Abstract
Introduction
Medulloblastoma, the largest group of embryonal brain tumors, has historically been classified into five variants based on histopathology. More recently, epigenetic and transcriptional analyses of primary tumors have sub-classified medulloblastoma into four to six subgroups, most of which are incongruous with histopathological classification.
Discussion
Improved stratification is required for prognosis and development of targeted treatment strategies, to maximize cure and minimize adverse effects. Several mouse models of medulloblastoma have contributed both to an improved understanding of progression and to developmental therapeutics. In this review, we summarize the classification of human medulloblastoma subtypes based on histopathology and molecular features. We describe existing genetically engineered mouse models, compare these to human disease, and discuss the utility of mouse models for developmental therapeutics. Just as accurate knowledge of the correct molecular subtype of medulloblastoma is critical to the development of targeted therapy in patients, we propose that accurate modeling of each subtype of medulloblastoma in mice will be necessary for preclinical evaluation and optimization of those targeted therapies.
Keywords: Medulloblastoma, Mouse models, Molecular subgroups, Targeted therapies, Preclinical testing
Introduction
Medulloblastoma, a highly aggressive WHO-grade IV embryonal tumor of the cerebellum [1, 2], is the most common malignant brain tumor in children. Medulloblastoma accounts for 20% of pediatric central nervous system tumors [3], and the incidence is 0.6 per 100,000 children in patients 0–19 years [4–6], decreasing with age. Seventy percent of cases occur in children younger than 10 years of age [6].
Leptomeningeal dissemination occurs in 30% of cases at presentation and is the strongest predictor of poor prognosis [7, 8]. The extent of surgical resection, which is affected by involvement of the brainstem, and the degree of tumor dissemination are also highly predictive of outcome [7, 9]. Multimodal surgical resection, radiation, and chemotherapy have led to modest improvements in overall survival over the last decade. Five-year survival rates are now as high as 70–80% in standard-risk patients [10–12], but survival is often achieved at the cost of treatment-induced morbidities [13–16]. Moreover, survivors suffer the continued risk of secondary tumors, relapse, and metastasis [17]. Despite the improved outcomes for standard-risk patients, the prognosis for high-risk patients (younger than 3 years old, or significant residual post-operative tumor, or leptomeningeal dissemination at presentation) remains dismal at only 25–40% 5-year event-free survival [18, 19].
Classification of medulloblastoma
Medulloblastoma arises in the posterior fossa [20]. Medulloblastoma tumor cells can invade the cerebellar cortex and white matter and spread, via the cerebrospinal fluid, to the leptomeningeal membranes that cover the CNS as well as to the spinal cord. Medulloblastoma cells typically appear undifferentiated, reminiscent of stem or progenitor cells. The WHO separates medulloblastoma into five variants based on histopathological features: (a) classic, (b) desmo-plastic/nodular, (c) medulloblastoma with extensive nodularity, (d) large cell medulloblastoma, and (e) anaplastic medulloblastoma [21].
Biomarkers and molecular profiling
Several biomarkers with prognostic significance (some of which have been validated in clinical trials) have been identified for specific subtypes of medulloblastoma. The Sonic Hedgehog (SHH) signaling pathway, which is required for normal cerebellar development [21], was first implicated in medulloblastoma when patients with Gorlin’s syndrome, who develop nevoid basal cell carcinoma [22, 23] and sporadic medulloblastoma [24–27], were found to harbor germline inactivating mutations of Patched1 (PTCH1). Subsequently, mutations in downstream SHH pathway components, such as Smoothened (SMO) and suppressor of fused (SUFU), and amplification of GLI1 and GLI2 and the miR17–92 complex were also identified in sporadic medulloblastoma [28–32]. SHH-dysregulated medulloblastomas comprise ~25% of all cases and may show desmoplastic or classic histopathology; desmoplastic tumors in particular have been associated with better prognoses [21, 33].
The canonical WNT pathway was first implicated in medulloblastoma based on observations that a subset of patients with germline mutations in the tumor suppressor APC (i.e., patients with Turcot syndrome) develops medulloblastoma [34, 35]. Subsequently, 5–10% of patients with sporadic medulloblastoma were also shown to harbor activating point mutations in the β-catenin gene CTNNB1, resulting in aberrantly activated WNT signaling [36, 37]. Other abnormalities found in WNT tumors include promoter methylation (hence gene silencing) of the secreted frizzled-related protein 1 family of WNT inhibitors and monosomy of chromosome 6 [4, 38]. WNT-associated tumors account for ~18–25% of all cases and are usually of classic histology [37, 39].
Amplification of MYC genes (c-MYC and MYCN, v-myc myelocytomatosis viral-related oncogene) correlates with poor prognosis and is often found in tumors with large cell anaplastic histopathology [40]. Amplification of c-MYC has been reported in 5–15% of medulloblastoma overall, while amplification of MYCN has been found in ~10% of cases [40–42]. TP53 loss or mutation contributes to 10–15% of cases, and Li–Fraumeni patients with germline mutations in TP53 have increased risk of cancers, including medulloblastoma [43–45]. In addition, loss of the tumor suppressor ARF due to homozygous deletion or promoter hypermethylation (with wild-type TP53) occurs in ~10% of tumors [44]. Analysis of a small subset of human tumors has found TP53 mutations and methylation and deletion of ARF in aggressive LCA tumors, demonstrating the importance of the ARF/TP53 pathway in promoting malignancy [44]. Curiously, the highest frequency of TP53 mutations is found in WNT medulloblastomas, which have the best relative prognosis [46].
Isochromosome 17q, the most frequent chromosomal aberration in medulloblastoma, is present in 30–50% of tumors [11, 38], and together with copy number abnormalities in chromosome 17, including 17q gains, and 17p deletions, is associated with poor prognosis [40]. Two candidate tumor suppressors, hypermethylated in cancer 1 (HIC1), a POZ domain transcriptional repressor, and RENKCTD11, an E3 ubiquitin ligase component that works with Cullin3 to deacetylate GLI1 and GLI2 [47–49], localize to this region. In medulloblastomas, HIC1 is frequently silenced in tumors due to promoter hypermethylation [50–52], while loss of RENKCTD11 during 17p deletion enhances SHH signaling [53].
Hypermethylation of promoters for tumor suppressor genes leads to gene silencing and may contribute to development of medulloblastoma. Implicated genes include ras association domain family protein 1, isoform A (RASSF1A) [54–56], serine protease inhibitor kunitz-type 2 (SPINT2), a tumor suppressor gene that inhibits HGF/MET signaling [57], and Kruppel-like factor 4 (KLF4) [58]. Finally, the NOTCH and phosphatidylinositol 3′-kinase (PI3K) signaling pathways may also contribute to medulloblastoma. Activation of the NOTCH pathway may occur as a result of overexpression or amplification of NOTCH1, NOTCH2, and downstream targets HES1 and HES5 [59, 60] or silencing of miR199b-5p, a negative regulator of NOTCH signaling [61]. The PI3K pathway has also been shown to contain aberrations in medulloblastoma; mutations in the catalytic subunit PIK3CA are found in ~5% of cases [62], while deletions or mutations in PTEN (a negative regulator of PI3K) occur in 30–35% of cases [63].
Several transcriptome analyses of human medulloblastoma samples have sub-classified this disease [38, 64–66]. Two subclasses of tumors, with dysregulated WNT or SHH signaling, have been consistently identified [38, 64–66], suggesting that these tumors arise and develop differently from other subtypes. Independent groups have classified non-WNT, non-SHH tumors into two to four subgroups; expression of neuronal and photoreceptor markers determines the precise number of subgroups. These subgroups may be best represented as a spectrum of tumor types, with one end of the spectrum expressing a “neuronal/glutamatergic” signature, while the other end is characterized by expression of a photoreceptor/GABAergic signature. Importantly, a subset of tumors on the “photoreceptor/GABAergic” end of the spectrum also exhibits a MYC activation signature; these MYC-associated tumors are the most aggressive and display the worst prognosis.
A comparison of the broad histopathological WHO classification of classic, desmoplastic, and large cell/anaplastic tumors with the molecular/transcriptional subclasses indicates some correlation but also highlights inconsistencies in these subclassification schemes. The common classic tumors and the rare LCA tumors span all molecular subtypes, while true desmoplastic tumors are almost entirely restricted to the SHH subgroup.
Mouse models of medulloblastoma
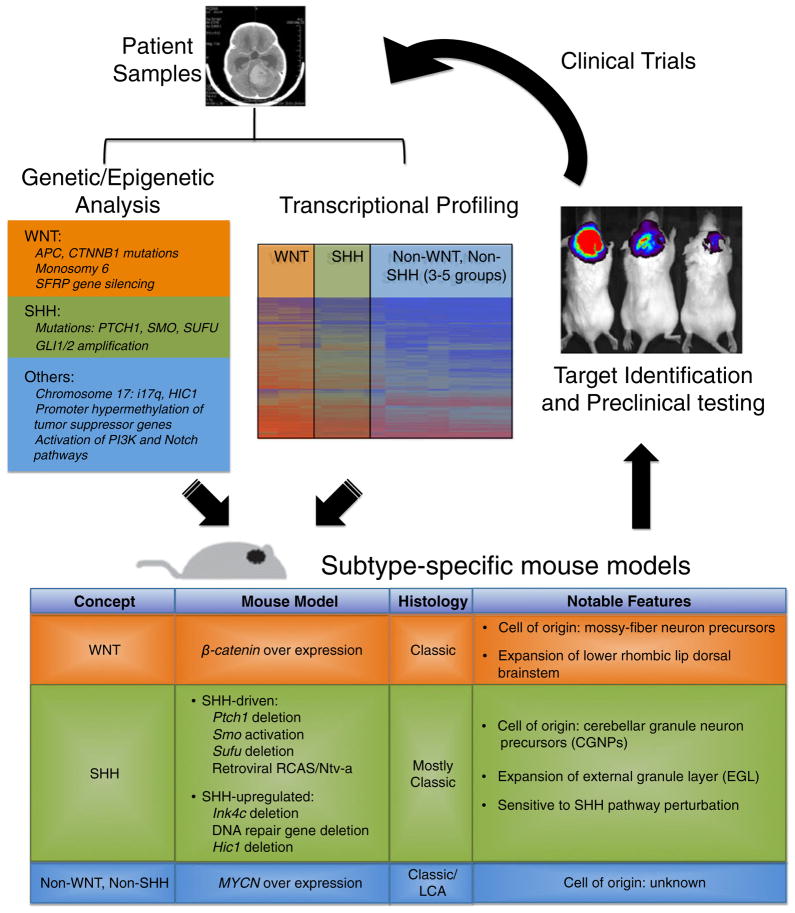
Medulloblastoma cell lines generally lack cellular heterogeneity and components of the microenvironment, and acute transplantation of tumor cells into mice does not recapitulate the malignancy and alterations in the microenvironment that develop in situ in patients. The use of genetically engineered mouse (GEM) models provides physiologically relevant insights into human tumor settings, albeit with limitations. The first models of medulloblastoma were derived by inoculating viral agents such as adenoviruses and polyoma viruses into rodents early during postnatal cerebellar development. Among the early successes were studies using Simian adenovirus SA7 [67, 68], the JC human polyoma virus [69, 70], or the JC virus T antigen (viral early regulatory protein). Since then, GEM models of medulloblastoma have been developed using two main strategies: germline modifications via transgenes (genetic knockins or knockouts) and somatic cell gene transfer by viral transduction (Fig. 1).
Fig. 1.
Utility of genetically engineered mouse models in the development of therapeutics. Knowledge of genetic abnormalities (mutations or pathway aberrations) and transcriptional signatures in human medulloblastoma and hypotheses regarding the cell of origin can be used to develop mouse models of the disease. Depicted are some of the current mouse models available and which human medulloblastoma subgroups they represent. Mouse models can be used to study tumor biology, and cells isolated from these models can be used for high-throughput or candidate screening to identify novel approaches to therapy. Mouse models can also be used to test the efficacy of these therapies in vivo. Subgroup-specific therapies can be translated back to the clinic to improve treatment strategies for patients
SHH pathway models
Because the SHH pathway has been shown to be important in normal cerebellar development and implicated in Gorlin’s syndrome, many models have been developed to manipulate components of this pathway. These manipulations include deletion of Ptch1, activation of Smo, and deletion of Sufu. While homozygous deletion of Ptch1 results in early embryonic lethality due to cardiac and neural tube defects, heterozygous mice are viable. Medulloblastoma develops in 14–19% of these mice by 10 months of age, with peak occurrence at 16–25 weeks [71–73] (systemic heterozygosity of Ptch1 also results in other tumors, notably sarcomas). Some groups have observed the retention of wild-type Ptch1 allele in these Ptch1+/− tumors [71, 73], while others report loss of heterozygosity or mutation of the remaining Ptch1 allele [74, 75]. Loss of Ptch2 (which has 73% amino acid similarity to Ptch1) can cooperate with Ptch1 heterozygosity to promote medulloblastoma progression [76], and complete loss of p53 in Ptch1+/− mice has also been shown to increase the incidence of tumors (95–100%) and reduce the latency to 4–16 weeks [77, 78]. Loss of Ptch1 results in increased activity of downstream genes Gli1 and Cyclin D1 in cerebellar granule neuronal precursors (CGNPs) and accordingly, complete loss of Gli1 or CyclinD1 in Ptch1+/− mice results in reduced tumor incidence, demonstrating that these genes contribute significantly to SHH-driven medulloblastoma [79, 80]. In addition, Ptch1+/− mice crossed into an Igf2-null background fail to develop tumors, demonstrating that IGF2 is essential for SHH-driven medulloblastoma [81, 82].
Transgenic mouse models have also been generated using activated SMO, either with the mutation W535L (SmoM2) in human SMO or the corresponding mutation W539L in mouse Smo (SmoA1). The mutation resides in the trans-membrane domain of SMO and results in SHH ligand-independent activation of downstream signaling. The SmoM2 mouse model uses a ubiquitously expressed CreER transgene to allow for tamoxifen-mediated activation of mutant SmoM2 and induction of downstream SHH signaling [83]. Sporadic leakiness of Cre activity results in 27% of mice developing medulloblastoma, and acute activation of SmoM2 at postnatal day 10 increases medulloblastoma incidence to 40%. The SmoA1 mouse model uses the NeuroD2 (ND2) promoter to drive SmoA1 expression in CGNPs [60, 84]. Hemizygous mice develop medulloblastoma at a median age of 26 weeks with 48% incidence, while 94% of homozygous mice develop tumors at 8 weeks of age.
Both the Ptch1 and SmoM2 models have been further engineered to restrict activated SHH signaling to cerebellar neural stem cells or CGNPs. When hGFAP-Cre or Math1-Cre drivers were used to delete Ptch1 conditionally, tumors developed in 100% of mice by 1–3 months of age [85]. Similarly, Schüller et al. expressed activated SmoM2 using hGFAP-, Math1-, Olig2-, and Tlx3-Cre drivers [86], where Olig2-Cre and Tlx3-Cre are specifically expressed in neuronal progenitors in the posterior EGL and remain expressed in the IGL. Medulloblastoma developed in 100% of mice using all four drivers, with an average survival of about 1–2 months. The authors also targeted SmoM2 using the Gli1 promoter. However, incidence of tumors was only 40%, and the animals displayed prolonged survival, suggesting that driving tumors using promoters of genes downstream from SHH less potently promotes oncogenesis [86]. Finally, a model targeting downstream Sufu has also been reported, in which Sufu+/−; p53−/− mice develop medulloblastomas with an incidence of 58% and rhabdomyosarcomas at 9% incidence by 10 months [87].
The other main strategy for generating GEM models of medulloblastoma is somatic cell gene transfer. Early studies used SHH-expressing Moloney murine leukemia viruses injected in utero into embryonic day 13.5 mouse cerebellum. Medulloblastoma arose in 76% of animals by P14–21, suggesting that direct overexpression of SHH alone is sufficient to initiate tumors [88, 89]. SHH-expressing retroviruses have also been introduced in the cerebellum of Gli1−/−mice. Medulloblastoma still formed, indicating that Gli1 is not critical in this model [87]. Gli2 was shown to be expressed in the tumors, suggesting that Gli2 may compensate for Gli1 loss.
To enable cell-type specific infection, the RCAS/tv-a system is now commonly used [90, 91]. The avian retrovirus RCAS (replication competent ASLV long terminal repeat with splice acceptor) only infects murine cells that are engineered to express the avian RCAS receptor tumor virus-A (tv-a), which is not normally expressed in mammalian cells [91]. RCAS-SHH retroviruses injected into Nestin promoter driving tv-a (Ntv-a) mice induce medulloblastoma at 9–39% incidence within 3 months [92–96]. Several genes of the IGF2/PI3K pathways were each introduced with SHH, including (a) IGF2, (b) an activated transforming form of AKT (Akt-Myr-Δ11–60), and (c) a stabilized, non-degradable T58A mutant NMYC [94, 95]. Additionally, RCAS-SHH system was combined with RCAS-Cre; PTEN-floxed mice to delete PTEN [97]. Viruses encoding the anti-apoptotic protein BCL-2 were also combined with SHH to block cell death mechanisms without affecting cell proliferation [93]. Combinations with all genes tested showed at least a doubling in incidence of tumors. Of note, viruses encoding IGF2, activated AKT, wild-type or mutant NMYC, and BCL-2 were not able to drive medulloblastoma in the absence of SHH, and combining NMYC with GLI1, AKT, IGF2, or BCL-2 was also insufficient to drive tumor formation. Together, these experiments suggest that the pro-tumorigenic effect of SHH is not solely mediated via NMYC and that other downstream effectors of SHH cooperate with IGF2/PI3K signaling to drive tumorigenesis.
Hepatocyte growth factor (HGF) has been shown to regulate expression of c-MYC and can cooperate with c-MYC to drive cell proliferation and apoptosis [98, 99]. High levels of HGF/c-MET receptor and amplification of c-MYC in human medulloblastoma are independently associated with LCA tumors and poor prognosis [99]. When expressed together with SHH using the RCAS/Ntv-a system, c-MYC and HGF each cooperated with SHH to increase penetrance, aggressiveness, and regional desmoplasia [92, 96]. However, it is not clear that this combination of genes mirrors cooperative expression in human tumors [38, 98, 99].
Other models with upregulated SHH signaling include deletion of cell cycle-associated genes Rb [100, 101] and cyclin-dependent kinase inhibitor Ink4c [102]; deletion of DNA repair enzymes Lig4 [103], Xrcc4 [104], KU80 [103], Brca2 [105], and Parp1 [106]; and candidate gene on chromosome 17 Hic1 [49]. The pathology of tumors in all of these SHH-associated models is mostly classic. Only the SHH/PTEN loss and SHH + HGF RCAS/Ntv-a models show some desmoplastic pathology, a feature characteristic of many SHH-driven human medulloblastoma tumors.
Non-SHH models
A limited number of non-SHH mouse models have also been developed. A model for WNT tumors was initiated from Blbp-expressing cells in the lower rhombic lip/dorsal brainstem, via activated β-catenin combined with p53 loss [107]. Classic tumors formed in 10 months, at 4% or 15% incidence dependent on partial or complete loss of p53. Tumors formed due to accumulation of Zic1-positive post-mitotic mossy-fiber neuron precursors that failed to migrate from the dorsal brainstem to form pontine gray nuclei in the ventral brainstem. The MRIs, location, and transcriptome of the murine tumors mirrored human WNT medulloblastoma, providing evidence that the WNT subclass of tumors likely originates from the lower rhombic lip/dorsal brainstem region, distinct from SHH tumors that originate from the CGNPs in the upper rhombic lip/EGL region.
In another model, the glutamate transporter (Glt1) promoter and a bidirectional Tet-operator were used to drive bidirectional and simultaneous expression of human MYCN and luciferase in the cerebellum (GTML), resulting in primarily SHH-independent tumors with classic or LCA histopathology [108]. These MYCN-driven tumors demonstrated recurrent genomic aberrations, suggesting that additional events cooperate with MYCN. Notably, a rare number of mice showed spinal metastases, consistent with leptomeningeal spread of the malignant primary brain tumor. MYC/MYCN amplification is correlated with poor prognosis and aggressive LCA subtype in human tumors [40], thus supporting the use of this model in pursuing investigations of high-risk patients with poor prognosis.
Current and future use of medulloblastoma models
The early implication of SHH pathway in medulloblastoma and the vast efforts of SHH tumor modeling have facilitated the preclinical testing of a number of SHH inhibitors, often using allografted tumors from variants of the Ptch1+/− model (Table 1). Cyclopamine is a steroidal alkaloid isolated from the corn lily Veratrum californicum that was discovered to inhibit tissue response to SHH signaling [109, 110] and was effective in blocking growth of SHH-driven medulloblastoma cells [74, 111]. Cyclopamine acts by binding directly to SMO, inhibiting activation of downstream SHH signaling [112, 113]. Treatment of mice with subcutaneous allograft tumors (derived from Ptch1+/−; p53+/− murine tumors) with cyclopamine led to decreased tumor mass and decreased proliferation [74].
Table 1.
Use of SHH-associated medulloblastoma models for testing of targeted therapy
| GEM | Drug | Target | GEM study outcome | Status in human clinical trials |
|---|---|---|---|---|
| Ptch1+/−; p53+/−Ptch1+/−; p53−/− allografts | Cyclopamine | SMO | Tumor regression | Preclinical only |
| Ptch1+/−; p53−/− | HhAntag-691 | SMO | Tumor inhibition, prolonged survival | Preclinical only |
| Ptch1+/−; Hic1+/−allograft | IPI-926 | SMO | Tumor regression | Phase I |
| Ptch1+/−Ptch1+/−; p53−/−Ptch1+/−; Hic1+/− allografts | NVP-LDE225 | SMO | Tumor regression, regrowth | Phase I |
| Ptch1+/− allograft | GDC-0449 | SMO | Tumor regression | Phase II |
| Ptch1+/−; p53−/− allograft | Bis-amide compound 5 | SMO | Tumor regression; regression of GDC-0449 resistant tumors | Preclinical |
| Ptch1+/−; p53−/− allograft | Arsenic trioxide | GLI | Tumor inhibition | Phase II completed |
| Ptch1+/−; p53−/− allograft | Itraconazole | SMO | Tumor inhibition | Phase I in children |
| SmoA1 | Suberoylanilide hydroxamic acid | Histone deacetylase | Increased apoptosis | |
| Ptch1+/− | Valproic acid | Histone deacetylase | Combined with 5-azaC | Phase II |
| Ptch1+/− | 5-azaC | DNA methylating agent | Prevented tumors, prolonged survival when combined with VPA | |
| Picropodophyllin | IGF-1R | Not in vivo | Preclinical | |
| SmoA1 | Gamma-secretase inhibitors | NOTCH signaling | Not in vivo? | Phase II completed |
| RCAS/tv-a SHH + HGF | HGF neutralizing antibody L2G7 | HGF | Prolonged survival | Preclinical |
Improved SMO antagonists [114, 115] include the benzimidazole derivative HhAntag-691 (HhAntag) [115], the semisynthetic cyclopamine analog IPI-926 [116], and the biphenyl carboxamide compound NVP-LDE225 [117, 118]. HhAntag treatment in young mice was reported to have permanent bone growth defects (presumably due to inhibition of Indian hedgehog signaling in the developing bone), calling into question the safety of using of this drug in young patients [119]. Although NVP-LDE225 treatment in mice showed initial success, extended analyses showed that tumors developed resistance to the drug and regrew [118].
GDC-0449 was identified from another screen of benzimidazole derivatives that inhibits SMO at a higher efficacy than cyclopamine. Treatment of allograft models of Ptch1+/− tumor cells at doses of 12.5 to 100 mg/kg resulted in regression [114, 120]. Nominal success was reported when a medulloblastoma patient with metastatic disease, harboring an inactivating mutation of Ptch1, was treated with GDC-0449. Treatment initially led to tumor regression [120], but tumors recurred, and biopsy analysis revealed a missense mutation in Smo resulting in an amino acid change (D473H) that disrupted drug binding to SMO and induced tumor cell resistance to GDC-0449 treatment [121]. This study highlights the need to identify agents or strategies that reduce emergence of resistant mutations. Further screens of inhibitors have since identified bis-amide compound 5 that could inhibit tumor growth of this resistant tumor strain [122]. First results from phase I clinical trials tested in patients with refractory, locally advanced, or metastatic solid tumors have reported acceptable safety profiles. Notably, of the 68 patients treated, 19 of 33 patients with basal cell carcinoma (a disease often displaying upregulated SHH pathway) showed partial or complete response [123].
In addition to SMO antagonists, other compounds have shown efficacy in causing tumor regression of SHH tumors in mice. Arsenic trioxide was found to be a GLI inhibitor [124]. The systemic antifungal, itraconazole, was found to be a SMO inhibitor [125]. The glucocorticoid dexamethasone was found to activate GSK-3β and thereby downregulate N-myc, which is an important effector downstream of SHH signaling [126]. Additionally, agents targeting epigenetic regulation also have efficacy in murine medulloblastoma, including the DNA methylating agent 5-azaC [74], and histone deacetylase inhibitors suberoylanilide hydroxamic acid [127] and valproic acid [128]. Lastly, other classes of compounds not targeting the SHH pathway have been reported to be effective in treating SHH-associated tumors. In a model driven by Ptch1 and p53 mutations, IGF signaling was found to be upregulated, and accordingly, picropodophyllin, an inhibitor of IGF1R tyrosine phosphorylation, effectively inhibited proliferation of tumor cells isolated from the murine tumors [129]. In the SmoA1 mouse model, tumors were found to concurrently upregulate the NOTCH pathway along with the SHH pathway. Inhibition of NOTCH signaling using soluble Delta ligand or gamma-secretase inhibitors could decrease proliferation and induce apoptosis in vitro, suggesting that SHH tumors that exhibit concurrent NOTCH activation may be effectively treated by targeting the NOTCH pathway [60]. Lastly, in the RCAS/tv-a SHH + HGF model, HGF neutralizing antibody L2G7 was more effective than SHH antagonists in increasing median survival time, suggesting potential use of HGF-targeted therapy for patients with elevated HGF levels [92, 130].
Conclusion
The development and use of relevant mouse models that closely reflect human medulloblastoma is critical to understand malignant progression in this disease and to test developmental therapeutics. Extensive progress has been made in modeling SHH tumors, with inhibitors of SHH signaling now entering clinical trials. Follow-up studies are critical to examine the mechanisms of resistance to these drugs, so that newer, more effective drugs can be developed. As WNT antagonists become available, it will be important to test the efficacy of these drugs in animal models of WNT-associated medulloblastoma. Finally, additional studies are needed to identify the drivers of non-SHH and non-WNT tumors (which comprise approximately half of all cases), so that models can be developed for these tumors as well. With robust animal models for each subtype of medulloblastoma, it should be possible to develop appropriate targeted therapies that eradicate tumor cells while sparing patients the devastating side effects of conventional radiation and chemotherapy.
Acknowledgments
We acknowledge support from the Alex’s Lemonade Stand and Pediatric Brain Tumor Foundations and from NIH grants R01CA159859, R01CA133091, R01CA148699, and R01CA122759.
Contributor Information
Jasmine Lau, Department of Neurology, University of California, San Francisco, CA, USA. Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA. Department of Neurological Surgery and Brain Tumor Research Center, University of California, San Francisco, CA, USA. Department of Pediatrics, University of California, San Francisco, CA, USA.
Christin Schmidt, Department of Neurology, University of California, San Francisco, CA, USA. Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA. Department of Neurological Surgery and Brain Tumor Research Center, University of California, San Francisco, CA, USA. Department of Pediatrics, University of California, San Francisco, CA, USA.
Shirley L. Markant, Tumor Development Program, Sanford-Burnham Medical Research Institute, La Jolla, CA, USA. Department of Pharmacology & Cancer Biology, Duke University Medical Center, Durham, NC, USA
Michael D. Taylor, Division of Neurosurgery, Hospital for Sick Children, University of Toronto, Toronto, ON, Canada. Arthur and Sonia Labatt Brain Tumour Research Centre, Program in Developmental and Stem Cell Biology, Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
Robert J. Wechsler-Reya, Tumor Development Program, Sanford-Burnham Medical Research Institute, La Jolla, CA, USA. Department of Pharmacology & Cancer Biology, Duke University Medical Center, Durham, NC, USA
William A. Weiss, Email: wweiss@ucsf.edu, Department of Neurology, University of California, San Francisco, CA, USA. Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA. Department of Neurological Surgery and Brain Tumor Research Center, University of California, San Francisco, CA, USA. Department of Pediatrics, University of California, San Francisco, CA, USA
References
- 1.Rossi A, Caracciolo V, Russo G, Reiss K, Giordano A. Medulloblastoma: from molecular pathology to therapy. Clin Cancer Res. 2008;14(4):971–976. doi: 10.1158/1078-0432.CCR-07-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol. 2004;5(4):209–218. doi: 10.1016/S1470-2045 (04)01424-X. [DOI] [PubMed] [Google Scholar]
- 3.Saran A. Medulloblastoma: role of developmental pathways, DNA repair signaling, and other players. Curr Mol Med. 2009;9(9):1046–1057. doi: 10.2174/156652409789839080. [DOI] [PubMed] [Google Scholar]
- 4.Pfister SM, Korshunov A, Kool M, Hasselblatt M, Eberhart C, Taylor MD. Molecular diagnostics of CNS embryonal tumors. Acta Neuropathol. 2010;120(5):553–566. doi: 10.1007/s00401-010-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Momota H, Holland EC. Mouse models of CNS embryonal tumors. Brain Tumor Pathol. 2009;26(2):43–50. doi: 10.1007/s10014-009-0253-0. [DOI] [PubMed] [Google Scholar]
- 6.CBTRUS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2006. Central Brain Tumor Registry of the United States; Hinsdale: 2010. www.cbtrus.org. [Google Scholar]
- 7.von Hoff K, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C, Benesch M, Warmuth-Metz M, Soerensen N, Zwiener I, Goette H, Schlegel PG, Pietsch T, Kortmann RD, Kuehl J, Rutkowski S. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer. 2009;45(7):1209–1217. doi: 10.1016/j.ejca.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, Allen JC, Stevens KR, Stanley P, Li H, Wisoff JH, Geyer JR, McGuire-Cullen P, Stehbens JA, Shurin SB, Packer RJ. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17(3):832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 9.Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P. Effects of medulloblastoma resections on outcome in children: a report from the Children’s Cancer Group. Neurosurgery. 1996;38(2):265–271. doi: 10.1097/00006123-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt AL, Brunetto AL, Schwartsmann G, Roesler R, Abujamra AL. Recent therapeutic advances for treating medulloblastoma: focus on new molecular targets. CNS Neurol Disord Drug Targets. 2010;9(3):335–348. doi: 10.2174/187152710791292602. [DOI] [PubMed] [Google Scholar]
- 11.Huse JT, Holland EC. Genetically engineered mouse models of brain cancer and the promise of preclinical testing. Brain Pathol. 2009;19(1):132–143. doi: 10.1111/j.1750-3639.2008.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol. 2008;65(11):1419–1424. doi: 10.1001/archneur.65.11.1419. [DOI] [PubMed] [Google Scholar]
- 13.Eberhart CG. Molecular diagnostics in embryonal brain tumors. Brain Pathol. 2011;21(1):96–104. doi: 10.1111/j.1750-3639.2010.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boman KK, Hovén E, Anclair M, Lannering B, Gustafsson G. Health and persistent functional late effects in adult survivors of childhood CNS tumours: a population-based cohort study. Eur J Cancer. 2009;45(14):2552–2561. doi: 10.1016/j. ejca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Mulhern RK, Palmer SL, Merchant TE, Wallace D, Kocak M, Brouwers P, Krull K, Chintagumpala M, Stargatt R, Ashley DM, Tyc VL, Kun L, Boyett J, Gajjar A. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(24):5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 16.Frange P, Alapetite C, Gaboriaud G, Bours D, Zucker JM, Zerah M, Brisse H, Chevignard M, Mosseri V, Bouffet E, Doz F. From childhood to adulthood: long-term outcome of medulloblastoma patients. The Institut Curie experience (1980–2000) J Neurooncol. 2009;95(2):271–279. doi: 10.1007/s11060-009-9927-z. [DOI] [PubMed] [Google Scholar]
- 17.Oyharcabal-Bourden V, Kalifa C, Gentet JC, Frappaz D, Edan C, Chastagner P, Sariban E, Pagnier A, Babin A, Pichon F, Neuenschwander S, Vinchon M, Bours D, Mosseri V, Le Gales C, Ruchoux M, Carrie C, Doz F. Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: a French Society of Pediatric Oncology Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(21):4726–4734. doi: 10.1200/JCO.2005.00.760. [DOI] [PubMed] [Google Scholar]
- 18.Pizer BL, Clifford SC. The potential impact of tumour biology on improved clinical practice for medulloblastoma: progress towards biologically driven clinical trials. Br J Neurosurg. 2009;23(4):364–375. doi: 10.1080/02688690903121807. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RE, Bailey CC, Robinson KJ, Weston CL, Walker DA, Ellison D, Ironside J, Pizer BL, Lashford LS. Outcome for patients with metastatic (M2-3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer. 2005;41(5):727–734. doi: 10.1016/j.ejca.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annual review of pathology. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 22.Gailani MR, Bale SJ, Leffell DJ, DiGiovanna JJ, Peck GL, Poliak S, Drum MA, Pastakia B, McBride OW, Kase R. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell. 1992;69(1):111–117. doi: 10.1016/0092-8674(92)90122-s. [DOI] [PubMed] [Google Scholar]
- 23.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale AE. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85(6):841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 24.Dong J, Gailani MR, Pomeroy SL, Reardon D, Bale AE. Identification of PATCHED mutations in medulloblastomas by direct sequencing. Hum Mutat. 2000;16(1):89–90. doi: 10.1002/1098-1004(200007)16:1<89::AID-HUMU18>3.0.CO;2–7. [DOI] [PubMed] [Google Scholar]
- 25.Vorechovský I, Tingby O, Hartman M, Strömberg B, Nister M, Collins VP, Toftgård R. Somatic mutations in the human homologue of Drosophila patched in primitive neuroectodermal tumours. Oncogene. 1997;15(3):361–366. doi: 10.1038/sj.onc.1201340. [DOI] [PubMed] [Google Scholar]
- 26.Pietsch T, Waha A, Koch A, Kraus J, Albrecht S, Tonn J, Sörensen N, Berthold F, Henk B, Schmandt N, Wolf HK, von Deimling A, Wainwright B, Chenevix-Trench G, Wiestler OD, Wicking C. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57(11):2085–2088. [PubMed] [Google Scholar]
- 27.Raffel C, Jenkins RB, Frederick L, Hebrink D, Alderete B, Fults DW, James CD. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57(5):842–845. [PubMed] [Google Scholar]
- 28.Taylor MD, Liu L, Raffel C, C-c H, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, Hao A, Goldstein AM, Stavrou T, Scherer SW, Dura WT, Wainwright B, Squire JA, Rutka JT, Hogg D. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31(3):306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 29.Zurawel RH, Allen C, Chiappa S, Cato W, Biegel J, Cogen P, de Sauvage F, Raffel C. Analysis of PTCH/SMO/SHH pathway genes in medulloblastoma. Genes Chromosomes Cancer. 2000;27(1):44–51. doi: 10.1002/(sici)1098-2264(200001)27:1<44::aid-gcc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Uziel T, Karginov FV, Xie S, Parker JS, Wang Y-D, Gajjar A, He L, Ellison D, Gilbertson RJ, Hannon G, Roussel MF. The miR-17 92 cluster collaborates with the Sonic Hedgehog pathway in medulloblastoma. Proc Natl Acad Sci USA. 2009;106(8):2812–2817. doi: 10.1073/pnas.0809579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Northcott PA, Fernandez-L A, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, Grundy R, Van Meter T, Rutka JT, Croce CM, Kenney AM, Taylor MD. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69(8):3249–3255. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23(23):2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellison DW. Childhood medulloblastoma: novel approaches to the classification of a heterogeneous disease. Acta Neuropathol. 2010;120(3):305–316. doi: 10.1007/s00401-010-0726-6. [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Mahler-Araujo BM, Sankila A, Chimelli L, Yonekawa Y, Kleihues P, Ohgaki H. APC mutations in sporadic medulloblastomas. Am J Pathol. 2000;156(2):433–437. doi: 10.1016/S0002-9440(10)64747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paraf F, Jothy S, Van Meir EG. Brain tumor-polyposis syndrome: two genetic diseases? Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1997;15 (7):2744–2758. doi: 10.1200/JCO.1997.15.7.2744. [DOI] [PubMed] [Google Scholar]
- 36.Zurawel RH, Chiappa SA, Allen C, Raffel C. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 1998;58(5):896–899. [PubMed] [Google Scholar]
- 37.Eberhart CG, Tihan T, Burger PC. Nuclear localization and mutation of beta-catenin in medulloblastomas. J Neuropathol Exp Neurol. 2000;59(4):333–337. doi: 10.1093/jnen/59.4.333. [DOI] [PubMed] [Google Scholar]
- 38.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD. Medulloblastoma comprises four distinct molecular variants. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, Pearson AD, Clifford SC Committee UKCsCSGBT . beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children’s Cancer Study Group Brain Tumour Committee. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(31):7951–7957. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 40.Pfister S, Remke M, Benner A, Mendrzyk F, Toedt G, Felsberg J, Wittmann A, Devens F, Gerber NU, Joos S, Kulozik A, Reifenberger G, Rutkowski S, Wiestler OD, Radlwimmer B, Scheurlen W, Lichter P, Korshunov A. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(10):1627–1636. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 41.Eberhart CG, Kratz JE, Schuster A, Goldthwaite P, Cohen KJ, Perlman EJ, Burger PC. Comparative genomic hybridization detects an increased number of chromosomal alterations in large cell/anaplastic medulloblastomas. Brain Pathol. 2002;12(1):36–44. doi: 10.1111/j.1750-3639.2002.tb00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aldosari N, Bigner SH, Burger PC, Becker L, Kepner JL, Friedman HS, McLendon RE. MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children’s Oncology Group. Arch Pathol Lab Med. 2002;126(5):540–544. doi: 10.5858/2002-126-0540-MAMOAI. [DOI] [PubMed] [Google Scholar]
- 43.Kleihues P, Schäuble B, zur Hausen A, Estève J, Ohgaki H. Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol. 1997;150(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 44.Frank AJ, Hernan R, Hollander A, Lindsey JC, Lusher ME, Fuller CE, Clifford SC, Gilbertson RJ. The TP53-ARF tumor suppressor pathway is frequently disrupted in large/cell anaplastic medulloblastoma. Brain Res Mol Brain Res. 2004;121(1–2):137–140. doi: 10.1016/j.molbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Tabori U, Baskin B, Shago M, Alon N, Taylor MD, Ray PN, Bouffet E, Malkin D, Hawkins C. Universal poor survival in children with medulloblastoma harboring somatic TP53 mutations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(8):1345–1350. doi: 10.1200/JCO.2009.23.5952. [DOI] [PubMed] [Google Scholar]
- 46.Pfaff E, Remke M, Sturm D, Benner A, Witt H, Milde T, von Bueren AO, Wittmann A, Schöttler A, Jorch N, Graf N, Kulozik AE, Witt O, Scheurlen W, von Deimling A, Rutkowski S, Taylor MD, Tabori U, Lichter P, Korshunov A, Pfister SM. TP53 mutation is frequently associated with CTNNB1 mutation or MYCN amplification and is compatible with long-term survival in medulloblastoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(35):5188–5196. doi: 10.1200/JCO.2010.31.1670. [DOI] [PubMed] [Google Scholar]
- 47.Mendrzyk F, Korshunov A, Toedt G, Schwarz F, Korn B, Joos S, Hochhaus A, Schoch C, Lichter P, Radlwimmer B. Iso-chromosome breakpoints on 17p in medulloblastoma are flanked by different classes of DNA sequence repeats. Genes Chromosomes Cancer. 2006;45(4):401–410. doi: 10.1002/gcc.20304. [DOI] [PubMed] [Google Scholar]
- 48.Di Marcotullio L, Ferretti E, De Smaele E, Argenti B, Mincione C, Zazzeroni F, Gallo R, Masuelli L, Napolitano M, Maroder M, Modesti A, Giangaspero F, Screpanti I, Alesse E, Gulino A. REN(KCTD11) is a suppressor of Hedgehog signaling and is deleted in human medulloblastoma. Proc Natl Acad Sci USA. 2004;101(29):10833–10838. doi: 10.1073/pnas.0400690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briggs KJ, Corcoran-Schwartz IM, Zhang W, Harcke T, Devereux WL, Baylin SB, Eberhart CG, Watkins DN. Cooperation between the Hic1 and Ptch1 tumor suppressors in medulloblastoma. Genes Dev. 2008;22(6):770–785. doi: 10.1101/gad.1640908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waha A, Waha A, Koch A, Meyer-Puttlitz B, Weggen S, Sörensen N, Tonn JC, Albrecht S, Goodyer CG, Berthold F, Wiestler OD, Pietsch T. Epigenetic silencing of the HIC-1 gene in human medulloblastomas. J Neuropathol Exp Neurol. 2003;62(11):1192–1201. doi: 10.1093/jnen/62.11.1192. [DOI] [PubMed] [Google Scholar]
- 51.Rood BR, Zhang H, Weitman DM, Cogen PH. Hyper-methylation of HIC-1 and 17p allelic loss in medulloblastoma. Cancer Res. 2002;62(13):3794–3797. [PubMed] [Google Scholar]
- 52.Lindsey JC, Anderton JA, Lusher ME, Clifford SC. Epigenetic events in medulloblastoma development. Neurosurg Focus. 2005;19(5):E10. doi: 10.3171/foc.2005.19.5.11. [DOI] [PubMed] [Google Scholar]
- 53.Canettieri G, Di Marcotullio L, Greco A, Coni S, Antonucci L, Infante P, Pietrosanti L, De Smaele E, Ferretti E, Miele E, Pelloni M, De Simone G, Pedone EM, Gallinari P, Giorgi A, Steinkühler C, Vitagliano L, Pedone C, Schinin ME, Screpanti I, Gulino A. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nature Cell Biology. 2010;12(2):132–142. doi: 10.1038/ncb2013. [DOI] [PubMed] [Google Scholar]
- 54.Peters I, Rehmet K, Wilke N, Kuczyk MA, Hennenlotter J, Eilers T, Machtens S, Jonas U, Serth J. RASSF1A promoter methylation and expression analysis in normal and neoplastic kidney indicates a role in early tumorigenesis. Mol Cancer. 2007;6:49. doi: 10.1186/1476-4598-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindsey JC, Lusher ME, Anderton JA, Bailey S, Gilbertson RJ, Pearson ADJ, Ellison DW, Clifford SC. Identification of tumour-specific epigenetic events in medulloblastoma development by hypermethylation profiling. Carcinogenesis. 2004;25(5):661–668. doi: 10.1093/carcin/bgh055. [DOI] [PubMed] [Google Scholar]
- 56.Lusher ME, Lindsey JC, Latif F, Pearson ADJ, Ellison DW, Clifford SC. Biallelic epigenetic inactivation of the RASSF1A tumor suppressor gene in medulloblastoma development. Cancer Res. 2002;62(20):5906–5911. [PubMed] [Google Scholar]
- 57.Kongkham PN, Northcott PA, Ra YS, Nakahara Y, Mainprize TG, Croul SE, Smith CA, Taylor MD, Rutka JT. An epigenetic genome-wide screen identifies SPINT2 as a novel tumor suppressor gene in pediatric medulloblastoma. Cancer Res. 2008;68(23):9945–9953. doi: 10.1158/0008-5472.CAN-08-2169. [DOI] [PubMed] [Google Scholar]
- 58.Nakahara Y, Northcott PA, Li M, Kongkham PN, Smith C, Yan H, Croul S, Ra Y-S, Eberhart C, Huang A, Bigner D, Grajkowska W, Van Meter T, Rutka JT, Taylor MD. Genetic and epigenetic inactivation of Kruppel-like factor 4 in medulloblastoma. Neoplasia. 2010;12(1):20–27. doi: 10.1593/neo.91122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64(21):7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 60.Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, Russell TL, Ellenbogen RG, Bernstein ID, Beachy PA, Olson JM. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64(21):7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 61.Garzia L, Andolfo I, Cusanelli E, Marino N, Petrosino G, De Martino D, Esposito V, Galeone A, Navas L, Esposito S, Gargiulo S, Fattet S, Donofrio V, Cinalli G, Brunetti A, Vecchio LD, Northcott PA, Delattre O, Taylor MD, Iolascon A, Zollo M. MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS One. 2009;4(3):e4998. doi: 10.1371/journal.pone.0004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broderick DK, Di C, Parrett TJ, Samuels YR, Cummins JM, McLendon RE, Fults DW, Velculescu VE, Bigner DD, Yan H. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 2004;64(15):5048–5050. doi: 10.1158/0008-5472.CAN-04-1170. [DOI] [PubMed] [Google Scholar]
- 63.Inda MM, Mercapide J, Muñoz J, Coullin P, Danglot G, Tuñon T, Martínez-Peñuela JM, Rivera JM, Burgos JJ, Bernheim A, Castresana JS. PTEN and DMBT1 homozygous deletion and expression in medulloblastomas and supratentorial primitive neuroectodermal tumors. Oncol Rep. 2004;12(6):1341–1347. [PubMed] [Google Scholar]
- 64.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS-v, Caron HN, Cloos J, Mrsić A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM, Kellie SJ, Taylor MD, Curran T, Gajjar A, Gilbertson RJ. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(12):1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 66.Cho Y-J, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen TT, Mora EC, Mealey J. Cultivation of medulloblastoma cells derived from simian adenovirus SA7-induced hamster brain tumor. Cancer Res. 1975;35(12):3566–3570. [PubMed] [Google Scholar]
- 68.Rapp F, Pauluzzi S, Waltz TA, Burdine JA, Matsen FA, Levy B. Induction of brain tumors in newborn hamsters by simian adenovirus SA7. Cancer Res. 1969;29(6):1173–1178. [PubMed] [Google Scholar]
- 69.Zu Rhein GM, Varakis JN. Perinatal induction of medulloblastomas in Syrian golden hamsters by a human polyoma virus (JC) Natl Cancer Inst Monogr. 1979;51:205–208. [PubMed] [Google Scholar]
- 70.Nagashima K, Yasui K, Kimura J, Washizu M, Yamaguchi K, Mori W. Induction of brain tumors by a newly isolated JC virus (Tokyo-1 strain) Am J Pathol. 1984;116(3):455–463. [PMC free article] [PubMed] [Google Scholar]
- 71.Zurawel RH, Allen C, Wechsler-Reya R, Scott MP, Raffel C. Evidence that haploinsufficiency of Ptch leads to medulloblastoma in mice. Genes Chromosomes Cancer. 2000;28(1):77–81. [PubMed] [Google Scholar]
- 72.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 73.Wetmore C, Eberhart DE, Curran T. The normal patched allele is expressed in medulloblastomas from mice with heterozygous germ-line mutation of patched. Cancer Res. 2000;60(8):2239–2246. [PubMed] [Google Scholar]
- 74.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, Beachy PA. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297(5586):1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 75.Oliver TG, Read TA, Kessler JD, Mehmeti A, Wells JF, Huynh TTT, Lin SM, Wechsler-Reya RJ. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development. 2005;132(10):2425–2439. doi: 10.1242/dev.01793. [DOI] [PubMed] [Google Scholar]
- 76.Lee Y, Miller HL, Russell HR, Boyd K, Curran T, McKinnon PJ. Patched2 modulates tumorigenesis in patched1 heterozygous mice. Cancer Res. 2006;66(14):6964–6971. doi: 10.1158/0008-5472.CAN-06-0505. [DOI] [PubMed] [Google Scholar]
- 77.Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61(2):513–516. [PubMed] [Google Scholar]
- 78.Romer J, Curran T. Targeting medulloblastoma: small-molecule inhibitors of the Sonic Hedgehog pathway as potential cancer therapeutics. Cancer Res. 2005;65(12):4975–4978. doi: 10.1158/0008-5472.CAN-05-0481. [DOI] [PubMed] [Google Scholar]
- 79.Pogoriler J, Millen K, Utset M, Du W. Loss of cyclin D1 impairs cerebellar development and suppresses medulloblastoma formation. Development. 2006;133(19):3929–3937. doi: 10.1242/dev.02556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura H, Stephen D, Joyner A, Curran T. Gli1 is important for medulloblastoma formation in Ptc1+/− mice. Oncogene. 2005;24(25):4026–4036. doi: 10.1038/sj.onc.1208567. [DOI] [PubMed] [Google Scholar]
- 81.Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4(5):619–622. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- 82.Hahn H, Wojnowski L, Specht K, Kappler R, Calzada-Wack J, Potter D, Zimmer A, Müller U, Samson E, Quintanilla-Martinez L, Zimmer A. Patched target Igf2 is indispensable for the formation of medulloblastoma and rhabdomyosarcoma. J Biol Chem. 2000;275(37):28341–28344. doi: 10.1074/jbc.C000352200. [DOI] [PubMed] [Google Scholar]
- 83.Mao J, Ligon KL, Rakhlin EY, Thayer SP, Bronson RT, Rowitch D, McMahon AP. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 2006;66(20):10171–10178. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hatton BA, Villavicencio EH, Tsuchiya KD, Pritchard JI, Ditzler S, Pullar B, Hansen S, Knoblaugh SE, Lee D, Eberhart CG, Hallahan AR, Olson JM. The Smo/Smo model: hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 2008;68(6):1768–1776. doi: 10.1158/0008-5472.CAN-07-5092. [DOI] [PubMed] [Google Scholar]
- 85.Yang Z-J, Ellis T, Markant SL, Read T-A, Kessler JD, Bourboulas M, Schüller U, Machold R, Fishell G, Rowitch DH, Wainwright BJ, Wechsler-Reya RJ. Medulloblastoma can be initiated by deletion of patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14(2):135–145. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schüller U, Heine VM, Mao J, Kho AT, Dillon AK, Han Y-G, Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14(2):123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T, McKinnon PJ. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26(44):6442–6447. doi: 10.1038/sj.onc.1210467. [DOI] [PubMed] [Google Scholar]
- 88.Gaiano N, Kohtz JD, Turnbull DH, Fishell G. A method for rapid gain-of-function studies in the mouse embryonic nervous system. Nat Neurosci. 1999;2(9):812–819. doi: 10.1038/12186. [DOI] [PubMed] [Google Scholar]
- 89.Weiner HL, Bakst R, Hurlbert MS, Ruggiero J, Ahn E, Lee WS, Stephen D, Zagzag D, Joyner AL, Turnbull DH. Induction of medulloblastomas in mice by sonic hedgehog, independent of Gli1. Cancer Res. 2002;62(22):6385–6389. [PubMed] [Google Scholar]
- 90.Orsulic S. An RCAS-TVA-based approach to designer mouse models. Mamm Genome. 2002;13(10):543–547. doi: 10.1007/s00335-002-4003-4. [DOI] [PubMed] [Google Scholar]
- 91.Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci USA. 1998;95(3):1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Binning MJ, Niazi T, Pedone CA, Lal B, Eberhart CG, Kim KJ, Laterra J, Fults DW. Hepatocyte growth factor and sonic Hedgehog expression in cerebellar neural progenitor cells costimulate medulloblastoma initiation and growth. Cancer Res. 2008;68(19):7838–7845. doi: 10.1158/0008-5472.CAN-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCall TD, Pedone CA, Fults DW. Apoptosis suppression by somatic cell transfer of Bcl-2 promotes Sonic Hedgehog-dependent medulloblastoma formation in mice. Cancer Res. 2007;67(11):5179–5185. doi: 10.1158/0008-5472.CAN-06-4177. [DOI] [PubMed] [Google Scholar]
- 94.Browd SR, Kenney AM, Gottfried ON, Yoon JW, Walterhouse D, Pedone CA, Fults DW. N-myc can substitute for insulin-like growth factor signaling in a mouse model of sonic hedgehog-induced medulloblastoma. Cancer Res. 2006;66(5):2666–2672. doi: 10.1158/0008-5472.CAN-05-2198. [DOI] [PubMed] [Google Scholar]
- 95.Rao G, Pedone CA, Del Valle L, Reiss K, Holland EC, Fults DW. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23(36):6156–6162. doi: 10.1038/sj.onc.1207818. [DOI] [PubMed] [Google Scholar]
- 96.Rao G, Pedone CA, Coffin CM, Holland EC, Fults DW. c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. Neoplasia. 2003;5(3):198–204. doi: 10.1016/S1476-5586(03)80052-0. NO_DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22(4):436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Lal B, Kwon S, Fan X, Saldanha U, Reznik TE, Kuchner EB, Eberhart C, Laterra J, Abounader R. The scatter factor/hepatocyte growth factor: c-met pathway in human embryonal central nervous system tumor malignancy. Cancer Res. 2005;65(20):9355–9362. doi: 10.1158/0008-5472.CAN-05-1946. [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Guessous F, Johnson EB, Eberhart CG, Li X-N, Shu Q, Fan S, Lal B, Laterra J, Schiff D, Abounader R. Functional and molecular interactions between the HGF/c-Met pathway and c-Myc in large-cell medulloblastoma. Lab Invest. 2008;88(2):98–111. doi: 10.1038/labinvest.3700702. [DOI] [PubMed] [Google Scholar]
- 100.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14(8):994–1004. [PMC free article] [PubMed] [Google Scholar]
- 101.Shakhova O, Leung C, van Montfort E, Berns A, Marino S. Lack of Rb and p53 delays cerebellar development and predisposes to large cell anaplastic medulloblastoma through amplification of N-Myc and Ptch2. Cancer Res. 2006;66(10):5190–5200. doi: 10.1158/0008-5472.CAN-05-3545. [DOI] [PubMed] [Google Scholar]
- 102.Zindy F, Nilsson LM, Nguyen L, Meunier C, Smeyne RJ, Rehg JE, Eberhart C, Sherr CJ, Roussel MF. Hemangiosarcomas, medulloblastomas, and other tumors in Ink4c/p53-null mice. Cancer Res. 2003;63(17):5420–5427. [PubMed] [Google Scholar]
- 103.Lee Y, McKinnon PJ. DNA ligase IV suppresses medulloblastoma formation. Cancer Res. 2002;62(22):6395–6399. [PubMed] [Google Scholar]
- 104.Yan CT, Kaushal D, Murphy M, Zhang Y, Datta A, Chen C, Monroe B, Mostoslavsky G, Coakley K, Gao Y, Mills KD, Fazeli AP, Tepsuporn S, Hall G, Mulligan R, Fox E, Bronson R, De Girolami U, Lee C, Alt FW. XRCC4 suppresses medulloblastomas with recurrent translocations in p53-deficient mice. Proc Natl Acad Sci USA. 2006;103(19):7378–7383. doi: 10.1073/pnas.0601938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frappart P-O, Lee Y, Lamont J, McKinnon PJ. BRCA2 is required for neurogenesis and suppression of medulloblastoma. EMBO J. 2007;26(11):2732–2742. doi: 10.1038/sj.emboj.7601703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tong W-M, Ohgaki H, Huang H, Granier C, Kleihues P, Wang Z-Q. Null mutation of DNA strand break-binding molecule poly(ADP-ribose) polymerase causes medulloblastomas in p53(−/−) mice. Am J Pathol. 2003;162(1):343–352. doi: 10.1016/S0002-9440(10)63825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, Finkelstein D, Pounds S, Weiss A, Patay Z, Scoggins M, Ogg R, Pei Y, Yang Z-J, Brun S, Lee Y, Zindy F, Lindsey JC, Taketo MM, Boop FA, Sanford RA, Gajjar A, Clifford SC, Roussel MF, McKinnon PJ, Gutmann DH, Ellison DW, Wechsler-Reya R, Gilbertson RJ. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010 doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swartling FJ, Grimmer MR, Hackett CS, Northcott PA, Fan Q-W, Goldenberg DD, Lau J, Masic S, Nguyen K, Yakovenko S, Zhe X-N, Gilmer HCF, Collins R, Nagaoka M, Phillips JJ, Jenkins RB, Tihan T, Vandenberg SR, James CD, Tanaka K, Taylor MD, Weiss WA, Chesler L. Pleiotropic role for MYCN in medulloblastoma. Genes Dev. 2010;24(10):1059–1072. doi: 10.1101/gad.1907510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280(5369):1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 110.Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125(18):3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 111.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406(6799):1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 112.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16(21):2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99(22):14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, Gould SE, Guichert O, Gunzner JL, Halladay J, Jia W, Khojasteh C, Koehler MFT, Kotkow K, La H, Lalonde RL, Lau K, Lee L, Marshall D, Marsters JC, Murray LJ, Qian C, Rubin LL, Salphati L, Stanley MS, Stibbard JHA, Sutherlin DP, Ubhayaker S, Wang S, Wong S, Xie M. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. 2009;19(19):5576–5581. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 115.Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, Curran T. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6(3):229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 116.Tremblay MR, Lescarbeau A, Grogan MJ, Tan E, Lin G, Austad BC, Yu L-C, Behnke ML, Nair SJ, Hagel M, White K, Conley J, Manna JD, Alvarez-Diez TM, Hoyt J, Woodward CN, Sydor JR, Pink M, MacDougall J, Campbell MJ, Cushing J, Ferguson J, Curtis MS, McGovern K, Read MA, Palombella VJ, Adams J, Castro AC. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926) J Med Chem. 2009;52(14):4400–4418. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- 117.Pan S, Wu X, Jiang J, Gao W, Wan Y, Cheng D, Han D, Liu J, Englund NP, Wang Y, Peukert S, Miller-Moslin K, Yuan J, Guo R, Matsumoto M, Vattay A, Jiang Y, Tsao J, Sun F, Pferdekamper AC, Dodd S, Tuntland T, Maniara W, Kelleher JF, Y-m Y, Warmuth M, Williams J, Dorsch M. Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Med Chem Lett. 2010;1(3):130–134. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A, Hsiao K, Yuan J, Green J, Ospina B, Yu Q, Ostrom L, Fordjour P, Anderson DL, Monahan JE, Kelleher JF, Peukert S, Pan S, Wu X, Maira S-M, García-Echeverría C, Briggs KJ, Watkins DN, Yao Y-m, Lengauer C, Warmuth M, Sellers WR, Dorsch M. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2(51):51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kimura H, Ng JMY, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13(3):249–260. doi: 10.1016/j. ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 120.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yauch RL, Dijkgraaf GJP, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, Bazan JF, Kan Z, Seshagiri S, Hann CL, Gould SE, Low JA, Rudin CM, de Sauvage FJ. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326(5952):572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dijkgraaf GJP, Alicke B, Weinmann L, Januario T, West K, Modrusan Z, Burdick D, Goldsmith R, Robarge K, Sutherlin D, Scales SJ, Gould SE, Yauch RL, de Sauvage FJ. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71(2):435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 123.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, Graham RA, Zerivitz KL, Low JA, Von Hoff DD. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(8):2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beauchamp EM, Ringer L, Bulut G, Sajwan KP, Hall MD, Lee Y-C, Peaceman D, Ozdemirli M, Rodriguez O, MacDonald TJ, Albanese C, Toretsky JA, Uren A. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. 2011;121(1):148–160. doi: 10.1172/JCI42874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, Reya T, Liu JO, Epstein EH, Stevens DA, Beachy PA. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17(4):388–399. doi: 10.1016/j. ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heine VM, Priller M, Ling J, Rowitch DH, Schüller U. Dexamethasone destabilizes Nmyc to inhibit the growth of hedgehog-associated medulloblastoma. Cancer Res. 2010;70(13):5220–5225. doi: 10.1158/0008-5472.CAN-10-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spiller SE, Ravanpay AC, Hahn AW, Olson JM. Suberoylanilide hydroxamic acid is effective in preclinical studies of medulloblastoma. J Neurooncol. 2006;79(3):259–270. doi: 10.1007/s11060-006-9142-0. [DOI] [PubMed] [Google Scholar]
- 128.Ecke I, Petry F, Rosenberger A, Tauber S, Mönkemeyer S, Hess I, Dullin C, Kimmina S, Pirngruber J, Johnsen SA, Uhmann A, Nitzki F, Wojnowski L, Schulz-Schaeffer W, Witt O, Hahn H. Antitumor effects of a combined 5-aza-2′deoxycytidine and valproic acid treatment on rhabdomyosarcoma and medulloblastoma in Ptch mutant mice. Cancer Res. 2009;69(3):887–895. doi: 10.1158/0008-5472.CAN-08-0946. [DOI] [PubMed] [Google Scholar]
- 129.Ohshima-Hosoyama S, Hosoyama T, Nelon LD, Keller C. IGF-1 receptor inhibition by picropodophyllin in medulloblastoma. Biochem Biophys Res Commun. 2010;399(4):727–732. doi: 10.1016/j.bbrc.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 130.Coon V, Laukert T, Pedone CA, Laterra J, Kim KJ, Fults DW. Molecular therapy targeting Sonic hedgehog and hepatocyte growth factor signaling in a mouse model of medulloblastoma. Mol Cancer Ther. 2010;9(9):2627–2636. doi: 10.1158/1535-7163. MCT-10-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]