Abstract
Dendritic cells (DCs) play a key role in the initial infection and cell-to-cell transmission events that occur upon HIV-1 infection. DCs interact closely with CD4+ T cells, the main target of HIV-1 replication. HIV-1 challenged DCs and target CD4+ T cells form a virological synapse that allows highly efficient transmission of HIV-1 to the target CD4+ T cells, in the absence of productive HIV-1 replication in the DCs. Immature and subsets of mature DCs show distinct patterns of HIV-1 replication and cell-to-cell transmission, depending upon the maturation stimulus that is used. The cellular and viral mechanisms that promote formation of the virological synapse have been the subject of intense study and the most recent progress is discussed here. Characterizing the cellular and viral factors that affect DC-mediated cell-to-cell transmission of HIV-1 to CD4+ T cells is vitally important to understanding, and potentially blocking, the initial dissemination of HIV-1 in vivo.
4.1 Dendritic Cell-Mediated HIV-1 Transmission
4.1.1 Immature and Mature DCs and Their Roles in HIV-1 Infection
DCs are important cells in the defense against invading pathogens. DCs act as a bridge between the innate and adaptive immune responses. Immature DCs (iDCs) are present at all mucosal surfaces and come into contact with pathogens, including HIV-1. Once pathogen contact with DCs is established, DCs can undergo maturation and migrate to the lymph node, where they present processed antigens to T cells and B cells, triggering an adaptive immune response to the invading pathogen. Many stimuli can induce maturation of DCs and these can be broadly grouped into pathogenic and immunological factors.
Pathogenic factors that induce DC maturation are factors that are expressed by invading pathogens, referred to as pathogen-associated molecular patterns (PAMPs). Due to the wide range of pathogenic bacteria, viruses, and fungi, PAMPs are specific for groups of pathogens. DCs express a range of receptors for these PAMPs, including toll-like receptors (TLRs) (Kawai and Akira 2010, 2011), a family of molecules in which each member recognizes a specific PAMP. For example, lipopolysaccharide (LPS) is a PAMP expressed by gram-negative bacteria. LPS interacts with TLR4, along with the TLR4 co-receptors MD-2 and CD14, on the cell surface and induces a response to the invading bacteria via a complex signaling cascade (Kumar et al. 2011). LPS stimulation causes DC maturation, leading to increased DC migration, decreased DC endocytosis, and increased expression of co-stimulatory molecules required for interactions with CD4+ T cells on the DCs (Iwasaki and Medzhitov 2004). In the study of HIV-1 interactions with DCs, LPS activation of DCs is important because there is an association between gram-negative bacterial translocation and high levels of LPS in the serum and the systemic immune activation observed in chronic HIV-1 infection (Brenchley et al. 2006). In addition, there is a possibility of coinfection with gram-negative bacteria along with HIV-1 infection (Gringhuis et al. 2010 ; Hernandez et al. 2011 ), which may facilitate HIV-1 spread by enhancing LPS-stimulated maturation of DC and, therefore, DC-mediated HIV-1 transmission to CD4+ T cells.
DCs and other immune cells respond to pathogens by releasing cytokines, chemokines, and other soluble factors into the extracellular milieu. Release of these immunological factors is important for preventing spread of infection within the host, as these molecules can act on surrounding naïve cells to promote immune cell activation or to protect surrounding cells by upregulating cellular factors that restrict pathogen spread. In the case of DCs, some immunological factors lead to DC maturation. For example, type I interferons (IFN) are antiviral cytokines produced as part of the innate immune response to an infection. The two main types of type I IFN are IFNα and IFNβ, both of which can prevent virus dissemination, trigger adaptive immune responses to clear the virus, and protect against reinfection (Stetson and Medzhitov 2006). IFNα can inhibit the replication of HIV-1 in CD4+ T cells, DCs, and macrophages in vitro (Coleman et al. 2011; Goujon and Malim 2010; Poli et al. 1989). IFNα can also inhibit the cell-to-cell transmission of HIV-1 between CD4+ T cells and DC-mediated HIV-1 transmission to CD4+ T cells (Coleman et al. 2011; Vendrame et al. 2009). The type I IFN inhibition of HIV-1 replication in DCs can be relieved by factors such as the Vpx proteins from HIV-2 or certain simian immunodeficiency viruses (SIV) (Pertel et al. 2011), which may allow the identification of type I IFN-inducible HIV-1 restriction factors in DCs. Altogether, these data demonstrate the importance of DCs matured by immunological factors in the prevention of replication and spread of HIV-1.
DCs may also act as important HIV-1 reservoirs and maintain a significant pool of HIV-1 during long-term viral infection. Given the low levels of HIV-1 replication and high levels of DC-mediated transmission of HIV-1 observed in some DC subtypes, it is possible that DC subtypes, particularly those in the lymph node, may act as significant pools of HIV-1 during long-term HIV-1 infection (reviewed in Coleman and Wu 2009).
4.1.2 HIV-1 Proteins Have the Potential to Promote Infection and Transmission Processes
Viruses need to ensure efficient replication and transmission within a host, as there is often a delicate balance between viruses and immune response. Viruses, particularly those with RNA genomes, have limited genomic capacity to encode proteins that can promote viral replication and cell-to-cell transmission. Therefore, viral proteins are often multifunctional and able to promote infection and/or cell-to-cell transmission using many mechanisms.
The HIV-1 genome encodes 15 individual proteins, the structural polyproteins Gag (consisting of matrix, capsid, nucleocapsid, and p6 proteins), Pol (consisting of protease, reverse transcriptase, and integrase proteins), and envelope (Env; consisting of gp120 and gp41); two regulatory proteins (Tat and Rev); and four accessory proteins (Vif, Vpr, Vpu, and Nef). The Gag and Pol polyproteins are cleaved into their constitutive parts during maturation of the virion, making them available to carry out their respective functions upon infection of a new cell. The Env polyprotein is cleaved within the cell and a complex is formed by the association of gp41 and gp120. The Env complexes then form trimers in the endoplasmic reticulum, are heavily glycosylated in the trans -golgi network, and are subsequently transported to the cell surface for incorporation into virions (Earl et al. 1991). On the cell surface, gp41 forms the trans-membrane portion and the gp120 forms the extracellular portion.
HIV-1 structural proteins and some accessory proteins are present in the virion and have the potential to interact with cellular factors and promote HIV-1 transmission during the initial stages of infection. Furthermore, HIV-1 proteins produced during replication may promote cell-to-cell transmission after the initial infection. Viral proteins may also act in both DC-mediated cis- and trans-infection; for example, Env is present on the surface of the virion and is able to interact directly with HIV-1 receptors and other cell surface molecules to promote cell-to-cell transmission of the virus (van Montfort et al. 2011) and newly synthesized Env can promote CD4+ T cell-mediated cell-to-cell transmission (Jolly et al. 2004).
4.1.3 Capture of HIV-1 by DCs
The first step in the DC-mediated transmission of HIV-1 to CD4+ T cells is the capture of HIV-1 from the extracellular milieu. iDCs express the HIV-1 receptor CD4 and low levels of the HIV-1 co-receptors CCR5 and CXCR4. Expression levels of CD4 are not significantly affected by DC maturation and small differences in expression of CCR5 and CXCR4 do not correlate with HIV-1 binding or DC-mediated HIV-1 transmission (Dong et al. 2007; Sanders et al. 2002). It has been shown that LPS-matured DCs capture significantly more HIV-1 than iDCs, without any significant upregulation of CD4 or CCR5 (Dong et al. 2007; Izquierdo-Useros et al. 2007). Other cellular factors that affect HIV-1 capture and binding to DCs will be discussed later.
LPS-matured DCs capture significantly more HIV-1 than iDCs (Dong et al. 2007; Izquierdo-Useros et al. 2007; Wang et al. 2007b), suggesting that mature DCs (mDCs) can act as a significant pool of infectious virus during the initial stages of infection. HIV-1 capture in LPS-matured mDCs, unlike in iDCs, has been shown to occur independently of HIV-1 gp120 engagement of CD4 and C-type lectin receptors (Hanley et al. 2010; Izquierdo-Useros et al. 2007). Studies using three-dimensional microscopy and real-time imaging of HIV-1 transfer from LPS-matured mDCs to CD4+ T cells analyzed both cell-free and cell-associated capture of HIV-1 virus-like particles (VLPs) by mDCs. Cell-free HIV-1 capture was found to occur in three distinct phases: HIV-1 VLPs randomly bind to the mDC plasma membrane, then “surf” to a polarized region of the cell, and are eventually concentrated into a distinct, invaginated pocket on the DC surface (Izquierdo-Useros et al. 2011). Coculture of mDCs with an HIV-1-producing T-cell line (MOLT-4) or HIV-1-infected primary CD4+ T cells demonstrates that HIV-1 transfer from infected cells to mDCs is more efficient than cell-free HIV-1 capture by mDCs (Izquierdo-Useros et al. 2011). These results suggest that LPS maturation of DCs enhances capture of HIV-1 released from infected CD4+ T cells and that this could promote spread of HIV-1 within the host and subsequently promote viral pathogenesis.
4.1.4 Mechanisms of DC-Mediated Spread of HIV-1: Cis- and Trans-infection
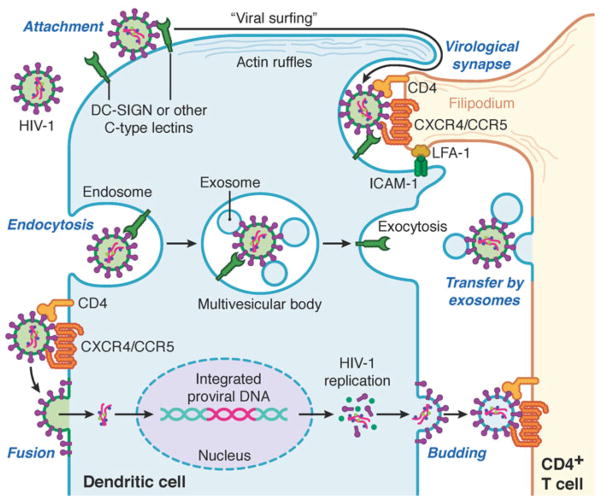
During HIV-1 replication, there are two major mechanisms of viral transmission between cells (reviewed in Wu and KewalRamani 2006). First, HIV-1 can infect target cells, and productively replicate and produce progeny virions that are released to infect new target cells; this is cis-infection. Second, the virus is retained at or near the cell surface of a donor cell and transmitted to a different type of target cell via the close contact and formation of a virological synapse (VS) or via the exosome secretion pathway; this is trans-infection. The proposed mechanisms of DC-mediated HIV-1 transmission are summarized in Fig. 4.1.
Fig. 4.1.
Summary of the mechanisms of DC-mediated HIV-1 transmission to CD4+ T cells. Incoming HIV-1 (virions with green membrane) can be transmitted from DCs to CD4+ T cells by three distinct mechanisms. First: Trans-infection via the virological synapse: HIV-1 can bind to DC-SIGN on the DC surface, “surf” along actin ruffles, and become polarized to a pocket structure on the cell surface. When DC contact with a CD4+ T cell is established, the virological synapse is formed and stabilized through interactions between ICAM-1 and LFA-1. The CD4+ T cell extends a filipodium, via actin cytoskeleton rearrangements, into the pocket on the DC surface to capture the HIV-1. Second: Trans-infection via the exosome secretion pathway: Endocytosed HIV-1 can converge with the exosome secretion pathway in the multi-vesicular bodies and HIV-1 can be released in association with exosomes to infect nearby CD4+ T cells. Third: Cis-infection: HIV-1 can enter the DC by fusion and undergo its complete replication cycle and newly formed infectious HIV-1 (virions with blue membrane) bud from the DC and is able to infect nearby CD4+ T cells. DC-SIGN dendritic cell-specific intercellular adhesion molecule 3-non-grabbing integrin, ICAM-1 intercellular adhesion molecule-1, LFA-1 leukocyte function-associated molecule-1
HIV-1 cis-infection can result in the transmission of HIV-1 by the completion of all of the steps of the replication cycle within the cell and release of fully infectious new virions to go on and infect new cells. This process is similar in many different cell types, though it can be blocked by the expression of cell type-specific restriction factors and can be affected by expression levels of molecules that are required for productive HIV-1 infection, such as HIV-1 restriction factors, which will be discussed in other chapters.
HIV-1 trans-infection is most prominently associated with DCs, as productive HIV-1 replication is relatively inefficient in all DC subtypes compared to more permissive cell types, such as macrophages and CD4+ T cells (Dong et al. 2007). Phagocytosis of pathogens by DCs usually leads to degradation via the lysosome or proteasome and destruction of the pathogens. By contrast, internalized HIV-1 is rerouted and polarized at the cell surface to escape canonical degradation routes via the lysozyme or proteasome, allowing ef fi cient transmission to CD4+ T-cells (Yu et al. 2008). Once the HIV-1 is concentrated on the cell surface, the infected DC comes into close contact with a target CD4+ T cell and forms the VS, enabling efficient transfer of surface-bound HIV-1 to the target CD4+ T cell (McDonald et al. 2003). The VS is structurally similar to the immunological synapse formed between DCs and CD4+ T cells during an immune response, but does display distinct morphological features and dynamics (reviewed in Vasiliver-Shamis et al. 2010). Formation of the VS involves adhesion molecule interactions (Jolly et al. 2007a; Wang et al. 2009) along with recruitment of HIV-1 receptors and co-receptors (McDonald et al. 2003) to the cellular junction. By being rerouted away from degradation pathways, therefore, HIV-1 is able to avoid destruction and be efficiently transmitted to target CD4+ T-cells.
The 3D architecture of the VS formed between DCs and CD4+ T cells has been visualized using ion abrasion scanning electron microscopy and electron tomography (Felts et al. 2010). These studies reveal that HIV-1 is localized to deep invaginations, or compartments, contiguous with the DC cell surface. Furthermore, two distinct types of contact between the DC and CD4+ T cell at the VS are observed. During the first contact event, sheets of membrane extensions covering the CD4+ T cell provide a secluded region on the cell surface for formation of the VS. The second type of contact occurs within the VS, where filopodial extensions from the CD4+ T cells reach out and penetrate the surface-accessible compartments in which HIV-1 is contained. These observations suggest that transfer of HIV-1 from mDCs to CD4+ T cells occurs, at least in part, via the native antigen capture and presentation capabilities of DCs. These studies also demonstrate that complex interactions between DCs and CD4+ T cells occur at the VS and these cell–cell contacts involve extensive rearrangement of the cellular membranes and cytoskeletal network to allow efficient HIV-1 transmission.
4.1.5 Effects of DC Maturation on Cis- and Trans-infections of HIV-1
Immature DCs and mDCs have differential interactions with HIV-1 with respect to productive virus replication and DC-mediated transmission of HIV-1 to CD4+ T cells (Coleman et al. 2011 ; Dong et al. 2007 ; Sanders et al. 2002). It has been proposed that iDCs capture HIV-1 at the mucosal surface and migrate to local lymph nodes, maturing to mDCs in transit, and efficiently transmit HIV-1 to CD4+ T cells in the lymph node, which are the major replication site of HIV-1 (Wu and KewalRamani 2006).
Cis- and trans-infection mediated by DCs are distinct and dissociable events (Dong et al. 2007). iDCs support productive replication of HIV-1 to a relatively higher level than mDCs (Dong et al. 2007). The effect of DC maturation on the ability of mDCs to support productive HIV-1 replication and to transmit HIV-1 to CD4+ depends upon the maturation stimulus used (Dong et al. 2007; Sanders et al. 2002).
DC-mediated HIV-1 transmission to CD4+ T cells has been modeled in vitro using a range of factors that activate or mature DCs and thereby mimic the normal responses of DCs to PAMPs and cytokines/chemokines. In Table 4.1, a summary of the experiments using a number of different stimuli is presented. LPS is used to model DC stimulation of TLR4 by gram-negative bacteria; Poly I:C is a synthetic RNA analog which mimics the double-stranded RNAs produced during some viral infections and stimulates TLR3; IFNα and tumor necrosis factor α (TNF-α) are innate immune cytokines produced in response to infections; CD40 ligand (CD40L) is a factor released as part of the immune response and binds to CD40 on the cell surface of the DCs to activate the DCs.
Table 4.1.
Relative efficiencies of DC-mediated HIV-1 uptake, viral replication, and transmission of HIV-1 to CD4+ T cells
| Maturation stimulus | HIV-1 uptake | Productive HIV-1 infection | Transmission of HIV-1 to target CD4+ T cells | References |
|---|---|---|---|---|
| None (immature DCs) | + | ++ | + | Dong et al. (2007) and Wang et al. (2007b) |
| LPS | ++++ | − | +++ | Dong et al. (2007), Pertel et al. (2011) and Sanders et al. (2002) |
| PolyI:C | n/a | n/a | ++++ | Sanders et al. (2002) |
| IFNα | ++ | − | + | Coleman et al. (2011) and Pertel et al. (2011) |
| TNFα | + | ++ | ++ | Dong et al. (2007) |
| CD40L | + | +++ | ++++ | Dong et al. (2007) and Sanders et al. (2002) |
In these experiments, immature DCs were treated with the maturation stimuli for 24–48 h and infected with HIV-1 or HIV-1 cell-to-cell transmission assays were performed as described in the noted references. Relative efficiencies of HIV-1 infection and transmission are scored with a “+”; restricted HIV-1 infection is indicated by a “−.” n/a = not assessed in current literature. LPS lipopolysaccharide, IFNα interferon-α, CD40L CD40 ligand, TNF-α tumor necrosis factor α
4.2 Specific Cellular Factors and Processes That Affect DC-Mediated HIV-1 Transmission
Host cellular molecules have been demonstrated to affect DC-mediated transmission of HIV-1 to CD4+ T cells, including dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), CD4, and intercellular adhesion molecules (ICAMs). Viral factors can also affect DC-mediated transmission of HIV-1 to CD4+ T cells, largely by directly interacting with and/or altering the expression of the host cell molecules. As such, the effects of viral factors on DC-mediated transmission of HIV-1 to CD4+ T cells will be described in the context of their effects on the host cell.
HIV-1 replication within DCs, as in any other cell types that are susceptible to HIV-1 infection, can be regulated in a number of ways and therefore affect DC-mediated cis-transmission of HIV-1. This chapter does not discuss in detail the range of host factors that are known to promote or restrict HIV-1 replication in DCs; however, some key molecules are presented and are discussed further. Each viral protein expressed as part of the HIV-1 life cycle has a specific role in ensuring productive viral replication and some specific effects of HIV-1 proteins that affect DC-mediated cis-transmission will be discussed.
DC-mediated HIV-1 trans-infection of CD4+ T cells is largely dependent on cellular factors that are expressed by the DC and interact directly with HIV-1 at initial stages of infection, or during the course of infection.
4.2.1 DC-SIGN
DC-SIGN is a C-type lectin expressed on DCs and functions as an adhesion molecule (van Kooyk and Geijtenbeek 2003). DC-SIGN on DCs is required for the stabilization of the DC:T cell immunological synapse by binding ICAM-3 on the T cells with high affinity (van Gisbergen et al. 2005). DC-SIGN also binds to virion-associated HIV-1 Env (Geijtenbeek et al. 2000a ) and DC-SIGN over-expression in DCs can promote HIV-1 entry and infection (Lee et al. 2001). HIV-1 binding to DC-SIGN on the DC surface triggers a signaling cascade that promotes HIV-1 replication in DCs (Gringhuis et al. 2010 ). These data suggest that DC-SIGN enhances HIV-1 infection of DCs by promoting HIV-1 binding to DCs and through DC-SIGN signaling and promoting cis-infection.
DC-SIGNalsopromotesHIV-1transmissionfromDCstoCD4+Tcells(Geijtenbeek et al. 2000a; Gurney et al. 2005) by direct binding of the HIV-1 envelope protein, gp120 (Geijtenbeek et al. 2000a). DC-SIGN also plays a role in the initial internalization of HIV-1 (Kwon et al. 2002 ), perhaps to protect the virus from degradation or loss into the extracellular milieu prior to VS formation and CD4+T cell engagement. Taken together, these data suggest that DC-SIGN promotes HIV-1 trans-infection by concentrating large amounts of HIV-1 on the cell surface for ef fi cient transmission to CD4+ T cells. Furthermore, DC-SIGN enhancement of HIV-1 transmission is not dependent on the normal interaction of DC-SIGN with ICAM-3 expressed on the CD4+ T cells as DC-mediated HIV-1 transmission cannot be significantly blocked by soluble ICAM-3 or neutralizing antibodies to ICAM-3 (Wu et al. 2002), suggesting that ICAM-3 does not play a role in DC-SIGN-mediated HIV-1 transmission.
DC-SIGN-mediated enhancement of HIV-1 transmission to CD4+ T cells is cell type dependent. In iDCs (Wang et al. 2007b) and in primary activated B lymphocytes (Rappocciolo et al. 2006), blocking DC-SIGN causes a significant impairment of HIV-1 transmission to CD4+ T cells. Furthermore, DC-SIGN over-expression in B cell lines causes significant enhancement of HIV-1 transmission to CD4+ T cells (Wu et al. 2004). Conversely, LPS-matured DCs express lower levels of DC-SIGN than iDCs (Geijtenbeek et al. 2000b) and blocking DC-SIGN on LPS-matured DCs does not significantly affect transmission of HIV-1 to CD4+ T cells (Wang et al. 2007b) and does not promote HIV-1 uptake by mDCs (Izquierdo-Useros et al. 2007). Taken together, these data suggest that the high levels of LPS-matured DC-mediated HIV-1 transmission (Table 4.1) is DC-SIGN independent (Wang et al. 2007b). These data indicate that DC-SIGN plays an important role in iDC-mediated HIV-1 transmission to CD4+ T cells by binding and localizing incoming HIV-1 to a highly concentrated area on the cell surface that can be used for efficient transmission to CD4+ T cells, but does not play a significant role in LPS-matured DC transmission of HIV-1 to CD4+ T cells.
4.2.2 Langerin
Langerhans cells are a specialized DC subset present in mucosal epithelia. In contrast to other DC subtypes, instead of DC-SIGN, langerhans cells express the related C-type lectin receptor, langerin. Langerin efficiently binds HIV-1; however, lan-gerin blocks HIV-1 infection by causing endocytosis of the virion leading to targeted degradation of incoming virions (de Witte et al. 2007). In contrast to DC-SIGN-enhanced HIV-1 transmission, these studies suggest that langerin inhibits DC-mediated HIV-1 transmission.
4.2.3 CD4
CD4 is a cell surface molecule that is highly expressed on a subtype of T cells, on which its main biological function is within the T cell receptor signaling complex. CD4 also acts as the primary receptor of HIV-1 and is expressed at a low level on DCs (Dong et al. 2007; Janas et al. 2008). CD4 expression on DCs inhibits DC-mediated HIV-1 transmission to CD4+ T cells (Wang et al. 2007a). When CD4 is blocked on iDCs using a neutralizing antibody, there is a significant increase in DC-mediated HIV-1 transmission to CD4+ T cells (Wang et al. 2007a). Furthermore, the effect of CD4 can be recapitulated in DC-SIGN expressing Raji B cells. In Raji cells, CD4 over-expression significantly inhibits DC-SIGN-mediated HIV-1 transmission to CD4+ T cells and over-expression of both DC-SIGN and CD4 promotes HIV-1 intracellular retention (Wang et al. 2007a), suggesting that CD4 inhibits DC-SIGN-mediated HIV-1 transmission to CD4+ T cells by causing internalization of the virion, rather than retention of HIV-1 at the cell surface. Furthermore, DC-SIGN expression significantly increases the affinity of HIV-1 gp140 for CD4 by stabilizing the CD4:gp120 complex (Hijazi et al. 2011), suggesting that, in the presence of high levels of both DC-SIGN and CD4, HIV-1 binding and internalization of HIV-1 are favored over cell-to-cell transmission of HIV-1 to CD4+ T cells. Interestingly, langerin is not able to stabilize the CD4 and gp120 complex (Hijazi et al. 2011), demonstrating that the DC-SIGN:CD4:gp120 complex is specific for DC-SIGN and not a general function of C-type lectins.
4.2.4 ICAMs
ICAMs are cell surface molecules that facilitate contact between cells by binding to integrins. In CD4+ T cells, the major integrin is leukocyte function-associated molecule-1 (LFA-1), which binds to ICAMs expressed on DCs to facilitate cell-to-cell contact during formation of the immunological synapse. A number of ICAM molecules have been investigated for their role in DC-mediated transmission of HIV-1 to CD4+ T cells.
ICAM-1 is significantly upregulated upon maturation of DCs with poly I:C (Sanders et al. 2002) or LPS (Sanders et al. 2002; Wang et al. 2009), correlating with enhanced transmission of HIV-1 to CD4+ T cells (Table 4.1). However, ICAM-1 does not directly enhance HIV-1 infection of DCs (Wang et al. 2009) or CD4+ T cells (Sanders et al. 2002; Wang et al. 2009), indicating that it does not play a role in HIV-1 cis-infection of DCs. Blocking of ICAM-1 function on DCs signi fi cantly inhibits DC-mediated HIV-1 transmission to CD4+ T cells (Sanders et al. 2002; Wang et al. 2009) and blocking of LFA-1 on CD4+ T cells significantly inhibits LPS-matured DC-mediated HIV-1 transmission to CD4+ T cells (Wang et al. 2009). Conversely, blocking ICAM-1 on CD4+ T cells or LFA-1 on DCs has no significant effect on DC-mediated HIV-1 transmission to CD4+ T cells (Sanders et al. 2002; Wang et al. 2009). Furthermore, the ICAM-1 and LFA-1 interaction that promotes DC-mediated HIV-1 transmission to CD4+ T cells is dependent on cell contact between the DCs and the CD4+ T cells and not on any factor released from the DCs that might indirectly promote HIV-1 replication in the CD4+ T cells (Sanders et al. 2002), suggesting that the ICAM-1 and LFA-1 interaction is vital for stabilization of the VS and promotion of HIV-1 transmission. Finally, ICAM-1 and LFA-1 do not play a signi fi cant role in HIV-1 transmission between CD4+ T cells (Puigdomenech et al. 2008), indicating that this interaction is likely specific for DC-mediated transmission of HIV-1 to CD4+ T cells.
ICAM-2 also binds to LFA-1, but ICAM-2 is not highly expressed on monocyte-derived DCs or primary CD4+ T cells and is not upregulated by LPS maturation of DCs (Wang et al. 2009). Blocking ICAM-2 on DCs or CD4+ T cells does not have any significant effect on DC-mediated transmission of HIV-1 to CD4+ T cells (Wang et al. 2009).
ICAM-3 binds strongly to DC-SIGN (Geijtenbeek et al. 2000b) and given the latter’s role in DC-mediated transmission of HIV-1 to CD4+ T cells, the role of ICAM-3 has been specifically investigated as a mediator of DC-mediated cell-to-cell transmission of HIV-1. ICAM-3-negative CD4+ T cells show increased replication of HIV-1, suggesting that ICAM-3 plays a role in promoting HIV-1 replication (Biggins et al. 2007) and, therefore, may play a role in HIV-1 cis-infection. However, blocking the DC-SIGN interaction with ICAM-3 does not affect HIV-1 cell-to-cell transmission (Wu et al. 2002), nor does blocking of ICAM-3 on DCs and CD4+ T cells have any significant effect on DC-mediated cell-to-cell transmission of HIV-1 (Wang et al. 2009). Taken together with the studies that describe the role of DC-SIGN, these data suggest that the DC-SIGN and ICAM-3 interaction plays no significant role in DC-mediated HIV-1 transmission to CD4+ T cells.
4.2.5 Tetraspanins
Tetraspanins are a broad family of cell-surface proteins, so named because they cross the plasma membrane four times. Tetraspanins facilitate aggregation of proteins into membrane microdomains (Levy and Shoham 2005a) and have mul-tifunctional roles in cell–cell fusion, cell adhesion, cell motility, and formation of immunological signaling complexes (Hemler 2003; Levy and Shoham 2005b).
In LPS-matured DCs, HIV-1 localizes to a surface-accessible (Yu et al. 2008) compartment containing tetraspanins, such as CD9, CD63, CD81, and CD82 (Garcia et al. 2005; Izquierdo-Useros et al. 2007). This compartmentalization of HIV-1 to tetraspanin-rich areas of the membrane is not observed in HIV-1-infected iDCs (Izquierdo-Useros et al. 2007). This suggests a correlation between DC-mediated transmission of HIV-1 to CD4+ T cells and HIV-1 localization to tetraspanin-containing compartments on the cell surface. However, the specific role of each of these molecules in DC-mediated HIV-1 transmission to CD4+ T cells has not been extensively investigated. It appears most likely that the localization of incoming HIV-1 to a tetraspanin-rich area of the host cell membrane is a consequence of DC maturation. Furthermore, tetraspanins may serve as markers for the subcellular localization and traf fi cking of HIV-1, rather than possessing a functional role in DC-mediated HIV-1 transmission to CD4+ T cells (Garcia et al. 2005; Izquierdo-Useros et al. 2009).
A study using HeLa cells as donor cells to transmit HIV-1 to the CD4+ CEM T cell line has suggested that CD9 and CD63 promote HIV-1 cell-to-cell transmission and CD81 inhibits HIV-1 cell-to-cell transmission without affecting the infectivity of the cell-free virus (Krementsov et al. 2009). However, tetraspanin-mediated HIV-1 transmission to CD4+ T cells has not been demonstrated in DCs. The role of each of these tetraspanins in DC-mediated HIV-1 transmission to CD4+ T cells remains to be investigated.
4.2.6 TLRs
DCs express pathogen-pattern-recognition receptors including TLRs. It has been shown that HIV-1 infection of DCs causes upregulation of TLRs in DCs (Hernandez et al. 2011) and that HIV-1 induces TLR signaling pathways in DCs to promote replication and cell-to-cell transmission (Gringhuis et al. 2010).
HIV-1 infection causes upregulation of TLR2 and TLR4 in DCs and monocytes from individuals co-infected with Mycobacterium tuberculosis and HIV-1 (Hernandez et al. 2011). There is a positive correlation between expression of TLR2 and TLR4 and an elevated HIV-1 viral load in these individuals, which suggests that upregulation of these TLRs may enhance HIV-1 replication and/or transmission (Hernandez et al. 2011). However, the direct effects of HIV-1 interactions with TLR2 and TLR4 on HIV-1 replication and DC-mediated cell-to-cell transmission have not been demonstrated. However, LPS-maturation of DCs via TLR4 does promote DC-mediated HIV-1 transmission (Dong et al. 2007; Sanders et al. 2002). It is possible that high levels of TLR4 may render DCs more susceptible to LPS maturation and, therefore, promote DC-mediated HIV-1 transmission to CD4+ T cells. Furthermore, HIV-1 coinfection with M. tuberculosis or Candida albicans, which stimulate TLR2 homo- or heterodimers, enhances HIV-1 replication in a manner dependent on the cellular kinase raf-1, via phosphorylation of the p65 subunit of NF-κB (Gringhuis et al. 2010), suggesting that TLR2 signaling promotes HIV-1 infection of DCs and DC-mediated transmission of HIV-1. Interestingly, when DCs are treated with the fungus Penicillium marneffei, DC-mediated HIV-1 transmission to CD4+ T cells is increased and HIV-1 infection of the DCs is blocked (Qin et al. 2011). Fungal PAMPs are recognized by TLR2 dimers; therefore it is possible that TLR2 stimulation can activate DCs to promote DC-mediated HIV-1 transmission, either by direct signaling or by upregulation of molecules that promote DC-mediated HIV-1 transmission; for example, ICAM-1 upregulation was observed in P. marneffei-treated DCs (Qin et al. 2011).
HIV-1 RNA selectively triggers TLR8 signaling, allowing initiation of transcription of HIV-1 genes via NF-κB activation, as knockdown of TLR8 in DCs abrogates HIV-1 transcription and causes abortive transcription of HIV-1 proviral DNA (Gringhuis et al. 2010). For transcriptional elongation to occur, a second signaling pathway is required: binding of HIV-1 gp120 to DC-SIGN activates Raf-1-dependent phosphorylation of the NF-κB p65 subunit. This allows elongation of HIV-1 transcripts, resulting in the production of full-length transcripts and productive HIV-1 infection in DCs and subsequent transmission to CD4+ T cells (Gringhuis et al. 2010). Previous studies suggested that the endocytosis of HIV-1 by DCs and subsequent trafficking through the endosomal pathway target HIV-1 for lysosomal degradation (Yu et al. 2008). By contrast, the study by Gringhuis et al. demonstrated that HIV-1 can use the endosomal route to allow initiation of transcription and ensure active replication, via a mechanism that uses the innate TLR8 and Raf-1 signaling pathways in DCs (Gringhuis et al. 2010 ). Taken together with the observation that endo-somal traf fi cking does not promote DC-mediated trans -infection (Yu et al. 2008), these data suggest that TLR8 signaling, induced by HIV-1 RNA in the endosome, promotes HIV-1 cis -infection, but not trans-infection.
4.2.7 Glycosphingolipids
The role of host cell-derived glycosphingolipids (GSLs) incorporated into HIV-1 particles in DC-mediated trans-infection has been assessed in both iDCs and mDCs. A novel mechanism has been defined, in which DCs capture HIV-1 independently of the envelope glycoprotein, gp120 (Hatch et al. 2009). Blocking of the GSL biosynthesis pathways using specific inhibitors suggests that host cell-derived GSLs are incorporated into HIV-1 virions and play a key role in HIV-1 interactions with DCs. Furthermore, depletion of the host cell GSLs results in HIV-1 particles that are deficient in GSLs and consequently cannot be efficiently captured or transmitted to CD4+ T cells by the DCs (Hatch et al. 2009). The specific roles of GSL in DC-mediated HIV-1 transmission and interactions will be discussed in more detail in Chap. 6.
4.2.8 The Exosome Secretion Pathway
Exosomes are cellular vesicles that are released from cells for transmission of signaling molecules between cells. In iDCs, there is evidence that the capture of HIV-1 and transmission to CD4+ T cells occur through a cell contact-free transmission event, involving a process called exocytosis (Wiley and Gummuluru 2006). After endocytosis of HIV-1, virus particles are localized to tetraspanin-rich compartments (Garcia et al. 2005; Izquierdo-Useros et al. 2007), postulated to be multi-vesicular bodies (MVBs). From the MVBs, HIV-1 particles are targeted directly to the exosome secretion pathway for release into the extracellu-lar milieu in association with the exosomes (Wiley and Gummuluru 2006). It was hypothesized that HIV-1 uses an internal DC trafficking pathway, wherein fusion between the HIV-1 containing MVB and the intracellular plasma membrane enables HIV-1 particles to escape lysosomal degradation. The fully infectious HIV-1 released from the DCs was confirmed to be a consequence of the release of intact, previously endocytosed HIV-1 virions, and not the result of productive replication within the DCs (Wiley and Gummuluru 2006). But, the infectivity of the HIV-1 released in association with exosomes to CD4+ T cells is lower than that observed in DC:CD4+ T cell cocultures (Wiley and Gummuluru 2006), indicating that the exosome secretion pathway is not as efficient at transmitting HIV-1 from DCs to CD4+ T cells as VS-dependent DC-mediated HIV-1 transmission to CD4+ T cells. Thus, the exosome secretion pathway may play a role as an alternative transmission route that adds to the overall DC-mediated HIV-1 transmission to CD4+ T cells. The transmission of HIV-1 via exosomes is the first demonstration of a cell contact-free DC-mediated trans-infection without de novo viral replication (Wiley and Gummuluru 2006), making exo-some-mediated transmission distinct from cis-infection of HIV-1.
4.2.9 Cytoskeleton-Dependent Macromolecular Movement
One of the mechanisms that retroviruses have evolved to circumvent host cell obstacles to productive replication, such as lysosomal degradation, is cytoskeleton-dependent macromolecular movement within cells (Fackler and Krausslich 2006; Naghavi and Goff 2007). HIV-1, in particular, is adept at using the host cell cytoskeleton to promote its own replication and cell-to-cell transmission (Lehmann et al. 2011). Numerous studies utilizing a wide variety of techniques have established roles for components of the cytoskeleton in the entry, trafficking, and DC-mediated HIV-1 transmission to CD4+ T-cells, including actin, microtubules, and membrane extensions (Fahrbach et al. 2007; Garcia et al. 2005; Izquierdo-Useros et al. 2007; McDonald et al. 2003; Trumpfheller et al. 2003; Turville et al. 2004; Wang et al. 2007b; Wiley and Gummuluru 2006). Transmission of HIV-1 between CD4+ T cells is an actin-dependent process. Actin allows concentration of HIV-1 at the VS to promote efficient cell-to-cell transmission (Jolly et al. 2007b) and actin-rich membrane extensions confer up to 90% of HIV-1 transmission from iDCs to CD4+ T cells (Nikolic et al. 2011). However, it is unknown if HIV-1 exploits a similar pathway to ensure that the virus is concentrated at the mDC:CD4+ T cell junction during mDC-mediated HIV-1 transmission to CD4+ T cells.
Both HIV-1 and SIV can be endocytosed into DCs through a variety of mechanisms, including clathrin-mediated endocytosis (Frank et al. 2002; Garcia et al. 2005; Wang et al. 2007b ) and receptor-mediated endocytosis (de Witte et al. 2007; Kwon et al. 2002). Furthermore, macropinocytosis, an actin-dependent form of nonselec-tive endocytosis, partially contributes to endocytosis of HIV-1 by LPS-matured DCs. Disruption of both macropinocytosis and cytoskeleton, using specific inhibitors, alters HIV-1 trafficking and inhibits DC-mediated HIV-1 transmission to CD4+ T cells (Wang et al. 2008). DC-mediated HIV-1 transmission to CD4+ T cells is inhibited because of impaired formation of the VS (Wang et al. 2008), indicating that HIV-1 exploits macropinocytosis and the cytoskeletal network to promote formation of the VS, allowing efficient HIV-1 transmission and dissemination.
Although studies indicate the importance of the cytoskeletal network in DC-mediated transmission of HIV-1 to CD4+ T cells, it is also evident that some components of the cytoskeletal network function to prevent HIV-1 from successfully trafficking and transmitting to CD4+ T cells. Leukocyte-specific protein (LSP)-1 is a cellular F-actin-binding protein localized on the plasma membrane. LSP-1 specifically interacts with C-type lectins, such as DC-SIGN, and targets HIV-1 particles to the proteasome for degradation. Knockdown of LSP-1 in DCs enhances transmission of HIV-1 to CD4+ T-cells (Smith et al. 2007), indicating that LSP-1 is responsible for targeting the incoming HIV-1 particles for degradation and, therefore, inhibits the trafficking of the incoming virus towards the VS.
4.2.10 Autophagy
Autophagy is a process of cellular self-digestion in which proteins and organelles are degraded. HIV-1 is able to inhibit autophagy to promote viral replication and DC-mediated trans-infection (Blanchet et al. 2010). Autophagy also plays an important role in the regulation of innate and adaptive immune responses to intracellular pathogens (reviewed in Levine and Deretic 2007 ). It has been shown that HIV-1-mediated inhibition of autophagy impairs the innate and adaptive immune functions of DCs (Blanchet et al. 2010). The details of the role of autophagy in HIV-1 interactions with DCs will be discussed in detail in Chap. 10.
4.3 HIV-1 Proteins That Affect DC-Mediated Cell-to-Cell Transmission of HIV-1
4.3.1 HIV-1 Env Glycosylation Affects DC-SIGN-Meditated HIV-1 Transmission to CD4+ T Cells
Glycosylation of Env plays a key role in ensuring efficient DC-mediated HIV-1 transmission to CD4+ T cells. HIV-1 Env is heavily glycosylated, with N-linked glycans contributing to almost half of the molecular mass of Env (van Montfort et al. 2011). N-Linked glycans are a family of carbohydrate moieties that are added to the nitrogen atoms in the side chain of asparagine amino acids in proteins (Schwarz and Aebi 2011). The N-linked glycan composition of Env is heterogeneous, with complex carbohydrate moieties present throughout the Env protein (van Montfort et al. 2011). N-Linked glycans on Env are important for correct Env folding (Walker et al. 1987), incorporation of Env into virions (Walker et al. 1987), and HIV-1 immune evasion (Reitter et al. 1998; Sanders et al. 2008).
The direct binding of Env to DC-SIGN is dependent on the carbohydrate moieties in Env (Hong et al. 2002) and this interaction has been mapped to specific N-glycans within the 2G12 epitope of Env (Hong et al. 2007). Interestingly, the specific N-glycan composition of the Env glycoprotein promotes interactions between Env and DC-SIGN and specifically promotes DC-mediated transmission of HIV-1 to CD4+ T cells (van Montfort et al. 2011). In fact, the Env protein requires a mix of N-glycan residues, as changing the glycans to universal oligomannose N-glycan promotes DC-SIGN binding but reduces DC-mediated HIV-1 transmission to CD4+ T cells, as the oligomannose residues on Env promote HIV-1 endocytosis and degradation (van Montfort et al. 2011).
4.3.2 HIV-1 Nef Promotes DC-Mediated HIV-1 Transmission to CD4+ T Cells
HIV-1 Nef is a multifunctional accessory protein encoded by HIV-1. It has a range of functions and contributes to HIV-1 pathogenesis in vivo, including modulation of immune evasion (Kirchhoff 2010) and HIV-1 persistence (Arhel and Kirchhoff 2009). Nef interacts with a range of host cell molecules to achieve its diverse effects on HIV-1 pathogenesis (Foster and Garcia 2008).
HIV-1 Nef protein expression causes down-regulation of the main HIV-1 receptors, CD4 and CCR5, on the surface of infected cells (Michel et al. 2005; Wang et al. 2007a). Thus, Nef protein promotes cis-infection of HIV-1 by preventing superinfection of the initially infected cell and forcing HIV-1 to infect other cells. Nef also promotes HIV-1 replication in DC and lymphocyte cocultures (Petit et al. 2001) and CD4 inhibits DC-mediated HIV-1 transmission to CD4+ T cells (Wang et al. 2007a); therefore, HIV-1 Nef protein may promote trans-infection mediated by DCs by down-regulation of CD4 expression.
HIV-1 Nef expression in iDCs promotes clustering of DCs and CD4+ T cells, DC maturation, and CD4+ T cell activation (Messmer et al. 2002a; Messmer et al. 2002b; Sol-Foulon et al. 2002). HIV-1 Nef causes up-regulation of DC-SIGN in HIV-1-infected DCs (Sol-Foulon et al. 2002) and over-expression of HIV-1 Nef protein into HeLa cells causes up-regulation of surface DC-SIGN expression, by preventing DC-SIGN endocytosis (Sol-Foulon et al. 2002). Nef-upregulated DC-SIGN expression promotes HIV-1 transmission from HeLa cells to cocultured lymphocytes (Sol-Foulon et al. 2002), but DC-SIGN may play only a partial role in DC-mediated HIV-1 transmission to CD4+ T cells (Boggiano et al. 2007; Gurney et al. 2005; Wu et al. 2002). HIV-1 Nef expression in the context of virus infection promotes DC-mediated transmission of HIV-1 to CD4+ T cells, which correlates with decreased CD4 expression and only modest increases in DC-SIGN expression (St Gelais et al. 2012). Therefore, Nef-dependent CD4 downregulation and Nef-dependent clustering of HIV-1-bearing DCs and uninfected target CD4+ T cells via the DC-SIGN:ICAM-3 interaction may both promote highly efficient HIV-1 transmission CD4+ T cells. Furthermore, Nef expression in CD4+ T cells causes increased CD4+ T cell activation and proliferation, which correlates with enhanced HIV-1 replication (St Gelais et al. 2012), suggesting that Nef is important for promoting HIV-1 replication in CD4+ T cells after DC-mediated transmission has occurred.
4.3.3 Viral Factors That Affect the Host Cell Cytoskeletal Network
HIV-1 binding to DC-SIGN on DCs can promote HIV-1 transmission to CD4+ T cells by promoting formation of the VS. It has been suggested that HIV-1 can hijack the cytoskeletal network to enhance its transmission to CD4+ T cells (Arrighi et al. 2004; Wang et al. 2009). Recent studies have demonstrated that binding of DC-SIGN by HIV-1 activates Rho-GTPases and other signaling molecules (Nikolic et al. 2011). It is suggested that HIV-1 induces activation of C-type lectin receptors and affects cytoskeletal remodeling, in particular through activation of Rho-GTPases (Nikolic et al. 2011), which can affect a range of DC functions including cell migration, trafficking, and cell polarity (Heasman and Ridley 2008).
A recent study indicated that HIV-1 is capable of triggering the formation of actin-based protrusions at the surface of iDCs (Nikolic et al. 2011). It was demonstrated that transmission from iDCs to CD4+ T cells occurs in a two-step transfer process in which HIV-1 is bound and concentrated at the VS and then transferred to CD4+ T cells in a Cdc42-dependent manner. Cdc42 is a small GTPase of the Rho-subfamily which acts as a signaling molecule with a range of functions in the cell, including regulation of the cell cycle, cell migration, and endocytosis (Heasman and Ridley 2008). The Cdc42-dependent transmission process is dependent on Env binding to DC-SIGN, causing subsequent activation of Cdc42 (Nikolic et al. 2011). When Cdc42 function is blocked in iDCs, there is a significant decrease in the amount of iDC-mediated HIV-1 transmission to CD4+ T cells (Nikolic et al. 2011). The number of VSs formed and the concentration of HIV-1 at the zone of contact between cells are not affected by blocking Cdc42 activation, indicating that polarization of HIV-1 at the contact point occurs independently of Cdc42 (Nikolic et al. 2011). Furthermore, HIV-1 Env engagement of DC-SIGN at the cell surface leads to Cdc42 activation and other signaling molecules that have been previously implicated in membrane extension formation or DC-SIGN signaling cascades in DCs; Pak1, Wasp, and Src kinases are activated in the presence of HIV-1 Env (Nikolic et al. 2011). These data demonstrate that HIV-1 Env binding to DC-SIGN triggers signaling that results in activation of Cdc42 in iDCs. Efficient DC-mediated HIV-1 transmission to CD4+ T cells occurs in the presence of activated Cdc42, but Cdc42 signaling is not required for the physical formation of the VS between the DCs and CD4+ T cells.
4.4 Conclusions and Future Directions
DCs are, potentially, one of the important cell types in the early transmission of HIV-1 to CD4+ T cells. The cellular and viral factors that affect the process of early-stage HIV-1 transmission have been extensively studied and characterized. Many cell surface molecules affect DC-mediated HIV-1 transmission to CD4+ T cells, but none of these molecules appear to be exclusively responsible for differential transmission between different DC subsets. For example, both DC-SIGN-dependent and independent transmission can occur in DCs.
HIV-1 exploits normal DC cellular process, such as macropinocytosis, and natural interactions with CD4+ T cells, such as ICAM-1 binding to LFA-1, to promote viral infection and cell-to-cell transmission. HIV-1 expresses proteins which promote the process of DC-mediated HIV-1 transmission to CD4+ T cells. In particular, the multifunctional pathogenic accessory protein Nef plays a key role in promoting DC-mediated HIV-1 transmission to CD4+ T cells by activating CD4+ T cells and modulating DC interactions with T cells. Overall, the cellular and viral factors that promote DC-mediated HIV-1 transmission to CD4+ T cells ensure efficient HIV-1 transmission and spread within the host and establishment of long-term infection.
Future work will involve further characterizations of cellular and viral factors that regulate DC-mediated HIV-1 transmission to CD4+ T cells, particularly those that act specifically in different DC subsets. A major area of study is the characterization of the physical interaction that occurs at the VS that promotes DC-mediated HIV-1 transmission to CD4+ T cells, for example, how CD4+ T cells efficiently retrieve HIV-1 from the deep invagination on the DC cell surface. These studies will help to better understand the mechanisms underlying HIV-1 cell-to-cell transmission.
Cellular and viral factors that affect DC-mediated transmission of HIV-1 to CD4+ T cells are also, potentially, important targets for therapeutic strategies, such as drugs that specifically target the interactions that promote formation of the VS and/ or strategies to target the HIV-1 pool associated with DCs. Drugs that target the initial interactions that occur between HIV-1 and DCs have the potential as topical treatments at the mucosal surfaces to prevent the initial DC-mediated HIV-1 transmission events that lead to establishment of persistent infection.
Acknowledgments
We thank members of the Wu laboratory for helpful discussions and Tim Vojt for illustration. The research in the Wu laboratory was supported by grants (AI068493, AI078762, and AI098524) to L.W. from the National Institutes of Health and by the program of Public Health Preparedness for Infectious Diseases of The Ohio State University. The authors apologize to all whose work has not been cited as a result of space limitations.
Contributor Information
Christopher M. Coleman, Department of Veterinary Biosciences, Center for Retrovirus Research, The Ohio State University, Columbus, OH, USA
Corine St Gelais, Department of Veterinary Biosciences, Center for Retrovirus Research, The Ohio State University, Columbus, OH, USA
Li Wu, Department of Veterinary Biosciences, Center for Retrovirus Research, The Ohio State University, Columbus, OH, USA
References
- Arhel NJ, Kirchhoff F. Implications of Nef: host cell interactions in viral persistence and progression to AIDS. Curr Top Microbiol Immunol. 2009;339:147–175. doi: 10.1007/978-3-642-02175-6_8. [DOI] [PubMed] [Google Scholar]
- Arrighi JF, Pion M, Garcia E, Escola JM, van Kooyk Y, Geijtenbeek TB, Piguet V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J Exp Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins JE, Biesinger T, Yu Kimata MT, Arora R, Kimata JT. ICAM-3 influences human immunodeficiency virus type 1 replication in CD4(+) T cells independent of DC-SIGN-mediated transmission. Virology. 2007;364:383–394. doi: 10.1016/j.virol.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet FP, Moris A, Nikolic DS, Lehmann M, Cardinaud S, Stalder R, Garcia E, Dinkins C, Leuba F, Wu L, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–669. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano C, Manel N, Littman DR. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J Virol. 2007;81:2519–2523. doi: 10.1128/JVI.01661-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Spearman P, Wu L. Tetherin does not significantly restrict dendritic cell-mediated HIV-1 transmission and its expression is upregulated by newly synthesized HIV-1 Nef. Retrovirology. 2011;8:26. doi: 10.1186/1742-4690-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology. 2009;6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TB. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- Dong C, Janas AM, Wang JH, Olson WJ, Wu L. Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J Virol. 2007;81:11352–11362. doi: 10.1128/JVI.01081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Moss B, Doms RW. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991;65:2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler OT, Krausslich HG. Interactions of human retroviruses with the host cell cytoskel-eton. Curr Opin Microbiol. 2006;9:409–415. doi: 10.1016/j.mib.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Fahrbach KM, Barry SM, Ayehunie S, Lamore S, Klausner M, Hope TJ. Activated CD34-derived Langerhans cells mediate transinfection with human immunodeficiency virus. J Virol. 2007;81:6858–6868. doi: 10.1128/JVI.02472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts RL, Narayan K, Estes JD, Shi D, Trubey CM, Fu J, Hartnell LM, Ruthel GT, Schneider DK, Nagashima K, et al. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci U S A. 2010;107:13336–13341. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JL, Garcia JV. HIV-1 Nef: at the crossroads. Retrovirology. 2008;5:84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I, Piatak M, Jr, Stoessel H, Romani N, Bonnyay D, Lifson JD, Pope M. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J Virol. 2002;76:2936–2951. doi: 10.1128/JVI.76.6.2936-2951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E, Pion M, Pelchen-Matthews A, Collinson L, Arrighi JF, Blot G, Leuba F, Escola JM, Demaurex N, Marsh M, et al. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic. 2005;6:488–501. doi: 10.1111/j.1600-0854.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000a;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000b;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Goujon C, Malim MH. Characterization of the alpha interferon-induced postentry block to HIV-1 infection in primary human macrophages and T cells. J Virol. 2010;84:9254–9266. doi: 10.1128/JVI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- Gurney KB, Elliott J, Nassanian H, Song C, Soilleux E, McGowan I, Anton PA, Lee B. Binding and transfer of human immunodeficiency virus by DC-SIGN + cells in human rectal mucosa. J Virol. 2005;79:5762–5773. doi: 10.1128/JVI.79.9.5762-5773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley TM, Blay Puryear W, Gummuluru S, Viglianti GA. PPARgamma and LXR signaling inhibit dendritic cell-mediated HIV-1 capture and trans-infection. PLoS Pathog. 2010;6:e1000981. doi: 10.1371/journal.ppat.1000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch SC, Archer J, Gummuluru S. Glycosphingolipid composition of human immundeficiency virus type-1 particles is a crucial determinant for dendritic cell-mediated HIV-1 trans infection. J Virol. 2009;83:3496–3506. doi: 10.1128/JVI.02249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- Hernandez JC, Arteaga J, Paul S, Kumar A, Latz E, Urcuqui-Inchima S. Up-regulation of TLR2 and TLR4 in dendritic cells in response to HIV type 1 and coinfection with opportunistic pathogens. AIDS Res Hum Retroviruses. 2011;27:1099–1109. doi: 10.1089/aid.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijazi K, Wang Y, Scala C, Jeffs S, Longstaff C, Stieh D, Haggarty B, Vanham G, Schols D, Balzarini J, et al. DC-SIGN increases the affinity of HIV-1 envelope glycoprotein interaction with CD4. PLoS One. 2011;6:e28307. doi: 10.1371/journal.pone.0028307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong PW, Flummerfelt KB, de Parseval A, Gurney K, Elder JH, Lee B. Human immunodeficiency virus envelope (gp120) binding to DC-SIGN and primary dendritic cells is carbohydrate dependent but does not involve 2G12 or cyanovirin binding sites: implications for structural analyses of gp120-DC-SIGN binding. J Virol. 2002;76:12855–12865. doi: 10.1128/JVI.76.24.12855-12865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong PW, Nguyen S, Young S, Su SV, Lee B. Identification of the optimal DC-SIGN binding site on human immunodeficiency virus type 1 gp120. J Virol. 2007;81:8325–8336. doi: 10.1128/JVI.01765-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Blanco J, Erkizia I, Fernandez-Figueras MT, Borras FE, Naranjo-Gomez M, Bofill M, Ruiz L, Clotet B, Martinez-Picado J. Maturation of blood-derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. J Virol. 2007;81:7559–7570. doi: 10.1128/JVI.02572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Esteban O, Rodriguez-Plata MT, Erkizia I, Prado JG, Blanco J, Garcia-Parajo MF, Martinez-Picado J. Dynamic imaging of cell-free and cell-associated viral capture in mature dendritic cells. Traffic. 2011;12:1702–1713. doi: 10.1111/j.1600-0854.2011.01281.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Naranjo-Gomez M, Archer J, Hatch SC, Erkizia I, Blanco J, Borras FE, Puertas MC, Connor JH, Fernandez-Figueras MT, Moore L, Clotet B, Gummuluru S, Martinez-Picado J. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113(12):2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas AM, Dong C, Wang JH, Wu L. Productive infection of human immunodeficiency virus type 1 in dendritic cells requires fusion-mediated viral entry. Virology. 2008;375:442–451. doi: 10.1016/j.virol.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Mitar I, Sattentau QJ. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol. 2007a;81:13916–13921. doi: 10.1128/JVI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Mitar I, Sattentau QJ. Requirement for an intact T-cell actin and tubulin cytoskel-eton for efficient assembly and spread of human immunodeficiency virus type 1. J Virol. 2007b;81:5547–5560. doi: 10.1128/JVI.01469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe. 2010;8:55–67. doi: 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Krementsov DN, Weng J, Lambele M, Roy NH, Thali M. Tetraspanins regulate cell-to-cell transmission of HIV-1. Retrovirology. 2009;6:64. doi: 10.1186/1742-4690-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16:135–144. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- Lee B, Leslie G, Soilleux E, O’Doherty U, Baik S, Levroney E, Flummerfelt K, Swiggard W, Coleman N, Malim M, et al. cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J Virol. 2001;75:12028–12038. doi: 10.1128/JVI.75.24.12028-12038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Nikolic DS, Piguet V. How HIV-1 takes advantage of the cytoskeleton during replication and cell-to-cell transmission. Viruses. 2011;3:1757–1776. doi: 10.3390/v3091757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S, Shoham T. Protein–protein interactions in the tetraspanin web. Physiology (Bethesda) 2005a;20:218–224. doi: 10.1152/physiol.00015.2005. [DOI] [PubMed] [Google Scholar]
- Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005b;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- Messmer D, Bromberg J, Devgan G, Jacque JM, Granelli-Piperno A, Pope M. Human immunodeficiency virus type 1 Nef mediates activation of STAT3 in immature dendritic cells. AIDS Res Hum Retroviruses. 2002a;18:1043–1050. doi: 10.1089/08892220260235407. [DOI] [PubMed] [Google Scholar]
- Messmer D, Jacque JM, Santisteban C, Bristow C, Han SY, Villamide-Herrera L, Mehlhop E, Marx PA, Steinman RM, Gettie A, et al. Endogenously expressed nef uncouples cytokine and chemokine production from membrane phenotypic maturation in dendritic cells. J Immunol. 2002b;169:4172–4182. doi: 10.4049/jimmunol.169.8.4172. [DOI] [PubMed] [Google Scholar]
- Michel N, Allespach I, Venzke S, Fackler OT, Keppler OT. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregu-late cell-surface CCR5 and CD4. Curr Biol. 2005;15:714–723. doi: 10.1016/j.cub.2005.02.058. [DOI] [PubMed] [Google Scholar]
- Naghavi MH, Goff SP. Retroviral proteins that interact with the host cell cytoskeleton. Curr Opin Immunol. 2007;19:402–407. doi: 10.1016/j.coi.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic DS, Lehmann M, Felts R, Garcia E, Blanchet FP, Subramaniam S, Piguet V. HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood. 2011;118:4841–4852. doi: 10.1182/blood-2010-09-305417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel T, Reinhard C, Luban J. Vpx rescues HIV-1 transduction of dendritic cells from the antiviral state established by type 1 interferon. Retrovirology. 2011;8:49. doi: 10.1186/1742-4690-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C, Buseyne F, Boccaccio C, Abastado JP, Heard JM, Schwartz O. Nef is required for efficient HIV-1 replication in cocultures of dendritic cells and lymphocytes. Virology. 2001;286:225–236. doi: 10.1006/viro.2001.0984. [DOI] [PubMed] [Google Scholar]
- Poli G, Orenstein JM, Kinter A, Folks TM, Fauci AS. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989;244:575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- Puigdomenech I, Massanella M, Izquierdo-Useros N, Ruiz-Hernandez R, Curriu M, Bofill M, Martinez-Picado J, Juan M, Clotet B, Blanco J. HIV transfer between CD4 T cells does not require LFA-1 binding to ICAM-1 and is governed by the interaction of HIV envelope glycoprotein with CD4. Retrovirology. 2008;5:32. doi: 10.1186/1742-4690-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Li Y, Liu W, Tian R, Guo Q, Li S, Li H, Zhang D, Zheng Y, Wu L, et al. Penicillium marneffei-stimulated dendritic cells enhance HIV-1 trans-infection and promote viral infection by activating primary CD4+ T cells. PLoS One. 2011;6:e27609. doi: 10.1371/journal.pone.0027609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Piazza P, Fuller CL, Reinhart TA, Watkins SC, Rowe DT, Jais M, Gupta P, Rinaldo CR. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Pathog. 2006;2:e70. doi: 10.1371/journal.ppat.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitter JN, Means RE, Desrosiers RC. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- Sanders RW, de Jong EC, Baldwin CE, Schuitemaker JH, Kapsenberg ML, Berkhout B. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J Virol. 2002;76:7812–7821. doi: 10.1128/JVI.76.15.7812-7821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, van Anken E, Nabatov AA, Liscaljet IM, Bontjer I, Eggink D, Melchers M, Busser E, Dankers MM, Groot F, et al. The carbohydrate at asparagine 386 on HIV-1 gp120 is not essential for protein folding and function but is involved in immune evasion. Retrovirology. 2008;5:10. doi: 10.1186/1742-4690-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol. 2011;21:576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Smith AL, Ganesh L, Leung K, Jongstra-Bilen J, Jongstra J, Nabel GJ. Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells. J Exp Med. 2007;204:421–430. doi: 10.1084/jem.20061604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol-Foulon N, Moris A, Nobile C, Boccaccio C, Engering A, Abastado JP, Heard JM, van Kooyk Y, Schwartz O. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity. 2002;16:145–155. doi: 10.1016/s1074-7613(02)00260-1. [DOI] [PubMed] [Google Scholar]
- St Gelais C, Coleman C, Wang J-H, Wu L. Nef enhances dendritic cell-mediated viral transmission to CD4+ T cells and promotes T-cell activation. PLoS One. 2012;7(3):e34521. doi: 10.1371/journal.pone.0034521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Trumpfheller C, Park CG, Finke J, Steinman RM, Granelli-Piperno A. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. Int Immunol. 2003;15:289–298. doi: 10.1093/intimm/dxg030. [DOI] [PubMed] [Google Scholar]
- Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, Dable J, Stossel H, Romani N, Piatak M, Jr, et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- van Gisbergen KP, Paessens LC, Geijtenbeek TB, van Kooyk Y. Molecular mechanisms that set the stage for DC-T cell engagement. Immunol Lett. 2005;97:199–208. doi: 10.1016/j.imlet.2004.11.008. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- van Montfort T, Eggink D, Boot M, Tuen M, Hioe CE, Berkhout B, Sanders RW. HIV-1 N-glycan composition governs a balance between dendritic cell-mediated viral transmission and antigen presentation. J Immunol. 2011;187:4676–4685. doi: 10.4049/jimmunol.1101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliver-Shamis G, Dustin ML, Hioe CE. HIV-1 virological synapse is not simply a copy-cat of the immunological synapse. Viruses. 2010;2:1239–1260. doi: 10.3390/v2051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrame D, Sourisseau M, Perrin V, Schwartz O, Mammano F. Partial inhibition of human immunodeficiency virus replication by type I interferons: impact of cell-to-cell viral transfer. J Virol. 2009;83:10527–10537. doi: 10.1128/JVI.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BD, Kowalski M, Goh WC, Kozarsky K, Krieger M, Rosen C, Rohrschneider L, Haseltine WA, Sodroski J. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc Natl Acad Sci U S A. 1987;84:8120–8124. doi: 10.1073/pnas.84.22.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Janas AM, Olson WJ, KewalRamani VN, Wu L. CD4 coexpression regulates DC-SIGN-mediated transmission of human immunodeficiency virus type 1. J Virol. 2007a;81:2497–2507. doi: 10.1128/JVI.01970-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Janas AM, Olson WJ, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol. 2007b;81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Kwas C, Wu L. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2 and -3, is important for dendritic cell-mediated human immunodeficiency virus type-1 transmission. J Virol. 2009;83:4195–4204. doi: 10.1128/JVI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Wells C, Wu L. Macropinocytosis and cytoskeleton contribute to dendritic cell-mediated HIV-1 transmission to CD4+ T cells. Virology. 2008;381:143–154. doi: 10.1016/j.virol.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Martin TD, Carrington M, KewalRamani VN. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology. 2004;318:17–23. doi: 10.1016/j.virol.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wu L, Martin TD, Vazeux R, Unutmaz D, KewalRamani VN. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J Virol. 2002;76:5905–5914. doi: 10.1128/JVI.76.12.5905-5914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HJ, Reuter MA, McDonald D. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 2008;4:e1000134. doi: 10.1371/journal.ppat.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]