Synopsis
With the introduction of new materials and changes in manufacturing practices, occupational health investigators continue to uncover associations between novel exposures and chronic forms of diffuse parenchymal lung disease and terminal airways disease. In order to discern exposure disease relationships, clinicians must maintain a high index of suspicion for the potential toxicity of occupational and environmental exposures. This article details several newly recognized chronic parenchymal and terminal airways. Diseases related to exposure to Indium, Nylon Flock, Diacetyl used in the flavorings industry, nanoparticles, and the World Trade Center disaster are reviewed. Additionally, this article will review methods in worker surveillance as well as the potential use of biomarkers in the evaluation of exposure disease relationships.
Keywords: Occupational Lung Disease, Environmental Lung Disease, Interstitial Lung Disease, Diffuse Parenchymal Lung Disease, Indium Lung, Nylon Flock Worker’s Lung, Diacetyl lung, Flavorings Related Lung Disease, “Popcorn Worker’s Lung”, World-Trade Center Lung, Constrictive Bronchiolitis, Nanoparticles
Introduction
Chronic parenchymal lung disease comprises a heterogeneous group of disorders that have overlapping clinical, physiologic, and radiologic features. Exposure-related chronic parenchymal lung diseases were thought to be limited to the pneumoconioses and hypersensitivity pneumonitis. However, recent studies have linked new causative occupational and environmental agents with both terminal airways disease and parenchymal lung disease. This research has also elucidated the contribution of these exposures to the burden of the so-called idiopathic interstitial lung diseases.[1]
Exploring causality in patients who develop an acute parenchymal process immediately after a high-intensity exposure is usually straightforward; however, inferring causality when chronic lower-level exposures occur over many months to years is challenging. Inferring such associations requires a high index of suspicion, a careful exposure history in individual patients and a meticulous evaluation of respiratory surveillance data for larger worker cohorts.
Over the years, agencies such as the National Institute for Occupational Safety and Health (NIOSH) have worked with industry toto conduct exposure assessments, review historical and current medical surveillance data, and implement prospective medical surveillance strategies.
This article reviews selected newly identified occupational and environmental causes of chronic terminal airways disease and diffuse parenchymal lung disease over the past twenty years, including indium lung, nylon workers lung, diacetyl–induced bronchiolitis obliterans, and respiratory disorders related to exposure to toxicants at the site of the attack on the World Trade Center. The potential toxicity of emerging technologies, such as nanoparticles, is also discussed. Newly recognized causes of acute lung injury, hypersensitivity pneumonitis, pneumoconiosis, and disease related to military service are reviewed elsewhere in this edition. Although the term diffuse parenchymal lung disease is preferable for these disorders, given that many affect anatomic structures other than the interstitium, the commonly used term interstitial lung disease (ILD) will also be used.
Emerging Diseases
Indium Lung
The recent story of indium lung illustrates that new occupational diseases can emerge with the novel use of existing materials. Although the US Bureau of Mines listed indium as a commodity in 1936[2], the industrial use of this malleable and fusible post-transition metal was limited to production of bearing and dental alloys, nuclear reactor control rods, and semiconductor research until the 1990s. The use of indium-tin oxide for the production of transparent conductive coatings for liquid crystal display and plasma display televisions stimulated an increase in worldwide demand for indium from 371 tons in 1999 to 1340 tons in 2007.[3]
In 2003, Homma and colleagues published the first case report of indium lung in a 27-year-old previously healthy Japanese man who worked on wet-surface polishing of indium-tin oxide targets used for the transparent coatings. The patient developed interstitial pneumonitis three years after he began employment and died four years after clinical presentation.[4] This dramatic case prompted Japanese investigators to conduct epidemiologic investigations to better characterize the burden of disease among workers. Cases have not been limited to Japan. For example, NIOSH concluded that lung disease occurred as a consequence of hazardous levels of indium-tin oxide in a Rhode Island factory prompting the development of formal recommendations to improve the safety of the workers.[5]
Subsequently, investigators have evaluated the prevalence of respiratory symptoms along with physiologic and radiographic abnormalities among indium workers. For example, Chonan and colleagues reported radiographic interstitial changes in 21% of indium workers (23 of 108)[5]. In another evaluation conducted in a liquid manufacturing display facility, 53% of workers (8 of 15) in the same job as an employee with documented indium lung left employment before receiving a diagnosis.[6–9]
A multidisciplinary panel consisting of a chest radiologist, a pulmonologist, epidemiologists, and industrial hygienists reviewed the 10 cases of indium lung disease known as of May 2010 (seven in Japan, two in the United States, and one in China). These patients were employed in production, use and reclamation jobs. [4]The two primary findings were pulmonary alveolar proteinosis (PAP) and pulmonary fibrosis. The patients were all men, had a median age at diagnosis of 35 years, and presented with the insidious onset of cough, dyspnea, and sputum production; one patient had hemoptysis. The latency period from initial employment to diagnosis was six years. Autoantibodies to granulocyte-macrophage colony-stimulating factor which have been implicated in the pathogenesis of PAP were also detected in one patient. Although indium is not known to be carcinogenic, lung cancer has been reported.[10]
The disease process stabilized or improved in only 2 of the 10 patients, one treated with whole-lung lavage and the second without treatment. Two of the 8 patients whose condition deteriorated died. Only one of seven patients treated with inhaled or oral corticosteroids had objective improvement, although it was not sustained. Only one of the three patients receiving whole-lung lavage—a treatment used for PAP—had sustained improvement.[7]
Radiographic features of patients with indium lung include PAP patterns and interstitial fibrosis patterns. Chest CT scan of patients with PAP showed the classic “crazy paving pattern-” consisting of ground-glass opacities superimposed on interlobular septal thickening (Figure 1). CT scan of patients with interstitial fibrosis showed traction bronchiectasis, bronchiolectasis, and septal thickening.
Figure 1.
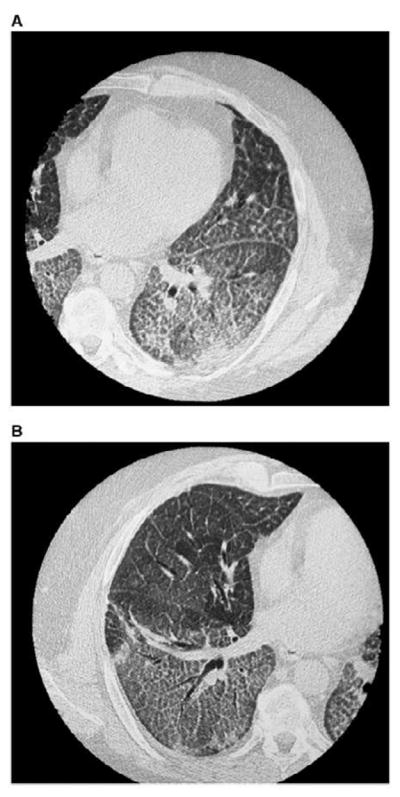
High resolution computed tomography scan of Indium Lung. The (A) left and (B) right chest showing bilateral ground glass opacities, centrilobular nodules, and intralobular and interlobular septal thickening. (from Cummings, K. Donat W. Ettensohn, David. Pulmonary Alveolar Proteinosis at an Indium Processing Facility. Am J Respir Crit Care Med. 181; 2010: 458–64, with permission)
Histopathologic evaluation in patients classified as PAP and interstitial fibrosis showed common features. Transbronchial biopsies or surgical biopsies were almost universally obtained. Although only three cases were initially diagnosed histopathologically as PAP, most of the cases deemed to be interstitial lung disease also had the granular eosinophilic and intraalveolar exudates characteristic of PAP. In addition, fibrosis was noted in all cases, even those initially diagnosed as PAP. Cholesterol clefts with associated granulomas were also noted in all cases (Figure 2). Lung tissue particle analysis confirmed the presence of indium in six patients. Inductively-coupled mass spectrometry conducted in one case showed an indium concentration 29.3 μg/g of lung tissue.
Figure 2.
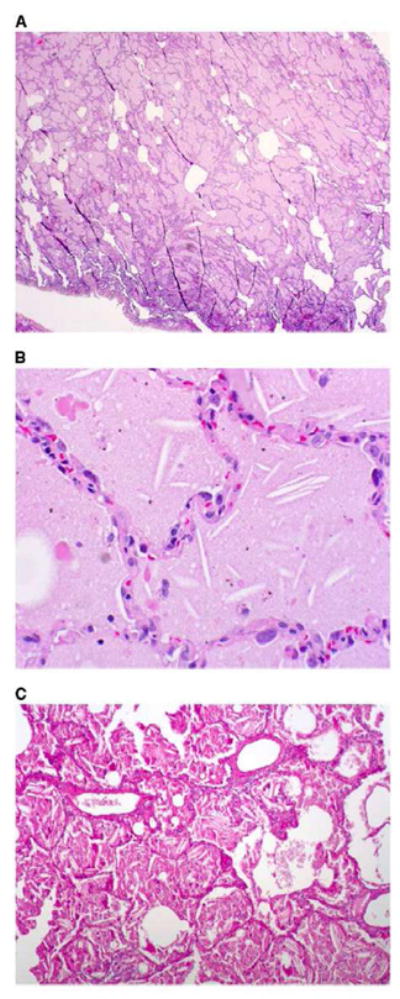
Histopathological sections of lung biopsy, hematoxylin and eosin stain. (A) Low-power overview showing filling of alveolar spaces by eosinophilic material (magnification 310). (B) High-power view showing granular eosinophilic material and cholesterol clefts. (magnification 3200). Birefrigent particles were identified with polarizing microscopy, consistent with the presence of crystalline indium-tin oxide. (C) Periodic acid-Schiff (PAS) stain after diastase digestion, showing granular, PAS-positive intraalveolar material, and cholesterol clefts (magnification X100). from Cummings, K. Donat W. Ettensohn, David. Pulmonary Alveolar Proteinosis at an Indium Processing Facility. Am J Respir Crit Care Med. 181; 2010: 458–464, with permission).
A relationship between indium exposure and disease has been suggested based on biomarkers of ILD. For example, KL-6 and lung surfactant protein D, which have been shown to be increased in patients exposed to indium in a dose-dependent manner.[10]
Recently, the Japanese Society for Occupational Health has recommended a comparatively much lower serum indium occupational exposure limit of less than 3 μg/L. Routine medical monitoring with symptom surveys and spirometry as well as baseline chest CT scan was also recommended. [11, 12]
Nylon Flock Worker’s Lung
In 1996 NIOSH and Brown University’s Program in Occupational Medicine launched an epidemiologic investigation after two young male workers employed in the same Rhode Island nylon flocking plant presented with interstitial lung disease. The employer requested the assistance of NIOSH through the Health Hazard Evaluation Program to conduct a formal worksite evaluation. Further investigation identified a cluster of eight workers who worked with rotary cut flock. Subsequent study detected affected workers in Rhode Island, Massachussetts, North Carolina, and Ontario as well as internationally. [13] [14] [15] [16] Even workers who had not sought medical evaluation had evidence of subclinical disease. For example, in one study, 19 of 32 asymptomatic workers had radiographic abnormalities on chest CT scan. [15]
The nylon flock exposed workers mostly commonly presented with chronic respiratory symptoms over several years, but subacute presentations also occurred. For example, in a Canadian outbreak, 5 of 88 exposed workers developed disease after exposure occurring over several days.[17] A temporal relationship between work and symptoms has not consistently been reported, although many workers have had clinical improvement within weeks to months after leaving work.
Clinical assessment reveals characteristic radiographic and histopathologic patterns. CT scan shows diffuse micronodular opacities, patchy ground glass opacities, patchy consolidation, and honeycombing (Figure 3, 4). Restrictive ventilatory defects are most common, but obstructive defects have been reported. Histopathologic evaluation commonly shows a nonspecific interstitial pneumonia pattern with characteristic lymphocytic bronchiolitis with peribronchovascular interstitial lymphoid infiltrates with or without germinal centers. Kern and colleagues reported one case of desquamative interstitial pneumonia and another case of bilateral synchronous adenocarcinoma in patients exposed to nylon flock.[14]
Figure 3.
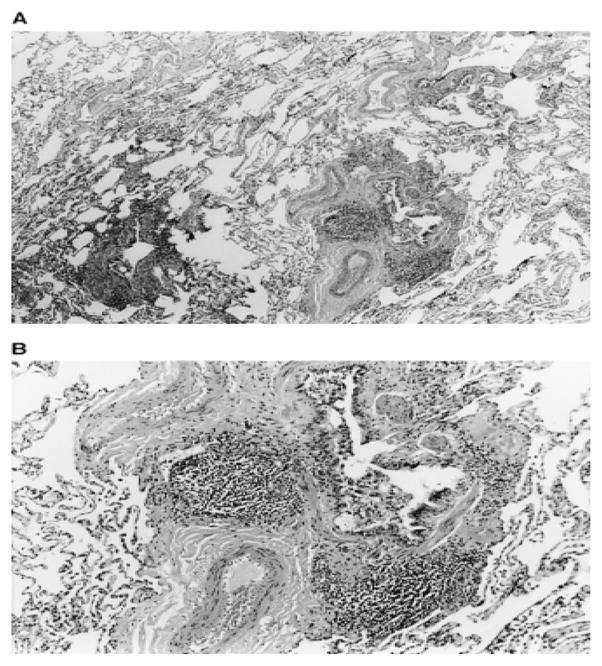
Nylon Flock Worker’s Lung Histopathology: Photomicrographs of thoracoscopic lung biopsy specimen from nylon flock plant worker. Histology reveals lymphocyte predominant infiltrate surrounding bronchiole in center of lobule. Original magnification of photomicrograph: (A) 3100; (B) 3250. Eschenbacher W, Kreiss, K, Lougheed, D. et al. Nylon Flock-Associated Interstitial Lung Disease. Am J Respir Crit Care Med 1999; 159: 2003–2008.
Figure 4.
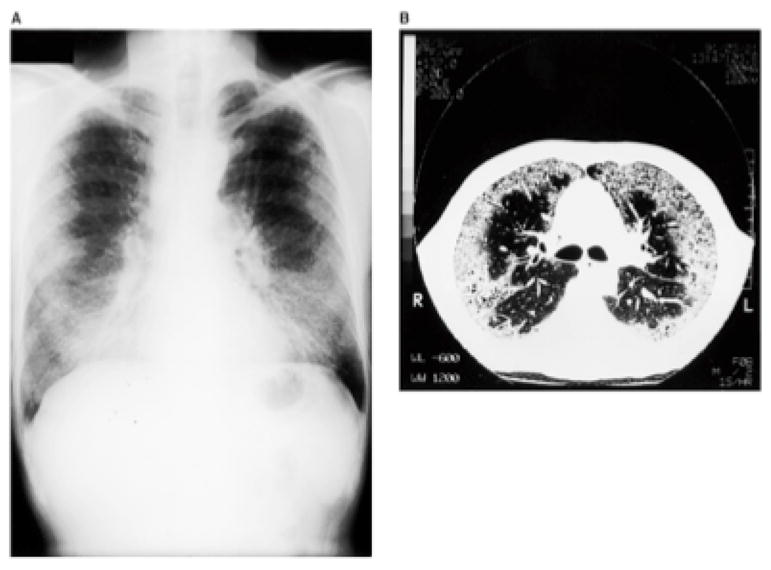
Radiographic imaging in Nylon Flock Worker’s Lung: Eschenbacher W, Kreiss, K, Lougheed, D. et al. Nylon Flock-Associated Interstitial Lung Disease. Am J Respir Crit Care Med 1999; 159: 2003–2008.
NIOSH investigators conducted qualitative and quantitative exposure assessments and medical surveillance that implicated respirable nylon fibers as the causative agent. Toxicologic study showed that rats exposed to intratracheal instillation of nylon flock developed bronchiolocentric inflammation.[16]
Pulmonary disease has been reported only in workers exposed to rotary cut flock as opposed to guillotines, which are most frequently used in this industry. When cutters are not appropriately sharpened and become dull, melting and tailing of the nylon flock ends occur and tend to break off during milling. High levels of these small respirable particles were found in the flocking room. [18]
The flock workers lung story demonstrates the effect of a comprehensive industrial hygiene assessment and control strategy. NIOSH investigations helped lead to the the implementation of exposure control measures that have reduced the incidence of the disease over recent years. After initial reports, the American Flock Association established an Occupational Health Committee for the approximately 3000 US employees. Industry efforts to reduce exposure including exhausting of process cyclones to outside, reduction in the use of compressed air for cleaning, improved cutter maintenance, and implementation of medical surveillance programs have reduced the number of reported cases.
World Trade Center–Related Lung Disease
The destruction of the World Trade Center towers on September 11, 2001 resulted in unprecedented respiratory exposure for thousands of rescue workers and residents. For those exposed, irritant-induced asthma or asthmatic bronchitis has received the greatest attention; however, terminal airways disease and ILD, including sarcoidosis and acute eosinophilic pneumonia, have been reported.
Prezant and colleagues reported the phenomenon of “World Trade Center cough” in 332 firefighters and demonstrated a dose-dependent response: 8% of firefighters with peak high exposure (those present at the time of the collapse of the towers), versus 3% with moderate exposure (those present within the first 2 days) and 1% with low exposure (those present within 3 to 7 days) developed the cough. A prospective cohort of 20,834 responders enrolled in the World Trade Center Medical Monitoring and Treatment Program had an increased lifetime prevalence of asthma from 3% in 2000 to 19% in 2007. An increased prevalence of respiratory symptoms has also been reported in persons living near the towers. [19–21]
Bronchiolitis has also been reported in persons exposed to World Trade Center dust. Mann and colleagues reported a pathologically confirmed case of chronic bronchiolitis with focal obliterative bronchiolitis that stabilized and improved after azithromycin therapy. [22] In 2010, Wu and colleagues reported the histopathologic presence of small airways disease among seven previously healthy first responders who developed respiratory impairment or radiologic abnormalities. [23]
Like asthma, bronchiolitis may present with dyspnea and cough. Also as in asthma, diagnostic assessment may reveal physiologic airway obstruction and normal chest radiographs. Radiography may show air trapping, a feature commonly overlooked if high resolution chest CT scans with inspiratory and expiratory images are not performed. Other possible findings include bronchial wall thickening, bronchiectasis, ground-glass opacities, and centrilobular nodules with tree-in-bud appearance. Investigational impulse oscillometry in persons exposed to World Trade Center dust has shown increased airway resistance, reflecting the distal airway abnormalities that occur in terminal airways disease.[24]
Other ILDs reported in patients exposed to the dust include two cases of eosinophilic pneumonitis, including a sentinel case of acute eosinophilic pneumonia in a New York City firefighter whose disorder responded to systemic corticosteroid therapy. Bronchoalveolar lavage revealed 70% eosinophils, and CT scan showed patchy ground-glass density, thickened bronchial walls, and bilateral pleural effusion. Minerologic analysis revealed commercial asbestosis fibers, fly ash, and degraded fiberglass.[25]
Imaging and histopathologic studies have suggested the presence of interstitial fibrosis after exposure to World Trade Center dust. Caplan-Shaw and colleagues reported diverse pathologic findings of patchy interstitial fibrosis as well as small airway findings with scant lymphoid aggregates.[26] Wu and colleagues reported four cases of diffuse interstitial fibrosis.[23]
Among the terminal airways and interstitial diseases reported in association with World Trade Center dust exposure, sarcoidosis has received the greatest attention. Izbicki and colleagues reported 26 cases of sarcoidosis among New York Fire Department rescue workers within five years of September 11, 2001, half of whom presented within the first year. The World Trade Center Registry and the World Trade Center Medical Monitoring and Treatment Program have also reported sarcoidosis, including such extrapulmonary findings as uveitis, dermatologic involvement, arthralgias, seizures, and cardiac arrhythmias. All sarcoidosis stages have been reported, including patients with stage I disease with intrathoracic lymphadenpathy and patients with stage II and III disease who had parenchymal disease. In Izbicki’s series four of eight patients improved or resolved with corticosteroid therapy.[27, 28]
Establishing causal links between exposure to World Trade Center dust and disease is complicated by a number of considerations, including limited exposure data, the latency period between exposure and onset of disease, and concerns regarding detection and surveillance bias. General prevalence and incidence data for ILDs is limited, and, therefore, comparing prevalence rates of interstitial lung disease in WTC exposed vs. unexposed persons is difficult.
Additionally, World Trade Center dust is a complex amalgam, and it is difficult to identify the specific toxic components. There exists a complexity of different exposures at different time points as the fires burned during the ensuing months and rescue efforts resuspended settled dust. Because the event was unanticipated and unprecedented, air samples representing the peak exposure at the time of collapse are unavailable. Existing environmental air monitoring stations set up to provide air pollution monitoring surveillance did not capture volatile gaseous materials and ultrafine particles.[29]
Analysis of the coarse medium-sized and large respirable particles of alkaline pH has revealed a mixture of fiberglass, asbestos, aluminum, calcium silicates and polycyclic hydrocarbons. Asbestos was used only in the early part of the construction of World Trade Center Tower 1 and not at all in Tower 2. In addition to asbestos, the dust contained other materials with fibrogenic potential, such as silica and man-made vitreous fibers. The dust analysis has not demonstrated metals such as beryllium, zirconium, and tungsten that have been associated with granulomatous and/or fibrotic lung disease. [28, 30, 31]
Lung tissue analyses support causal relationships between exposure to World Trade Center dust exposure and disease. Mineralogic analysis of the bronchoalveolar lavage fluid from the sentinel case of eosinophilic pneumonia revealed asbestos fibers, degraded fiberglass, and fly ash particles.[25] Induced sputum from New York City firefighters demonstrated particles with minerals, including titanium.[32] In another study, tissue mineralogic analyses from seven responders revealed aluminum, magnesium silicates, asbestos, phosphate, and calcium sulfate as well as shards of glass containing silica and magnesium. Nanomaterials, such as carbon nanotubes, were detected in three patients. Carbon nanotubes were unlikely present in the building structure before 2001; however, investigators postulated that high temperatures from fuel combustion may have generated large number of carbon nanotubes.[23] Finally, Caplan-Shaw and colleagues reported a study of twelve patients undergoing surgical lung biopsy who demonstrated opaque and birefingent particles within macrophages, particles that containing silica, aluminum silicates, titanium dioxide, talc, and metals.[26]
Toxicology studies in animals and in cultured cells further support the biological plausibility of the toxicity of World Trade Center dust. Mice exposed to the dust developed a slight increase in bronchoalveolar neutrophils, although the study dust exposure dose principally simulates the high exposure levels present at peak exposure. Studies of cultured human alveolar macrophages and type II cells exposed to the dust showed a dose-dependent increase in proinflammatory cytokines, such as tumor necrosis factor–alpha (TNF-α), interleukin-6 and -8, and gamma-glutamyl transpeptidase.[30, 31]
Flavoring-Related Lung Disease (Popcorn Workers Lung)
In 2000, the Missouri Department of Health received a report of bronchiolitis obliterans in eight workers formerly employed in a microwave popcorn production facility.[33] The Missouri Department of Health in collaboration with industry subsequently enlisted the assistance of NIOSH to develop a protocol to protect the safety of current workers, measure the disease burden among other workers, and investigate and identify the respiratory intoxicant. Mixers and microwave packaging workers were found to be at highest risk. Industrial hygiene sampling demonstrated more than 100 volatile organic compounds found the greatest risk of airflow obstruction in workers exposed to high levels of diacetyl, and a water soluble volatile diketone that readily vaporizes and that is used in popcorn production and other food flavoring industries. [34]
Monitoring programs of food flavoring workers expanded, and the California Department of Public Health and the California Division of Occupational Safety and Health (Cal/OSHA) implemented a major public health surveillance program for workers from twenty different flavoring manufacturing companies. Of the 677 workers evaluated, 23% had abnormal spirometry, 4.9% had airways obstruction, and approximately 9.6% had excessive FEV1 decline with rates of decline greater in companies using more than 800 lbs of diacetyl per year. One patient lost one liter of FEV1 after approximately four months of exposure, a finding that suggests that annual spirometry may not be sufficiently frequent to detect disease.[35] There have been additional case reports from other food plants using flood flavorings that contain diacetyl, such as a British worker at a potato chip factory.[36] Lung disease has also been reported in workers employed at a chemical plant in the Netherlands that produced diacetyl but not other food flavorings, supporting the conclusion that diacetyl is the most likely causative agent. [37]
Studies in animals experimentally exposed to diacetyl have shown evidence of airway tissue injury and necrosis. Continuous exposure to high and subchronic diacetyl concentrations as well as high brief intense bursts have been associated with injury.[38, 39]
Preliminary NIOSH studies suggest that of diacetyl substitutes such as 2,3 hexanedione, 2,3 heptanedione, and diacetyl trimer may also have respiratory toxicity. Preliminary NIOSH studies have demonstrated potential toxicity. [40]
Workers with diacetyl lung disease commonly present with cough and exertional dyspnea. Irritation of the eye, nose, and throat and skin involvement may also occur in exposed workers. Both the insidious and rapid onset of disease has been reported. Diagnostic workup includes pulmonary function testing that shows evidence of obstruction without bronchodilator response. Recent evidence suggests using a cutoff of a 15% decline in FEV1 per year may not be adequately sensitive to screen for disease and alternative methods such as calculating the longitudinal limit of decline which incorporates data precision may allow for earlier detection of excessive lung function loss[41]
Chest radiography is unremarkable, whereas high-resolution CT scans shows subtle findings of bronchial wall thickening as well as air trapping detectable only on inspiratory and expiratory images (Figure 5). Histopathologic evaluation shows constrictive bronchiolitis obliterans characterized by inflamed and scarred small airways (Figure 6). Induced sputum from workers with high levels of diacetyl exposure show inflammatory responses as evidenced by higher neutrophil counts and levels of interleukin-8 and eosinophilic cationic protein. [42] Non-smokers may be at higher risk than smokers.
Figure 5.
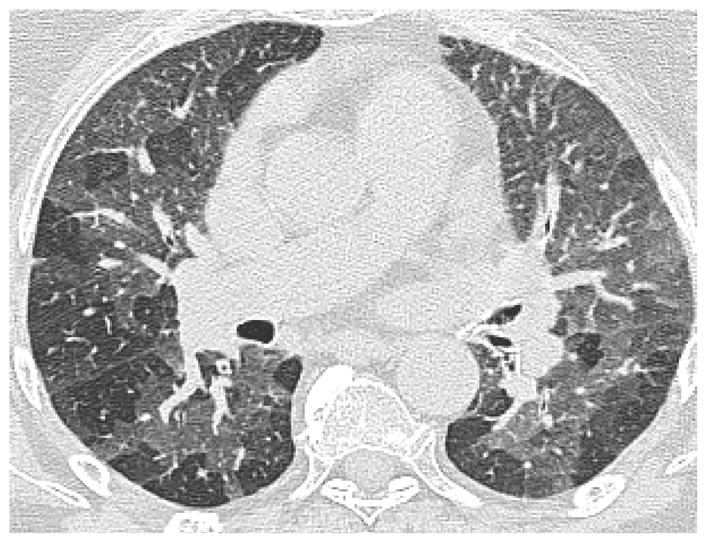
Bronchiolitis Obliterans: High-resolution computed tomography chest scan: E Expiratory, showing patchy air trapping. (Courtesy of Ami Rubinowitz, MD Yale School of Medicine)
Figure 6.
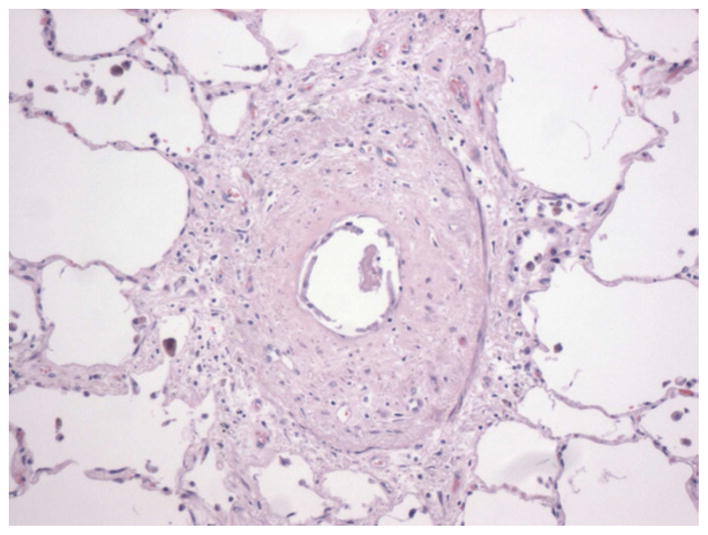
Constrictive bronchiolitis: Marked submucosal fibrosis causing severe narrowing of the airway lumen (Courtesy of Robert Homer, MD/PHD, Yale School of Medicine)
OSHA issued a Hazard Communication regarding diacetyl that did not establish an occupational exposure limit but did require that manufacturers supply workers with updated toxicologic information and health effects information. With respect to control measures, OSHA suggested effective respiratory protection for workers with higher exposure, including air purifying respirators, and suggested that manufacturers consider industrial hygiene sampling and medical surveillance. In 2011, NIOSH issued a draft criteria document for diacetyl exposure, proposing a recommended exposure limit of 5 parts per billion as an 8-hour time-weight average and a short-term exposure limit of 25 parts per billion. Recommendations for 2, 3-pentanedione were also proposed.[43]
Nanoparticles
The implementation of nanoscale materials has the potential to revolutionize multiple industries. Investigators are currently studying the toxicity of nanoparticles as a potential source of occupational lung disease. Nanoparticles have at least one dimension smaller than 100 nm and are further characterized by such physicochemical properties as size, surface area, structure, agglomerativity, and solubility. The various categories of nanoparticles include carbon-based (nanotubes), metal-based (for example, titanium dioxide), and biological (for example, viruses designd for drug delivery). These nanoparticles may lead to more efficient water purification, stronger and lighter building materials, increased computing power, and new nanomedical devices. However, the small size of nanoparticles may result in a range of toxicity.
The interest in nanoparticle toxicity as evolved over time. In the past, the term ultrafine particles has been used to refer to both unintentionally generated nanoparticles such as those found in air pollution and nanoparticles that have been intentionally manufactured. Ambient air pollution studies have suggested an association between exposure to the unintentionally generated ultrafine particles and increased cardiopulmonary toxicity.[44] This has rcontributed to an interest in the potential toxicity of the manufactured nanoparticles. Oberdorster published a highly cited paper on the emerging discipline of nanotoxicology[45] and the journal Nanotoxicology began publication in 2007. NIOSH and various international agencies have funded hazard and risk assessments of nanomaterials. The Project on Emerging Technologies at the Woodrow Wilson International Center for Scholars (www.wilsoncenter.org/nano) maintains an updated list of such particles. [46–48]
Although nanoparticles may be ingested or penetrate the skin, nanoparticles easily penetrate the alveoli and can enter the blood circulation reaching the liver, heart, and nervous system within hours. Nanoparticles may be ineffectively cleared by alveolar macrophages if the nanoparticles are agglomerated. The adherence of metals or other organic compounds to nanoparticles may also contribute to toxicity. There are few human studies of the effects of nanoparticles. Computer models have suggested increased deposition of nanoparticles in diseased or constricted airways. Animal studies have demonstrated low levels of nanoparticles distal to the lung. Some animal studies have shown lung toxicity. [49] Studies have focused on carbon nanotubes, carbon black, fullerenes, silica, and metal-based nanoparticles including titanium dioxide. The method and route of exposure can affect toxicity. Route of exposure includes dermal and gastrointestinal exposure in addition to inhalational exposure through the respiratory tract. These factors may affect agglomerativity and the potential for translocation to other organs distal to the lung. Carbon nanotubes have been shown to induce fibrotic and inflammatory responses.[45–48]
There are several pathophysiologic mechanisms through which nanoparticles can cause toxicity. After being ingested, nanoparticles may activate macrophages that then release such proinflammatory mediators as IL-1, IL-6, TNF-α, macrophage inhibitory protein, and monocyte chemotactic protein. Nanoparticles can also lead to the generation of reactive oxygen species and oxidative stress. The proinflammatory and oxidative stress induced by nanoparticles contribute to a milieu that may promote the development of diffuse interstitial lung disease.
Recently, a case of bronchiolitis obliterans organizing pneumonia was reported in a 58-year-old man after a 3-month exposure at a polyester powder plant. Transmission electron microscopy of the lung tissue demonstrated the presence of titanium dioxide. With the explosion of nanomaterials in manufacturing, medical surveillance in the workplace is recommended. [50]
Idiopathic Interstitial Pneumonias
The contribution of occupational and environmental exposures to “idiopathic” diseases is likely underappreciated. However, epidemiologic studies have been hampered by the relatively low prevalence and heterogeneity of ILD, limited exposure data, and variability in individual susceptibility to exposure.[51]
Exposure to some agents can cause patterns similar to those seen in specific “idiopathic” interstitial pneumonias. For example, asbestos can cause radiographic changes indistinguishable from idiopathic pulmonary fibrosis, the most common form of idiopathic interstitial pneumonia. A careful occupational history and an evaluation for markers of asbestos exposure, such as pleural plaques on chest CT scans or asbestos bodies on histopathologic examination, can differentiate asbestosis from idiopathic pulmonary fibrosis.[52] Nonspecific interstitial pneumonia may represent the pathologic manifestation of hypersensitivity pneumonitis from exposure to organic antigens, such as avian proteins in bird fanciers lung.[53]
The epidemiologic and pathologic evidence supporting the link between chronic occupational and environmental exposures and the broader group of “idiopathic” interstitial pneumonias has evolved over the years. In the 1980s, several case reports demonstrated a relationship between ILD and exposures in aluminum welders, dairy workers, domestic wood burning, dental technicians and diamond polishing. [54, 55] Lung mineral analyses have also supported the relationship between exposures to mineral dusts and parenchymal lung disease. [56–58]
In 2006 Taskar and Coultas reviewed epidemiologic evidence supporting the causal link between occupational exposures and “idiopathic” interstitial pneumonia. The literature, predominantly based on case-control studies in the United States, Japan, and the United Kingdom showed an increased risk of ILD was associated with agricultural exposures, livestock, wood dust, metal dust, stone/sand/silica, and smoking.[59] Inconsistent associations between exposure and disease have been noted with textile dust, mold, and wood fires. Dose-dependent associations have been shown for cigarette smoke, metal, and wood exposure. [59–63] More recent studies include patients in a Swedish oxygen registry suggested associations of interstitial lung disease in patients with exposure to birch and hardwood dust [64] and a 2011 Mexican study showed that patients with idiopathic pulmonary fibrosis were more likely than unaffected persons to be former smokers as well as more likely to have been exposed to “dusts, smokes, gases and chemicals.” [65]
Chronic silica exposure has traditionally been linked to the development of simple silicosis or progressive massive fibrosis, but two recent Japanese studies have linked chronic silica exposure to the development of clinicoradiographic patterns characteristic of the idiopathic interstitial pneumonias. Arakawa reported that 12% of patients with mixed dust pneumoconioses or silicosis had radiographic evidence of chronic interstitial pneumonia, including idiopathic pulmonary fibrosis.[66] Kitamura reported the presence of inorganic dust particles, including silica, in the hilar lymph nodes of patients with idiopathic pulmonary fibrosis.[67] Finally, a recent autopsy study of California farm workers detected increased small airways disease and pneumoconiosis as well as findings of interstitial fibrosis. Crystalline silica and aluminum silicate particles as demonstrated by scanning electron microscopy and X-ray spectrometry were more prevalent in farm workers than in non-farm workers.[56]
Future Directions in Occupational and Environmental Terminal Airways and Diffuse Parenchymal Lung Disease
Evaluating Causality
Recent discoveries of new causes for interstitial and small airway disease highlight some of the difficulties in recognizing the role of occupational and environmental exposures, including clinician awareness and recognition, misdiagnosis and limited information on work and environmental exposures, and the presentation of variable clinical phenotypes in response to a single exposure.
The contribution of occupational and environmental exposures should be considered in all patients with diffuse pulmonary diseases. (Table 1) In fact, individual workers with small airways disease and ILD disease are often misdiagnosed with chronic obstructive pulmonary disease or idiopathic pulmonary fibrosis.
Table 1.
Methods for Exploring Suspected Exposure-Disease Relationships
| Methods | Benefits | Limitations |
|---|---|---|
Full Environmental and Occupational History
|
|
|
Imaging
|
|
|
Pulmonary Function Testing
|
|
|
| Lung Volumes and DLCO |
|
|
Exposure Assessment
|
|
|
| Animal Toxicology and In-vitro studies |
|
|
| Biomarkers |
|
|
Most important, a high index of suspicion and a thorough occupational and environmental history is essential. For more chronic diseases and those with a long latency between exposure the development of disease, such asbestosis, it is important to ask about past jobs, which is subject to recall bias. A unique or unusual presentation of disease, such as the presentation of ILD in a younger patient, should prompt a careful exposure history. Investigators should inquire about respiratory symptoms among coworkers or other individuals sharing similar exposures. Clinicians can solicit crude yet effective exposure information from patients by asking simple questions, such as whether visible dust, gases, or fumes are present in the work environment and whether personal protective equipment, such as respirators, is used.
Routine evaluation tools, such as plain chest radiography or office spirometry, may be insufficient to detect terminal airways disease or interstitial disease. For example, air trapping in patients with indium lung or subtle reticular markings or ground glass in patients with other forms of interstitial disease, easily missed on chest x-ray, can be detected on high resolution CT scan. Restrictive ventilatory defects and diffusion impairments require full pulmonary function testing and will be missed by routine office spirometry.
When an index case of possible work-related lung disease is identified, the possibility that co-workers may also be affected should always be considered. Ideally, regulatory agencies, industrial hygienists specializing in exposure assessments, and pulmonary medicine and occupational health providers will collaborate to investigate the possibility of work-related disease among co-workers or other cohorts with similar exposures. For example, recent reports of indium lung and flavoring-related lung disease in individual patients prompted further investigation by NIOSH, and implementation of medical surveillance, that revealed a greater burden of clinical and subclinical disease among larger cohorts of workers.
When one suspects a correlation between exposure and disease has occurred in one individual patient, it can be difficult to determine if there is a more widespread effect of any particular exposure. Exposures rarely occur without other confounding exposures and can be difficult to measure. The goal of epidemiologic studies is to estimate the relevant exposure and try to find association with disease. Case-control studies often use measures of exposure, such as self-report or job exposure or tasks from administrative datasets but these may be crude or inaccurate. Quantitative exposure assessments that establish dose relationships are preferable but such measurements are often lacking and not mandated or performed in industry. For example, quantitative exposure assessments of respiratory intoxicants at the time of collapse of the World Trade Centers were not available; however, a qualitative measure of exposure—the physical presence of a patient at the actual time of the collapse—has been shown to be an effective means to classify individuals with the highest level of exposure. When new causes of occupational diseases are identified, the causative agent is not always evident. For example, the specific component of World Trade Center dust responsible for disease is still not clear given the number of different exposures that occurred. Industries are required to conduct routine industrial hygiene sampling only for certain specific exposures. Therefore, historical database of exposures are often unavailable. Finally, even, when a novel occupational disease is suspected to correlate with one specific exposure, recommended or required exposure limits for a respiratory toxicant may be completely lacking or far above what has actually caused toxicity in susceptible workers
Animal studies can support the biological plausibility of a given exposure causing disease and better understand dose-response relationships and possible mechanisms. Such studies have been performed, for example, in the case of Indium lung, Nylon flock worker’s lung and diacetyl-induced lung disease.
Detection of foreign material in lung tissue using methods such as polarizable light microscopy or scanning electron microscopy and energy dispersive X-ray spectroscopy can be used to help evaluate inhalational exposures. For example, scanning electron microscopy and energy dispersive X-ray spectroscopy revealed opaque and birefringent particles with macrophages that contained silica, aluminum silicates, titanium oxide, talc, and metals in a series of patients with ILD exposed to World Trade Center dust.[26] However, the significance of these findings is unclear, given lack of controls and the small sample size. In the case of indium lung, biopsy specimens have confirmed presence of indium in lung tissue (put first). Such methods may also advance our understanding of “idiopathic” lung diseases.
Respiratory Surveillance Programs
Workplace medical surveillance programs can help detect early lung disease and lead to improved preventive strategies.
Such programs are based on serial periodic spirometry and symptom surveys and can help detect disease in an individual patient or identify risk factors for disease in an at-risk cohort of workers, such as certain tasks or processes. When lung disease presents in a single or few workers, medical surveillance can help estimate the burden of disease among other workers with similar exposures.
Detecting disease in a working population can be particularly challenging as many workers have “supranormal” lung function or above average levels of spirometric function. This is due to the healthy worker phenomenon that arises because individuals entering the working force are in general healthier than the general population. The evaluation of longitudinal changes in lung function can help identify workers with excessive declines in lung function, despite apparently ‘normal’ appearing lung function. Given the significant variability between individuals, longitudinal changes in individual workers compares an individual to him/herself. Ideally longitudinal spirometry also includes baseline spirometric testing prior to the onset of exposure. When individuals experience accelerated declines in lung function particularly after the introduction of a new exposure. The case of diacetyl induced lung disease as described above, for example, clearly demonstrated excessive declines in lung function.[35]
The performance and evaluation of spirometry in workers over time is challenging, including issues related to the quality of the spirometry testing and analysis and interpretation of the results, recently reviewed by Hnizdo. [68] Defining excessive declines of lung function over time has been challenging and is dependent on the quality of the spirometry obtained. Recommendations have varied from greater than 15% yearly FEV1 loss, absolute loss of 60 mL/year, or 90 mL/year. Recently NIOSH has developed a program called Spirometry Longitudinal Data Analysis (SPIROLA) that can help determine excessive longitudinal lung function loss. This program takes into account the quality and precision of the spirometry testing and calculates a longitudinal limit of normal for lung function decline.
One difficulty with spirometry is the lack of sensitivity or specificity for restrictive disease, which is common in ILD. A reduced forced vital capacity may suggest restrictive disease; however, a formal measurement of total lung capacity is required to make a formal diagnosis. It should be remembered that spirometry and questionnaires performed in the work setting are designed to identify those with possible early lung disease who may need further evaluation, such as full pulmonary function tests and diffusing capacity, and chest imaging. A normal spirometry does not rule out lung disease, and should be interpreted in the context of symptom questionnaire and other relevant information.
Plain chest radiography is insensitive for detecting subtle changes, such as air trapping, ground-glass, or reticular markings common in patients with terminal airways disease and interstitial disease. High resolution chest CT Scan can be helpful and detecting subtle reticular markings or ground glass opacities, for example, Inspiratory and expiratory imaging can reveal mosaic or air trapping in individuals with terminal airways disease.
Biomarkers
The use of biomarkers of disease in conjunction with epidemiologic data may improve diagnostic capabilities and understanding of occupational disease. Such markers can measure exposure, susceptibility, and effect. Biomarkers of exposure, for example serum indium concentrations, confirm the presence of a biological dose and decrease the possibility of exposure misclassification.
Many biomarkers have been evaluated in fibrotic lung disease. KL-6 is a mucin-like glycoprotein that serves as a chemotactictactic for fibroblasts and has considerable accuracy in ILD diagnosis. Surfactant proteins and matrix metalloproteases have also been shown to be elevated in ILD. Other implicated proteins include certain chemokines, such as CCL2, YKL-40, and osteopontin. Recent investigations of newly diagnosed terminal airways disease and ILD have used these biomarkers as evidence of lung injury in exposed workers with and without clinically apparent disease. Investigators seeking to characterize the toxicity of indium, for example, use KL-6 and surfactant proteins as biomarkers of effect. Multiple biomarkers have been suggested for silica and coal workers pneumoconioses, including such markers of inflammation as TNF-ά, Il-1B, and IL-8 and smarkers of oxidant injury such as 8-isoprostanes and glutathione peroxidase activity. [69] Nonetheless, the field is still in its infancy. The validity of using such biomarkers to indicate of disease or disease severity must still be established.
Conclusion
Over the past twenty years a number of important new causes of occupational and environmental terminal airways disease and diffuse parenchymal lung disease have been recognized, including indium lung, flock-worker’s lung, diacetyl lung, the spectrum of World Trade Center lung diseases, World-trade center lung, and nanoparticle related lung disease. Yet despite the increased recognition of occupation hazards in the workplace, these examples highlight the difficulty in evaluating causality despite advances in our understanding of diffuse parenchymal lung disease. Given that new potential hazards such as engineered nanoparticles and unanticipated exposures such as after the collapse of the World Trade Center continue to occur, the individual clinician must carefully consider the potential role of occupational exposures in the diagnosis of chronic parenchymal lung and terminal airways disease.
Key Points.
Occupational health investigators continue to uncover associations between novel exposures and chronic forms of diffuse parenchymal lung disease and terminal airways disease.
This article details several newly recognized chronic parenchymal and terminal airways. Diseases related to exposure to Indium, Nylon Flock, Diacetyl used in the flavorings industry, nanoparticles, and the World Trade Center disaster are reviewed.
Additionally, this article will review methods in worker surveillance as well as the potential use of biomarkers in the evaluation of exposure disease relationships.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blanc PD, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax. 2009;64(1):6–12. doi: 10.1136/thx.2008.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyler PM. Minor Metals, in Minerals. US Bureau of Mines; 1937. pp. 759–786. [Google Scholar]
- 3.Minami H. Kinzoku-Shigen Report. 2010. Trend of demand, supply and price of indium and gallium; pp. 81–93. [Google Scholar]
- 4.Homma T, et al. Interstitial pneumonia developed in a worker dealing with particles containing indium-tin oxide. J Occup Health. 2003;45(3):137–9. doi: 10.1539/joh.45.137. [DOI] [PubMed] [Google Scholar]
- 5.Cummings KJ, et al. Pulmonary alveolar proteinosis in workers at an indium processing facility. Am J Respir Crit Care Med. 2010;181(5):458–64. doi: 10.1164/rccm.200907-1022CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chonan T, Taguchi O, Omae K. Interstitial pulmonary disorders in indium-processing workers. Eur Respir J. 2007;29(2):317–24. doi: 10.1183/09031936.00020306. [DOI] [PubMed] [Google Scholar]
- 7.Cummings KJ, et al. Indium lung disease. Chest. 141(6):1512–21. doi: 10.1378/chest.11-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakano M, et al. Causal relationship between indium compound inhalation and effects on the lungs. J Occup Health. 2009;51(6):513–21. doi: 10.1539/joh.l9077. [DOI] [PubMed] [Google Scholar]
- 9.Nogami H, et al. Pulmonary disorders in indium-processing workers. Nihon Kokyuki Gakkai Zasshi. 2008;46(1):60–4. [PubMed] [Google Scholar]
- 10.Omae K, et al. Indium lung--case reports and epidemiology. Int Arch Occup Environ Health. 2011;84(5):471–7. doi: 10.1007/s00420-010-0575-6. [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Health L and Welfare. Technical guidelines for preventing health impairment in the indium tin oxide handling process. 2010 [cited 2012 July 28]; Available from: htt://wwwhourei.mhlw.go.jp/cgi-
- 12.(ACGIH), A.C.o.G.I.H.; A.C.o.I.G.I. Hygienists. Documentation of the threshold limit values and biologicla exposure indices. Cincinnati: 2001. [Google Scholar]
- 13.Kern DG, et al. Flock worker’s lung: chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129(4):261–72. doi: 10.7326/0003-4819-129-4-199808150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kern DG, et al. Flock worker’s lung: broadening the spectrum of clinicopathology, narrowing the spectrum of suspected etiologies. Chest. 2000;117(1):251–9. doi: 10.1378/chest.117.1.251. [DOI] [PubMed] [Google Scholar]
- 15.Weiland DA, et al. Thin-section CT findings in flock worker’s lung, a work-related interstitial lung disease. Radiology. 2003;227(1):222–31. doi: 10.1148/radiol.2271011063. [DOI] [PubMed] [Google Scholar]
- 16.Porter DW, et al. Acute inflammatory reaction in rats after intratracheal instillation of material collected from a nylon flocking plant. J Toxicol Environ Health A. 1999;57(1):25–45. doi: 10.1080/009841099157845. [DOI] [PubMed] [Google Scholar]
- 17.Lougheed MD, et al. Desquamative interstitial pneumonitis and diffuse alveolar damage in textile workers. Potential role of mycotoxins. Chest. 1995;108(5):1196–200. doi: 10.1378/chest.108.5.1196. [DOI] [PubMed] [Google Scholar]
- 18.Eschenbacher WL, et al. Nylon flock-associated interstitial lung disease. Am J Respir Crit Care Med. 1999;159(6):2003–8. doi: 10.1164/ajrccm.159.6.9808002. [DOI] [PubMed] [Google Scholar]
- 19.Prezant DJ, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med. 2002;347(11):806–15. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 20.Reibman J, et al. The World Trade Center residents’ respiratory health study: new-onset respiratory symptoms and pulmonary function. Environ Health Perspect. 2005;113(4):406–11. doi: 10.1289/ehp.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidotti TL, Prezant D, de la Hoz R, Miller A. The evolving spectrum of pulmonary disease in responders to the World Trade Center tragedy. American Journal of Industrial Medicine. 2011;54(9):649–660. doi: 10.1002/ajim.20987. [DOI] [PubMed] [Google Scholar]
- 22.Mann JM, et al. World Trade Center dyspnea: bronchiolitis obliterans with functional improvement: a case report. Am J Ind Med. 2005;48(3):225–9. doi: 10.1002/ajim.20196. [DOI] [PubMed] [Google Scholar]
- 23.Wu M, et al. Case report: Lung disease in World Trade Center responders exposed to dust and smoke: carbon nanotubes found in the lungs of World Trade Center patients and dust samples. Environ Health Perspect. 118(4):499–504. doi: 10.1289/ehp.0901159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman SM, et al. Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am J Respir Crit Care Med. 184(5):582–9. doi: 10.1164/rccm.201011-1909OC. [DOI] [PubMed] [Google Scholar]
- 25.Rom WN, et al. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am J Respir Crit Care Med. 2002;166(6):797–800. doi: 10.1164/rccm.200206-576OC. [DOI] [PubMed] [Google Scholar]
- 26.Caplan-Shaw CE, et al. Lung pathologic findings in a local residential and working community exposed to World Trade Center dust, gas, and fumes. J Occup Environ Med. 53(9):981–91. doi: 10.1097/JOM.0b013e31822fff60. [DOI] [PubMed] [Google Scholar]
- 27.Crowley LE, et al. “Sarcoid like” granulomatous pulmonary disease in World Trade Center disaster responders. Am J Ind Med. 2011;54(3):175–84. doi: 10.1002/ajim.20924. [DOI] [PubMed] [Google Scholar]
- 28.Izbicki G, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131(5):1414–23. doi: 10.1378/chest.06-2114. [DOI] [PubMed] [Google Scholar]
- 29.McGee JK, et al. Chemical analysis of World Trade Center fine particulate matter for use in toxicologic assessment. Environ Health Perspect. 2003;111(7):972–80. doi: 10.1289/ehp.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavett SH, et al. World Trade Center fine particulate matter causes respiratory tract hyperresponsiveness in mice. Environ Health Perspect. 2003;111(7):981–91. doi: 10.1289/ehp.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lioy PJ, Georgopoulos P. The anatomy of the exposures that occurred around the World Trade Center site: 9/11 and beyond. Ann N Y Acad Sci. 2006;1076:54–79. doi: 10.1196/annals.1371.002. [DOI] [PubMed] [Google Scholar]
- 32.Fireman EM, et al. Induced sputum assessment in New York City firefighters exposed to World Trade Center dust. Environ Health Perspect. 2004;112(15):1564–9. doi: 10.1289/ehp.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreiss K, et al. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347(5):330–8. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- 34.From the Centers for Disease Control and Prevention. Fixed obstructive lung disease in workers at a microwave popcorn factory--Missouri, 2000–2002. JAMA. 2002;287(22):2939–40. [PubMed] [Google Scholar]
- 35.Kreiss K, et al. Longitudinal lung function declines among California flavoring manufacturing workers. Am J Ind Med. doi: 10.1002/ajim.21013. [DOI] [PubMed] [Google Scholar]
- 36.Hendrick DJ. “Popcorn worker’s lung” in Britain in a man making potato crisp flavouring. Thorax. 2008;63(3):267–8. doi: 10.1136/thx.2007.089607. [DOI] [PubMed] [Google Scholar]
- 37.van Rooy FG, et al. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am J Respir Crit Care Med. 2007;176(5):498–504. doi: 10.1164/rccm.200611-1620OC. [DOI] [PubMed] [Google Scholar]
- 38.Morgan DL, et al. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol Sci. 2008;103(1):169–80. doi: 10.1093/toxsci/kfn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hubbs Ann F, WTG, Kashon Michael L, Frazer David, Mercer Robert R, Battelli Lori A, Kullman Gregory J, Schwegler-Berry Diane, Friend Sherri, Castranova Vincent. Respiratory Toxicologic Pathology of Inhaled Diacetyl in Sprague-Dawley Rats. Toxicologic Pathology. 36(2):330–344. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- 40.Flake GP, PJK, Price HC, Gage PS, Kelly FL, Palmer SM, Foley JF, Morgan DL. Bronchiolitis obliterans-like lesions in rats treated with diacetyl, acetoin, or acetyl propionyl by intratracheal instillation. The Toxicologist--An official journal of the society of toxicology. 2010;114 [Google Scholar]
- 41.Chaisson NF, et al. Evaluation of methods to determine excessive decline of forced expiratory volume in one second in workers exposed to diacetyl-containing flavorings. J Occup Environ Med. 2010;52(11):1119–23. doi: 10.1097/JOM.0b013e3181f84577. [DOI] [PubMed] [Google Scholar]
- 42.Akpinar-Elci M, et al. Induced sputum evaluation in microwave popcorn production workers. Chest. 2005;128(2):991–7. doi: 10.1378/chest.128.2.991. [DOI] [PubMed] [Google Scholar]
- 43.document, n.d., 2011.
- 44.Donaldson K, et al. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109(Suppl 4):523–7. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonner JC. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc Am Thorac Soc. 2010;7(2):138–41. doi: 10.1513/pats.200907-061RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morimoto Y, et al. Hazard assessments of manufactured nanomaterials. J Occup Health. 2010;52(6):325–34. doi: 10.1539/joh.r10003. [DOI] [PubMed] [Google Scholar]
- 48.Castranova V. Overview of current toxicological knowledge of engineered nanoparticles. J Occup Environ Med. 2011;53(6 Suppl):S14–7. doi: 10.1097/JOM.0b013e31821b1e5a. [DOI] [PubMed] [Google Scholar]
- 49.Choi HS, et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010;28(12):1300–3. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng TH, et al. Bronchiolitis obliterans organizing pneumonia due to titanium nanoparticles in paint. Ann Thorac Surg. 2012;93(2):666–9. doi: 10.1016/j.athoracsur.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 51.Glazer CS, Newman LS. Occupational interstitial lung disease. Clin Chest Med. 2004;25(3):467–78. vi. doi: 10.1016/j.ccm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med. 2004;170(6):691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]
- 53.Vourlekis JS, et al. Nonspecific interstitial pneumonitis as the sole histologic expression of hypersensitivity pneumonitis. Am J Med. 2002;112(6):490–3. doi: 10.1016/s0002-9343(02)01046-x. [DOI] [PubMed] [Google Scholar]
- 54.Pujol JL, et al. Interstitial pulmonary disease induced by occupational exposure to paraffin. Chest. 1990;97(1):234–6. doi: 10.1378/chest.97.1.234. [DOI] [PubMed] [Google Scholar]
- 55.Sherson D, Maltbaek N, Heydorn K. A dental technician with pulmonary fibrosis: a case of chromium-cobalt alloy pneumoconiosis? Eur Respir J. 1990;3(10):1227–9. [PubMed] [Google Scholar]
- 56.Schenker MB, et al. Pneumoconiosis from agricultural dust exposure among young California farmworkers. Environ Health Perspect. 2009;117(6):988–94. doi: 10.1289/ehp.0800144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pariente R, et al. A study of pulmonary dust deposits using the electron microscope in conjunction with the electron sound analyser. Thorax. 1972;27(1):80–2. doi: 10.1136/thx.27.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monso E, et al. Mineralogical microanalysis of idiopathic pulmonary fibrosis. Arch Environ Health. 1990;45(3):185–8. doi: 10.1080/00039896.1990.9936714. [DOI] [PubMed] [Google Scholar]
- 59.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 2006;3(4):293–8. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- 60.Baumgartner KB, et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. Am J Epidemiol. 2000;152(4):307–15. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 61.Baumgartner KB, et al. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155(1):242–8. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 62.Hubbard R, et al. Risk of cryptogenic fibrosing alveolitis in metal workers. Lancet. 2000;355(9202):466–7. doi: 10.1016/S0140-6736(00)82017-6. [DOI] [PubMed] [Google Scholar]
- 63.Hubbard R, et al. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet. 1996;347(8997):284–9. doi: 10.1016/s0140-6736(96)90465-1. [DOI] [PubMed] [Google Scholar]
- 64.Gustafson T, et al. Occupational exposure and severe pulmonary fibrosis. Respir Med. 2007;101(10):2207–12. doi: 10.1016/j.rmed.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Sancho C, et al. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir Med. 2011;105(12):1902–7. doi: 10.1016/j.rmed.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 66.Arakawa H, et al. Chronic interstitial pneumonia in silicosis and mix-dust pneumoconiosis: its prevalence and comparison of CT findings with idiopathic pulmonary fibrosis. Chest. 2007;131(6):1870–6. doi: 10.1378/chest.06-2553. [DOI] [PubMed] [Google Scholar]
- 67.Kitamura H, et al. Inhalation of inorganic particles as a risk factor for idiopathic pulmonary fibrosis--elemental microanalysis of pulmonary lymph nodes obtained at autopsy cases. Pathol Res Pract. 2007;203(8):575–85. doi: 10.1016/j.prp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Hnizdo E, Glindmeyer HW, Petsonk EL. Workplace spirometry monitoring for respiratory disease prevention: a methods review. Int J Tuberc Lung Dis. 2010;14(7):796–805. [PubMed] [Google Scholar]
- 69.Gulumian M, et al. Mechanistically identified suitable biomarkers of exposure, effect, and susceptibility for silicosis and coal-worker’s pneumoconiosis: a comprehensive review. J Toxicol Environ Health B Crit Rev. 2006;9(5):357–95. doi: 10.1080/15287390500196537. [DOI] [PubMed] [Google Scholar]


