient in α-CGRP. Circ Res. 2001;89:983–90. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- (14).Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–33. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Koltzenburg M. The role of TRP channels in sensory neurons. Novartis Found Symp. 2004;260:206–13. [PubMed] [Google Scholar]
- (16).Jancsó G, Kiraly E, Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurons. Nature. 1977;270:741–3. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- (17).Wang DH, Li J, Qiu J. Salt-sensitive hypertension induced by sensory denervation: introduction of a new model. Hypertension. 1998;32:649–53. doi: 10.1161/01.hyp.32.4.649. [DOI] [PubMed] [Google Scholar]
- (18).Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–6. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- (19).Cohen RA. The endothelium-derived hyperpolarizing factor puzzle: a mechanism without a mediator? Circulation. 2005;111:724–7. doi: 10.1161/01.CIR.0000156405.75257.62. [DOI] [PubMed] [Google Scholar]
- (20).Köhler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilation. Arterioscler Thromb Vasc Biol. 2006;26:1495–502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- (21).Harmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Köhler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE. 2007;2:e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Schaeffer P, Laplace MC, Prabonnaud V, Bernat A, Gully D, Lespy L, Herbert JM. Neurotensin induces the release of prostacyclin from human umbilical vein endothelial cells in vitro and increases plasma prostacyclin levels in the rat. Eur J Pharmacol. 1997;323:215–21. doi: 10.1016/s0014-2999(97)00041-1. [DOI] [PubMed] [Google Scholar]
- (23).Travis MD, Hoque A, Bates JN, Lewis SJ. Blockade of voltage-sensitive Ca2+-channels markedly diminishes nitric oxide-but not L-S-nitrosocysteine- or endothelium-dependent vasodilation in vivo. Eur J Pharmacol. 2000;408:289–98. doi: 10.1016/s0014-2999(00)00792-5. [DOI] [PubMed] [Google Scholar]
- (24).Izuta M, Clavier N, Kirsch JR, Traystman RJ. Cerebral blood flow during inhibition of brain nitric oxide synthase activity in normal, hypertensive, and stroke-prone rats. Stroke. 1995;26:1079–85. doi: 10.1161/01.str.26.6.1079. [DOI] [PubMed] [Google Scholar]
- (25).Brooks VL, Clow KA, Welch LS, Giraud GD. Does nitric oxide contribute to the basal vasodilation of pregnancy in conscious rabbits? Am J Physiol Regul Integr Comp Physiol. 2001;281:R1624–32. doi: 10.1152/ajpregu.2001.281.5.R1624. [DOI] [PubMed] [Google Scholar]
- (26).Kawabata A, Nakaya Y, Kuroda R, Wakisaka M, Masuko T, Nishikawa H, Kawai K. Involvement of EDHF in the hypotension and increased gastric mucosal blood flow caused by PAR-2 activation in rats. Br J Pharmacol. 2003;140:247–54. doi: 10.1038/sj.bjp.0705433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Sankaralingam S, Desai KM, Wilson TW. Clofibrate acutely reverses saline-induced endothelial dysfunction: role of calcium-activated potassium channels. Am J Hypertens. 2006;19:1167–73. doi: 10.1016/j.amjhyper.2006.04.005. [DOI] [PubMed] [Google Scholar]
- (28).Wang Y, Wang DH. Increased depressor response to N-arachidonoyl-dopamine during high salt intake: role of the TRPV1 receptor. J Hypertens. 2007;25:2426–33. doi: 10.1097/HJH.0b013e3282efd1bf. [DOI] [PubMed] [Google Scholar]
- (29).Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Högestätt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br J Pharmacol. 2000;130:1483–8. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–7. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- (31).Wang Y, Kaminski NE, Wang DH. VR1-mediated depressor effects during high-salt intake: role of anandamide. Hypertension. 2005;46:986–91. doi: 10.1161/01.HYP.0000174596.95607.fd. [DOI] [PubMed] [Google Scholar]
- (32).Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41:757–62. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- (33).Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective Role for TRPV4 Ion Channels in Visceral Sensory Pathways. Gastroenterology. 2008;134(7):2059–69. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Watson RE, Supowit SC, Zhao H, Katki KA, Dipette DJ. Role of sensory nervous system vasoactive peptides in hypertension. Braz J Med Biol Res. 2002;35(9):1033–45. doi: 10.1590/s0100-879x2002000900004. [DOI] [PubMed] [Google Scholar]
- (35).Ma W, Eisenach JC. Intraplantar injection of a cyclooxygenase inhibitor ketorolac reduces immunoreactivities of substance P, calcitonin gene-related peptide, and dynorphin in the dorsal horn of rats with nerve injury or inflammation. Neuroscience. 2003;121(3):681–90. doi: 10.1016/s0306-4522(03)00497-4. [DOI] [PubMed] [Google Scholar]
- (36).Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–15. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- (37).Poblete IM, Orliac ML, Briones R, Adler-Graschinsky E, Huidobro-Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol. 2005;568:539–51. doi: 10.1113/jphysiol.2005.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).de Wit C, Wölfle SE. EDHF and gap junctions: important regulators of vascular tone within the microcirculation. Curr Pharm Biotechnol. 2007;8:11–25. doi: 10.2174/138920107779941462. [DOI] [PubMed] [Google Scholar]
- (39).Köhler R, Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney International. 2007;72:145–150. doi: 10.1038/sj.ki.5002303. [DOI] [PubMed] [Google Scholar]
- (40).Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–25. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- (41).Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–9. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- (42).Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117:1065–74. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- (43).Kotlikoff MI. EDHF redux: EETs, TRPV4, and Ca2+ sparks. Circ Res. 2005;97:1209–10. doi: 10.1161/01.RES.0000196741.99904.e4. [DOI] [PubMed] [Google Scholar]