Obesity / excessive body weight leads to morbidity known collectively as metabolic syndrome and afflicts perhaps 1 billion people worldwide.[1] Reduction in caloric input continues to be the most effective means of treatment with approaches ranging from simple dieting to the extreme of bariatric surgery.[2] Regulating the desire to eat through pharmaceutical intervention is a lofty goal with a troubled history mostly because of an inability to separate regulation of satiety from the body’s fundamental reward system. The end product, depending on mechanism, is that potential therapeutic agents become drugs of abuse or lead to depression.[2b]
The profound role ghrelin plays in feelings of satiety and that it is considerably upstream of the body’s reward system has lead to intense interest in its biochemical pathway. Logically, therapeutic intervention in ghrelin regulation has attracted the interest of the pharmaceutical industry in the pursuit of anti-obesity agents.[2b, 3]
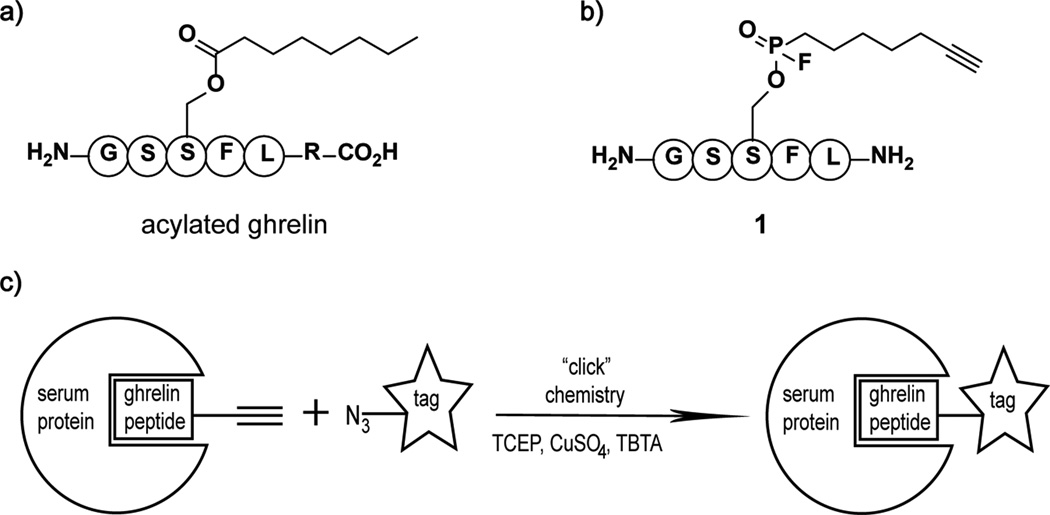
Ghrelin is an appetite-stimulating hormone secreted from endocrine cells in the stomach. Ghrelin was first identified in 1999 by Kojima et al. as the endogenous ligand for the growth-hormone receptor 1a (GHSR1a), a G-coupled receptor.[4] It has been well established that this potent growth-hormone secretagogue plays an important metabolic role in regulating food intake and energy homeostasis[5]; more recently, ghrelin has been implicated in the regulation of glucose metabolism[6]. Ghrelin is synthesized as an 117 amino acid polypeptide (preproghrelin), which is translocated through the endoplasmic reticulum membrane followed by proteolytic cleavage of the N-terminal 23 amino acid signal sequence to produce proghrelin (94 amino acids). Subsequent posttranslational modification events include cleavage of proghrelin after residue Arg28 and octanoylation of residue Ser3 with an eight carbon fatty acid chain to generate the mature ghrelin protein, 28 amino acids in length. Ghrelin is the only known protein to contain an n-octanoyl moiety and importantly, this posttranslational modification is essential for binding to and activation of GHSRla (Figure 1a).
Figure 1.
a. Mature ghrelin (R = 23 amino acids). b. Ghrelin activity-based probe, phosphonofluoridate 1. c. Cartoon depicting labeling of serum proteins via a copper(I)-catalyzed azide-alkyne cycloaddition reaction.
Under fasting conditions ghrelin production is increased and correlates with a higher level of circulating ghrelin in serum; conversely, serum levels of ghrelin decrease immediately after food intake. The enzyme responsible for post-translationally acylating ghrelin and thereby activating it, was recently identified as the acyltransferase enzyme ghrelin O-acyltransferase.[7] However, enzymes and pathways involved in ghrelin deacylation and proteolysis are poorly understood.
Ghrelin has been reported to be deacylated and proteolyzed into smaller peptide fragments by serum and tissue homogenates.[8] Two enzymes have been proposed to participate in the deacylation of ghrelin in serum, liver carboxylesterase (rat serum) and butyrylcholinesterase (human serum).[8] Additionally, acyl-protein thioesterase 1/lysophospholipase I has been isolated from both rat stomach homogenates and bovine serum; a recombinant form of this enzyme deacylates ghrelin in vitro.[9]
The above studies relied on the identification of potential deacylating enzymes from analytically separated serum or homogenate fractions followed by analysis of proteins present in the active samples. Our laboratory pursued a different approach by use of a mechanism-based probe that could irreversibly bind to ghrelin deacylating enzymes within serum directly. Upon conjugation of a reporter tag to the now enzyme-bound probe, deacylating enzymes could be identified via proteomics.
Within serum, inactivation of ghrelin is thought to occur predominately by way of deacylation. In addition, the work of De Vriese et al. in which ghrelin serum stability was monitored in the presence of a variety of known hydrolase inhibitors strongly implicated serine hydrolases as the dominant participants in ghrelin degradation.[8] The serine hydrolases are a large family of enzymes representing more than 2% of the eukaryotic proteome that share a common chemical mechanism involving an activated serine as part of a catalytic diad or triad. These enzymes hydrolyze ester and amine bonds in small-molecule and protein substrates. Within the serine hydrolase family are carboxylesterases and cholinesterases.
By virtue of their catalytic mechanism, serine hydrolases are susceptible to the fluorophosphonate warhead when it is incorporated within a chemical structure with sufficient recognition elements for the target enzyme to attempt to hydrolyze it. Thus fluorophosphonates have found wide use in the design of irreversible inhibitors of serine hydrolases and has become a valuable tool in the production of activity-based probes for this family of enzymes.[10] By use of this approach, we generated a “bait” ghrelin-like molecule containing a fluorophosphonate warhead to capture serum-based serine hydrolase enzymes participating in the deacylation of circulating ghrelin.
To design an appropriate ghrelin analog, we noted that the amino acid sequence of ghrelin is highly conserved among mammals and the first 10 amino acids are identical. Short truncated peptides containing the first 4 to 5 residues of ghrelin are potent agonist of GHSR1a with efficiencies similar to the full length protein; the tetrapeptide Gly-Ser-Ser(n-octanoyl)-Phe is considered to be the active core of ghrelin.[11] However, while the activity of the tetra- and penta- ghrelin peptides are essentially equivalent, binding affinity of the tetrapeptide is increased approximately 16-fold by the addition of the fifth residue Leu.[11] Another important binding interaction occurs between GHSR1a and the octanoyl chain of ghrelin which is accommodated in a hydrophobic pocket of the receptor.[12] Therefore, ghrelin probe 1 was designed as a short peptide containing the first 5 amino acids of ghrelin with a fluorophosphonate warhead at Ser3 in place of the physiological n-octanoyl modification (Figure 1b). A key feature of the probe is the conservation of the hydrocarbon chain length within 1 which may also be important for the binding of and selectivity toward ghrelin deacylating enzymes. Lastly, an alkyne group at the end of the hydrocarbon chain allows for the conjugation of an azide reporter tag via a copper(I)-catalyzed azide-alkyne cycloaddition reaction or "click chemistry" for subsequent analysis and protein identification (Figure 1c).[13]
Chemical synthesis of 1 was achieved as follows (Scheme 1). Primary alcohol 2 was prepared from 2-heptyn-1-ol by the procedure of O’Doherty et al.,[14] followed by tosylation to yield 3.[15] The tosyl group of 3 was displaced with LiBr to yield the intermediate bromide, which was without further purification heated with tris (trimethylsilyl) phosphite to yield phosphonic acid 4 after trimethylsilyl hydrolysis. The TBDMS serine protecting group of solid-phase resin attached to peptide 5 was selectively removed using TBAF, followed by Mitsunobu coupling of partially deprotected peptide 6 with phosphonic acid 4 to give resin-attached phosphonic acid modified peptide 7. Phosphonic acid 7 was then converted to the phosphonofluoridate with DAST, followed by TFA mediated resin cleavage to give crude phosphonofluoridate modified peptide 1 after peptide precipitation by diethyl ether. While phosphonofluoridate 1 could be purified by preparative HPLC, all attempts to concentrate the HPLC fractions containing phosphonofluoridate product resulted in hydrolysis to the corresponding phosphonic acid modified peptide. As a result, crude phosphonofluoridate 1 was used for all labeling experiments.
Scheme 1.
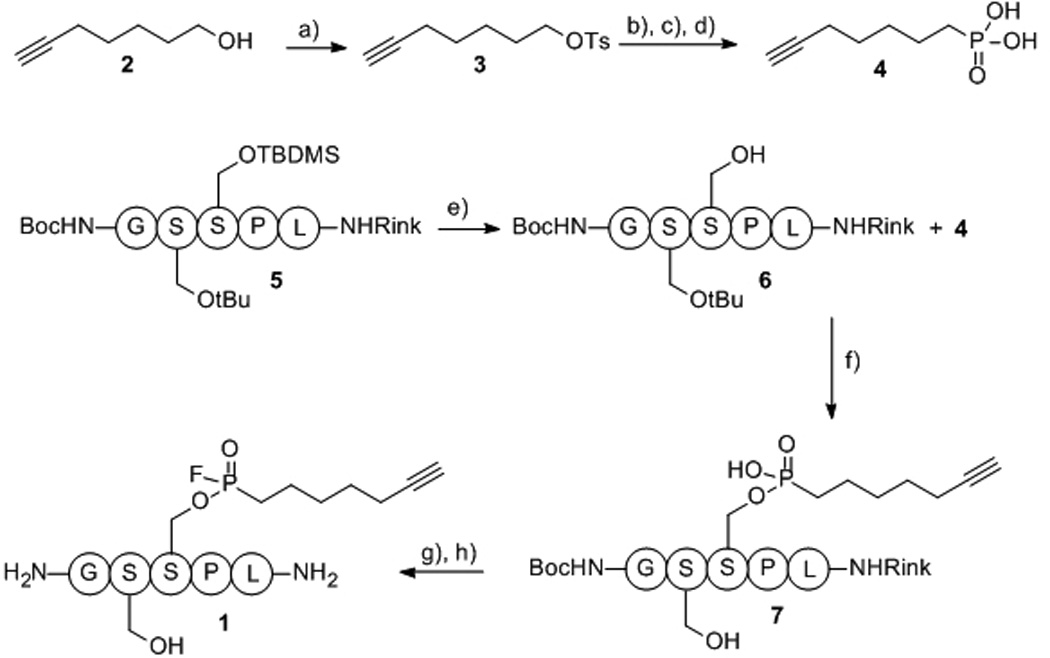
Synthesis of phosphonofluoridate 1. Reagents and conditions: a) TsCl (1.1 equiv), NEt3 (2.1 equiv), DMAP (catalytic), CH2Cl2 to rt, 16 hr 80%; b) LiBr (4 equiv), acetone, 14 hr; c) P(OSiMe3)3 (1.0 equiv), Δ, 8 hr; d) H2O, rt, 4 hr, 22% (2 steps); e) 0.1 M TBAF, DMF, rt, 2 × 15 min; f) 3 (5.5 equiv), DIC (12 equiv), DIPEA (5 equiv), DMAP (5.5 equiv), DMF, rt, 2 days; g) DAST (4.0 equiv), CH2Cl2, rt, 2 hr; h) 95% TFA, 2.5% TIPS, 2.5% H2O.
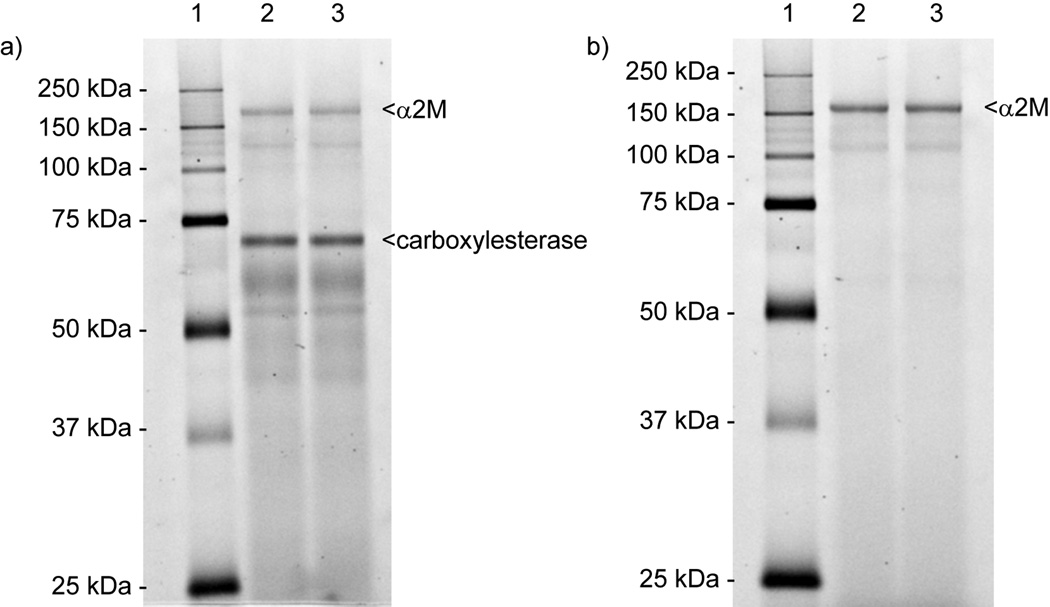
For the enzyme capture phase, probe 1 was incubated with albumin depleted rat serum for 90 min at 37 °C to allow cross-linking between the mechanism-based probe and reactive serum enzymes. The samples were then subjected to click chemistry with a rhodamine-azide tag (RhN3) and subsequently, labeled proteins within the serum sample were separated by SDS-PAGE and visualized by fluorescence imaging (Figure 2a). [13b] Two dominate bands were excised, in-gel trypsin digested, and the resulting peptide fragments were analyzed by nanoLC-MS/MS. The results were searched against protein databases and the two labeled proteins were putatively identified.
Figure 2.
Protein labeling with P-F probe 1. Serum samples (a) or purified human α2M (b) were incubated with 1 and reacted with RhN3 using click chemistry after which the reactions were analyzed by SDS-PAGE and visualized by in-gel fluorescence scanning. Lanes: 1, molecular weight marker; 2 and 3, duplicate labeling reactions.
One of the proteins identified was rat liver carboxylesterase (E.C.3.1.1.1). Notably, this enzyme has been proposed previously to play a major role in the deacylation of ghrelin in rat serum.[8] Studies conducted with commercially purified porcine liver carboxylesterase validated the ability of this enzyme to catalyze the deacylation reaction.[8] The identification of liver carboxylesterase in our study illustrated the proof of concept and we were encouraged by this positive result. The second protein identified was α2-macroglobulin (α2M), a large homotetrameric plasma glycoprotein with a molecular weight of approximately 725 kDa for the human form.[16] α2M can inhibit all mechanistic classes of proteinases through a serpin-like mechanism involving proteinase-mediated proteolysis of a "bait" region within α2M, followed by immediate and major conformational changes of α2M, ultimately "trapping" the enzyme.[17] This proteinase-induced conformational change then triggers the exposure of binding sites for proteins such as cytokines, growth factors, and hormones.[17] Additionally, the hidden receptor binding domain of α2M is revealed, leading to receptor-mediated endocytosis and rapid clearance of the complex from circulation.[17] In general, α2M plays an important role in the regulation of numerous biological processes.
The capture of α2M by probe 1 from rat serum (Figure 2a) suggests that α2M possesses a ghrelin recognition site containing an appropriately positioned reactive serine. Yet, to our knowledge such hydrolytic activity has never been identified for α2M. To further delineate this previously undocumented esterolytic activity, we employed our HPLC-based ghrelin hydrolase assay previously reported.[18] The substrate for this assay is full length acylated ghrelin containing the fluorophore 7-methoxycoumarin-4-acetic acid (MCA) at the C-terminus of the protein. Solutions containing purified human α2M (2 µM) and low micromolar concentrations of the ghrelin-MCA substrate demonstrated that α2M can indeed catalyze the deacylation of ghrelin. To further characterize this reaction, steady-state kinetic studies were conducted with α2M and varying concentrations of ghrelin-MCA. Well-behaved Michaelis-Menten kinetics were observed with α2M displaying a Km of 24 ± 3 µM and kcat of 2.3 × 10−2 ± 0.1 × 10−2 min−1. Probe 1 used to initially isolate the α2M protein from labeling studies was further investigated as an inhibitor of this reaction. Studies were conducted at approximately Km of the ghrelin-MCA with varying concentrations of 1; a dose-dependent inhibition response was observed thereby verifying that this fluorophosphonate pentapeptide probe was an effective mimic of the acylated ghrelin substrate. Additionally, labeling studies containing purified human α2M and probe 1 further validated the ability of the human protein to react with the acylated ghrelin mimic (Figure 2b).
A number of protease inhibitors known to inhibit hydrolytic enzymes in serum were evaluated.[8] The serine hydrolase mechanism-based probes phenylmethylsulfonyl fluoride (PMSF), 4-(aminophenyl) methanesulfonyl fluoride (p-APMSF), and bis-p-nitrophenyl-phosphate (BNPP) were evaluated as inhibitors of α2M's deacylating catalytic activity. Other compounds such as EDTA, eserine, and NaF were not expected to inhibit α2M's deacylating activity, thereby providing additional evidence that α2M is a serine hydrolase. All compounds were tested at approximately Km of substrate. Only two compounds displayed significant inhibition at a concentration of 1 mM or less following pre-incubation for 30 min. BNPP, a carboxylesterase inhibitor, showed 40 % inhibition at 1 mM and 0 % inhibition at 100 µM. p-APMSF, a serine protease inhibitor, showed 100% inhibition at 50 µM and 80% inhibition at 5 µM, making p-APMSF the most potent inhibitor of the screen. p-APMSF is an irreversible inhibitor mechanistically analogous to fluorophosphonate compounds. While it was not explicitly characterized, we can place a lower limit on the p-APMSF α2M specificity constant of 470 M−1 s−1 (supporting information).
Irreversible inhibitors will titrate the number of catalytic sites present in an enzyme sample making them a reliable method for quantitating the amount of active enzyme present in a sample.[19] The instability of ghrelin probe 1 made its precise quantitation difficult therefore we selected p-APMSF as a titrant to identify the number of catalytic sites within the α2M tetramer. The protein α2M is a homotetramer and in principle each subunit could contain a functional active site. Titration experiments conducted at 2 µM tetramer/8 µM monomer of α2M and varying concentrations of p-APMSF (0 – 10 µM) are illustrated in Figure 3. Extrapolation of velocity to the x-intercept yields an active site concentration of ~ 6 µM. Within experimental uncertainty, there are 4 active sites per α2M tetramer.
Figure 3.
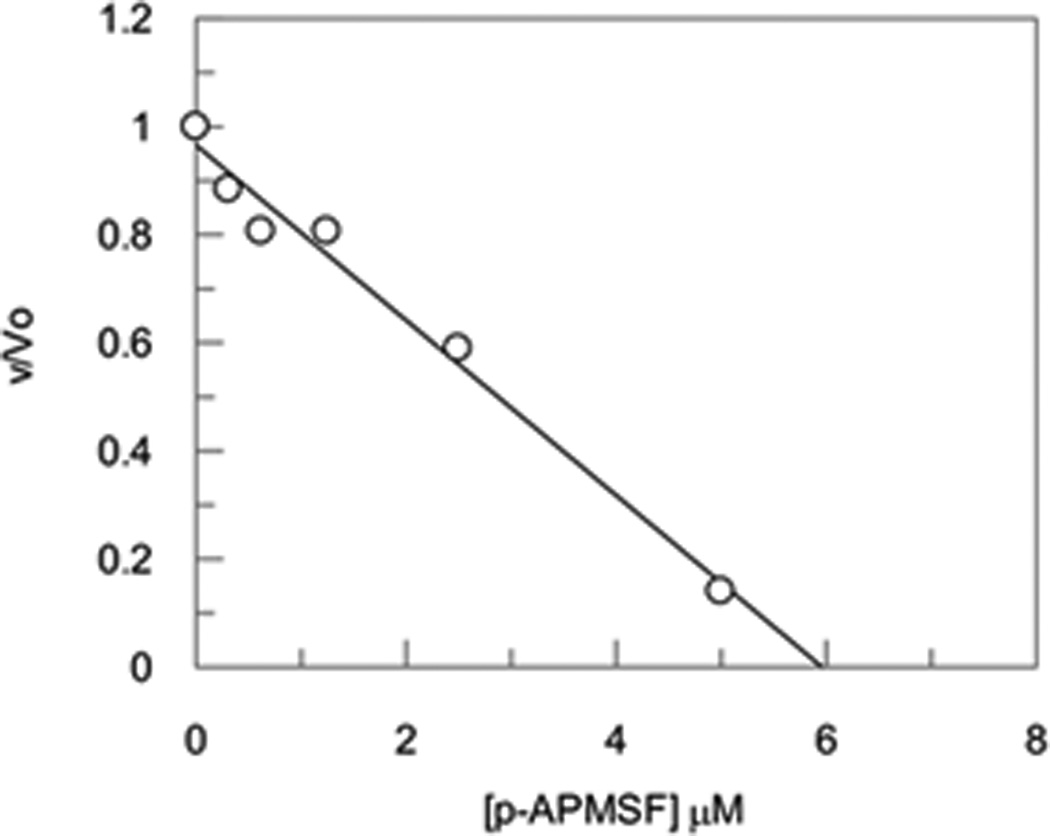
Titration studies with the inhibitor p-APMSF and α2M.
Could α2M ghrelin esterase activity be of physiologically importance? Ghrelin’s plasma half life has been estimated to be 240 minutes affording a first order rate constant of 0.003 min−1 thus its physiological concentration must be low relative to the Km’s of any preferred substrates for reported candidate esterases.[8] As such, each esterase is operating under substrate limiting (1st order) conditions. The overall half-life of ghrelin then is governed by the sum of the concentrations of each participating enzyme multiplied by their (kcat/Km)ghrelin. The concentration of plasma α2M has been reported in the range of 0.25 g/l to 2 g/l. Taking a midrange value of 1 g/l gives a plasma concentration of 1.4 µM, which when coupled with the ghrelin specificity constants vide supra, gives a plasma first order rate constant of 0.0014 min−1 which accounts for one half the total rate. This calculation, thus, places α2M as an important participant in ghrelin metabolism.
Mechanism-based probes offer an objective approach to the identification of physiologically relevant enzyme activities. The method confirmed the alternative approach of De Vriese et al. by capturing carboxylesterase.[8] On the other hand, the capture of α2M by ghrelin probe 1 and the subsequent discovery of its ghrelin esterase activity were unanticipated and unprecedented, as there are no prior reports of α2M possessing esterase activity. The studies reported here constitute one step towards the goal of better understanding nutrient-hormone interactions contributing to metabolic disease states.
Supplementary Material
Footnotes
This work was supported by The Skaggs Institute for Chemical Biology and the National Institute of Health under grant number R01-DK092191.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Lisa M. Eubanks, Departments of Chemistry and Immunology, The Skaggs Institute for Chemical Biology and The Worm Institute for Research and Medicine, The Scripps Research Institute 10550 North Torrey Pines Road, La Jolla CA 92037 (USA)
G. Neil Stowe, Departments of Chemistry and Immunology, The Skaggs Institute for Chemical Biology and The Worm Institute for Research and Medicine, The Scripps Research Institute 10550 North Torrey Pines Road, La Jolla CA 92037 (USA).
Sandra De Lamo Marin, Departments of Chemistry and Immunology, The Skaggs Institute for Chemical Biology and The Worm Institute for Research and Medicine, The Scripps Research Institute 10550 North Torrey Pines Road, La Jolla CA 92037 (USA).
Alexander V. Mayorov, Departments of Chemistry and Immunology, The Skaggs Institute for Chemical Biology and The Worm Institute for Research and Medicine, The Scripps Research Institute 10550 North Torrey Pines Road, La Jolla CA 92037 (USA)
Mark S. Hixon, Department of Discovery Biology Takeda San Diego, Inc. 10410 Science Center Drive, San Diego, CA 92121
Kim D. Janda, Departments of Chemistry and Immunology, The Skaggs Institute for Chemical Biology and The Worm Institute for Research and Medicine, The Scripps Research Institute 10550 North Torrey Pines Road, La Jolla CA 92037 (USA).
References
- 1.Yach D, Stuckler D, Brownell KD. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 2.a Wilson T, Bray GA, Temple NJ, Struble MB. In: Nutrition Guide for Physicians. Bendich A, editor. New York: Humana Press; 2010. pp. 253–274. [Google Scholar]; b Yao F, MacKenzie RG. Pharmaceuticals. 2010;3:3494–3521. [Google Scholar]
- 3.Nieforth KA, Cohen ML. In: Principles of Medicinal Chemistry. 3rd ed. Foye WO, editor. Philadelphia: Lea & Febiger; 1989. pp. 277–309. [Google Scholar]
- 4.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 5.Cheng KC, Li YX, Asakawa A, Inui A. Int J Mol Med. 2010;26:771–778. doi: 10.3892/ijmm_00000524. [DOI] [PubMed] [Google Scholar]
- 6.Heppner KM, Tong J, Kirchner H, Nass R, Tschop MH. Curr Opin Endocrinol Diabetes Obes. 2011;18:50–55. doi: 10.1097/MED.0b013e328341e1d3. [DOI] [PubMed] [Google Scholar]
- 7.a Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]; b Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Proc Natl Acad Sci U S A. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. Endocrinology. 2004;145:4997–5005. doi: 10.1210/en.2004-0569. [DOI] [PubMed] [Google Scholar]
- 9.a Satou M, Nishi Y, Yoh J, Hattori Y, Sugimoto H. Endocrinology. 2010;151:4765–4775. doi: 10.1210/en.2010-0412. [DOI] [PubMed] [Google Scholar]; b Shanado Y, Kometani M, Uchiyama H, Koizumi S, Teno N. Biochem Biophys Res Commun. 2004;325:1487–1494. doi: 10.1016/j.bbrc.2004.10.193. [DOI] [PubMed] [Google Scholar]
- 10.Evans MJ, Cravatt BF. Chem Rev. 2006;106:3279–3301. doi: 10.1021/cr050288g. [DOI] [PubMed] [Google Scholar]
- 11.Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV. J Med Chem. 2000;43:4370–4376. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- 12.Pedretti A, Villa M, Pallavicini M, Valoti E, Vistoli G. J Med Chem. 2006;49:3077–3085. doi: 10.1021/jm058053k. [DOI] [PubMed] [Google Scholar]
- 13.a Best MD. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]; b Speers AE, Cravatt BF. Chem Biol. 2004;11:535–546. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Li M, O'Doherty GA. Org Lett. 2006;8:6087–6090. doi: 10.1021/ol062595u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapp FF, Jr, Srivastava PC, Callahan AP, Cunningham EB, Kabalka GW, Sastry KA. J Med Chem. 1984;27:57–63. doi: 10.1021/jm00367a011. [DOI] [PubMed] [Google Scholar]
- 16.Sottrup-Jensen L, Stepanik TM, Kristensen T, Wierzbicki DM, Jones CM, Lonblad PB, Magnusson S, Petersen TE. J Biol Chem. 1984;259:8318–8327. [PubMed] [Google Scholar]
- 17.Borth W. FASEB J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 18.Mayorov AV, Amara N, Chang JY, Moss JA, Hixon MS, Ruiz DI, Meijler MM, Zorrilla EP, Janda KD. Proc Natl Acad Sci U S A. 2008;105:17487–17492. doi: 10.1073/pnas.0711808105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. Hoboken, NJ: Wiley & Sons; 1975. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.