Abstract
Background
Individuals with a negative screening colonoscopy are recommended to repeat colonoscopy in ten years.
Objective
To assess the effectiveness and costs of colonoscopy versus other rescreening strategies following a negative colonoscopy.
Design
Microsimulation model.
Data Sources
Literature and SEER.
Target Population
50-year-olds who had no adenomas or cancer detected at screening colonoscopy.
Time Horizon
Lifetime.
Perspective
Societal.
Interventions
No further screening and rescreening at age 60 with ten-yearly colonoscopy, yearly highly-sensitive guaiac fecal occult blood testing (HSFOBT), yearly fecal immunochemical testing (FIT), or five-yearly computed tomographic colonography (CTC).
Outcome Measures
Lifetime number of colorectal cancer cases, life expectancy, and lifetime costs per 1000 individuals assuming: 1) perfect adherence and 2) imperfect adherence.
Results of Base-Case Analysis
Rescreening with any modality yielded sizable reductions in colorectal cancer risk compared to no further screening (range 7.7 to 12.6 lifetime cases per 1000 (perfect adherence) and 17.7 to 20.9 lifetime cases per 1000 (imperfect adherence), versus 31.3 lifetime cases per 1000 with no further screening). For both adherence scenarios, the differences in life-years across rescreening strategies were small (range 30,893 to 30,902 per 1000 (perfect adherence) and 30,865 to 30,869 per 1000 (imperfect adherence)). Compared to continuing colonoscopy, rescreening with HSFOBT, FIT, or CTC had fewer complications and was less costly.
Results of Sensitivity Analysis
Results were sensitive to test-specific adherence rates.
Limitations
Limited data on adherence with rescreening.
Conclusions
Compared with the currently-recommended strategy of continuing ten-yearly colonoscopy after an initial negative exam, rescreening at age 60 with yearly HSFOBT, yearly FIT, or five-yearly CTC provides approximately the same benefit in life-years with fewer complications and at a lower cost. Therefore it is reasonable to rescreen individuals with a negative colonoscopy with other modalities.
INTRODUCTION
Screening has been shown to reduce colorectal cancer incidence (1–3) and mortality (1–6). Screening rates have increased substantially over the past decade (7–8). Although alternative screening approaches are sanctioned by guidelines (9–10), much of the rise in screening was driven by increased use of colonoscopy (7).
Colonoscopy is a recommended method for routine colorectal cancer screening (9–10) and is used for follow-up of individuals with positive results on other screening tests (9) such as fecal occult blood tests (FOBTs), and for surveillance of individuals with a family or personal history of adenomas or colorectal cancer (11–13). Although safe, colonoscopy can cause complications (14–16) that in rare cases may be fatal (14–15, 17). Moreover, it requires considerable resources. Thus, strategic use of colonoscopy should be a priority for healthcare delivery.
Population-based registry and claims database studies have shown that the risk of developing colorectal cancer for individuals with a negative colonoscopy is substantially lower than the risk for those who are unscreened (18–19). This has prompted consideration of whether the guideline recommendation to repeat colonoscopy ten years following a negative exam (9–10) is indeed necessary. These guidelines (and mathematical models that have evaluated them) have assumed that people use only one screening test throughout their lifetimes. We assessed whether alternative rescreening strategies for individuals following a negative screening colonoscopy could maximize benefits and minimize costs and harms.
METHODS
SimCRC Model
We used the Simulation Model of Colorectal Cancer (SimCRC) to evaluate management strategies for 50-year-olds with neither adenomas nor colorectal cancer detected at their first screening colonoscopy. SimCRC is one of the models in the National Cancer Institute-sponsored Cancer Intervention and Surveillance Modeling Network (CISNET) and has been used to inform the US Preventive Services Task Force's colorectal cancer screening guidelines (20) and the Centers for Medicare and Medicaid Services coverage decisions for stool deoxyribonucleic acid testing (21) and computed tomographic colonography (CTC) screening (22).
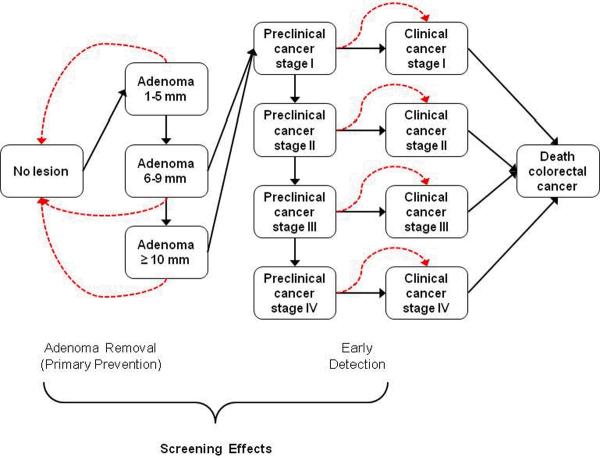
The SimCRC model is programmed in Microsoft Visual C++ 2010 Express (Seattle, Washington). The model specifications have been described (23–24). Briefly, the model's natural history component tracks the development of adenomas and their possible progression to invasive colorectal cancer in the absence of screening. An individual enters the model at birth and over time, may develop one or more adenomas (Figure 1). Adenomas may grow in size from small (1–5mm) to medium (6–9mm) to large (≥10mm); some may progress to preclinical colorectal cancer. Preclinical colorectal cancer may progress in stage (I–IV) and may be detected by symptoms. Relative survival following cancer diagnosis depends on age and tumor site and stage (25). Individuals may die from causes other than colorectal cancer at any age (26).
Figure 1.
Schematic of the SimCRC natural history model (solid lines) with the effect of screening noted (dashed lines). Individuals enter the model at birth free of colorectal cancer and adenomas. Over time they are at risk of forming one or more adenomas, each of which has the chance of growing in size and progressing to preclinical (ie, undiagnosed) and ultimately, clinical (ie, diagnosed), colorectal cancer. Screening has the ability to interrupt the natural history by detecting preclinical cancers before they progress to a more advanced stage, and detecting adenomas for removal, thereby preventing their potential to transition to colorectal cancer.
SimCRC's screening component allows adenoma(s) and/or preclinical colorectal cancer(s) to be detected based on the sensitivity of the screening test for lesion(s) of that type/size and, for endoscopic tests, the depth of endoscope insertion. Non-adenomatous polyps are not explicitly modeled but are reflected in test false-positive rates, allowing individuals to be referred for follow-up and undergo polypectomy for non-adenomatous polyps. We assume each detected adenoma is removed, thereby preventing its potential progression to colorectal cancer. Individuals with screen-detected colorectal cancer may face a lower risk of cancer death if it is detected at an earlier stage.
Model Calibration
Because the natural history of colorectal cancer is largely unobserved, there are limited data to directly inform some model parameters. We inferred their values by calibrating the model to data on the prevalence, size, location and multiplicity of adenomas from autopsy studies (27–36) and the incidence of colorectal cancer from the Surveillance, Epidemiology, and End Results (SEER) Program (25). We used SEER data from 1975–1979 since colorectal cancer screening was rarely performed during this period. The calibration approach and fit of the model to these data are provided elsewhere (22–24).
Rescreening Strategies
We evaluated five rescreening strategies for individuals with a negative colonoscopy at age 50: no further screening; continuing ten-yearly colonoscopy; or rescreening with a yearly highly-sensitive guaiac FOBT (HSFOBT), yearly fecal immunochemical test (FIT), or five-yearly CTC. These strategies are guideline-sanctioned options for routine screening for 50-year-olds (9–10) and are therefore reasonable alternatives for rescreening individuals with a negative colonoscopy. Rescreening was assumed to begin at age 60 (that is, ten years after the negative colonoscopy) for all strategies.
Follow-up, Surveillance, and Adherence
We assumed individuals with a positive HSFOBT or FIT or a CTC indicating lesion(s) 6mm or larger were referred for follow-up colonoscopy. Due to the possibility of systematic positive HSFOBTs or FITs due, for example, to persistent gastrointestinal bleeding unrelated to adenomas or colorectal cancer, individuals with no adenomas or colorectal cancer detected at follow-up were assumed to discontinue HSFOBT or FIT and resume screening with ten-yearly colonoscopy; individuals with a positive CTC but no adenomas or colorectal cancer detected at follow-up were assumed to continue CTC screening. If an adenoma was detected and removed at colonoscopy, the individual began colonoscopy surveillance consistent with guidelines (13). We assumed screening of individuals with no history of adenomas or colorectal cancer ended after age 75 (10), but surveillance of individuals with a history of adenoma(s) continued for life.
Reliable estimates for adherence are limited, yet adherence rates may have a major impact on results. Therefore, we considered two adherence scenarios: perfect and imperfect. Perfect adherence meant 100% participation with each test. With imperfect adherence, adherence following the initial negative colonoscopy varied by test and incorporated within-subject correlation for adherence with rescreening (Appendix Table 1). Adherence with HSFOBT was based on Department of Veterans Affairs' data (37). Among men who exclusively received guaiac FOBT, 42% received one over a five-year period, 26% received two, 18% received three, and 14% received four or more. For FIT, we assumed per-test adherence was 24% higher than with HSFOBT, based on the relative increase in uptake with FIT vs. guaiac FOBT in a Dutch screening program (38). For the first rescreening colonoscopy we assumed 52% average adherence based on adherence with a five-year repeat colonoscopy among those with a negative initial exam (39). We further assumed that individuals had, on average, only one of the two recommended rescreening colonoscopies (at age 60 or 70). For each follow-up and surveillance colonoscopy we assumed 94% average adherence (2, 38). In the absence of data for CTC, we assumed the average chance of adhering with the first CTC was the same as for repeat colonoscopy (that is, 52%) and that individuals had, on average, two CTCs by age 75.
Test Characteristics, Complications, and Costs
The sensitivities and specificity for each screening modality are shown in Table 1. We assumed that 5% of individuals would require two colonoscopies to achieve a complete exam and that the cecum was eventually examined in 95% of individuals. The model incorporated the risks of complications, including perforation, bleeding, and other gastrointestinal events (Table 2). We assumed there were 51.9 deaths per 1000 perforations (17).
Table 1.
Screening test characteristics and costs used in the analyses.
| Sensitivity* for adenomas by size and for CRC (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Analysis/screening test | Small (1–5mm) | Medium (6–9mm) | Large (≥10mm) | CRC | Specificity (%) | Source | Test cost, $ | Cost description |
| Base-case analysis | ||||||||
| HSFOBT | 7 | 12 | 24 | 70 | 93 | (40) | 23 | 2007 national average Medicare reimbursement for fecal occult blood assay (HCPCS G0394) adjusted to 2010 dollars, plus cost of one hour of patient time¶ |
| FIT | 5 | 10 | 22 | 70 | 95 | (40) | 46 | 2007 national average Medicare reimbursement and beneficiary copayment for immunoassay-based fecal occult blood test (HCPCS G0328) adjusted to 2010 dollars, plus cost of one hour of patient time¶ |
| Colonoscopy | 75 | 85 | 95 | 95 | 84† | (39,41) | 1,153 without polypectomy | Without polypectomy: weighted average of 2007 national average Medicare payments and beneficiary copayments for diagnostic colonoscopy (CPT 45378), colon screen in high-risk individual (CPT G0105), and colon cancer screening for non-high-risk individual (CPT G0121) (40) adjusted to 2010 dollars, plus the costs of colonic preparation** and 24 hours of patient/escort time¶ |
| 1,347 with polypectomy | With polypectomy: weighted average of 2007 national average Medicare payments and beneficiary copayments for colonoscopy and biopsy (CPT 45380), colonoscopy with submucosal injection (CPT 45381), colonoscopy/control bleeding (CPT 45382), lesion removal colonoscopy — fulguration (CPT 45383), lesion removal colonoscopy-hot biopsy (CPT 45384), and lesion removal colonoscopy-snare polypectomy (CPT 45385) (40) adjusted to 2010 dollars, plus the costs of colonic preparation** and 24 hours of patient/escort time¶ | |||||||
| CTC | -- | 57‡ | 84 | 84§ | 88∥ | (42) | 530 | 2010 national average Medicare reimbursement†† and beneficiary copayment for a diagnostic CTC (CPT 74261), plus the costs of colonic preparation** and eleven hours of patient time¶ |
| Sensitivity analysis ‡‡ | ||||||||
| Colonoscopy§§ | 60 | 68 | 76 | 76 | 84† | assumption | 577/770 to 5,765/5,959 | 0.5–5 times the base-case estimate of colonoscopy without polypectomy, plus the incremental cost of polypectomy |
| CTC | -- | 84‡ | 92 | 95 | 80∥ | (43–44) | 530 | Cost estimate was not varied from the base-case value |
CPT = Current Procedural Terminology code; CRC = colorectal cancer; CTC = computed tomographic colonography with ≥6mm threshold for colonoscopy referral; HSFOBT = highly-sensitive guaiac fecal occult blood test; HCPCS = Healthcare Common Procedure Coding System code; FIT = fecal immunochemical test, -- indicates sensitivity is not provided because size is smaller than the referral threshold for a colonoscopy.
Sensitivity is provided per individual for HSFOBT and FIT and per lesion for colonoscopy and CTC.
The lack of specificity with colonoscopy reflects the detection of non-adenomatous lesions. Non-adenomatous lesions induce polypectomy and biopsy costs.
Sensitivity for CTC for medium adenomas was calculated from published tables (42).
Sensitivity for CRC was assumed to be the same as for large adenomas.
The lack of specificity with CTC reflects detection of non-adenomatous lesions, artifacts, and adenomas smaller than the lesion size threshold for referral to colonoscopy of 6mm
The value of an hour of patient and/or caregiver time was assumed to equal the 2010 US median hourly wage rate for the civilian population, $18(45). The amounts of patient and escort time assumed for each test are detailed in Appendix Table 2.
Estimated at $23 (2010 average wholesale price of GoLYTELY® (46)).
With implementation of the Out-patient Prospective Payment cap on the technical component of imaging procedures (47).
The sensitivity analysis on colonoscopy test characteristics was performed separately from the sensitivity analysis on colonoscopy cost.
Assuming a 20% reduction from the base-case values. To account for the possibility that colonoscopy may not be as protective against right-sided disease, in the sensitivity analysis on colonoscopy test characteristics, we assumed that only 80% of colonoscopies are compete to the cecum (vs. 95% in the base-case analysis)
Table 2.
Complication rates, complication costs, and stage- and phase-specific costs of colorectal cancer care.
| Risks per 100,000 individuals by age group |
Annual costs of cancer care by phase†,$ |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis/test/complication | 50–65y* | 66–69y | 70–74y | 75–79y | 80–84y | ≥85y | Source | Cost, $ | Source | Analysis/Stage | Initial | Continuing | Terminal, CRC death | Terminal, Non-CRC death | Source |
| Base-case analysis | Base-case analysis | ||||||||||||||
| Colonoscopy | I | 34,547 | 2,907 | 59,719 | 18,473 | Personal communication R. Yabroff & M. Brown of the NCI | |||||||||
| Perforation | 36 | 36 | 42 | 52 | 64 | 87 | (16) | 15,985 | (40) | II | 46,145 | 2,734 | 59,484 | 16,720 | |
| Bleeding with transfusion | 89 | 89 | 103 | 127 | 156 | 214 | (16) | 7,784 | (40) | III | 55,870 | 3,793 | 62,705 | 20,581 | |
| Bleeding without transfusion | 245 | 245 | 284 | 351 | 430 | 589 | (16) | 1,775 | (40) | IV | 72,533 | 11,352 | 82,413 | 46,834 | |
| Other gastrointestinal events | 320 | 320 | 400 | 540 | 730 | 880 | (16) | 1,195 | (40) | ||||||
| CTC | |||||||||||||||
| – Perforation | 5 | 5 | 5 | 5 | 5 | 5 | (48) | 15,985 | (40) | ||||||
| Sensitivity analysis | Sensitivity analysis | ||||||||||||||
| Colonoscopy | I | 43,184 | 3,634 | 74,648 | 23,091 | Assumption‡ | |||||||||
| Perforation | 28 | 28 | 32 | 40 | 49 | 67 | (16) | II | 57,681 | 3,417 | 74,355 | 20,901 | |||
| Bleeding with transfusion | 16 | 16 | 18 | 22 | 27 | 37 | (16) | III | 69,838 | 4,741 | 78,382 | 25,726 | |||
| Bleeding without transfusion | 43 | 43 | 50 | 61 | 75 | 103 | (16) | IV | 90,667 | 14,189 | 103,017 | 58,542 | |||
| Other gastrointestinal events | 34 | 34 | 42 | 57 | 77 | 93 | (16) | ||||||||
CRC = colorectal cancer; CTC = computed tomographic colonography; NCI = National Cancer Institute; y = years.
The risks of colonoscopy complications were based on a study by of Medicare beneficiaries (16). We assumed the risks for individuals 65 years and younger were the same as those for 66–69-year-olds, that the proportion of serious gastrointestinal events that were perforations (vs. bleeding) did not vary by age, and that bleeding alone was never fatal.
Estimates include beneficiary copayments and patient time costs. The initial phase is the first 12 months following diagnosis, the terminal phase is the final 12 months of life, and the continuing phase is all months between the initial and terminal phases, annualized. For simulated individuals who survived 12 months or less, only terminal costs (or a fraction thereof) were assigned; those who survived more than 12 months but less than or equal to 24 months were assigned terminal costs and initial costs (or a fraction thereof). Those who survived more than 24 months were assigned terminal costs, initial costs, and continuing costs.
We assumed a 25% increase in stage- and phase-specific costs, compared to the base-case estimates.
Costs of screening tests (Table 1) and complications (Table 2) were based on 2007 national-average Medicare payments and beneficiary co-payments (assuming these payments were applicable to 50-64-year-olds) and patient time costs (see Appendix Table 2). Since Medicare does not currently reimburse for a screening CTC, we used the payment for a diagnostic study. The cost of bowel preparation was estimated at $23 (46). An hour of time was valued at the 2010 median hourly wage rate for civilians, $18 (45).
The stage and phase specific costs of colorectal cancer care (Table 2) were based on analyses of SEER-Medicare linked data. The analyses used the methodology reported by Yabroff (49), with stage reclassified using the American Joint Committee on Cancer staging algorithm and costs in the last year of life stratified by cause of death. The estimates incorporate patient time costs and copayments (50).
All costs were expressed in 2010 dollars and were inflation-adjusted as needed using the Consumer Price Index (51).
Analysis
We used SimCRC to estimate the number of colorectal cancer cases and deaths, life-years, perforations and other complications, procedures requiring bowel preparation, and lifetime colorectal screening- and cancer-related costs for a hypothetical cohort of 50-year-olds with a negative screening colonoscopy under two adherence scenarios and five rescreening strategies. Outcomes were tallied from the time of the negative colonoscopy at age 50 until death. Costs were tallied from the societal perspective.
We performed sensitivity analyses on: colonoscopy test characteristics and cecal intubation rate; CTC test characteristics; colonoscopy complication rates; colonoscopy cost; costs of cancer care; and adherence rates (see Tables 1 and 2 and Appendix Table 1 for values used in the sensitivity analyses).
Role of the Funding Source
The National Cancer Institute funded this research. The funding source had no role in the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication. Drs. M. Brown and R. Yabroff of the National Cancer Institute provided the costs of colorectal cancer care used in the base-case analysis.
RESULTS
Base-case Analysis
Perfect adherence
SimCRC predicts that 15% of those with a negative colonoscopy at age 50 would have adenomas or colorectal cancer detected by colonoscopy at age 60. The corresponding estimate for those with a positive colonoscopy at age 50 is 31%, assuming no surveillance colonoscopies are performed between ages 50 and 60.
With no further screening, 31.3 per 1000 50-year-olds with a negative screening colonoscopy would be diagnosed with colorectal cancer in their lifetimes and 11.9 per 1000 would die from the disease. Compared to no further screening, all rescreening strategies yielded sizable reductions in colorectal cancer risk. With perfect adherence, continuing ten-yearly colonoscopy screening yielded the fewest cancer cases (7.7 per 1000) and deaths (2.4 per 1000), but the largest number of perforations and other complications (1.1 and 20.9 per 1000, respectively) (Table 3). Rescreening with CTC yielded slightly more cases (9.3 per 1000) and deaths (2.7 per 1000) than continuing colonoscopy and nearly halved the rates of perforation and other complications (0.7 and 10.1 per 1000, respectively), but had the largest number of procedures requiring bowel preparation (3,982 per 1000 vs. 2,592 with colonoscopy). Rescreening with HSFOBT or FIT yielded 11.4 and 12.6 cases and 3.2 and 3.5 deaths per 1000 respectively, with complication rates similar to CTC. The number of procedures requiring bowel preparation was 1,557 per 1000 for HSFOBT and 1,282 per 1000 for FIT. All rescreening strategies yielded comparable life-years, ranging from 30,893 per 1000 for FIT to 30,902 per 1000 for colonoscopy (Table 3), a difference of 3 days per person.
Table 3.
Colorectal cancer cases, life-years, perforations, other complications, and lifetime costs per 1000 50-year-old individuals with a negative screening colonoscopy, by adherence scenario and rescreening strategy.
| Outcomes per 1000 individuals with a negative colonoscopy at age 50 |
|||||||
|---|---|---|---|---|---|---|---|
| Adherence scenario/Rescreening strategy* | CRC cases | CRC deaths† | Life-years | Perforations | Other complications‡ | Procedures equiring bowel preparation§ | Lifetime costs,∥ thousands $ |
| Perfect adherence | |||||||
| Continue with COL | 7.7 | 2.4 | 30,902 | 1.1 | 20.9 | 2,592 | 3,840 |
| Switch to CTC | 9.3 | 2.7 | 30,899 | 0.7 | 10.1 | 3,982 | 3,673 |
| Switch to HSFOBT | 11.4 | 3.2 | 30,895 | 0.7 | 13.0 | 1,557 | 3,069 |
| Switch to FIT | 12.6 | 3.5 | 30,893 | 0.6 | 10.9 | 1,282 | 3,059 |
| No further screening | 31.3 | 11.9 | 30,821 | 0.0 | 0.0 | 31 | 2,446 |
| Imperfect adherence | |||||||
| Continue with COL | 17.7 | 6.4 | 30,867 | 0.6 | 11.0 | 1,361 | 3,084 |
| Switch to CTC | 17.8 | 6.1 | 30,869 | 0.4 | 5.6 | 2,135 | 2,993 |
| Switch to HSFOBT | 20.9 | 6.7 | 30,865 | 0.3 | 5.6 | 672 | 2,588 |
| Switch to FIT | 20.5 | 6.4 | 30,868 | 0.3 | 5.2 | 626 | 2,634 |
| No further screening | 31.3 | 11.9 | 30,821 | 0.0 | 0.0 | 31 | 2,446 |
COL = ten-yearly colonoscopy; CRC = colorectal cancer; CTC = five-yearly computed tomographic colonography; HSFOBT = yearly highly-sensitive guaiac fecal occult blood test; FIT = yearly fecal immunochemical test.
Assuming screening resumes at age 60 years, screening ends after age 75 years, and surveillance of individuals with a history of adenoma(s) continues until death.
Includes deaths from screening complications.
Bleeding and other gastrointestinal events.
Includes computed tomographic colonographies and screening, diagnostic, and surveillance colonoscopies. Does not include procedures performed after cancer diagnosis.
Includes costs of screening, follow-up, surveillance, complications, diagnosis of symptomatic cases, and cancer care.
With lifetime screening- and cancer-related costs of $3,840 per person, continuing colonoscopy screening was the most costly strategy (Table 3). Compared to continuing colonoscopy, cost-savings were $166 per person with CTC, $771 per person with HSFOBT, and $781 per person with FIT. Discounted life-years and costs are available in Appendix Table 3.
Imperfect adherence
With imperfect adherence, continuing colonoscopy yielded the fewest colorectal cancer cases (17.7 per 1000), followed closely by switching to CTC (17.8 per 1000) (Table 3). Switching to CTC yielded the fewest colorectal cancer deaths (6.1), compared with 6.4 deaths with colonoscopy, 6.4 deaths with FIT, and 6.7 deaths with HSFOBT (all deaths are per 1000 with a negative colonoscopy at age 50). Continuing colonoscopy yielded the highest rate of perforation and other complications (0.6 and 11.0 per 1000 respectively). Rates for CTC were 0.4 and 5.6 per 1000 respectively, but CTC required more procedures with bowel preparation (2,135 per 1000 vs. 1,361 with colonoscopy). The FOBT strategies had perforation and complication risks similar to CTC (0.3 and 5.2–5.6 per 1000 respectively) and required fewer procedures with bowel preparation (626–672 per 1000). The differences in life-years across rescreening strategies were small, ranging from 30,865 per 1000 for HSFOBT to 30,869 per 1000 for CTC, a difference of 1 day per person.
All other strategies yielded lower screening- and cancer-related costs than continuing colonoscopy ($3,084 per person with a negative colonoscopy at age 50 (Table 3)), with cost-savings from switching from colonoscopy to CTC of $91 per person, to FIT $450 per person, and to HSFOBT $495 per person.
Sensitivity Analyses
While the absolute number of life-years changed with assumptions about colonoscopy test characteristics and cecal intubation rate (Appendix Table 4), CTC test characteristics (Appendix Table 5), and colonoscopy risks (data not shown), the differences in life-years across rescreening strategies remained small, 4 days or fewer per person. The cost-savings from rescreening with a strategy other than colonoscopy fell.
Since colonoscopies are performed in all rescreening strategies, the lifetime costs of all strategies changed with colonoscopy cost. If colonoscopy cost was half the base-case estimate, all rescreening strategies, including continuing colonoscopy, yielded similar lifetime costs. As colonoscopy cost increased above the base-case estimate, the cost-savings from rescreening with methods other than colonoscopy increased (Appendix Figure 1). Due to the small number of cancer cases, the findings were relatively insensitive to higher costs of cancer care (Appendix Figure 2).
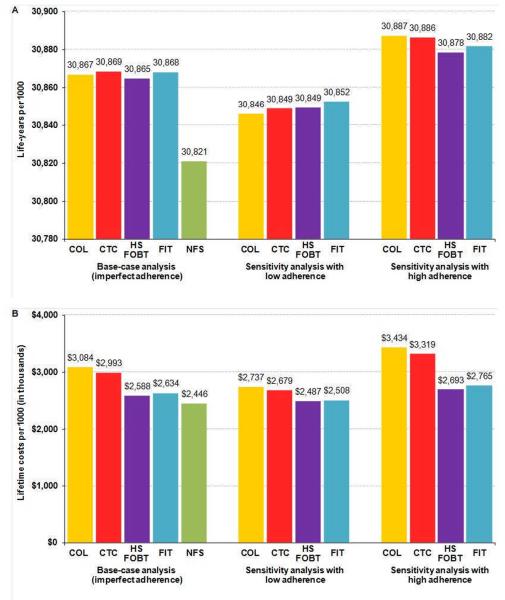
The results were sensitive to test-specific (imperfect) adherence rates. If adherence with each rescreening strategy was lower (higher) than the base-case, the life-years remained similar across tests (Figure 2, A). However, if adherence with some strategies was higher than the base-case values while adherence with others was lower, the differences in life-years across tests increased to a maximum of 40 years per 1000 (30,846 life-years per 1000 if continue colonoscopy with low adherence versus 30,886 if rescreen with CTC with high adherence), which is 15 days per person. In such a case, switching to CTC was no longer cost-saving (lifetime costs $2,737 per person if continue colonoscopy with low adherence versus $3,319 if rescreen with CTC with high adherence) (Figure 2, B).
Figure 2.
Life-years (A) and lifetime costs (B) per 1000 50-year-old individuals with a negative screening colonoscopy with imperfect adherence (see Appendix Table 1): sensitivity analysis on adherence rates.
COL = colonoscopy; CTC = computed tomographic colonography with ≥6mm threshold for colonoscopy referral; HSFOBT = highly-sensitive guaiac fecal occult blood test; FIT = fecal immunochemical test; NFS = no further screening.
DISCUSSION
Colonoscopy is a well-accepted strategy for prevention of colorectal cancer death (52) and efforts to promote its use have increased the proportion of Americans who report having had the procedure (7–8). However, the value of alternative rescreening strategies for those with a negative initial exam is uncertain. Ideally, a randomized trial would address this question, but such a study is unlikely to be performed. Results from a validated simulation model can therefore be informative.
Since it is debatable whether policy decisions and clinical recommendations should be informed by analyses that assume perfect adherence or those that incorporate more realistic, but poorly-described imperfect adherence rates, we evaluated both adherence scenarios. Notably, conclusions were similar across scenarios. Compared with the currently-recommended strategy of continuing ten-yearly colonoscopy after an initial negative exam, all of the other rescreening options we examined provide approximately the same benefit in life-years with fewer complications and at a lower cost. Therefore it is reasonable to rescreen individuals with a negative colonoscopy with other modalities.
Our findings have several implications. Colonoscopy has become the accepted standard for colorectal cancer screening in the US. However, there are not enough trained colonoscopists to perform all of the necessary screening procedures. Using modalities other than colonoscopy for rescreening may help to solve this shortage as it would free up scarce colonoscopy personnel to perform more primary screening exams.
From a policy perspective, the potential cost-savings (in 2010 dollars) from switching to FIT or HSFOBT following a negative screening colonoscopy rather than continuing colonoscopy are considerable. For every individual who switches, $450 to $495 is saved over their lifetime (assuming imperfect adherence). Data from the 2008 National Health Information Survey (53) indicate that approximately 40% of 50–54-year-olds had an endoscopy within the recommended intervals, and 92% reported their most recent endoscopy was a colonoscopy. On average, no adenomas or colorectal cancer are detected in 82% of initial screening colonoscopies (39). This suggests that if the estimated 6.6M 50–54-year-olds who had a negative screening colonoscopy in 2008 (that is, 40% × 92% × 82% × 21.5M 50–54-year-olds (54)) were rescreened with yearly FIT or yearly HSFOBT, $3 billion could be saved over the course of their lives. The cost-savings from switching to five-yearly CTC following a negative colonoscopy are lower yet still sizable ($0.6 billion), although these savings could be at least partially offset by the costs of working up extracolonic findings.
Our analysis has a number of limitations. We did not consider the risks and costs of radiation exposure from CTC because the radiation-related cancer risk was estimated to be very small in comparison to the reduction in colorectal cancer risk from CTC screening (55). We also did not include the risks, potential benefits, or costs associated with the detection of incidental findings by CTC. The prevalence of clinically-significant incidental findings in asymptomatic populations ranges from 7% to 11%, and the average cost of their work-up (in US settings) has been estimated at $28 to $99 per person screened (56). When these costs, as well as any potential cost-savings (and gains in life-expectancy) associated with earlier detection of clinically-significant disease are confirmed, they should be included in the assessment of a CTC strategy.
Data from several studies suggest that colonoscopy may not offer as much protection from right-sided compared with left-sided disease (19, 57–59). We did not incorporate this into our analysis because the reasons for the difference remain unclear, but likely involve a combination of technical and biological factors that may affect the location-specific effectiveness of colonoscopy as well as other screening modalities. When additional data become available that confirm the magnitude of the effect and elucidate the mechanism, they should be incorporated into an assessment of all modalities.
There are limited data on test-specific adherence, particularly among those who already had a colonoscopy and had no adenomas or colorectal cancers detected. Imperiale (39) reported adherence of 52% with a repeat colonoscopy five years after a negative exam. It is unclear if adherence ten years after a negative colonoscopy would differ. In the absence of data for CTC, we assumed adherence with the first CTC was equal to that with a repeat colonoscopy (that is, 52%) and that individuals on average have two CTCs by age 75. Many have suggested that adherence with CTC for initial screening may be higher than with colonoscopy (60–62), although such claims have been based on small single-institution studies. A Dutch population-based study found that screening uptake was higher for CTC vs. colonoscopy (63). However, CTC was performed without cathartic bowel preparation. It is unclear if uptake would be higher if individuals had cathartic bowel preparation (as modeled in our analysis). Our estimates of adherence with FOBT were based on data from a Veteran population over a five-year period; adherence among the general screening population (and over longer periods of time) may differ. Furthermore, adherence with FOBT may differ among those who already opted for colonoscopy.
In conclusion, compared with the currently-recommended strategy of continuing colorectal cancer screening with ten-yearly colonoscopy following an initial negative exam, rescreening at age 60 with yearly HSFOBT, yearly FIT, or five-yearly CTC yield comparable life-years with fewer complications and at a lower cost. Therefore it is reasonable to rescreen individuals with a negative colonoscopy with other modalities.
Supplementary Material
Acknowledgments
We acknowledge Martin Brown, PhD, and Robin Yabroff, PhD, of the NCI for their assistance with obtaining colorectal cancer treatment costs using SEER-Medicare linked data and Eric (Rocky) Feuer, PhD, of the NCI for continued support of the work and infrastructure of the CISNET consortium. We thank Carolyn M. Rutter, PhD, of the Group Health Research Institute and Ann G. Zauber, PhD, of Memorial Sloan-Kettering Cancer Center for helpful comments and review of earlier versions of this article. None of the individuals acknowledged above received compensation for their contributions.
Funding support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number RC1CA147256. The model used in this analysis was also supported by the National Cancer Institute (U01CA088204 and U01CA152959).The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Primary Funding Source: National Cancer Institute of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Disclosures: Dr. Gazelle is a consultant for GE Healthcare. The authors declare no conflicts of interest.
Reproducible Research Statement Protocol: Available to approved individuals with written agreement from Dr. Knudsen (aknudsen@mgh-ita.org).
Statistical code and data set: Simulation model available to approved individuals with written agreement from Dr. Knudsen.
REFERENCES
- 1.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 2.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 3.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366(25):2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronborg O, Jorgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol. 2004;39(9):846–51. doi: 10.1080/00365520410003182. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91(5):434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 6.Scholefield JH, Moss S, Sufi F, Mangham CM, Hardcastle JD. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut. 2002;50(6):840–4. doi: 10.1136/gut.50.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1611–21. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of Colorectal Cancer Test Use, Including CT Colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Preventive Services Task Force Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 11.Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001--testing for early lung cancer detection. CA Cancer J Clin. 2001;51(1):38–75. doi: 10.3322/canjclin.51.1.38. quiz 7–80. [DOI] [PubMed] [Google Scholar]
- 12.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124(2):544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 13.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'Brien MJ, Levin B, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130(6):1872–85. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145(12):880–6. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 15.Rabeneck L, Paszat LF, Hilsden RJ, Saskin R, Leddin D, Grunfeld E, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135(6):1899–906. 906 e1. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 16.Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150(12):849–57. W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 17.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95(3):230–6. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 18.Brenner H, Chang-Claude J, Seiler CM, Sturmer T, Hoffmeister M. Does a negative screening colonoscopy ever need to be repeated? Gut. 2006;55(8):1145–50. doi: 10.1136/gut.2005.087130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh H, Turner D, Xue L, Targownik LE, Bernstein CN. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA. 2006;295(20):2366–73. doi: 10.1001/jama.295.20.2366. [DOI] [PubMed] [Google Scholar]
- 20.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–69. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, Wilschut JA, Zauber AG, van Ballegooijen M. Stool DNA testing to screen for colorectal cancer in the medicare population: a cost-effectiveness analysis. Ann Intern Med. 2010;153(6):368–77. doi: 10.1059/0003-4819-153-6-201009210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Savarino JE, van Ballegooijen M, Kuntz KM, et al. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the Medicare population. J Natl Cancer Inst. 2010;102(16):1238–52. doi: 10.1093/jnci/djq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuntz KM, Lansdorp-Vogelaar I, Rutter CM, Knudsen AB, van Ballegooijen M, Savarino JE, et al. A systematic comparison of microsimulation models of colorectal cancer: the role of assumptions about adenoma progression. Med Decis Making. 2011;31(4):530–9. doi: 10.1177/0272989X11408730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Ballegooijen M, Rutter CM, Knudsen AB, Zauber AG, Savarino JE, Lansdorp-Vogelaar I, et al. Clarifying differences in natural history between models of screening: the case of colorectal cancer. Med Decis Making. 2011;31(4):540–9. doi: 10.1177/0272989X11408915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Public-Use, Nov 2003 Sub (1973–2001). Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov)
- 26.Arias E. National vital statistics reports. no 9. vol 59. National Center for Health Statistics; Hyattsville, MD: 2011. United States life tables, 2007. [PubMed] [Google Scholar]
- 27.Arminski TC, McLean DW. Incidence and Distribution of Adenomatous Polyps of the Colon and Rectum Based on 1,000 Autopsy Examinations. Dis Colon Rectum. 1964;7(4):249–61. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 28.Blatt L. Polyps of the colon and rectum: incidence and distribution. Dis Colon Rectum. 1961;4(4):277–82. [Google Scholar]
- 29.Bombi JA. Polyps of the colon in Barcelona, Spain. An autopsy study. Cancer. 1988;61(7):1472–6. doi: 10.1002/1097-0142(19880401)61:7<1472::aid-cncr2820610734>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 30.Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157(2):223–6. doi: 10.1097/00000658-196302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark JC, Collan Y, Eide TJ, Esteve J, Ewen S, Gibbs NM, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36(2):179–86. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 32.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33(11):1508–14. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989;24(7):799–806. doi: 10.3109/00365528909089217. [DOI] [PubMed] [Google Scholar]
- 34.Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43(5):1847–57. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 35.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49(4):819–25. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 36.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23(10):835–42. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gellad ZF, Stechuchak KM, Fisher DA, Olsen MK, McDuffie JR, Ostbye T, et al. Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am J Gastroenterol. 2011;106(6):1125–34. doi: 10.1038/ajg.2011.11. [DOI] [PubMed] [Google Scholar]
- 38.Hol L, Wilschut JA, van Ballegooijen M, van Vuuren AJ, van der Valk H, Reijerink JC, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. Br J Cancer. 2009;100(7):1103–10. doi: 10.1038/sj.bjc.6604961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359(12):1218–24. doi: 10.1056/NEJMoa0803597. [DOI] [PubMed] [Google Scholar]
- 40.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, van Ballegooijen M, Kuntz KM. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer: Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and SimCRC Models. 2007 Available at: https://www.cms.hhs.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp&id=212&. [PubMed]
- 41.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101(2):343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 42.Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207–17. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett, Hildebrandt HA, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349(23):2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 44.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology. 2011;259(2):393–405. doi: 10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bureau of Labor Statistics . National Compensation Survey: Occupational Earnings in the United States, 2010. U.S. Bureau of Labor Statistics; 2011. [Google Scholar]
- 46.Red Book: Pharmacy's Fundamental Reference. Physicians' Desk Reference Inc; Montvale, NJ: 2010. [Google Scholar]
- 47.Federal Register Vol. 71. No. 231, Friday, December 1, 2006, p 69624-70251. Medicare Program—Revisions to Payment Policies, etc.; Final Rule
- 48.Pickhardt PJ. Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology. 2006;239(2):313–6. doi: 10.1148/radiol.2392052002. [DOI] [PubMed] [Google Scholar]
- 49.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 50.Yabroff KR, Warren JL, Knopf K, Davis WW, Brown ML. Estimating patient time costs associated with colorectal cancer care. Med Care. 2005;43(7):640–8. doi: 10.1097/01.mlr.0000167177.45020.4a. [DOI] [PubMed] [Google Scholar]
- 51.U.S. Census Bureau, Statistical Abstract of the United States: 2012 (131st Edition) Washington, DC, 2011; Section 14: Prices, Table 725. [Accessed December 6, 2011];Consumer Price Indexes (CPI-U) by Major Groups: 1990 to 2010. < http://www.census.gov/compendia/statab/>.,
- 52.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Center for Health Statistics National Health Interview Survey (NHIS): Public-use data release. 2008 Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2008/srvydesc.pdf.
- 54.Population Division, U.S. Census Bureau [Accessed 15 September 2010];Table 1: Annual Estimates of the Resident Population by Sex and Five-Year Age Groups for the United States: April 1, 2000 to July 1, 2008 (NC-EST2008-01) Release data May 14, 2009. Available from http://www.census.gov/popest/national/asrh/NC-EST2008-sa.html.
- 55.de Gonzalez AB, Kim KP, Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Smith-Bindman R, et al. Radiation-related cancer risks from CT colonography screening: a risk-benefit analysis. AJR Am J Roentgenol. 2011;196(4):816–23. doi: 10.2214/AJR.10.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding A, Eisenberg JD, Pandharipande PV. The economic burden of incidentally detected findings. Radiol Clin North Am. 2011;49(2):257–65. doi: 10.1016/j.rcl.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 58.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102(2):89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 59.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 60.Ho W, Broughton DE, Donelan K, Gazelle GS, Hur C. Analysis of barriers to and patients' preferences for CT colonography for colorectal cancer screening in a nonadherent urban population. AJR Am J Roentgenol. 2010;195(2):393–7. doi: 10.2214/AJR.09.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moawad FJ, Maydonovitch CL, Cullen PA, Barlow DS, Jenson DW, Cash BD. CT colonography may improve colorectal cancer screening compliance. AJR Am J Roentgenol. 2010;195(5):1118–23. doi: 10.2214/AJR.10.4921. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz DC, Dasher KJ, Said A, Gopal DV, Reichelderfer M, Kim DH, et al. Impact of a CT colonography screening program on endoscopic colonoscopy in clinical practice. Am J Gastroenterol. 2008;103(2):346–51. doi: 10.1111/j.1572-0241.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 63.Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2011 doi: 10.1016/S1470-2045(11)70283-2. [DOI] [PubMed] [Google Scholar]
- 64.Jonas DE, Russell LB, Sandler RS, Chou J, Pignone M. Patient time requirements for screening colonoscopy. Am J Gastroenterol. 2007;102(11):2401–10. doi: 10.1111/j.1572-0241.2007.01387.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.