Abstract
The cholinergic neurons of the basal forebrain (BFCNs) in human and non-human primates are rich in the calcium binding protein calbindin-D28k (CB). We have shown a selective loss of CB from BFCNs in the course of normal aging, which appears to predispose these neurons to tangle formation and degeneration in Alzheimer’s disease. Our previous preliminary investigation demonstrated that rodent BFCNs are devoid of CB. Here we confirm that rat choline acetyltransferase-rich BFCNs are devoid of CB immunoreactivity. We then describe a method for adeno-associated viral vector (AAV) induced expression of CB in rat BFCNs in vivo. We constructed AAV vectors bearing the CB gene under the control of the CMV promoter, or neuron-specific enolase (NSE) promoter, to bias expression in neurons. Both vectors resulted in CB expression in mouse neuronal cultures, and in rat brain following injections. AAV-NSE-CB resulted in more robust expression in neurons. Injections of 10 μl of AAV-NSE-CB in the BFCNs component located within the internal segment of globus pallidus and internal capsule resulted in expression of CB in 84% of BFCNs. Expression was optimum at 14 days. Injections of AAV-NSE-LacZ resulted in robust β-galactosidase expression, but no CB immunoreactivity. Our results show that use of NSE promoter leads to high expression of genes in neurons and that the BFCNs can be targeted for expression of genes that are differentially expressed in the rodent and primate brains. These findings also have important implications for gene replacement therapy in human BFCNs.
Keywords: Adeno-Associated Viral Vector, Aging, Alzheimer’s Disease, Basal Forebrain Cholinergic Neurons, Calbindin-D28K
Introduction
While the presence of pathological proteins and their effects in neurodegenerative disorders have received extensive experimental attention, a major question remains unanswered: why are certain neuronal populations selectively vulnerable to degeneration in these disorders? The basal forebrain cholinergic neurons (BFCNs) represent one such population. The BFCNs are vulnerable to pathology and degeneration early in Alzheimer’s disease (AD) (Geula and Mesulam, 1999; Riascos et al., 2011) and in a number of other neurodegenerative disorders that afflict the elderly (Rogers et al., 1985), and their loss is correlated with severity of dementia in AD (Samuel et al., 1991; Lehericy et al., 1993). Age is the primary risk factor in these disorders, suggesting that age-related changes are permissive of selective vulnerability of BFCNs.
We have shown that BFCNs in the human and non-human primate brains are rich in the calcium binding protein calbindin-D28K (CB) (Geula et al., 1993b; Wu et al., 2000). Calbindin is a strong calcium buffer (Mattson et al., 1991; Miller, 1991), and its presence protects neurons against a number of insults (Figueredo-Cardenas et al., 1998; D'Orlando et al., 2001; Rintoul et al., 2001), presumably by regulating levels of intracellular calcium (Mattson et al., 1991). Of interest in the context of AD, we have demonstrated that the BFCNs display a regionally and biochemically selective and substantial loss of CB in the course of normal aging in both the human and in non-human primates (Wu et al., 1997; Geula et al., 2003a; Geula et al., 2003b; Wu et al., 2003). Importantly, nearly all of the remaining BFCNs in AD are CB-positive and the presence of CB protects the BFCNs from the formation of tangles, a hallmark of the degenerative process in AD (Riascos et al., 2011).
It is highly desirable to use animal models to dissect the mechanisms of neuronal vulnerability in neurodegenerative disorders. Rodents are ideal models for such investigations because they can be used in sufficiently high numbers and are convenient for genetic manipulations, such as over-expression or knockout of genes. However, molecular and neurochemical phenotypes of specific neuronal populations are not always identical in rodents when compared with primates (Mesulam and Geula, 1991; Geula et al., 1993a). In the case of CB in BFCNs, the rodent presents a challenge. In our earlier studies, we had noted that unlike the primate BFCNs, the rodent BFCNs appear to be completely devoid of CB (Geula et al., 1993b). Thus, to investigate the mechanisms through which CB confers protection to BFCNs against degeneration, it is necessary to induce rodent BFCNS to express CB.
Adeno-associated viral (AAV) vectors have been used for in vivo delivery of genes into various organs, including the CNS (Du et al., 1996; Daly, 2004; Tenenbaum et al., 2004). In this report we confirm absence of CB in the rat BFCNs. We then describe the use of an AAV vector bearing the gene for CB, under the control of the neuron specific enolase promoter, for ectopic and time-dependent expression of CB in the rodent BFCNs. In addition to allowing mechanistic studies in the rodent, AAV mediated expression of CB in BFCNs will have important therapeutic value in the human for replacing the CB lost in the aging process, with the aim of protecting the BFCNs in degenerative disorders.
Materials and Methods
Choice, Construction and Packaging of Vector
Viral vectors, such as herpes simplex virus (HSV) and adenovirus (AV), have been utilized for gene delivery into the CNS (Berns, 1990; Hermens and Verhaagen, 1998). The main drawbacks of these vectors are their cytotoxicity, immunogenicity (esp., AV) and instability of expression of the transfected genes. AAV vectors present with several advantages, including non-pathogenicity, low immunogenicity, ability to integrate into the host chromosome, and apparent anti-oncogenic activities (Berns, 1990; Daly, 2004; Tenenbaum et al., 2004). The genome of this small human parvovirus is a linear, single-stranded DNA molecule, 4,680 nucleotides in length, containing genes encoding the viral regulatory (rep) and structural (cap) proteins, with each gene cassette under the control of its own promoter. Because no promoter elements are contained in the viral terminal repeats, it has been relatively easy to design packaging systems for AAV vectors that do not permit homologous recombination.
In the studies reported here, a multi-use AAV vector system was employed for delivering the CB gene to the rat BFCNs. In one system, a cDNA encoding CB was inserted into pACP, a generic AAV vector in which expression is under the control of CMV immediate early gene promoter. This promoter element has been cloned into a unique Xba 1 site in a parental AAV vector, pAP, derived from plasmid pSSV9 (a gift from R.J. Samulski (Samulski et al., 1991)). A second AAV vector was constructed in which the expression of the CB gene at the level of transcription is under the control of a cell-type specific promoter element. A 1.5 kb promoter fragment derived from the gene for neuron-specific enolase (NSE) (gift from N. Muzyczka (Peel et al., 1997)), was used to bias expression to neurons. The CB cDNA was amplified by PCR to provide it with convenient cloning sites, and inserted into both NSE and CMV driven AAV vectors. Both vectors also bear a 0.6 kb SV40 fragment providing the polyadenylation function as well as an intron. The complete gene cassettes flanked by the AAV ITRs in these constructs are still smaller than 5.1 kb, the size limit for AAV packaging.
After packaging, AAV vector preparations were purified by banding through cesium gradients. Each vector preparation was titered by a modified infectious center assay (ICA), which provides a titer of infectious vector particles (Du et al., 1996). Titers used contained 108 infectious units per ml. Serial dilutions of vectors expressing the LacZ gene onto 293 cells followed by histochemical staining for β-galactosidase (β-Gal) yielded functional titers consistent with estimates obtained from the ICAs.
Cultures
Primary murine neuronal cultures were prepared from cortical tissue using standard protocols (Hilgenberg and Smith, 2007). Neurons were cultured on poly-D-lysine coated coverslips in Neurobasal Medium supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, glutamine and B27. Medium was partially changed every 3–4 days. Cultures were plated at a density of 105 cells per coverslip and allowed to adhere and grow for at least 10 days before exposure to AAV vectors or vehicle.
Intracranial Injections
Male Sprague-Dawley rats, weighing 225–250 grams were housed under 12 hours light/dark cycle with access to food and water ad libitum. Surgery was conducted under anesthesia (ketamine – xylazine, 80mg – 10 mg/Kg) using aseptic conditions. The scalp was shaved and the animal was mounted on a Kopf stereotaxic apparatus. After cleaning with iodine surgical soap followed by 70% ethanol, an incision was made in the scalp and the skull exposed and cleaned with saline. A hole was drilled in the skull, using a dental drill, at specific coordinates determined from the stereotaxic atlas of the rat brain according to Paxinos and Watson (Paxinos and Watson, 1986). The needle of a microsyringe containing AAV vector or media was lowered through the hole to the center of the Ch4. Each injection was made in 0.3–0.4 μl increments over 5 minutes using a 26-gauge sharp-tipped needle of a 10 μl syringe. The needle was left in place for 5 minutes after the injection, and then was withdrawn gradually. Injections were made bilaterally, with AAV injected in one hemisphere and either vehicle control, AAV control or a different dose of AAV injected in the opposite hemisphere. To determine the regional specificity of AAV mediated gene expression, trial injections were also made in the parietal cortex and thalamus.
Following injections, the skull was cleaned with saline and the scalp closed using surgical clips. After appropriate survival times, each animal was euthanized by an overdose of sodium pentobarbital (100 mg/kg, i.p.), and perfused intracardially with 50 ml of saline, followed sequentially by 250–300 ml of cold 4% paraformaldehyde and 100 ml of 10% sucrose. The brain was placed in 20% sucrose overnight at 4°C, and sectioned at 40 μm on a freezing microtome into 0.1 M phosphate buffer.
Immunohistochemistry
One in twelve series of sections from each hemisphere were processed immunohistochemically using the avidin-biotin-peroxidase (ABC) method as described elsewhere (Geula et al., 2003a), utilizing the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). The antibodies used were a polyclonal antibody against the specific cholinergic enzyme choline acetyltransferase (ChAT, gift of Dr. Louis Hersh, University of Kentucky Medical School, 1/500), a monoclonal antibody against CB (Swant, Switzerland, 1/1000) and a monoclonal antibody against the low affinity neurotrophin receptor (p75NTR, Advanced Targeting Systems, 1/500). To control for non-specific staining, sections stained using the above antibodies were compared with sections stained in the absence of primary antibodies or in the presence of non-specific IgG in place of primary antibodies.
For concurrent visualization of two antigens within the same section, the double-immunohistochemical method of Levy et al. (1986) was used. Tissue sections were first processed for one antigen using diaminobenzidine as chromogen. After the development of the DAB brown reaction product, sections were processed for the second antigen with the peroxidase labeling visualized using benzidine dihydrochloride (BDHC), which results in a granular blue reaction product. This method is superior to other chromogen based double immunohistochemical methods in that the smooth DAB and the granular BDHC reaction products can be easily distinguished, allowing visualization and identification of two antigens in the same neuron. The advantage of this method over fluorescent double immunohistochemical methods is that it results in permanent reaction products whereas fluorescence is subject to quenching over time. We have used this method successfully for studies of co-localization of ChAT and CB in human and non-human primate BFCNs (Geula et al., 1993b; Geula et al., 2003a; Geula et al., 2003b; Riascos et al., 2011).
β-Galactosidase Histochemistry
As a control, AAV-NSE vectors bearing the LacZ gene were injected within the above sites or used in cultures. In these experiments, β-Gal activity resulting from LacZ expression was visualized histochemically using protocols described previously (Debacq-Chainiaux et al., 2009). X-gal was used as substrate for histochemical visualization of β-Gal activity.
Analysis of AAV-Mediated CB Expression
Stained cultures and rat brain sections were examined for the presence of each antigen. Three animals were analyzed per group. Co-localization of CB immunoreactivity with the cholinergic marker ChAT and BFCNs marker p75NTR was determined in intact animals. AAV and control injection sites were included in the analysis only if a needle track and the center of the injection could be clearly identified. We first determined the expression of CB in neurons at the AAV injected sites when compared with the control injection sites in single stained sections. We then determined co-localization of CB with the cholinergic marker ChAT in double stained basal forebrain sections. All of the 1 in 12 sections passing through each injection site were used for this analysis. In addition, the number of cholinergic neurons with and without CB immunoreactivity within AAV injection sites was determined in double-stained sections using a counting box at 20X magnification and expressed as the percentage of cholinergic neurons that contain CB immunoreactivity.
Results
Rat Basal Forebrain Cholinergic Neurons Do Not Contain Calbindin
Choline acetyltransferase immunoreactivity was present in all the known cholinergic cell groups within the rat basal forebrain (Mesulam et al., 1983), i.e. medial septum (Ch1), vertical and horizontal limbs of the diagonal band of Broca (Ch2 & 3) and the nucleus basalis of Meynert (Ch4). The latter was the most extensive of the four cell groups, with a major component distributed within the internal globus pallidus (GPi) and the internal capsule (ic) (Fig. 1 A and B). Distribution of immunoreactivity for the neurotrophin receptor p75NTR, which in the forebrain is known to be enriched specifically within the Ch1-Ch4 group, was identical to that of ChAT immunoreactivity in the basal forebrain (not shown).
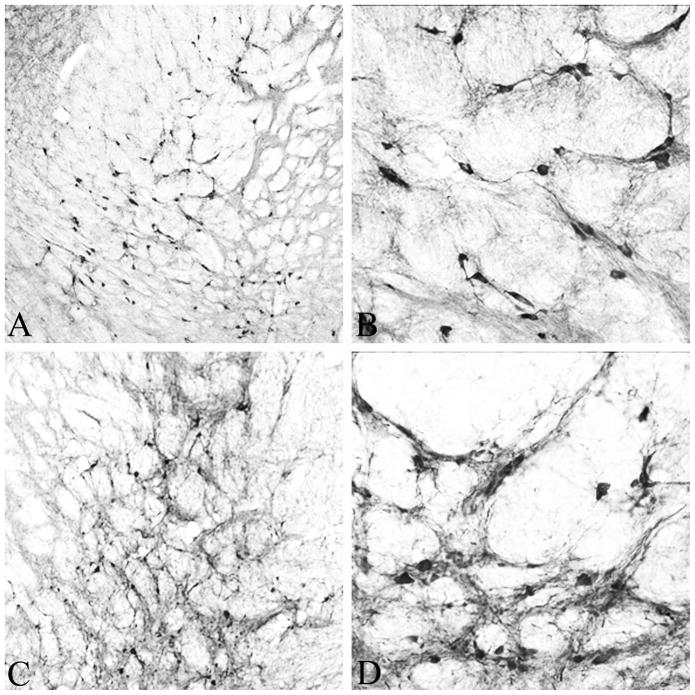
Figure 1.
Low (A) and high (B) power photomicrographs of ChAT-positive cholinergic neurons in the internal globus pallidus and internal capsule that form a major component of the nucleus basalis of Meynert in the rat brain. This region is virtually devoid of calbindin-positive neurons (see Fig. 2 & 3). Low (C) and high (D) power photomicrographs of calbindin positive neurons in the same region of the rat brain shown in (A) and (B), 30 days following injection of 10 μl of AAV-NSE-CB vector. Note the abundance of calbindin immunoreactive neurons with virtually identical morphology and distribution as ChAT-positive neurons in (A) and (B). Magnification in A & C is 10X and in B & D 20X.
Examination of basal forebrain regions within which the Ch1-Ch4 are located in single CB immunostained sections revealed few CB-positive neurons. In contrast, areas adjacent to these regions contained many CB-positive neurons. Nearly all CB-positive neurons in these regions were smaller than the magnocellular ChAT-positive neurons. The GPi/ic region within which BFCNs are located was nearly completely devoid of calbindin-positive neurons. An exception was posterior aspects of the Ch4 cell group, within which ChAT and CB immunoreactive neurons of similar morphology appeared to intermingle.
Double immunohistochemical procedures revealed distinct and separate ChAT and CB immunoreactive neurons within all basal forebrain sectors (Fig. 2 A). Again, virtually no CB-positive neurons were observed in the GPi/ic regions that house the BFCNS. Even within the posterior Ch4 sector, in which ChAT and CB immunoreactive neurons were intermingled in close proximity to each other, none of the ChAT positive neurons contained CB immunoreactivity (Fig. 2 B and C).
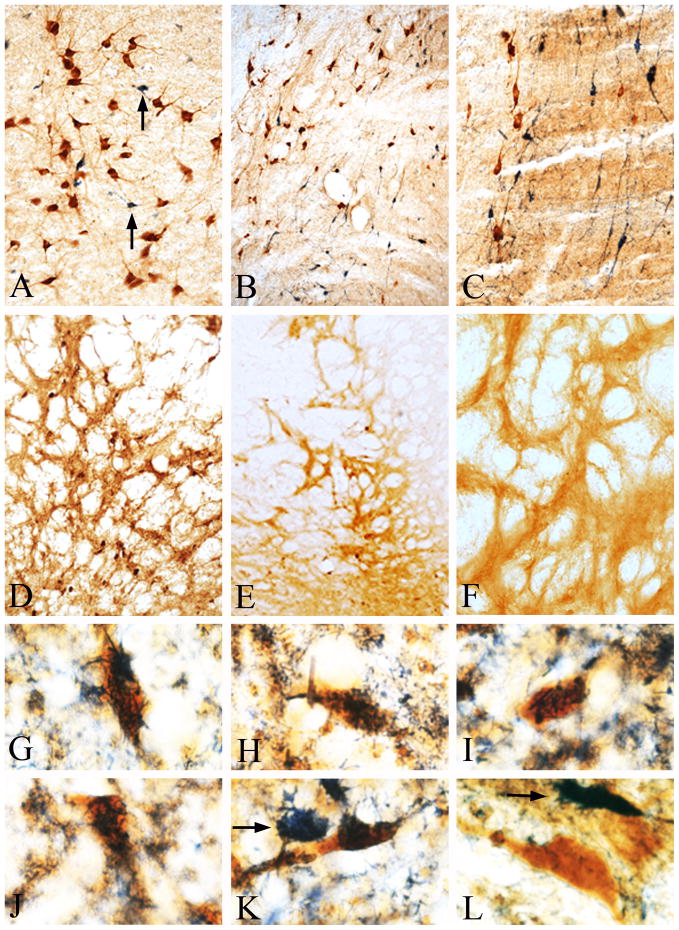
Figure 2.
(A–C) Double immunohistochemical staining for ChAT and calbindin, demonstrating lack of calbindin immunoreactivity in basal forebrain cholinergic neurons. (A) The cluster of ChAT immunoreactive neurons (brown) located beneath the anterior commissure is completely devoid of calbindin immunoreactivity (granular blue-black). Only occasional, small calbindin immunoreactive neurons (arrows) are seen intermingled with the cholinergic neurons. (B) Low power micrograph of the posterior aspects of the basal forebrain cholinergic system within which ChAT (brown) and calbindin (granular blue-black) immunoreactive neurons of similar size are intermingled. (C) Higher magnification of the region in (B) clearly demonstrates that ChAT and calbindin are present in distinct populations of neurons and the two immunoreactivities are never seen in the same neuron. (D) Calbindin immunoreactivity in the medial globus pallidus/internal capsule region within which cholinergic neurons are found 20 days after a 10 μl injection of AAV-NSE-CB. Note calbindin immunoreactive neurons with the morphology and distribution of cholinergic neurons (compare with Fig. 1A). (E) Low and (F) high power photomicrographs of an identical area as in (D), 20 days following a 10 μl injection of AAV-NSE-LacZ. Note the nearly complete absence of calbindin immunoreactive neurons. AAV-NSE-LacZ resulted in robust expression of β-galactosidase (see Fig. 2F), but not calbindin. This region contains a high density of ChAT-positive cholinergic neurons (see Fig. 1A and 1B), but is devoid of calbindin-positive neurons. (G–K) High power photomicrographs of calbindin immunoreactivity (granular blue-black) in cholinergic neurons (brown) in the region shown in (D) processed using double immunohistochemistry. (L) ChAT-positive neurons (brown), outside of the injection site were devoid of calbindin immunoreactivity in the same sections. Single stained calbindin immunoreactive neurons (arrows in K & L) were also observed in this region. Magnification in A, C and F is 20X, in B, D and E 10X and in G-L 60X.
AAV-Mediated Gene Transfer Results in Robust Expression of Calbindin in Neurons
We first confirmed CB expression in neuronal cultures mediated by our AAV vectors. Neuronal cultures were exposed to 5 μl of AAV-CMV-CB, AAV-NSE-CB or media. After 72 hours of exposure, cultures were fixed and immunostained for visualization of CB immunoreactivity in neurons. Background staining was observed in cultures exposed to media alone with occasional scattered CB-positive neurons consistent with the distribution of CB-positive cortical neurons in vivo. Similarly, a control AAV-NSE vector, bearing the LacZ gene, resulted in robust β-Gal staining but no CB immunoreactivity in cultured neurons (Fig. 3 A). In contrast, cultures exposed to AAV-CMV-CB and AAV-NSE-CB (Fig. 3 B and C) displayed clusters of CB immunoreactive neurons of varying staining intensity within the cytoplasm. Exposure to AAV-NSE-CB appeared to result in a higher number of the darkest CB immunoreactive neurons (Fig. 3 D and E) when compared with exposure to AAV-CMV-CB.
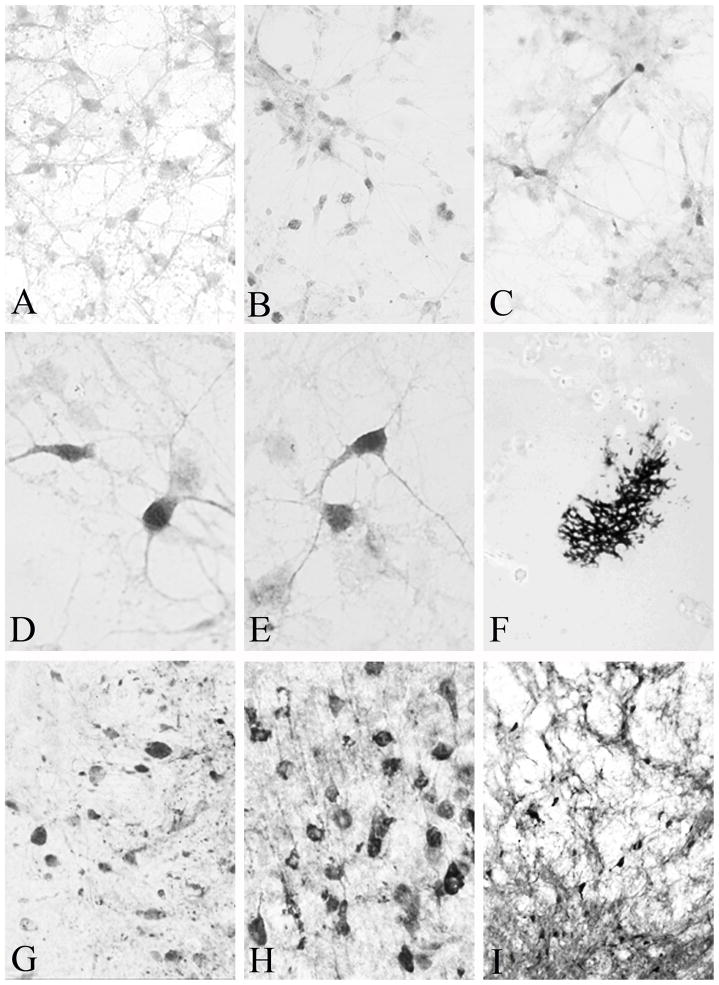
Figure 3.
(A) Exposure of neuronal cultures to the control AAV-NSE-LacZ vector did not result in expression of calbindin immunoreactivity. Only background staining was seen. In contrast, exposure of cultures to AAV-ACP-CB (B) or AAV-NSE-CB (C) resulted in robust expression of calbindin in neurons after 37 hours, visible at low power. (D & E) High power photomicrographs clearly demonstrate dark calbindin immunoreactivity, frequently seen in cultures exposed to AAV-NSE-CB. (F) β-galactosidase staining in the nucleus basalis of Meynert following a 10 μl injection of AAV-NSE-LacZ. This low-power micrograph demonstrates the extent of spread of the injected AAV. High (G and H) and low (I) power micrographs demonstrating calbindin expression in the lateral aspects of the thalamus (G), parietal cortex (H) and globus pallidus/internal capsule component of the nucleus basalis of Meynert 15 days following injection of 5μl of AAV-NSE-CB. Magnification in A-C is 40X, in D & E 60X, in F 4X, in G & H 40X, and in I 10X.
We then investigated expression of CB in rat brain neurons in vivo following injections of various volumes of the two vectors (3, 5 and 10 μl) in three different sites (basal forebrain, thalamus and parietal cortex). To examine the spread of the injected AAV vectors and as a control for specificity of CB gene transfer, equal volumes of AAV-NSE-LacZ were injected in the same areas of the opposite hemisphere. In some animals, media alone was injected in the opposite hemisphere as a control. Following survival times between 3–30 days, the presence of CB immunoreactivity within neurons was assessed in each injection site.
Injections of media alone did not result in CB immunoreactivity beyond control levels regardless of the volume, site of injection or survival time. Injections of AAV-NSE-LacZ resulted in a dose- and time-dependent expression of β-Gal, but not in CB immunoreactivity. β-Gal staining indicated that after a 10 μl injection, AAV spread approximately 3 mm (maximum of 5 mm) from the center of injection (Fig. 3 F).
Injections of 3 μl of AAV-ACP-CB or AAV-NSE-CB resulted in faint cellular expression of CB after 3 days of survival. Neuronal expression of CB in the lateral thalamus, parietal cortex and basal forebrain displayed an AAV dose and survival time dependent increase. The 10 μl injections resulted in the most robust and highest number of CB expressing neurons in all three sites. Significant CB expression was observed over and above background at 7 days of survival, and peak expression was achieved by 14 days (Fig. 3 G-I). AAV-NSE-CB injections resulted in more intense expression and in a greater number of neurons when compared with AAV-ACP-CB injections.
AAV-NSE-CB Results in Intense Calbindin Expression in Cholinergic Neurons
We used 10 μl injections of AAV-NSE-CB into the GPi/ic region containing the major component of the BFCNs, a region nearly devoid of CB-positive neurons, to investigate further specific expression of CB in the BFCNs. Examination of sections from intact animals, brains injected with control media or with AAV-NSE-LacZ revealed the nearly complete lack of CB immunoreactive neurons in this region (Fig. 2 E and F). In contrast, injections of AAV-NSE-CB resulted in robust neuronal CB immunoreactivity, with a distribution similar to ChAT-positive neurons, particularly within the GPi/ic (Fig. 1 C and D, Fig. 2 D). Of note, we did not detect CB expression in any cells with morphological characteristics of glia. CB immunoreactivity was restricted to neurons and neuronal processes.
Optimal CB expression was observed by 14 days of survival. We used brains from animals with a survival time of 30 days in double immunohistochemical procedures to determine the presence of CB immunoreactivity in ChAT-positive BFCNS. In intact animals, double immunohistochemistry showed that ChAT- and CB immunoreactive neurons in the basal forebrain comprised completely separate and non-overlapping populations. In contrast, at sites injected with AAV-NSE-CB, numerous ChAT-positive BFCNs also contained CB immunoreactivity (Fig. 2 G-L). In the 3 mm area surrounding the center of injections, nearly all ChAT-positive BFCNs also expressed CB. Counts of ChAT immunoreactive and ChAT/CB double stained neurons in this region revealed that on average 84% of the ChAT-positive BFCNs in each section also contained CB immunoreactivity (range 71–91%).
The best coordinates for injections aimed at the center of the GPi/ic component of the BFCNs were −1.2 mm from bregma, ±2.2 mm from midline and −7.2 mm from the skull. For CB expression in nearly all BFCNs located in the nbM, a second injection centered within the substantia innominata/ventral globus pallidus, with coordinates −0.8 mm from bregma, ±2.5 mm from midline and −8.0 from the skull is recommended.
Discussion
The results of the present study confirmed our earlier impression that unlike the human and non-human primate BFCNs, the rat BFCNs are devoid of CB (Geula et al., 1993b). In general, CB-positive neurons were rare in regions within which the BFCNs are located and were smaller than the BFCNs. Even in the posterior basal forebrain regions in which appreciable numbers of ChAT and CB immunoreactive neurons are located, the two markers were never co-localized in the same neuron.
We found that our AAV vector bearing the rodent CB gene under the control of the NSE promoter is effective in inducing CB expression both in vitro and in vivo. Regardless of the site of injection, AAV-NSE-CB resulted in robust expression of CB restricted to neurons. AAV induced CB expression was dose and time dependent. A 10 μl injection of the vector resulted in optimum CB expression locally following 14 days of survival. Significantly, injections of media or AAV-NSE-LacZ did not result in CB expression. Injections of AAV-CMV-CB also resulted in substantial neuronal expression of CB. However, in both culture and in vivo experiments, CB expression was stronger when AAV-NSE-CB was used.
The rodent BFCNs make up a system of neurons scattered in a wide region comprising the medial septum, the diagonal bands of Broca and the nbM (Mesulam et al., 1983). The cholinergic neurons in the nbM are the most extensive and are spread below the anterior commissure in the ventral globus pallidus and substantia innominata, and within the GPi/ic region. Thus, inducing expression of any protein in all of the BFCNs represents a significant challenge. Our results demonstrate that CB expression can be induced in an ectopic manner within the BFCNs following local AAV-NSE-CB injection. We concentrated on the component of nbM located within the GPi/ic because this region is virtually devoid of CB-positive neurons under natural conditions. To target the rest of the BFCNs in nbM, a second AAV injection aimed at the region below the anterior commissure would be necessary. The AAV-NSE-LacZ injections demonstrated that the AAV used spreads an average of 3 mm from the center of injection. Such spread is an advantage in the context of induced gene expression in the BFCNs, which are themselves spread across a wide region.
The BFCNs from the rodent brain can be isolated and cultured to nearly complete homogeneity (Schnitzler et al., 2008). Thus, AAV vector mediated CB expression can be induced in cholinergic neurons in culture to investigate mechanistically the effects on cholinergic neuronal survival from damage. However, a distinct advantage of AAV CB gene delivery to BFCNs in vivo is that the effects of CB expression can be investigated with intact brain microenvironment and neuronal connections. Nevertheless, in vivo AAV mediated expression has the limitation of lack of neuronal-type specificity. While the AAV-NSE vector used in this study resulted in CB expression in a substantial population of the BFCNs (up to 91%), we also observed CB expression beyond what is seen in intact brains in non-cholinergic neurons. However, the so called compact regions containing the BFCNs, located within each BFCNs sector including the nbM region under the anterior commissure and GPi/ic, contain nearly completely cholinergic neurons. This is most likely the reason why within the GPi/ic region we only observed occasional non-cholinergic neurons which contained CB immunoreactivity in vector injected animals. At present, there are no AAV vectors available that specifically target gene expression in the BFCNs. Therefore, targeting gene expression specifically to the BFCNs must await successful construction and packaging of vectors with gene promoters active only within these neurons.
We have shown a selective and substantial loss of CB from the human and non-human primate BFCNs in the course of normal aging (Wu et al., 1997; Geula et al., 2003a; Wu et al., 2003). It would be of considerable interest to know whether similar age-related changes are seen in AVV mediated expression of CB within the rodent BFCNs. However, the relatively short survival times employed in the present study do not allow such determination. Thus, addressing the potential age-related loss of AVV mediated CB expression from the rodent BFCNs must await future investigation.
The BFCNs are dependent on nerve growth factor (NGF) for survival during development and for protection against age-related changes and damage in adult brains (Gahwiler et al., 1990; Koliatsos et al., 1994; Kordower et al., 1994). Successful in vivo AAV mediated expression of NGF in rodent BFCNs has been reported by one group of investigators (Klein et al., 2000; Wu et al., 2005). Thus, our results represent AAV mediated gene transfer of a second and different protein namely CB, in the rodent BFCNs. The combined results indicate that the BFCNs can be successfully targeted for gene delivery, opening the way for mechanistic studies of the vulnerability of these neurons. As has been suggested (Mandel, 2010), successful gene delivery to the BFCNs also presents therapeutic possibilities, aimed at rescuing the BFCNs from degeneration in AD, and perhaps also in other neurodegenerative disorders that afflict the elderly.
Highlights.
Basal forebrain cholinergic neurons in the primate brain contain calbindin-D28K
Basal forebrain cholinergic neurons in the rat brain are devoid of calbindin-D28K
AAV-calbindin results in robust calbindin expression in vitro and in vivo
Neuron specific enolase (NSE) promoter enhances calbindin expression
AAV-NSE-calbindin results in robust expression of calbindin in cholinergic neurons
Acknowledgments
We are grateful to Sarah Burton for expert technical assistance. This work was supported in part by a grant from the National Institute on Aging (AG14706).
Abbreviations
- AV
adenovirus
- β-Gal
β-galactosidase
- BFCNs
basal forebrain cholinergic neurons
- CB
calbindin-D28K
- Ch
cholinergic cell group
- ChAT
choline acetyltransferase
- GPi
globus pallidus internal segment
- HSV
herpes simplex virus
- ic
internal capsule
- NSE
neuron-specific enolase
- p75NTR
low affinity neurotrophin receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicholas Nagykery, Email: nnagykery@yahoo.com.
Ernest F. Terwilliger, Email: eterwiligr@aol.com.
Changiz Geula, Email: c-geula@northwestern.edu.
References
- Berns KI. Parvovirus replication. Microbiol Rev. 1990;54:316–329. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orlando C, Fellay B, Schwaller B, Salicio V, Bloc A, Gotzos V, Celio MR. Calretinin and calbindin D-28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res. 2001;909:145–158. doi: 10.1016/s0006-8993(01)02671-3. [DOI] [PubMed] [Google Scholar]
- Daly TM. Overview of adeno-associated viral vectors. Meth Mol Biol. 2004;246:157–165. doi: 10.1385/1-59259-650-9:157. [DOI] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- Du B, Wu P, Boldt-Houle DM, Terwilliger EF. Efficient transduction of human neurons with an adeno-associated virus vector. Gene Ther. 1996;3:254–261. [PubMed] [Google Scholar]
- Figueredo-Cardenas G, Harris CL, Anderson KD, Reiner A. Relative resistance of striatal neurons containing calbindin or parvalbumin to quinolinic acid-mediated excitotoxicity compared to other striatal neuron types. Exp Neurol. 1998;149:356–372. doi: 10.1006/exnr.1997.6724. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Rietschin L, Knopfel T, Enz A. Continuous presence of nerve growth factor is required for maintenance of cholinergic septal neurons in organotypic slice cultures. Neurosci. 1990;36:27–31. doi: 10.1016/0306-4522(90)90348-8. [DOI] [PubMed] [Google Scholar]
- Geula C, Bu J, Nagykery N, Scinto LF, Chan J, Joseph J, Parker R, Wu CK. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol. 2003a;455:249–259. doi: 10.1002/cne.10475. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam MM. Cholinergic systems in Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. Vol. 2. Lippincott Williams and Wilkins; Philadelphia: 1999. pp. 269–292. [Google Scholar]
- Geula C, Mesulam MM, Tokuno H, Kuo CC. Developmentally transient expression of acetylcholinesterase within cortical pyramidal neurons. Dev Brain Res. 1993a;76:23–31. doi: 10.1016/0165-3806(93)90119-u. [DOI] [PubMed] [Google Scholar]
- Geula C, Nagykery N, Wu CK, Bu J. Loss of calbindin-D28K from aging human cholinergic basal forebrain: relation to plaques and tangles. J Neuropathol Exp Neurol. 2003b;62:605–616. doi: 10.1093/jnen/62.6.605. [DOI] [PubMed] [Google Scholar]
- Geula C, Schatz CR, Mesulam MM. Differential localization of NADPH-diaphorase and calbindin-D28k within the cholinergic neurons of the basal forebrain, striatum and brainstem in the rat, monkey, baboon and human. Neurosci. 1993b;54:461–476. doi: 10.1016/0306-4522(93)90266-i. [DOI] [PubMed] [Google Scholar]
- Hermens WT, Verhaagen J. Viral vectors, tools for gene transfer in the nervous system. Prog Neurobiol. 1998;55:399–432. doi: 10.1016/s0301-0082(98)00007-0. [DOI] [PubMed] [Google Scholar]
- Hilgenberg LG, Smith MA. Preparation of dissociated mouse cortical neuron cultures. Journal of visualized experiments J Vis Exp. 2007:562. doi: 10.3791/562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Hirko AC, Meyers CA, Grimes JR, Muzyczka N, Meyer EM. NGF gene transfer to intrinsic basal forebrain neurons increases cholinergic cell size and protects from age-related, spatial memory deficits in middle-aged rats. Brain Res. 2000;875:144–151. doi: 10.1016/s0006-8993(00)02634-2. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Price DL, Gouras GK, Cayouette MH, Burton LE, Winslow JW. Highly selective effects of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 on intact and injured basal forebrain magnocellular neurons. J Comp Neurol. 1994;343:247–262. doi: 10.1002/cne.903430206. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Winn SR, Liu YT, Mufson EJ, Sladek JR, Jr, Hammang JP, Baetge EE, Emerich DF. The aged monkey basal forebrain: rescue and sprouting of axotomized basal forebrain neurons after grafts of encapsulated cells secreting human nerve growth factor. Proc Natl Acad Sci USA. 1994;91:10898–10902. doi: 10.1073/pnas.91.23.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Hirsch EC, Cervera-Pierot P, Hersh LB, Bakchine S, Piette F, Duyckaerts C, Hauw JJ, Javoy-Agid F, Agid Y. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer's disease. J Comp Neurol. 1993;330:15–31. doi: 10.1002/cne.903300103. [DOI] [PubMed] [Google Scholar]
- Levey AI, Bolam JP, Rye DB, Hallanger AE, Demuth RM, Mesulam MM, Wainer BH. A light and electron microscopic procedure for sequential double antigen localization using diaminobenzidine and benzidine dihydrochloride. J Histochem Cytochem. 1986;34:1449–1457. doi: 10.1177/34.11.2430010. [DOI] [PubMed] [Google Scholar]
- Mandel RJ. CERE-110, an adeno-associated virus-based gene delivery vector expressing human nerve growth factor for the treatment of Alzheimer's disease. Curr Opin Mol Therap. 2010;12:240–247. [PubMed] [Google Scholar]
- Mattson MP, Rychlik B, Chu C, Christakos S. Evidence for calcium-reducing and excitoprotective roles for the calcium-binding protein calbindin-D 28K in cultured hippocampal neurons. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. Acetylcholinesterase-rich neurons of the human cerebral cortex: cytoarchitectonic and ontogenetic patterns of distribution. J Comp Neurol. 1991;306:193–220. doi: 10.1002/cne.903060202. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1-Ch6) Neurosci. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Miller RJ. The control of neuronal Ca2+ homeostasis. Prog Neurobiol. 1991;37:255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Peel AL, Zolotukhin S, Schrimsher GW, Muzyczka N, Reier PJ. Efficient transduction of green fluorescent protein in spinal cord neurons using adeno-associated virus vectors containing cell type-specific promoters. Gene Ther. 1997;4:16–24. doi: 10.1038/sj.gt.3300358. [DOI] [PubMed] [Google Scholar]
- Riascos D, de Leon D, Baker-Nigh A, Nicholas A, Yukhananov R, Bu J, Wu CK, Geula C. Age-related loss of calcium buffering and selective neuronal vulnerability in Alzheimer's disease. Acta Neuropathol. 2011;122:565–576. doi: 10.1007/s00401-011-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintoul GL, Raymond LA, Baimbridge KG. Calcium buffering and protection from excitotoxic cell death by exogenous calbindin-D28k in HEK 293 cells. Cell Calc. 2001;29:277–287. doi: 10.1054/ceca.2000.0190. [DOI] [PubMed] [Google Scholar]
- Rogers JD, Brogan D, Mirra SS. The nucleus basalis of Meynert in neurological disease: a quantitative morphological study. Ann Neurol. 1985;17:163–170. doi: 10.1002/ana.410170210. [DOI] [PubMed] [Google Scholar]
- Samuel WA, Henderson VW, Miller CA. Severity of dementia in Alzheimer disease and neurofibrillary tangles in multiple brain regions. Alz Dis Assoc Disor. 1991;5:1–11. doi: 10.1097/00002093-199100510-00001. [DOI] [PubMed] [Google Scholar]
- Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N, Hunter LA. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler AC, Lopez-Coviella I, Blusztajn JK. Purification and culture of nerve growth factor receptor (p75)-expressing basal forebrain cholinergic neurons. Nat Protoc. 2008;3:34–40. doi: 10.1038/nprot.2007.477. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J, Levivier M. Recombinant AAV-mediated gene delivery to the central nervous system. J Gen Med. 2004;6(Suppl 1):S212–222. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- Wu CK, Hersh LB, Geula C. Cyto- and chemoarchitecture of basal forebrain cholinergic neurons in the common marmoset (Callithirx jacchus) Exp Neurol. 2000;165:306–326. doi: 10.1006/exnr.2000.7468. [DOI] [PubMed] [Google Scholar]
- Wu CK, Mesulam MM, Geula C. Age-related loss of calbindin from human basal forebrain cholinergic neurons. Neurorep. 1997;8:2209–2213. doi: 10.1097/00001756-199707070-00024. [DOI] [PubMed] [Google Scholar]
- Wu CK, Nagykery N, Hersh LB, Scinto LF, Geula C. Selective age-related loss of calbindin-D28k from basal forebrain cholinergic neurons in the common marmoset (Callithrix jacchus) Neurosci. 2003;120:249–259. doi: 10.1016/s0306-4522(03)00248-3. [DOI] [PubMed] [Google Scholar]
- Wu K, Meyer EM, Bennett JA, Meyers CA, Hughes JA, King MA. AAV2/5-mediated NGF gene delivery protects septal cholinergic neurons following axotomy. Brain Res. 2005;1061:107–113. doi: 10.1016/j.brainres.2005.08.056. [DOI] [PubMed] [Google Scholar]