Abstract
The aryl hydrocarbon receptor (AHR) has physiological roles in the absence of exposure to exogenous ligands and mediates adaptive and toxic responses to the environmental pollutant, 2,3,7,8-tetracholorodibenzo-p-dioxin (TCDD). A readily metabolized AHR agonist, 3-methylcholanthrene, disrupts expression of mouse hepatic growth hormone (GH) signaling components and suppresses cytochrome P450 2D9 (Cyp2d9), a male-specific gene controlled by pulsatile GH via signal transducer and activator of transcription 5b (STAT5b). Using TCDD as an essentially non-metabolized AHR agonist and Ahr −/− mice as the preferred model to determine the AHR-dependence of biological responses, we now show that two mouse hepatic STAT5b target genes, Cyp2d9 and major urinary protein 2 (Mup2), are suppressed by TCDD in an AHR-dependent manner. TCDD also decreased hepatic mRNA levels for GH receptor, Janus kinase 2, and STAT5a/b with AHR-dependence. Without inducing selected hepatic inflammatory markers, TCDD caused AHR-dependent induction of Cyp1a1 and NADPH-cytochrome P450 oxidoreductase (Por) and suppression of Cyp3a11. In vehicle-treated mice, basal mRNA levels for CYP2D9, CYP3A11, POR, serum amyloid protein P, and MUP2 were influenced by Ahr genetic status. We conclude that AHR activation per se leads to dysregulation of hepatic GH signaling components and suppression of some, but not all, STAT5b target genes.
Keywords: aryl hydrocarbon receptor; 2,3,7,8-tetrachlorodibenzo-p-dioxin; cytochrome P450; growth hormone receptor; Janus kinase 2; signal transducer and activator of transcription 5b; cytokine-inducible Src homology 2 domain-containing protein; major urinary protein 2; inflammatory markers; NADPH-cytochrome P450 oxidoreductase
Introduction
The aryl hydrocarbon receptor (AHR) mediates both adaptive and toxic effects of halogenated aromatic hydrocarbons (HAHs) such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and polycyclic aromatic hydrocarbons (PAHs) such as benzo[a]pyrene and 3-methylcholanthrene (MC) (Schmidt and Bradfield 1996). The adaptive response to AHR agonists is typified by induction of a battery of drug-metabolizing enzymes including cytochrome P450 1A1 (CYP1A1) (Whitlock 1999). Persistent AHR activation by long-lived pollutants such as TCDD results in toxicities including a wasting syndrome, endocrine disruption, thymic atrophy, chloracne, tumor promotion, teratogenesis, and hepatotoxicity (Poland and Knutson 1982; Pohjanvirta and Tuomisto 1994). Mice homozygous for an Ahr-null allele (Ahr −/− mice) are not only extremely resistant to the adaptive and toxic effects of HAH and PAH exposure (Fernandez-Salguero et al. 1996; Shimizu et al. 2000), but they also display important deficits in hepatic, vascular, and hematopoietic development in the apparent absence of exposure to exogenous AHR ligands (Schmidt et al. 1996; Lahvis et al. 2000). This suggests that endogenous AHR activators play key roles in mammalian physiology and development (Denison and Nagy 2003; Nguyen and Bradfield 2008).
Although the induction of CYP1A1 is a well-characterized response to HAHs and PAHs, our recent focus has been on the poorly understood processes involved in the suppression of gene expression in response to these chemicals (Riddick et al. 2003; Riddick et al. 2004). CYP2C11 is the most abundant constitutive hepatic P450 in male rats and a primary physiological regulator of its expression is the pulsatile pattern of pituitary growth hormone (GH) secretion, with signal transducer and activator of transcription 5b (STAT5b) as an intracellular messenger at least partially responsible for the sexually dimorphic expression (Park and Waxman 2001; Ahluwalia et al. 2004; Clodfelter et al. 2006). Aromatic hydrocarbons cause CYP2C11 down-regulation via a transcriptional mechanism in male rats in vivo (Jones and Riddick 1996; Lee and Riddick 2000) and in cultured primary rat hepatocytes (Safa et al. 1997; Bhathena et al. 2002). AHR involvement in this suppression response was suggested by structure-activity relationship data (Safa et al. 1997) and we showed that the activated AHR binds to a putative dioxin-responsive element (DRE) in the CYP2C11 5′-flanking region (Bhathena et al. 2002). MC treatment causes down-regulation of luciferase reporter constructs driven by the CYP2C11 promoter and 5′-flanking region when delivered to the liver of living male rats via high-volume tail vein injection (Sawaya and Riddick 2008b), but no suppression of these reporter plasmids in response to MC or TCDD is observed in transfected continuous cell lines or primary rat hepatocytes (Bhathena et al. 2002; Sawaya and Riddick 2008a). Although MC interferes with the ability of GH to stimulate hepatic CYP2C11 expression in the liver of hypophysectomized male rats (Timsit and Riddick 2000), there were no apparent effects of MC on GH-stimulated STAT5b signaling in rat liver or in H4IIE rat hepatoma cells (Timsit and Riddick 2002).
Mouse Cyp2d9 encodes a male-specific steroid 16α-hydroxylase and the hepatic expression of this gene is clearly regulated by pulsatile GH in a STAT5b-dependent manner (Udy et al. 1997; Davey et al. 1999; Clodfelter et al. 2006). Similar to the suppression of rat CYP2C11 by aromatic hydrocarbons, we showed that MC treatment of male mice caused down-regulation of hepatic Cyp2d9 at the mRNA, protein and catalytic activity levels (Lee et al. 2006), and this was accompanied by decreased mRNA levels for the GH receptor (GHR) and major urinary protein 2 (MUP2), a GH-regulated and STAT5b-dependent transcript. Others have shown that treatment of mice with MC leads to an AHR-dependent decrease in hepatic levels of mRNA encoding GHR, Janus kinase 2 (JAK2) and two distinct STAT5b targets, MUP2 and cytokine-inducible Src homology 2 domain-containing protein (CIS) (Nukaya et al. 2004).
Most previous studies suggesting that aromatic hydrocarbons suppress the expression of sexually dimorphic constitutive hepatic P450s in rats and mice by disrupting the GHR-JAK2-STAT5b signaling pathway were conducted with MC as a prototypical PAH. The parent MC molecule binds AHR with relatively high affinity (Riddick et al. 1994) and alters expression of numerous AHR target genes (Kondraganti et al. 2005; Pansoy et al. 2010); however, studies with MC are complicated by time-dependent changes in biological potency caused by its rapid biotransformation to multiple metabolites (Poland and Glover 1974; Riddick et al. 1994), some of which are highly reactive and toxic (Mathieu et al. 2001). In contrast, TCDD is highly resistant to biotransformation and causes persistent AHR activation without being converted to reactive metabolites in the process, providing an opportunity for a cleaner assessment of the importance of AHR activation per se in a given biological response.
Using TCDD as an essentially non-metabolized AHR agonist and Ahr −/− mice as the preferred model to determine the AHR-dependence of biological responses, our goal was to answer the following questions with respect to levels of hepatic mRNAs encoding selected constitutive hepatic P450s, GH signaling components and STAT5b target genes. (1) Are basal expression levels in the absence of TCDD treatment influenced by Ahr genetic status? (2) Are expression levels altered by TCDD treatment? (3) Are responses elicited by TCDD treatment AHR-dependent or AHR-independent? (4) Are the observed effects of TCDD accompanied by induction of selected hepatic inflammatory markers?
Materials and methods
Animals and treatment
Mouse hepatic RNA samples were provided by Dr. Allan B. Okey (University of Toronto) and details of the original mouse gene expression profiling study were described previously (Tijet et al. 2006). A brief summary of the animal work is given here for context. Male Ahr −/− mice in a C57BL/6J background (Schmidt et al. 1996) and wild-type male C57BL/6J mice received a single dose of TCDD (1000 μg/kg) or corn oil vehicle by gavage. Liver was harvested 19 h after treatment and stored in liquid nitrogen until subsequent RNA isolation.
Analysis of mRNA levels by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Hepatic levels of mRNAs encoding CYP1A1, JAK2, STAT5a/b, CIS and MUP2, normalized to β-actin as the internal reference standard, were determined by measuring the intensity of PCR products on Vistra Green-stained polyacrylamide gels using the primers and experimental conditions previously described (Lee et al. 2006).
Analysis of mRNA levels by real-time quantitative RT-PCR
The reverse transcription step was carried out as described previously (Lee et al. 2006). Hepatic levels of mRNAs encoding CYP2D9, CYP3A11, NADPH-cytochrome P450 oxidoreductase (POR), serum amyloid protein P (SAP), suppressor of cytokine signaling 3 (SOCS3) and GHR, normalized to β-actin as the internal reference standard, were determined in triplicate for all samples using the ABI Prism 7500 Sequence Detection System. The efficiencies of the real-time PCR reactions for all targets relative to β-actin were validated as equivalent as required for the comparative threshold cycle (ΔΔCt) relative quantitation method. Each PCR reaction contained input cDNA derived from an optimized amount of RNA, optimized final primer concentrations and Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA). Primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA) and sequences are listed in Table 1. Cycling conditions were: initial cycle of 2 min at 50°C and 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. A wild-type vehicle-treated sample was used as the calibrator sample and β-actin mRNA was used as the endogenous control for each sample measured. Ct values for each sample were normalized to β-actin mRNA (ΔCt) and the calibrator sample (ΔΔCt). Relative fold-change (RQ) was calculated as 2−ΔΔCt and the mean RQ for each treatment group was expressed as a percentage of the wild-type vehicle-treated group.
Table 1.
Primer sequences for target genes measured at the mRNA level by real-time quantitative RT-PCR.
| Target | Forward and Reverse PCR Primer Sequences | PCR product size (bp) | Reference |
|---|---|---|---|
| CYP2D9 | 5′-AGTCTCTGGCTTAATTCCTGAGGTT-3′ 5′-CGCAAGAGTATCGGGAATGC-3′ |
63 | (Wiwi et al. 2004) |
| CYP3A11 | 5′-CTTTCCTTCACCCTGCATTCC-3′ 5′-CTCATCCTGCAGTTTTTTCTGGAT-3′ |
87 | (Wiwi et al. 2004) |
| POR | 5′-GCCTGCCTGAGATCGACAAG-3′ 5′-GGGTCGCCTTCTCCGTATGT-3′ |
64 | (Muguruma et al. 2006) |
| SAP | 5′-TACTGCTTTGGATGTTTGTCTTCAC-3′ 5′-TCAGCTTCACATGATCAGTTTCAG-3′ |
115 | (Charles et al. 2006) |
| SOCS3 | 5′-CACCTGGACTCCTATGAGAAAGTGA-3′ 5′-GGAGCATCATACTGATCCAGGAA-3′ |
74 | (Yang et al. 2005) |
| GHR | 5′-CAGTTCCAAAGATTAAAGGGATTGA-3′ 5′-TTATCATGAATGCCTAAGATGGTGTT-3′ |
88 | (Reddy et al. 2007) |
| β-actin | 5′-GATTACTGCTCTGGCTCCTAGCA-3′ 5′-GCCACCGATCCACACAGAGT-3′ |
82 | (Smith et al. 2003) |
Statistical analysis
All data are expressed as mean ± SD. All statistical analyses were performed on the original raw data and not on the percent control data presented in the figures. Data were analyzed initially using a randomized-design two-way ANOVA to identify significant influences of the two independent variables and their interaction (treatment, genotype, treatment x genotype interaction). Post test analyses for the planned comparisons (treatment effect, genotype effect) were performed to assess whether there were significant differences between particular groups. Post tests were Bonferroni-corrected for multiple comparisons and used the mean square residual (pooled variance) and corresponding degrees of freedom from the two-way ANOVA. A result was considered statistically significant if P < 0.05.
Results
We have examined the expression of selected constitutive P450s, GH signaling components and STAT5b target genes in the liver of male C57BL/6J mice and their Ahr −/− counterparts at 19 h following a single oral dose of TCDD (1000 μg/kg). The pathway under investigation is shown schematically in Fig. 1. We obtained hepatic RNA samples from a gene expression profiling study that was designed to identify the batteries of genes whose expression in vivo is affected by Ahr genetic status alone, by TCDD treatment alone, or by the combination of TCDD treatment and Ahr genetic status (Tijet et al. 2006). The dose of TCDD was intentionally high, about 5-times the single-dose oral LD50 for male C57BL/6J mice (Pohjanvirta and Tuomisto 1994), since the goal of the original study was to identify early, and hence likely to be primary, gene expression changes that could be responsible for major aspects of dioxin toxicity, such as hepatotoxicity, wasting and lethality.
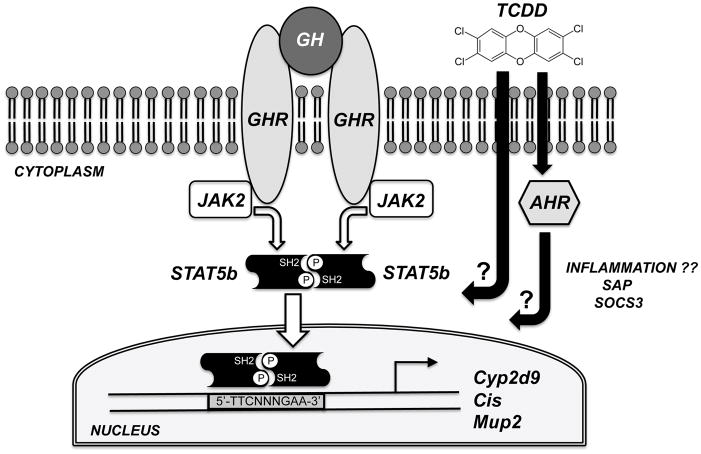
Fig. 1.
Schematic representation of the potential disruption of the hepatic GHR-JAK2-STAT5b signal transduction pathway by TCDD. GH, secreted from the pituitary gland in a pulsatile fashion in male rodents, triggers dimerization of the cell-surface GHR, followed by recruitment and activation of the tyrosine kinase JAK2, and phosphorylation of a specific tyrosine residue in STAT5b. A STAT5b homodimer forms via symmetrical and mutual SH2-phosphotyrosine interactions, and this dimer translocates to the nucleus and binds to specific DNA response elements to influence the expression of STAT5b target genes such as Cyp2d9, Cis, and Mup2. TCDD diffuses through the plasma membrane and may disrupt this signaling pathway via AHR-dependent or AHR-independent mechanisms. We also assessed whether TCDD’s effects were accompanied by induction of two hepatic inflammatory markers (SAP and SOCS3).
Cyp1a1 induction was monitored in this study as a well-characterized positive control response to TCDD treatment. The original published study demonstrated by both microarray analysis and real-time quantitative RT-PCR that CYP1A1 mRNA is strongly induced by TCDD in wild-type mice, whereas CYP1A1 mRNA is undetectable in Ahr −/− mice or in vehicle-treated wild-type mice (Tijet et al. 2006). We confirmed that only TCDD-treated wild-type mice displayed detectable CYP1A1 mRNA by semiquantitative RT-PCR analysis (data not shown).
Constitutive hepatic cytochromes P450 and POR
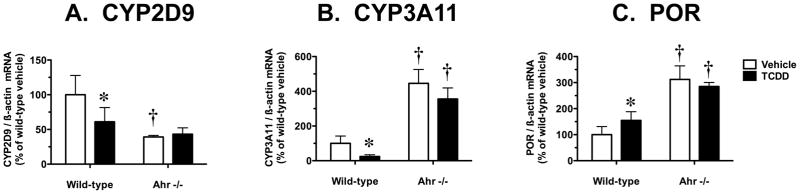
Cyp2d9 encodes a male-specific steroid 16α-hydroxylase and the hepatic expression of this gene is clearly regulated by pulsatile GH in a STAT5b-dependent manner (Udy et al. 1997; Davey et al. 1999; Clodfelter et al. 2006). TCDD caused a 39% decrease in hepatic CYP2D9 mRNA levels in wild-type mice and this suppression response was not observed in Ahr −/− mice (Fig. 2A). In vehicle-treated mice, the basal expression of CYP2D9 mRNA was 2.6-fold higher in wild-type mice compared to Ahr −/− mice (Fig. 2A).
Fig. 2.
Effect of TCDD treatment on hepatic mRNA levels for constitutive P450s and POR in wild-type and Ahr −/− mice. Real-time quantitative RT-PCR analysis of mRNA levels for (A) CYP2D9, (B) CYP3A11 and (C) POR, relative to β-actin. Results are expressed as a percentage of the mean for the vehicle-treated wild-type mice. Data are expressed as mean ± SD of determinations from six wild-type mice per group and three Ahr −/− mice per group. Data were analyzed initially using a randomized-design two-way ANOVA and the P values for the main effects are reported here: CYP2D9 (treatment, P = 0.1177; genotype, P = 0.0022; interaction, P = 0.0597), CYP3A11 (treatment, P = 0.0031; genotype, P < 0.0001; interaction, P = 0.7639), and POR (treatment, P = 0.4288; genotype, P < 0.0001; interaction, P = 0.0298). Planned comparisons to identify significant differences between particular groups utilized a post test Bonferroni-corrected for multiple comparisons. *, significantly different (P < 0.05) from genotype-matched vehicle control mice; †, significantly different (P < 0.05) from treatment-matched wild-type mice.
Cyp3a11 encodes the most abundant CYP3A subfamily protein in the liver of male mice (Yanagimoto et al. 1997) and the expression of this gene is sex-independent and GH-independent in males (Sakuma et al. 2002; Jarukamjorn et al. 2006). We showed previously that MC treatment caused a dramatic decrease in mouse hepatic CYP3A11 content, particularly at the protein level (Lee et al. 2006). TCDD caused a 76% decrease in hepatic CYP3A11 mRNA levels in wild-type mice and this suppression response was not observed in Ahr −/− mice (Fig. 2B). In vehicle-treated mice, the basal expression of CYP3A11 mRNA was 4.5-fold higher in Ahr −/− mice compared to wild-type mice (Fig. 2B).
The flavoprotein POR is the obligate electron-transfer partner protein for all reactions catalyzed by microsomal P450s. Our previous work showed that mouse hepatic POR catalytic activity was increased by up to 33% following MC treatment (Lee et al. 2006). POR mRNA levels were increased by 55% following TCDD treatment in wild-type mice and this small magnitude induction response was not see in Ahr −/− mice (Fig. 2C). In vehicle-treated mice, the basal expression of POR mRNA was 3.1-fold higher in Ahr −/− mice compared to wild-type mice (Fig. 2C).
Hepatic inflammatory markers
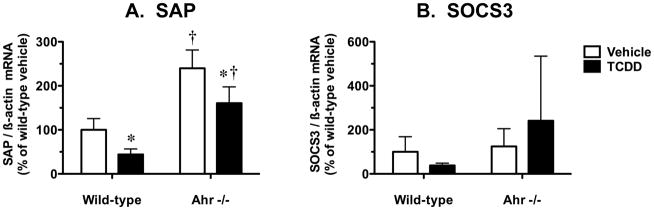
Down-regulated expression of constitutive hepatic P450s often occurs in response to inflammatory conditions (Riddick et al. 2004; Morgan et al. 2008). Since TCDD treatment in wild-type mice suppressed hepatic mRNA levels for both CYP2D9 and CYP3A11, we checked if TCDD increased expression of two established hepatic inflammatory markers: SAP, a hallmark reactant in the acute phase response to inflammation in mice (Charles et al. 2006), and SOCS3, an important feedback inhibitor of hepatic cytokine signaling known to be induced by pro-inflammatory cytokines including interleukin-6 (IL-6) (Yang et al. 2005). We found no evidence for induction of hepatic SAP mRNA levels (Fig. 3A) or SOCS3 mRNA levels (Fig. 3B) by TCDD at the 19-h time-point examined. In fact, TCDD treatment decreased SAP mRNA levels by 56% in wild-type mice and by 33% in Ahr −/− mice (Fig. 3A). In vehicle-treated mice, the basal expression of SAP mRNA was 2.4-fold higher in Ahr −/− mice compared to wild-type mice (Fig. 3A). Hepatic SOCS3 mRNA levels were not influenced by TCDD treatment or Ahr genotype (Fig. 3B).
Fig. 3.
Effect of TCDD treatment on hepatic mRNA levels for inflammatory markers in wild-type and Ahr −/− mice. Real-time quantitative RT-PCR analysis of mRNA levels for (A) SAP and (B) SOCS3, relative to β-actin. Results are expressed as a percentage of the mean for the vehicle-treated wild-type mice. Data are expressed as mean ± SD of determinations from six wild-type mice per group and three Ahr −/− mice per group. Data were analyzed initially using a randomized-design two-way ANOVA and the P values for the main effects are reported here: SAP (treatment, P = 0.0002; genotype, P < 0.0001; interaction, P = 0.3996), and SOCS3 (treatment, P = 0.6679; genotype, P = 0.0843; interaction, P = 0.1683). Planned comparisons to identify significant differences between particular groups utilized a post test Bonferroni-corrected for multiple comparisons. *, significantly different (P < 0.05) from genotype-matched vehicle control mice; †, significantly different (P < 0.05) from treatment-matched wild-type mice.
Components of the hepatic GHR-JAK2-STAT5b signal transduction pathway
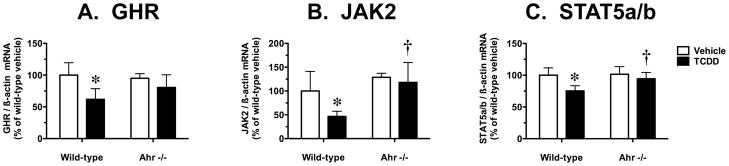
To determine if suppression of Cyp2d9 by TCDD may be related to disruption of hepatic GH signaling, we measured mRNA levels for the key components in the GHR-JAK2-STAT5b pathway known to control the male-specific hepatic expression of this P450. In wild-type mice, but not Ahr −/− mice, TCDD decreased hepatic mRNA levels for GHR, JAK2 and STAT5a/b by 38%, 53% and 25%, respectively (Fig. 4). In vehicle-treated mice, the basal mRNA levels for GHR, JAK2 and STAT5a/b did not differ between wild-type and Ahr −/− mice (Fig. 4).
Fig. 4.
Effect of TCDD treatment on hepatic mRNA levels for components of the GHR-JAK2-STAT5b signaling pathway in wild-type and Ahr −/− mice. Real-time quantitative RT-PCR analysis of mRNA levels for (A) GHR, and semiquantitative RT-PCR analysis of mRNA levels for (B) JAK2 and (C) STAT5a/b, relative to β-actin. Results are expressed as a percentage of the mean for the vehicle-treated wild-type mice. Data are expressed as mean ± SD of determinations from six wild-type mice per group and three Ahr −/− mice per group. Data were analyzed initially using a randomized-design two-way ANOVA and the P values for the main effects are reported here: GHR (treatment, P = 0.0089; genotype, P = 0.4350; interaction, P = 0.1948), JAK2 (treatment, P = 0.0511; genotype, P = 0.0050; interaction, P = 0.1771), and STAT5a/b (treatment, P = 0.0080; genotype, P = 0.0650; interaction, P = 0.1131). Planned comparisons to identify significant differences between particular groups utilized a post test Bonferroni-corrected for multiple comparisons. *, significantly different (P < 0.05) from genotype-matched vehicle control mice; †, significantly different (P < 0.05) from treatment-matched wild-type mice.
Other hepatic STAT5b target genes
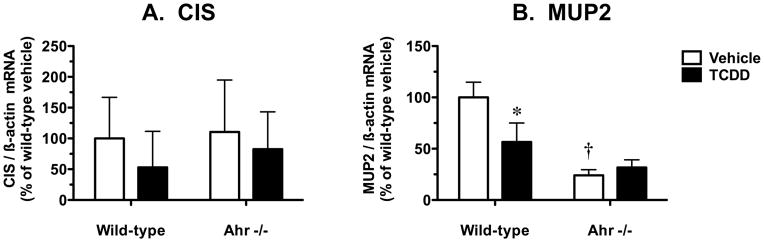
Disruption of the hepatic GHR-JAK2-STAT5b signal transduction cascade by TCDD could result in suppression of STAT5b target genes in addition to Cyp2d9. CIS is induced by GH via a STAT5b-dependent transcriptional mechanism and plays a role in negative feedback inhibition of GH signaling (Landsman and Waxman 2005). MUP2 belongs to the family of α2-microglobulin-related liver secretory proteins and is a significant protein component of mouse urine; pulsatile GH signaling via a STAT5b-dependent mechanism confers male-predominant expression of MUP2 (Udy et al. 1997). Hepatic CIS mRNA levels were not influenced by TCDD treatment or Ahr genotype (Fig. 5A). In contrast, TCDD caused a 43% decrease in hepatic MUP2 mRNA levels in wild-type mice and this suppression response was not observed in Ahr −/− mice (Fig. 5B). In vehicle-treated mice, the basal expression of MUP2 mRNA was 4.2-fold higher in wild-type mice compared to Ahr −/− mice (Fig. 5B).
Fig. 5.
Effect of TCDD treatment on hepatic mRNA levels for other STAT5b target genes in wild-type and Ahr −/− mice. Semiquantitative RT-PCR analysis of mRNA levels for (A) CIS and (B) MUP2, relative to β-actin. Results are expressed as a percentage of the mean for the vehicle-treated wild-type mice. Data are expressed as mean ± SD of determinations from six wild-type mice per group and three Ahr −/− mice per group. Data were analyzed initially using a randomized-design two-way ANOVA and the P values for the main effects are reported here: CIS (treatment, P = 0.2762; genotype, P = 0.5544; interaction, P = 0.7782), and MUP2 (treatment, P = 0.0286; genotype, P < 0.0001; interaction, P = 0.0036). Planned comparisons to identify significant differences between particular groups utilized a post test Bonferroni-corrected for multiple comparisons. *, significantly different (P < 0.05) from genotype-matched vehicle control mice; †, significantly different (P < 0.05) from treatment-matched wild-type mice.
Discussion
Although a historical emphasis in the AHR field has been on altered gene expression in response to xenobiotics such as TCDD, studies of Ahr −/− mice allow the investigation of genes whose expression is altered by Ahr genetic status independent of exposure to exogenous receptor agonists. Comparisons of hepatic gene expression profiles by microarray analysis show that expression levels for hundreds of genes differ between wild-type and Ahr −/− mice in the absence of TCDD treatment (Tijet et al. 2006). With our focus on constitutive hepatic P450s, inflammatory markers, GH signaling components and STAT5b target genes (Fig. 1), we have identified several genes whose basal expression is influenced by Ahr genetic status alone. On the one hand, basal mRNA levels for CYP2D9 and MUP2 are higher in wild-type versus Ahr −/− mice. A similar result was reported in the original microarray study for CYP2D9 mRNA (Tijet et al. 2006) and we now provide confirmation by real-time quantitative RT-PCR. On the other hand, basal mRNA levels for CYP3A11, POR, and SAP are lower in wild-type versus Ahr −/− mice. A similar result was reported in the original microarray study for CYP3A11 mRNA (Tijet et al. 2006) and we now provide confirmation by real-time quantitative RT-PCR.
The impact of Ahr genetic status on the basal hepatic expression of multiple genes suggests important roles for the AHR in normal physiology. However, three fundamental questions about this basal regulation persist: (a) does the AHR act directly or indirectly to control basal gene expression? (b) why is the presence of AHR associated with higher basal expression of some genes and lower basal expression of other genes? (c) do the actions of the AHR in the absence of an exogenous agonist involve receptor activation by endogenous ligands or ligand-independent functions of AHR? The classic example of a hepatic gene showing lowered basal expression in Ahr −/− mice is Cyp1a2 (Shimada et al. 2002; Tijet et al. 2006). Since this gene is induced by TCDD via AHR activation, it is generally thought that endogenous ligands interact with the AHR to drive higher basal expression of Cyp1a2 in wild-type mice. For genes such as Cyp2d9 and Mup2, whose expression is instead suppressed by TCDD in wild-type mice, it is difficult to envision mechanisms accounting for higher basal expression in wild-type versus Ahr −/− mice. Since Ahr −/− mice have lower expression of some hepatic drug-metabolizing enzymes (e.g. CYP1A2), endogenous and dietary substrates for such enzymes may accumulate and potentially activate other xenosensors such as the pregnane X receptor or the constitutive androstane receptor (CAR). An indirect mechanism of this nature might account for the elevated basal expression of genes such as Cyp3a11 and Por that we observed in Ahr −/− mice. Interestingly, CAR was shown to be more robustly activated by exogenous octachlorostyrene in the liver of Ahr −/− mice (Yanagiba et al. 2009). Mice with conditional deletion of the AHR nuclear translocator in the intestinal epithelium display markedly elevated CYP1A1 mRNA and catalytic activity in non-gut tissues due to impaired metabolism and accumulation of dietary inducers (Ito et al. 2007).
An important goal of the present study was to determine if the persistent AHR agonist, TCDD, affected the expression of constitutive mouse hepatic P450s, GH signaling components and STAT5b target genes in a similar manner to what we characterized previously for MC, a readily metabolized AHR agonist (Lee et al. 2006). The AHR-dependence of TCDD’s effects was also investigated using the Ahr −/− mouse model. Like MC, TCDD decreased hepatic mRNA levels for both Cyp2d9, a GH-dependent gene, and Cyp3a11, a GH-independent gene. A similar result was reported in the original microarray study for CYP3A11 mRNA (Tijet et al. 2006) and we now provide confirmation by real-time quantitative RT-PCR. The suppression of these two constitutive hepatic P450s by TCDD is clearly AHR-dependent, suggesting that these pretranslational responses are likely due to AHR activation per se rather than requiring bioactivation of MC to reactive metabolites. We are also pursuing this question by examining the effects of MC in mice deficient in hepatic POR activity.
Like Cyp2d9, Mup2 is a STAT5b target gene that was also down-regulated by TCDD at the mRNA level in an AHR-dependent manner. This raises the intriguing possibility that TCDD may disrupt the GHR-JAK2-STAT5b pathway, a shared physiological regulatory cascade for both genes. However, the hepatic expression of an additional STAT5b target gene, Cis, was not altered by TCDD treatment. Similar to what we reported for MC (Lee et al. 2006), it appears that some, but not all, hepatic genes regulated by the GHR-JAK2-STAT5b pathway may be targeted for suppression by TCDD. It is important to point out that our measurements of CIS mRNA levels were characterized by wide inter-animal variability that may have compromised our ability to discern an effect of TCDD.
Two previous studies (Nukaya et al. 2004; Lee et al. 2006) showed that MC decreases mRNA levels encoding key GH signaling components and that this can result in compromised binding of STAT5a/b to DNA response elements (Nukaya et al. 2004). Our current work shows that TCDD down-regulates hepatic GHR, JAK2, and STAT5a/b mRNA levels in an AHR-dependent manner. A similar result was reported in the original microarray study for GHR mRNA (Boutros et al. 2008) and we now provide confirmation by real-time quantitative RT-PCR. Our findings suggest that AHR activation by TCDD leads to lowered mRNA levels for GHR, JAK2, and STAT5a/b, and this may potentially play a role in the observed suppression of STAT5b target genes such as Cyp2d9 and Mup2. A limitation of the current study is that all assessments of the GH signaling components were performed at the mRNA level and it will be critical to examine the functional impacts of TCDD exposure by measuring the protein levels and the activation status of the players in this signaling cascade. It is currently not known if the genes encoding these GH signaling components represent direct transcriptional targets of the AHR, but this possibility is worth investigation since putative DREs have been identified in the 5′-flanking regions of the mouse Ghr and Stat5a/b genes (Nukaya et al. 2004).
Gene expression profiling experiments demonstrate that the AHR is required for nearly all transcriptional responses to TCDD in mouse liver (Tijet et al. 2006). The current study detected only one AHR-independent response to TCDD treatment, this being the suppression of SAP mRNA levels observed in both wild-type and Ahr −/− mice. The lack of induction of this gene in response to TCDD, along with the absence of TCDD-mediated induction of Socs3 expression, suggests that the down-regulation of constitutive hepatic P450s in response to TCDD is not accompanied by induction of selected hepatic inflammatory markers. Several roles of the AHR in the immune system are under active investigation (Stockinger et al. 2011). Exogenous AHR agonists appear to have dual effects on inflammatory signaling. On the one hand, TCDD exposure can induce inflammatory responses as exemplified by: (a) the synergistic induction of IL-6 expression caused by interleukin-1β and TCDD co-treatment via an AHR-dependent and DRE-mediated mechanism (Hollingshead et al. 2008); and (b) induction of Socs3 expression by TCDD via an AHR-dependent and protein kinase-dependent non-genomic pathway that does not require AHR nuclear localization (Li et al. 2010). On the other hand, AHR activation by TCDD can disrupt nuclear factor-κB signaling and suppress acute phase response genes such as serum amyloid protein A3 (Saa3) via a mechanism that does not require binding of AHR to a DRE (Patel et al. 2009).
It is informative to consider whether the changes in mouse hepatic gene expression observed here are also seen with orthologous rat genes in recent microarray studies. Compared to Long-Evans (Turku A/B) rats (L-E), which express a wild-type AHR and are sensitive to TCDD, Han/Wistar (Kuopio) rats (H/W) are extraordinarily resistant to acute TCDD lethality and express a variant AHR with a large deletion in the transactivation domain. Basal hepatic mRNA levels for POR, SAP, SOCS3, GHR, JAK2, STAT5a/b, and CIS did not differ by AHR genotype in a study comparing two rat strains with the variant AHR (H/W and Line-A) versus three rat strains with wild-type AHR (L-E, Line-C, and Sprague-Dawley) (Boutros et al. 2011a). The suppression of SAP and GHR mRNA by TCDD in mice are responses that are conserved in rats (Boutros et al. 2008), occur in selected rat strains bearing the variant or wild-type AHR (Moffat et al. 2010; Yao et al. 2012), and persist for up to 10 days after TCDD exposure (Boutros et al. 2011b). Microarray studies suggest that TCDD treatment in rats does not alter hepatic mRNA levels for SOCS3, JAK2, STAT5a/b, and CIS (Boutros et al. 2008; Moffat et al. 2010; Boutros et al. 2011b; Yao et al. 2012), providing examples of both inter-species similarity (in the case of SOCS3 and CIS) and difference (in the case of JAK2 and STAT5a/b).
In conclusion, acute exposure to a high dose of TCDD suppresses mouse hepatic Cyp2d9 and Mup2, two genes regulated by pulsatile GH in a STAT5b-dependent manner, and these responses are accompanied by decreased mRNA levels for key components of the GHR-JAK2-STAT5b signaling cascade. These actions of TCDD are AHR-dependent and not accompanied by induction of selected hepatic inflammatory markers. Combined with previous work using MC as a readily metabolized AHR agonist (Nukaya et al. 2004; Lee et al. 2006), this study using TCDD and the Ahr −/− mouse model suggests that AHR activation per se leads to dysregulation of hepatic GH signaling components and suppression of some, but not all, STAT5b target genes.
Acknowledgments
We thank Dr. Allan B. Okey (University of Toronto) for providing the mouse hepatic RNA samples utilized in this study. We thank Dr. Graham R. Robertson (University of Sydney) for helpful discussions regarding hepatic inflammatory markers.
Sources of funding
This work was supported by the Canadian Institutes of Health Research (MOP-93759 to DSR).
References
- Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol Endocrinol. 2004;18(3):747–760. doi: 10.1210/me.2003-0138. [DOI] [PubMed] [Google Scholar]
- Bhathena A, Lee C, Riddick DS. Suppression of cytochrome P4502C11 by aromatic hydrocarbons: mechanistic insights from studies of the 5′-flanking region of the CYP2C11 gene. Drug Metab Dispos. 2002;30(12):1385–1392. doi: 10.1124/dmd.30.12.1385. [DOI] [PubMed] [Google Scholar]
- Boutros PC, Moffat ID, Okey AB, Pohjanvirta R. mRNA levels in control rat liver display strain-specific, hereditary, and AHR-dependent components. PLoS One. 2011a;6(7):e18337. doi: 10.1371/journal.pone.0018337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros PC, Yan R, Moffat ID, Pohjanvirta R, Okey AB. Transcriptomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in liver: comparison of rat and mouse. BMC Genomics. 2008;9(1):419. doi: 10.1186/1471-2164-9-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros PC, Yao CQ, Watson JD, Wu AH, Moffat ID, Prokopec SD, Smith AB, Okey AB, Pohjanvirta R. Hepatic transcriptomic responses to TCDD in dioxin-sensitive and dioxin-resistant rats during the onset of toxicity. Toxicol Appl Pharmacol. 2011b;251(2):119–129. doi: 10.1016/j.taap.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Charles KA, Rivory LP, Brown SL, Liddle C, Clarke SJ, Robertson GR. Transcriptional repression of hepatic cytochrome P450 3A4 gene in the presence of cancer. Clin Cancer Res. 2006;12(24):7492–7497. doi: 10.1158/1078-0432.CCR-06-0023. [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20(6):1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- Davey HW, Park SH, Grattan DR, McLachlan MJ, Waxman DJ. STAT5b-deficient mice are growth hormone pulse-resistant: role of STAT5b in sex-specific liver P450 expression. J Biol Chem. 1999;274(50):35331–35336. doi: 10.1074/jbc.274.50.35331. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- Hollingshead BD, Beischlag TV, Dinatale BC, Ramadoss P, Perdew GH. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 2008;68(10):3609–3617. doi: 10.1158/0008-5472.CAN-07-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito SJ, Chen C, Satoh J, Yim SH, Gonzalez FJ. Dietary phytochemicals regulate wholebody CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest. 2007;117(7):1940–1950. doi: 10.1172/JCI31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarukamjorn K, Sakuma T, Jaruchotikamol A, Ishino Y, Oguro M, Nemoto N. Modified expression of cytochrome P450 mRNAs by growth hormone in mouse liver. Toxicology. 2006;219(1–3):97–105. doi: 10.1016/j.tox.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Jones EJ, Riddick DS. Regulation of constitutive rat hepatic cytochromes P450 by 3-methylcholanthrene. Xenobiotica. 1996;26(10):995–1012. doi: 10.3109/00498259609167418. [DOI] [PubMed] [Google Scholar]
- Kondraganti SR, Muthiah K, Jiang WW, Barrios R, Moorthy B. Effects of 3-methylcholanthrene on gene expression profiling in the rat using cDNA microarray analyses. Chem Res Toxicol. 2005;18(11):1634–1641. doi: 10.1021/tx050085n. [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci USA. 2000;97(19):10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman T, Waxman DJ. Role of the cytokine-induced SH2 domain-containing protein CIS in growth hormone receptor internalization. J Biol Chem. 2005;280(45):37471–37480. doi: 10.1074/jbc.M504125200. [DOI] [PubMed] [Google Scholar]
- Lee C, Riddick DS. Transcriptional suppression of cytochrome P4502C11 gene expression by 3-methylcholanthrene. Biochem Pharmacol. 2000;59(11):1417–1423. doi: 10.1016/s0006-2952(00)00249-5. [DOI] [PubMed] [Google Scholar]
- Lee C, Hutson JR, Tzau VKF, Riddick DS. Regulation of constitutive mouse hepatic cytochromes P450 and growth hormone signaling components by 3-methyl-cholanthrene. Drug Metab Dispos. 2006;34(9):1530–1538. doi: 10.1124/dmd.106.009936. [DOI] [PubMed] [Google Scholar]
- Li W, Vogel CF, Wu D, Matsumura F. Non-genomic action of TCDD to induce inflammatory responses in HepG2 human hepatoma cells and in liver of C57BL/6J mice. Biol Chem. 2010;391(10):1205–1219. doi: 10.1515/BC.2010.126. [DOI] [PubMed] [Google Scholar]
- Mathieu MC, Lapierre I, Brault K, Raymond M. Aromatic hydrocarbon receptor (AhR)•AhR nuclear translocator- and p53-mediated induction of the murine multidrug resistance Mdr1 gene by 3-methylcholanthrene and benzo[a]pyrene in hepatoma cells. J Biol Chem. 2001;276(7):4819–4827. doi: 10.1074/jbc.M008495200. [DOI] [PubMed] [Google Scholar]
- Moffat ID, Boutros PC, Chen H, Okey AB, Pohjanvirta R. Aryl hydrocarbon receptor (AHR)-regulated transcriptomic changes in rats sensitive or resistant to major dioxin toxicities. BMC Genomics. 2010;11(1):263. doi: 10.1186/1471-2164-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, Charles KA, Clarke SJ, Kacevska M, Liddle C, Richardson TA, Sharma R, Sinal CJ. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36(2):205–216. doi: 10.1124/dmd.107.018747. [DOI] [PubMed] [Google Scholar]
- Muguruma M, Nishimura J, Jin M, Kashida Y, Moto M, Takahashi M, Yokouchi Y, Mitsumori K. Molecular pathological analysis for determining the possible mechanism of piperonyl butoxide-induced hepatocarcinogenesis in mice. Toxicology. 2006;228(2–3):178–187. doi: 10.1016/j.tox.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21(1):102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukaya M, Takahashi Y, Gonzalez FJ, Kamataki T. Aryl hydrocarbon receptor-mediated suppression of GH receptor and Janus kinase 2 expression in mice. FEBS Lett. 2004;558(1–3):96–100. doi: 10.1016/S0014-5793(03)01528-X. [DOI] [PubMed] [Google Scholar]
- Pansoy A, Ahmed S, Valen E, Sandelin A, Matthews J. 3-Methylcholanthrene induces differential recruitment of aryl hydrocarbon receptor to human promoters. Toxicol Sci. 2010;117(1):90–100. doi: 10.1093/toxsci/kfq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Waxman DJ. Inhibitory cross-talk between STAT5b and liver nuclear factor HNF3β: impact on the regulation of growth hormone pulse-stimulated, male-specific liver cytochrome P-450 gene expression. J Biol Chem. 2001;276(46):43031–43039. doi: 10.1074/jbc.M107597200. [DOI] [PubMed] [Google Scholar]
- Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest. 2009;89(6):695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev. 1994;46 (4):483–549. [PubMed] [Google Scholar]
- Poland A, Glover E. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin, a potent inducer of aryl hydrocarbon hydroxylase, with 3-methylcholanthrene. Mol Pharmacol. 1974;10:349–359. [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Reddy GR, Pushpanathan MJ, Ransom RF, Holzman LB, Brosius FC, 3rd, Diakonova M, Mathieson P, Saleem MA, List EO, Kopchick JJ, Frank SJ, Menon RK. Identification of the glomerular podocyte as a target for growth hormone action. Endocrinology. 2007;148(5):2045–2055. doi: 10.1210/en.2006-1285. [DOI] [PubMed] [Google Scholar]
- Riddick DS, Huang Y, Harper PA, Okey AB. 2,3,7,8-Tetrachlorodibenzo-p-dioxin versus 3-methylcholanthrene: comparative studies of Ah receptor binding, transformation, and induction of Cyp1a1. J Biol Chem. 1994;269(16):12118–12128. [PubMed] [Google Scholar]
- Riddick DS, Lee C, Bhathena A, Timsit YE. The 2001 Veylien Henderson Award of the Society of Toxicology of Canada. Positive and negative transcriptional regulation of cytochromes P450 by polycyclic aromatic hydrocarbons. Can J Physiol Pharmacol. 2003;81(1):59–77. doi: 10.1139/y03-003. [DOI] [PubMed] [Google Scholar]
- Riddick DS, Lee C, Bhathena A, Timsit YE, Cheng PY, Morgan ET, Prough RA, Ripp SL, Michael Miller KK, Jahan A, Chiang JYL. Transcriptional suppression of cytochrome P450 genes by endogenous and exogenous chemicals. Drug Metab Dispos. 2004;32(4):367–375. doi: 10.1124/dmd.32.4.367. [DOI] [PubMed] [Google Scholar]
- Safa B, Lee C, Riddick DS. Role of the aromatic hydrocarbon receptor in the suppression of cytochrome P-450 2C11 by polycyclic aromatic hydrocarbons. Toxicol Lett. 1997;90(2–3):163–175. doi: 10.1016/s0378-4274(96)03843-x. [DOI] [PubMed] [Google Scholar]
- Sakuma T, Endo Y, Mashino M, Kuroiwa M, Ohara A, Jarukamjorn K, Nemoto N. Regulation of the expression of two female-predominant CYP3A mRNAs (CYP3A41 and CYP3A44) in mouse liver by sex and growth hormones. Arch Biochem Biophys. 2002;404(2):234–242. doi: 10.1016/s0003-9861(02)00329-6. [DOI] [PubMed] [Google Scholar]
- Sawaya RM, Riddick DS. Cytochrome P450 2C11 5′-flanking region and promoter: regulation by aromatic hydrocarbons in vitro. Toxicology. 2008a;248(2–3):104–112. doi: 10.1016/j.tox.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Sawaya RM, Riddick DS. Cytochrome P450 2C11 5′-flanking region and promoter mediate in vivo suppression by 3-methylcholanthrene. Drug Metab Dispos. 2008b;36 (9):1803–1811. doi: 10.1124/dmd.108.020966. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GHT, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA. 1996;93(13):6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Inoue K, Suzuki Y, Kawai T, Azuma E, Nakajima T, Shindo M, Kurose K, Sugie A, Yamagishi Y, Fujii-Kuriyama Y, Hashimoto M. Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice. Carcinogenesis. 2002;23(7):1199–1207. doi: 10.1093/carcin/23.7.1199. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2000;97(2):779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Davies R, Dalton TP, Miller ML, Judah D, Riley J, Gant T, Nebert DW. Intrinsic hepatic phenotype associated with the Cyp1a2 gene as shown by cDNA expression microarray analysis of the knockout mouse. EHP Toxicogenomics. 2003;111(1T):45–51. [PubMed] [Google Scholar]
- Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol. 2011;23(2):99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol Pharmacol. 2006;69(1):140–153. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- Timsit YE, Riddick DS. Interference with growth hormone stimulation of hepatic cytochrome P4502C11 expression in hypophysectomized male rats by 3-methyl-cholanthrene. Toxicol Appl Pharmacol. 2000;163(2):105–114. doi: 10.1006/taap.1999.8862. [DOI] [PubMed] [Google Scholar]
- Timsit YE, Riddick DS. Stimulation of hepatic signal transducer and activator of transcription 5b by GH is not altered by 3-methylcholanthrene. Endocrinology. 2002;143(9):3284–3294. doi: 10.1210/en.2002-220212. [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA. 1997;94(14):7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JP. Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Wiwi CA, Gupte M, Waxman DJ. Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4α-deficient mice. Mol Endocrinol. 2004;18(8):1975–1987. doi: 10.1210/me.2004-0129. [DOI] [PubMed] [Google Scholar]
- Yanagiba Y, Ito Y, Kamijima M, Gonzalez FJ, Nakajima T. Octachlorostyrene induces cytochrome P450, UDP-glucuronosyltransferase, and sulfotransferase via the aryl hydrocarbon receptor and constitutive androstane receptor. Toxicol Sci. 2009;111(1):19–26. doi: 10.1093/toxsci/kfp130. [DOI] [PubMed] [Google Scholar]
- Yanagimoto T, Itoh S, Sawada M, Kamataki T. Mouse cytochrome P450 (Cyp3a11): predominant expression in liver and capacity to activate aflatoxin B1. Arch Biochem Biophys. 1997;340(2):215–218. doi: 10.1006/abbi.1997.9900. [DOI] [PubMed] [Google Scholar]
- Yang XP, Schaper F, Teubner A, Lammert F, Heinrich PC, Matern S, Siewert E. Interleukin-6 plays a crucial role in the hepatic expression of SOCS3 during acute inflammatory processes in vivo. J Hepatol. 2005;43(4):704–710. doi: 10.1016/j.jhep.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Yao CQ, Prokopec SD, Watson JD, Pang R, P’ng C, Chong LC, Harding NJ, Pohjanvirta R, Okey AB, Boutros PC. Inter-strain heterogeneity in rat hepatic transcriptomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Appl Pharmacol. 2012;260(2):135–145. doi: 10.1016/j.taap.2012.02.001. [DOI] [PubMed] [Google Scholar]