Since the discovery of RNA interference (RNAi), by which the activity of specific genes can be efficiently suppressed, synthetic small interfering RNA (siRNA)-mediated RNAi has been of substantial interest for various disease treatments.[1] However, the safe and effective delivery of siRNA to target cells remains a major hurdle for its widespread clinical applications.[2] Due to its polyanionic and macromolecular characteristics, naked siRNA cannot readily cross the cell membrane, and thus requires specific delivery vehicles to facilitate its intracellular uptake and cytosolic delivery for bioactivity. These delivery vehicles are also expected to improve the pharmacological properties of siRNA by reducing its susceptibility to serum nucleases, renal filtration, and uptake by the mononuclear phagocyte system.
Among the numerous vehicles developed for RNAi delivery, cationic lipids and polymers can efficiently self-assemble with siRNAs to form nanoscale complexes (lipoplexes or polyplexes), and have shown potential in delivering siRNA.[3] Nevertheless, a substantial number of these materials may produce problems associated with toxicity, immune or inflammatory responses, and serum instability. We believe that, to optimally protect both siRNA and nanocomplexes from the physiological barriers in vivo, it would be ideal to completely entrap siRNA within nanocarriers that have a relatively neutral surface charge.[4a] To this end, several strategies have been adopted including pegylation of nanocomplexes and liposomal envelopment of polyplexes (lipopolyplexes).[4] For example, Huang and colleagues developed pegylated liposome-polycation nanoparticles (NPs) for the delivery of siRNA to inhibit tumor growth.[4c,d] Furthermore, Peer et al. designed a neutral lipid-enveloped siRNA-protamine polyplex that demonstrated significant silencing of cyclin D1 (CyD1) in leukocytes, revealing anti-CyD1 siRNA as a potential anti-inflammatory agent.[4e] In addition to avoiding cationic surface charge, it is also advantageous to use clinically validated biodegradable and biocompatible materials for RNAi delivery. One such material being studied is poly(lactic-co-glycolic acid) (PLGA), a specific polymer that has been widely used in FDA approved drug delivery and biomedical devices.[5] By pre-complexing siRNA with natural polyamines, Saltzman and colleagues have been able to densely load siRNA within PLGA NPs for safe and sustained gene silencing.[6]
Herein, we aim to develop a simple and robust NP platform for siRNA delivery, which could potentially express the unique features of both pegylated lipoplexes and PLGA NPs. Different from previous hybrid lipid-polymer systems,[4,7] we propose a differentially charged hollow core/shell lipid-polymer-lipid hybrid nanostructure (Figure 1a), which comprises four distinct components – a positively charged lipid layer forming the inner hollow core, a middle hydrophobic PLGA layer, and a relatively neutral charged lipid layer forming an interface between the PLGA and the outer PEG (polyethylene glycol) layer. These NPs, which are engineered through a modified double-emulsion solvent evaporation technique and self-assembly method, can be constructed with three functional features. The outer PEG-lipid layer is incorporated into the system with the intentions that it could allow the particles to escape immunological recognition, improve particle stability during circulation, and slow polymer degradation and drug release.[4a,7b] The middle PLGA polymer forms a barrier through which the sustained release of encapsulated siRNA is achieved, and can additionally serve to carry water-insoluble drugs.[8] Moreover, the inner positively charged hollow core, composed of the cationic lipids, can encapsulate siRNA much more efficiently than PLGA alone.
Figure 1.
a) Schematic representation of the lipid-polymer-lipid hybrid nanostructure. The particle is composed of an outer lipid-PEG surface, a middle polymer layer, and an inner cationic lipid hollow core entrapping aqueous siRNA. Representative TEM image b) of the hybrid nanoparticles in a), and confocal laser scanning fluorescence image c) of the hybrid microparticles demonstrated the existence of outer lipid-PEG layer (green) and inner lipid layer (red), separated by a PLGA layer (blue). The scale bar in c) is 10 μm.
In a first set of experiments, the lipid-polymer-lipid hybrid NPs were prepared via a modified double-emulsion method, and characterized by various techniques. In the first emulsion, small droplets of siRNA solution were dispersed in an organic solvent containing polymers and cationic ethylphosphocholine (EPC) lipids, by 25 sec probe sonication. Due to their amphiphilic nature, EPC lipids were able to self-assemble tightly at the water-oil interface with their hydrophilic headgroups oriented towards the aqueous droplets and lipophilic tails immersed in the polymer phase (Figure 1a).[9] This inner lipid layer could thus stabilize the aqueous droplets, and efficiently encapsulate siRNA in a similar way to which cationic liposomes entrap siRNA. Herein, ester-terminated PLGA was used as a model hydrophobic polymer to form the middle polymer layer. The inner lipid layer was composed of 1,2-dimyristoleoyl-sn-glycero-3-ethylphospho-choline (EPC14:1) which demonstrates low toxicity and is biodegradable.[10] Interestingly, EPC14:1 was shown to exhibit maximum DNA transfection activity among different cationic EPC derivatives, and is hypothesized to promote cubic phase formation and enhance cytosolic delivery.[11] In the second emulsion and subsequent solvent evaporation, DSPE-PEGm and lecithin self-assembled around the PLGA layer, forming the outer lipid-PEG surface. It should be noted that, to avoid liposome/micelle formation, both DSPE-PEGm and lecithin concentrations were far below their respective critical micelle concentration (CMC).[7b,12] DSPE-PEGm was used to coat the polymer layer in that NPs with methoxyl surface groups are considered ideal for drug delivery applications, inducing lower complement activation compared to NPs with other surface groups.[13]
To characterize the structure of the hybrid NPs, they were first incubated with negative staining solution of uranyl acetate, by which the electron density of the outer lipid layer could be enhanced. After washing and drying, the NPs were then imaged by TEM. Figure 1b shows that the spherical NPs have three layers with different electron density. The outer dark ring represents the stained DSPE-PEGm/lecithin layer, the middle polymer layer has thickness ~ 20 – 50 nm, and the inner core contains siRNA. To further demonstrate the inner lipid and outer lipid-PEG structure shown in Figure 1a, lipid-polymer-lipid microparticles were formulated and visualized under confocal laser scanning microscope. Three different dyes were encapsulated in the particles, with DSPE-PEG-CF embedded with DSPE-PEGm to coat the PLGA layer, hydrophobic BODIPY® 665/676 dye localized with PLGA, and DHPE-Texas Red self-assembling with EPC14:1 lipids at the water-polymer interface. The fluorescence image in Figure 1c clearly shows that each dye stains the outer lipid-PEG, middle PLGA, and inner cationic lipid layers, respectively, which indicates the three-layer structure of the NPs. It is worth noting that the three dyes were carefully chosen to avoid energy transfer among each other.
In addition, dynamic light scattering was used to measure the NP size and surface charge (zeta potential). The average particle diameter is 225 ± 8 nm and is consistent with the size range in TEM images. The average zeta potential ranged between - 10 mV and 0 mV, which suggests that the NPs could minimize phagocytosis and are less likely to cause immunological reaction in vivo.[4a,13] NP stability, another important factor for in vivo applications, was also tested by monitoring the size change of NPs in PBS and cell growth medium (DMEM supplemented with 10% FBS) over 2 days. Data in Figure S1 demonstrate that the NPs remain stable in PBS without significant change in size, and the NP size initially increases to 262 ± 10 nm in cell growth medium and remains constant thereafter. The results suggest the outer DSPE-PEGm and lecithin layer can provide substantial protection to the NPs in PBS and culture medium.
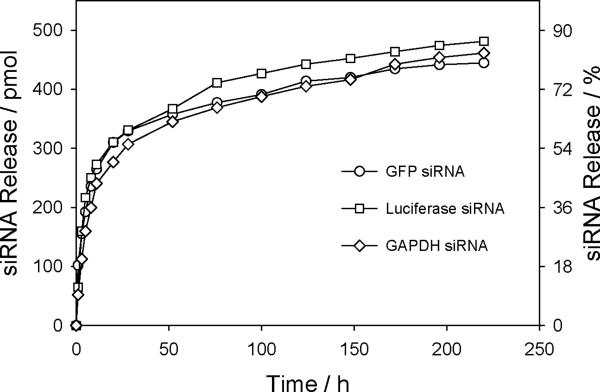
To examine the siRNA encapsulation efficiency and loading yield, Cy3-siRNA encapsulated hybrid NPs were prepared. When 1.4 nmol Cy3-siRNA was initially added for NP formation, calculations based on fluorescence measurements gave an encapsulation efficiency of ~ 78 – 82% and a loading efficiency of ~ 364 – 383 pmol siRNA/mg PLGA. As a comparison, PLGA and PLGA-PEG NPs can only encapsulate ~ 4 – 8% of applied siRNA, yielding a very low load of ~ 18 – 37 pmol siRNA/mg PLGA. This indicates that EPC14:1 can drastically enhance the entrapment of siRNA within the NPs. In the experiments used to determine the release rate of siRNA, three different kinds of siRNAs were encapsulated in NPs and the in vitro release kinetics for each siRNA was measured (Figure 2). As can be seen, it took the NPs ~ 12 – 20 h to release 50% of each siRNA, and the individual release profiles are similar to one another. The above results demonstrate that the sandwich structured lipid-polymer-lipid NPs are stable, and siRNA can be densely loaded and released in a sustained fashion.
Figure 2.
In vitro release profiles of GFP siRNA, GAPDH siRNA, and luciferase GL3 siRNA from respective hybrid NPs.
In a next set of experiments, we evaluated the in vitro bioactivity of the siRNA-encapsulated hybrid NPs. GFP expressing HeLa cells were transfected with NPs containing 60 pmol GFP siRNA, and then imaged by confocal laser scanning microscopy at one day post-transfection. Figures 3a and 3b show the GFP fluorescence images of HeLa cells treated with no siRNA and with NP(GFP siRNA), respectively. The much lower fluorescence intensity in Figure 3b implied that the expression of GFP was significantly silenced by NP(GFP siRNA). To determine the knockdown efficacy of GFP expression, flow cytometry was performed to obtain mean fluorescence intensity of GFP-HeLa cells and of HeLa cells serving as autofluorescent background (Figure 3c). The performance of naked siRNA, NP(neg. siRNA), and NP(GFP siRNA) was systemically tested as a function of siRNA dose in 12-well plate formats. Figure 4a demonstrated that naked siRNA and NP(neg. siRNA) showed little silencing under all conditions. On the other hand, the GFP expression was gradually silenced with the increasing of GFP siRNA dose in NPs. When 60 pmol siRNA/1 mL medium (60 nM GFP siRNA) was used, the NP(GFP siRNA) could achieve ~ 72% GFP knockdown, which is comparable to the commercially available liposome-based lipoplex (Lipo2000-siRNA complex).
Figure 3.
Confocal laser scanning fluorescence images of GFP-expressed HeLa cells treated with a) no siRNA and b) with NP(GFP siRNA); and c) flow cytometry histograms of autofluorescent HeLa cells (yellow), GFP-HeLa cells (black), and GFP-HeLa cells treated with NP(GFP siRNA) (blue) and with Lipofectamine 2000-GFP siRNA complex (red).
Figure 4.
a) GFP expression in GFP-HeLa cells and b) Firefly luciferase expression in Dual-Luc HeLa cells transfected with naked siRNA, NP(neg. siRNA), NP(siRNA), and Lipo2000-siRNA complex.
The applications of our hybrid NPs in delivering other siRNAs to different cell lines, including HepG2 hepatocytes and Dual-Luc HeLa cells, were also explored in 96-well plate formats. The expression of GAPDH enzyme in HepG2 hepatocytes was measured after treatment with naked siRNA, NP(neg. siRNA), NP(GAPDH siRNA), and Lipo2000-siRNA complex (Figure S2). In the case of delivering luciferase siRNA to Dual-Luc HeLa cells, which were engineered to simultaneously express firefly and Renilla luciferase, the GL3 siRNA was chosen to selectively suppress the expression of firefly luciferase. The gene knockdown efficacy of GL3 siRNA-encapsulated hybrid NPs was determined by the reduction of luminescence from firefly luciferase, relative to the control luminescence from Renilla luciferase.[14] Figure 4b shows the response of firefly luciferase expression to siRNA in different formulations. Both experiments demonstrate very similar results to the siRNA transfection of GFP-HeLa cells. Moreover, with the highest siRNA dose (10 pmol/100 μL medium in 96-well plate experiments), the gene knockdown efficiency of our NPs is better than that of Lipo2000. It should also be noted that no significant cellular toxicity was observed by MTT assay, under all the conditions used for in vitro experiments.
In a final set of experiments, we wished to exploit the lipid-polymer-lipid NPs to deliver siRNA in vivo. To do this, luciferase-expressing xenograft tumors were developed by subcutaneous injection of Dual-Luc HeLa cells in the flank of nude mice. Ten days later, comparative efficacy studies were performed by dividing animals into four groups (n = 4), minimizing animal weight and tumor size difference among the groups. After obtaining initial bioluminescence images of each mouse (day 0), four different regimens including GL3 siRNA, GL3 lipoplex, NP(neg. siRNA), and NP(GL3) were respectively administered into mice from each group by a single intratumoral injection. The mice bearing luciferase-expressing tumors were imaged every 2 days thereafter. Compared to day 0, the bioluminescence intensity from the tumor treated with NP(GL3) was almost identical at day 2, and slightly increased at day 4 (Figure 5a). On the other hand, the bioluminescence intensity in the tumor of mice injected with NP(neg. siRNA) drastically increased in the following days (Figure 5b). These results suggest that the hybrid NPs are capable of delivering GL3 siRNA to inhibit luciferase expression in vivo.
Figure 5.
In vivo bioluminescence imaging of mice-bearing luciferase expressing tumor treated with a) NP(GL3) and b) NP(neg. siRNA). Images were taken at day 0, 2, 4, and 6. Luminescence intensity is shown by the legend to the right. c) Luciferase expression of each group (n = 4) was evaluated by the luminescence intensity (mean ± SE) relative to day 0. *, P < 0.05 vs. NP(neg. siRNA) at day 2 and 4.
To quantitatively demonstrate the gene silencing efficacy of NP(GL3), relative to NP(neg. siRNA), the total bioluminescence intensity (photon/sec) obtained from each tumor at different imaging days was calibrated by normalizing the initial bioluminescence signal (at day 0) to equal 1. The relative luminescence intensity (n = 4, mean ± SE) was then plotted as a function of time (Figure 5c). As can be seen, the luciferase expression is ~ 42 – 45% less in mice transfected with NP(GL3) than with NP(neg. siRNA), at day 2 and day 4. For comparison, mice treated with GL3 siRNA alone and GL3 lipoplex were also imaged, and the relative luminescence intensity was calculated. The change of luciferase expression in these two cases was similar to each other, indicating the cationic liposome did not provide obvious benefits for siRNA delivery during the in vivo experiments. This result is indeed consistent with previous reported findings.[15] Among the three regimens containing GL3 siRNA, the NP(GL3) exhibited highest efficacy in suppressing luciferase expression. The enhanced in vivo gene silencing data imply that our lipid-polymer-lipid NPs may hold potential in the delivery of siRNA for inhibiting tumor growth.[4d]
In summary, a differentially charged hollow core/shell lipid- polymer-lipid hybrid NP system was developed by a simple and potentially scalable formulation strategy. The hybrid NPs, combining the unique characteristics of polymeric NPs and pegylated lipoplexes, can be densely loaded with siRNA and efficiently deliver such biomacromolecules in vitro and in vivo. Nonetheless, the NPs could be further optimized by screening various factors, such as inner cationic lipids, outer PEG chain length, middle polymer composition and molecular weight, as wel as formulation parameters. We also postulate that by modifying with targeting ligands, the NPs may offer higher efficacy in siRNA delivery and gene silencing, as targeted delivery has shown potential in enhancing intracellular uptake of nanoparticles via receptor-mediated endocytosis.[16] Beyond these studies currently in progress, our hybrid NPs may also be of interest in co-delivering siRNA and synergistic small molecule drugs carried within the hydrophobic PLGA layer for the treatment of a myriad of important diseases including multidrug resistant cancers.[17]
Supplementary Material
Footnotes
This work was supported by National Institute of Health (NIH) grants CA151884, EB003647 and N01 HV-08236, and the David Koch – Prostate Cancer Foundation Award in Nanotherapeutics. We also thank Dr. Akin Akinc (Alnylam Pharmaceuticals, Inc.) and Prof. Daniel G. Anderson (MIT) for providing Dual-Luc HeLa cells and GFP-HeLa cells.
References
- [1].a) Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]; b) Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]; c) Behlke MA. Mol. Ther. 2006;13:644–670. doi: 10.1016/j.ymthe.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Whitehead KA, Langer R, Anderson DG. Nat. Rev. Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Blow N. Nature. 2007;450:1117–1120. doi: 10.1038/4501117a. [DOI] [PubMed] [Google Scholar]; c) Shi J, Votruba AR, Farokhzad OC, Langer R. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. J. Intern. Med. 2009;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Tseng YC, Mozumdar S, Huang L. Adv. Drug Deliv. Rev. 2009;61:721–731. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Howard KA. Adv. Drug Deliv. Rev. 2009;61:710–720. doi: 10.1016/j.addr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- [4].a) Davis ME. Mol. Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]; b) Auguste DT, Furman K, Wong A, Fuller J, Armes SP, Deming TJ, Langer R. J. Control. Release. 2008;130:266–274. doi: 10.1016/j.jconrel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Li SD, Huang L. Mol. Pharm. 2006;3:579–588. doi: 10.1021/mp060039w. [DOI] [PubMed] [Google Scholar]; d) Li SD, Chen YC, Hackett MJ, Huang L. Mol. Ther. 2008;16:163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jain RA. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- [6].Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Nat. Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Troutier AL, Ladaviere C. Adv. Colloid Interface Sci. 2007;133:1–21. doi: 10.1016/j.cis.2007.02.003. [DOI] [PubMed] [Google Scholar]; b) Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ, Radovic-Moreno AF, Alexis F, Langer R, Farokhzad OC. ACS Nano. 2008;2:1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bershteyn A, Chaparro J, Yau R, Kim M, Reinherz E, Ferreira-Moita L, Irvine DJ. Soft Matter. 2008;4:1787–1791. doi: 10.1039/b804933e. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lee SM, Chen H, Dettmer CM, O'Halloran TV, Nguyen ST. J. Am. Chem. Soc. 2007;129:15096–15097. doi: 10.1021/ja070748i. [DOI] [PubMed] [Google Scholar]; e) Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- [8].Farokhzad OC, Cheng JJ, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pautot S, Frisken BJ, Weitz DA. Langmuir. 2003;19:2870–2879. doi: 10.1073/pnas.1931005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].MacDonald RC, Rakhmanova VA, Choi KL, Rosenzweig HS, Lahiri MK. J. Pharm. Sci. 1999;88:896–904. doi: 10.1021/js990006q. [DOI] [PubMed] [Google Scholar]
- [11].Koynova R, Tenchov B, Wang L, Macdonald RC. Mol. Pharm. 2009;6:951–958. doi: 10.1021/mp8002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Priev A, Zalipsky S, Cohen R, Barenholz Y. Langmuir. 2002;18:612–617. [Google Scholar]
- [13].Salvador-Morales C, Zhang L, Langer R, Farokhzad OC. Biomaterials. 2009;30:2231–2240. doi: 10.1016/j.biomaterials.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Akinc A, et al. Nat. Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Minakuchi Y, et al. Nucleic Acids Res. 2004;32:e109. doi: 10.1093/nar/gnh093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- [17].Saad M, Garbuzenko OB, Minko T. Nanomedicine. 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.