Abstract
Provider-assisted methods of partner notification increase testing and counseling among sexual partners of patients diagnosed with HIV, however they are resource-intensive. The sexual partners of individuals enrolled in a clinical trial comparing different methods of HIV partner notification were analyzed to identify who was unlikely to seek testing on their own. Unconditional logistic regression was used to identify partnership characteristics, which were assigned a score based on their coefficient in the final model, and a risk score was calculated for each participant. The risk score included male partner sex, relationship duration 6–24 months, and index education > primary. A risk score of ≥ 2 had a sensitivity of 68% and specificity of 78% in identifying partners unlikely to seek testing on their own. A risk score to target partner notification can reduce the resources required to locate all partners in the community while increasing the testing yield compared to patient-referral.
Keywords: HIV/AIDS, Partner Notification, Contact Tracing, sub-Saharan Africa
Introduction
HIV counseling and testing provides an opportunity for prevention and an entry point to clinical care. Few infected persons in sub-Saharan Africa know their HIV status[1,2]. The sexual partners of individuals diagnosed with HIV infection are an important population to target for counseling and testing; HIV serodiscordance within couples is common in Africa[3,4] and thus uninfected partners can take steps to stay uninfected. Conversely, if the partner is infected, they can benefit from evaluation for antiretroviral treatment. Providing counseling and testing to partners of individuals recently diagnosed with HIV infection is an important way to target prevention strategies and provide early care to a very high risk population.
HIV Partner notification is a strategy to increase counseling and testing among sexual partners of HIV-positive persons and involves informing partners that they have been exposed to HIV and encouraging them to seek counseling, testing and other diagnostic and preventive services[5]. Partner notification programs were developed in the 1930s and 1940s as part of the U.S. and British syphilis control efforts, and were later expanded to include gonorrhea, chlamydial infection, and HIV. Partner notification programs are implemented in every state in the United States, and programs in both the United States and Europe have been found effective in identifying previously undiagnosed HIV infections[6–9]. However, strategies to increase partner HIV testing have not been evaluated in developing countries[5] and little is known about partner testing behavior in these settings. To date, the standard of care for partner notification in sub-Saharan Africa is patient referral.
We conducted a randomized trial in Africa to determine the effectiveness of partner notification strategies to increase partner referral in Lilongwe, Malawi. The overall partner counseling and testing rate was 35% and provider-assisted referral methods of partner notification were twice as effective than when the patient was completely responsible for notification themselves (patient referral) and resulted in the most rapid evaluation of partners [10]. However, provider-assisted methods of referral are resource-intensive and usually require additional counseling and field staff and transportation. Ideally, only those partners who are unlikely to respond to patient referral should be targeted with provider-assisted referral, while partners more likely to report rapidly on their own for counseling and testing should be given the opportunity to do so. Understanding the characteristics of the partners and index patients associated with returning to the clinic for HIV counseling and testing will inform the implementation of partner notification programs in sub-Saharan Africa and suggest sub-populations to be targeted for future provider-assisted partner notification efforts.
We therefore sought to identify index patient and partner characteristics predicting partner uptake of HIV counseling and testing by creating a risk score algorithm to predict partners unlikely to report for counseling and testing on their own.
Methods
Study setting and population
Individuals with newly diagnosed HIV infection at the Kamuzu Central Hospital and the Bwaila Hospital outpatient STI clinics in Lilongwe, Malawi were recruited into a trial of HIV partner notification[10]. Patients from Lilongwe who had a positive HIV test result for the first time, were 18 years or older, report being sexually active in the last 90 days, were willing and able to provide locator information for their sexual partners, and agreed to be randomized to a method of partner notification were eligible to participate. Index patients were randomized to one of three methods of HIV partner notification: patient referral, contract referral, or provider referral. The patient referral group was responsible for notifying their partners themselves. The contract referral group was given 7 days to notify their partners, after which a health care provider contacted partners who had not reported for counseling and testing. In the provider referral group, a community counselor notified partners directly. Partners to index patients enrolled in the passive and contract referral arms were included in this analysis because they were the groups that represented partners who had the opportunity to report to the clinic on their own within 7 days.
Data collection
All index patients answered a short questionnaire that included items related to demographics and recent sexual behavior, including the number, type, and locations of sexual partners in the past three months. While index patients might have been infectious for years prior to their diagnosis, 3 months was chosen as a time period of eligibility in order to capture the most recent partners and to minimize recall bias by the index patients. Clinical staff performed a physical examination and patients received treatment for STI based on the Malawi Syndromic Management guidelines. All were provided referral cards to give to their partners and had blood drawn for CD4 counts using flow cytometry (Epics-XL, Coulter). Index patients received their randomization assignment at the end of their enrollment visit (after all partner data and locator information had been collected).
Partners were identified when they visited the clinic if they presented a partner referral card or by cross-checking a log of named partners with all incoming patients to the clinic. Partners were tested for HIV under the opt-out testing protocol that is standard of care. HIV antibody-negative or -indeterminate specimens were tested for the presence of HIV RNA using the ultrasensitive Roche Amplicor Monitor HIV RNA assay.
Data was double-entered into a Microsoft Access database and checked for accuracy.
Data analysis
The predictive model includes all partners with locator information provided by the enrolled HIV-positive index patients who had the opportunity to report to the clinic on their own accord (i.e. all partners of index patients enrolled in the patient and contract referral arms). The outcome for the model was failing to report to the clinic for counseling and testing on their own accord, without tracing by community counselors (for the contract referral arm only after 7 days). Partners of index patients in the contract referral arm who reported to the clinic after contact with a community counselor were considered not returning on their own. All partners were given 30 days to present for testing and counseling. The majority (81%) of partners who presented on their own did so within the first 7 days. While it is conceivable that partners in the contract referral arm who came after a visit from the health staff might have come on their own if they had been given enough time, analysis of the characteristics of these partners showed that they were more similar to the partners in the passive referral arm who never came[10].
All data was provided by the index during their enrollment visit.
Variables hypothesized a priori to be predictors of partner reporting for counseling and testing included index characteristics and partner characteristics. Index characteristics included age of the index, enrollment site (KCH vs. Bwaila STI clinics), diagnosis of genital ulcer disease on physical examination, and index education. Age of the index patient was considered to be more reliable than age of the partner as many index patients were unable to provide the age of their partner. Therefore age of the index was used rather than age of the partner in order to increase the accuracy of the model and minimize missing data. Partner characteristics included partner gender, partner type (main partner vs. non-main partner), duration of partnership, and transportation barriers. In this setting HIV transmission is predominantly through heterosexual sex, and in the study population male partner gender was associated exclusively with female index gender.
Main partners were defined as spouses and live-in partners, or boyfriend/girlfriend if the index did not name a spouse or live-in partner. Non-main partners included regular casual partners, infrequent casual partners, sex workers and boyfriend/girlfriend if the index already had a spouse. Duration of partnership was categorized at less than 6 months, 6 – 24 months, and greater than 24 months. This categorization was motivated to distinguish new partnerships, use information about the dose-response relationship between length of partnership and partner testing, and ensure adequate strata sizes for multivariate modeling. Age of the index was dichotomized at age less than 25 since younger age groups (15–19 and 20–24) in Malawi are more likely to report high-risk partnerships. Age of the index was used rather than age of the partner because the index was often unable to provide the age of their sexual partners. Genital ulcer disease (GUD) of the index was determined during physical examination by a clinician during the enrollment visit. The diagnosis of genital ulcer disease was hypothesized to influence partner testing because the partner may also have genital sores and be motivated to seek treatment for him or herself. GUD is also a know co-factor for HIV transmission, increasing the risk for both transmission and acquisition.
Index education was dichotomized at completed primary education or less compared to greater than primary education. Sentinel surveillance data on HIV infection in Malawi suggests higher education is associated with HIV infection and previous analysis of the KSU database showed individuals with greater than a primary education were less likely to consent to HIV testing (more likely to opt-out). Education was coded as a dichotomous variable with a cut-point at completion of primary education because of the similarity of participants within these binary strata of education. Since primary education is free in Malawi the greatest difference exists between persons with a primary education or less and persons with any secondary education.
Partner area of residence was described by the index when they provided locator information about the each partner. Transport barriers were considered high if the partner did not live in an area served by the public transport network in Lilongwe or if the partner would need to pay for more than one mode of transport in order to reach the clinic.
We first calculated unadjusted odds ratios for partner failing to report and all partner characteristics (male gender, non-main partner, relationship duration less than 6 months, relationship duration 6 – 24, months and transport barriers) and index characteristics (age less than 25 years, enrollment site, STI other than GUD diagnosed, and greater than primary education).
We used unconditional multiple logistic regression to develop a predictive model of the partner failing to report with a cluster robust variance estimator[11] to account for the possibility of multiple partners per index case. All variables and an interaction term for gender were entered into the full model. We constructed a simplified final model using backwards selection with a predetermined stopping rule of p = 0.10 to maintain predictive ability and reduce the likelihood of omitting important variables. Nested models were compared using likelihood ratio tests. We assessed model accuracy for all using area under the receiving operator characteristics curves.
In order to create a simple instrument that can be applied in the field we assigned each variable in the final model a predictor score equal to its beta coefficient (natural log of the adjusted odds ratio) rounded to the nearest integer[12]. We summed the risk scores to obtain a risk score for each participant. The sensitivity of the model is the probability a partner who needs tracing will be correctly identified by the model for tracing. The specificity of the model is the probability a partner who will come in on their own will not be traced unnecessarily. We calculated the proportion of partners requiring tracing by a community counselor under each risk score scenario as:
| (equation 1) |
Where Ptraced = proportion of partners traced, Se = sensitivity, Sp = specificity, and PNR= proportion failing to report
We calculated the proportion of partners traced unnecessarily as:
| (equation 2) |
Where Punnecessary = proportion of partners traced unnecessarily, Se = sensitivity, Sp = specificity, PNR= proportion failing to report, Ntraced = number of partners traced
In the clinical trial, the proportion of partners who tested under universal provider assisted referral was 51%[10], which is also consistent with the proportion of partners tested under provider-assisted referral in the only other randomized trial of partner notification[10]. The estimated proportion of partners tested under each risk score includes the proportion of partners who are tested after tracing plus the proportion of partners who come in on their own without tracing and was calculated as:
| (equation 3) |
where Ptested = estimated proportion of partners tested, Sp = specificity, PNR= proportion failing to report, Ptraced = proportion of partners traced
A false positive was a partner who was identified using the risk score algorithm for provider-assisted referral, but would have reported to the clinic on their own. A false negative is a partner who was not identified for provider-assisted referral using the risk score algorithm and did not receive testing. The relative costs of false negatives and false positives were compared at different model cut-points using the formula:
| (equation 4) |
where p is the prevalence of partner testing on their own (21%), Sp1 and Se1 are the specificity and sensitivity of universal provider-assisted referral (cut-point=0) and Sp2 and Se2 are the specificity and sensitivity of each cut-point.
The cases of HIV missed for each cut-point relative to universal provider-assisted referral was calculated as the prevalence of HIV among tested partners (64%)[10] times the number of false negatives.
We performed internal validation of the modeling strategy and model performance using 1000 bootstrap repetitions[13]. We used STATA v.10 (College Station, Texas) for all analyses.
Ethical considerations
The Institutional Review Board at the University of North Carolina, Chapel Hill and the National Health Sciences Research Committee in Malawi approved the protocol. Informed consent was obtained from all participants prior to participation.
Results
Among 187 partners to 159 index patients in the passive or contract referral arms who had the opportunity to report to the clinic on their own, 170 had locator information and were included in the analysis. Thirty-seven (21.8%) partners reported to the clinic on their own volition. Slightly more than half of partners were male most were classified as main partners, and most named only a single partner in the previous three months [Table 1].
Table 1.
Index and partner characteristics (n = 170)
| Characteristic | N | % |
|---|---|---|
| Partner Characteristics | ||
| Partner gender | ||
| Male | 93 | 45.3% |
| Female | 77 | 54.7% |
| Partner type | ||
| Main partner | 142 | 83.5% |
| Non-main partner | 26 | 16.5% |
| Length of partnership | ||
| < 6 months | 45 | 26.5% |
| 6 – 24 months | 42 | 24.7% |
| > 24 months | 83 | 48.8% |
| Face transport barriers | ||
| Yes | 84 | 49.1% |
| No | 86 | 50.9% |
| Index Characteristics | ||
| Age of index | ||
| < 25 years | 52 | 30.6% |
| ≥ 25 years | 118 | 69.4% |
| Enrollment site | ||
| Bwaila | 21 | 12.4% |
| Kamuzu Central Hospital | 149 | 87.7% |
| GUD diagnosed | ||
| Yes | 38 | 22.4% |
| No | 132 | 77.6% |
| Index Education | ||
| ≤ Primary education or less | 95 | 55.9% |
| > Primary education | 75 | 44.1% |
In bivariable analysis, male partners, being a non-main partner, relationship duration of less than 6 months, and an STI other than GUD diagnosed in the index was associated with a partner not reporting to the clinic without community tracing [Table 2]. Partner type was not included in multivariate analysis because of co-linearity with relationship duration. The final model predicting failure to report to the clinic included male partner gender, relationship duration < 6 months, relationship duration 6 –24 months and greater than primary education in the index. The area under the receiver operator characteristic curve for the final model was 0.76 (95% CI 0.67 – 0.83). Male gender, index education greater than primary, and relationship duration 6 – 24 months were assigned a score of 1 and relationship duration less than 6 months was assigned a score of 2 in the risk score algorithm [Table 2]. The area under this receiver operator characteristic curve for the risk score was 0.76 (95% CI 0.67 – 0.84).
Table 2.
Unadjusted and adjusted odds ratios and risk scores for index and partner characteristics predicting partner failing to present for HIV counseling and testing (n = 170)
| Predictor | OR | 95% CI | aOR | 95%CI | β | Score |
|---|---|---|---|---|---|---|
| Partner Characteristics | ||||||
| Gender | ||||||
| Female | 1.0 | 1.0 | ||||
| Male | 2.3 | 1.1 – 4.8 | 3.5 | 1.6 – 8.3 | 1.3 | 1 |
| Type of Partner | ||||||
| Main partner | 1.0 | |||||
| Non-main partner | 8.8 | 1.2 – 67.4 | ||||
| Partnership duration | ||||||
| < 6 months | 6.4 | 1.8 – 22.5 | 10.5 | 2.8 – 39.9 | 2.4 | 2 |
| 6 – 24 months | 2.3 | 0.9 – 5.8 | 2.6 | 1.0 – 6.9 | 0.9 | 1 |
| > 24 months | 1.0 | 1.0 | ||||
| Faced transport barriers | ||||||
| Yes | 1.0 | 0.5 – 2.0 | ||||
| No | 1.0 | |||||
| Index Characteristics | ||||||
| Age | ||||||
| < 25 years | 2.6 | 1.0 – 6.7 | ||||
| >= 25 years | 1.0 | |||||
| Enrollment site | ||||||
| Bwaila | 0.7 | 0.2 – 1.8 | ||||
| Kamuzu Central Hospital | 1.0 | |||||
| STI diagnosed | ||||||
| GUD | 2.1 | 0.9 – 4.7 | ||||
| Other | 1.0 | |||||
| Education status | ||||||
| <= Primary Education | 1.0 | |||||
| > Primary Education | 2.1 | 0.9 – 4.6 | 2.2 | 1.0 – 5.2 | 0.8 | 1 |
The model includes all partners with the opportunity to present on their own for HIV counseling and testing (passive and contract referral). The outcome is partners failing to present for HIV counseling and testing on their own.
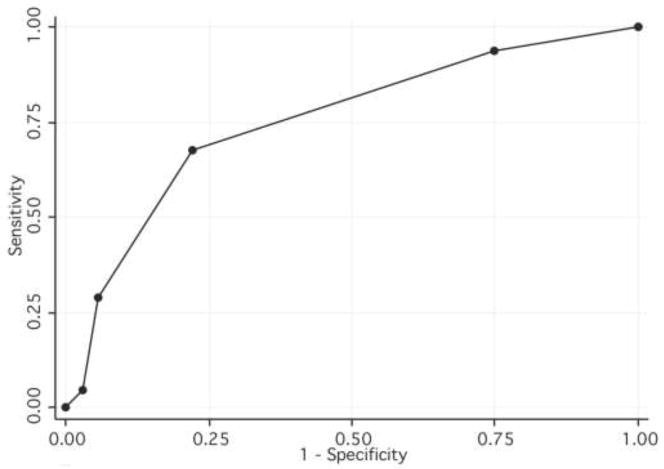
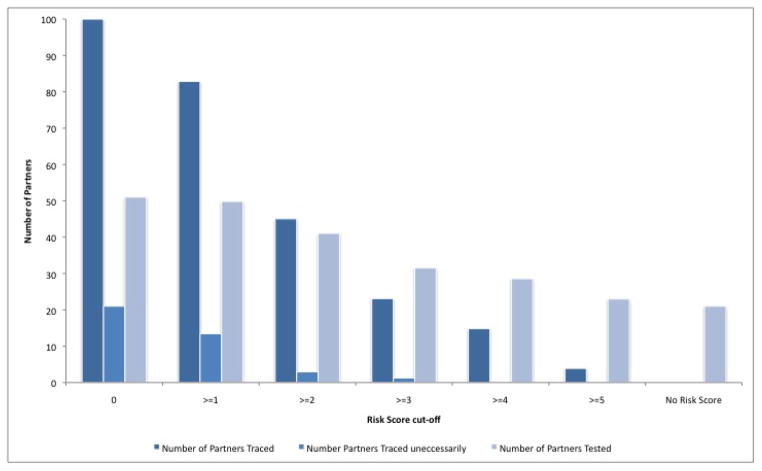
A risk score cut-off of ≥3 has a sensitivity of 29% and a specificity of 94% [Table 3, Figure 1] for identifying partners unlikely to report to the clinic on their own volition. Under this scenario, only 24% of all partners would be traced by a community counselor and 32% of all partners are expected to be tested [Table 3, Figure 2].
Table 3.
Sensitivity and specificity of risk score to predict partners unlikely to report for counseling and testing, proportion of partners traced and tested for each risk score cut-off, and errors in a population of 100 partners
| Risk Score Cut-off | Sensitivity* | Specificity† | Proportion of partners traced‡ | Proportion of partners traced unnecessarily§ | Proportion of partners tested# | False Positives** | False Negatives†† | Total Errors‡‡ | Cases of HIV missed relative to universal provider-assisted referral§§ | Relative cost of false negatives relative to false positives## |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100% | 0% | 100% | 21% | 51% | 21 | 0 | 21 | 0 | |
| >=1 | 94% | 25% | 84% | 17% | 51% | 16 | 0 | 16 | 0 | 15.7 |
| >=2 | 68% | 78% | 45% | 8% | 46% | 5 | 5 | 10 | 3 | 9.2 |
| >=3 | 29% | 94% | 23% | 5% | 32% | 1 | 19 | 20 | 12 | 5.0 |
| >=4 | 5% | 97% | 15% | 14% | 23% | 1 | 28 | 29 | 18 | 3.8 |
| No Tracing | 0% | 100% | 0% | 0% | 21% | 0 | 30 | 30 | 19 | 3.8 |
Sensitivity (Se) is the proportion of partners who require tracing (those who fail to report on their own) who are traced under each risk score cut-off
Specificity (Sp) is the proportion of partners who will report on their own who are not traced (correctly identified as not requiring tracing)
Proportion traced by counselor = Se*Pno report + (1−Sp)*(1−Pno report), where Pno report = proportion failing to report
Proportion of partners traced unnecessarily is (1−Sp)*(1−Pno report)/[Se*Pno report + (1−Sp)*(1−Pno report)] = (1−Sp)*(1−Pno report)/Npartners traced, where Se = sensitivity, Sp = specificity, Pno report = proportion failing to report, Npartners traced = number of partners traced
Proportion of partners tested = 0.51*NTtraced + Sp*(1 − Pno report)
A false positive was a partner who was identified using the risk score algorithm for provider-assisted referral, but would have reported to the clinic on their own.
A false negative is a partner who was not identified for provider-assisted referral using the risk score algorithm and did not receive testing.
Total errors is the number of false positives plus the number of false negatives
The cases of HIV missed for each cut-point relative to universal provider-assisted referral was calculated as the prevalence of HIV among tested partners (64%) [10] times the number of false negatives.
The relative costs of false negatives and false positives were compared at different model cut-points using the formula cost = (1−p)*[(1−Sp1) − (1−Sp2)]/p*[(1−Se2) − (1−Se1)] where p is the prevalence of partner testing on their own (21%), Sp1 and Se1 are the specificity and sensitivity of universal provider-assisted referral (cut-point=0) and Sp1 and Se1 are the specificity and sensitivity of each cut-point.
Figure 1. Receiver Operating Characteristics Curve for different risk score cut-offs.
ROC curves plot sensitivity versus 1 – specificity for all possible cut-offs of an algorithm. A perfect algorithm would arch to the upper left corner; an algorithm with no useful discrimination is a diagonal line connecting the lower left to upper left corners. The Area under the ROC curve for the algorithm is 0.76 (95% CI 0.67 – 0.84).
Figure 2. Number of partners traced, number of partners traced uneccessarily, and number of partners tested for different risk score cutoffs in a population of 100 partners.
In a population of 100 partners when no partners are traced 21 are expected to report to the clinic on their own and 79 are not expected to report to the clinic on their own volition. When all partners are traced 51% are expected to report for counseling and testing[10]. The number of partners traced is calculated as [sensitivity × 79 partners + (1 − specificity) × 21 partners]. The number of partners traced uneccessarily is the number of partners who are traced but would have reported to the clinic on their own accord and is calculated as (1 − specificity) × 21 partners. The total number of partners tested is calculated as 0.51 × [number of partners traced) + (specificity × 21 partners).
When the cut-off is ≥2, more partners would be referred for tracing immediately and the specificity still remains high. The sensitivity increases to 68% (95% CI 60%–75%), with specificity at 77% (95% CI 70%–84%); 58% of all partners would be traced with provider-assisted notification and 46% of all partners are expected to be tested using a risk score cut-off of ≥2. When the risk score cut-off is ≥2 all new partners in the last 6 months are targeted for provider-assisted referral.
At a risk score cut-off of ≥2, both the false negative and false positive rates are low. When all partners are referred for provider-assisted referral the false positive rate is 21%. If false positives and false negatives are weighted equally, then a cut-off of ≥2 minimizes both, and only misses 3 (9%) diagnoses of HIV relative to universal provider-assisted referral [Table 3]. In our setting and using the cutoff of ≥2, the relative costs of false negatives must be 9 times as costly as false positives to justify tracing all partners.
The bootstrapped samples yielded the same predictors following backward elimination. Confidence intervals derived from bootstrap validation were consistent with the original analysis.
Discussion
Reaching the sexual partners of persons testing positive for HIV is critical for both potential treatment and prevention. HIV partner notification is one method to reach this high-risk population. Despite its potential, HIV partner notification has yet to be implemented widely in sub-Saharan Africa[14] and many logistics must still be determined, including how and whom to target with provider-assisted referral.
We have shown that HIV partner notification, including provider-assisted techniques, is feasible in this setting. However, because provider-assisted HIV partner notification will require additional human resources to be effective, it is essential to determine best strategies and practices. Prediction models for HIV acquisition have been used to target counseling messages and interventions[15–17]. Here, we predict who is unlikely to respond to partner notification by the index patient alone in order to direct provider-assisted referral.
Using a risk score to target provider-assisted referral can reduce the resources required to trace clients in the community compared to universal provider-assisted referral and increase partner testing compared to patient-referral. For example, when the risk score cut-off is ≥2 less than two-thirds of the resources can be used to yield more than 90% of the partners tested under universal provider-assisted referral. Identifying populations to target provider-assisted referral will inform policy-makers and implementing agencies.
Rapid referral of partners is preferred, and our experience in the field suggests partners are more likely to be located the earlier they are traced. We have also observed that a high proportion of partners who are located and notified of their exposure present for counseling and testing. Urban populations in sub-Saharan Africa are highly mobile. Delay in partner tracing reduces the ability to locate and refer partners for counseling and testing. However, tracing all sexual partners may not be feasible, particularly in resource constrained settings. Given the resources involved in community tracing, the ideal risk score cut-off should have a sufficiently high specificity to minimize tracing partners who are likely to come in on their own, while simultaneously identifying a large proportion of those who would not come in on their own without provider assistance. In the Malawi setting, using an easy to implement risk score with a cut-off of ≥2 would result in almost 70% of partners who are unlikely to report for testing and counseling on their own to be referred to provider-assisted referral immediately and very few partners being traced unnecessarily.
The relative costs of false positives and false negatives need to be considered when determining the optimal cutoff, or whether universal referral should be implemented at all. The costs of a false positive are the resources involved in locating partners in the community and the potential social costs, such as stigma and loss of confidentiality, which in our Malawi experience were minimal. The costs of a false negative are the partner not receiving testing and perhaps not being notified, necessary prevention, and if the partner were HIV-infected, the costs of not accessing treatment services and the potential for onward transmission related to lack of awareness of one’s HIV status. If false negatives and false positives are weighted equally, then the cut-point of 2 minimizes both and the fewest errors are realized. If the costs of missing the opportunity to test a partner and prevent an HIV transmission event are weighted more strongly, then a lower cut-point or universal testing would be preferred. At a cut-point of 2 a false positive must be 9 times more costly than a false negative to justify immediate provider-assisted referral for all partners. The relative costs may differ based on the setting and must consider the HIV prevalence, the availability of prevention and treatment services, and the capacity to follow-up with partners in the community. Additionally, the proportion of partners tested under provider-assisted referral may vary between settings. Ultimately, the utility of targeted provider-assisted partner notification strategies depends on effective interruption of transmission to negative partners and effective linkage to care for the partners found to be infected. Future modeling that varies these parameters will estimate the projected impact in different settings.
Provider-assisted partner notification could be an important tool to increase referral among male partners, who we found are unlikely to be notified and be tested on their own. All female patients who test positive for HIV could be presented the option of provider-assisted referral during post-test counseling. The results of our predictive model suggest provider-assisted partner notification strategies could also help increase the proportion of new partners and casual partners who receive testing earlier. A clinic policy assigning provider-assisted referral for all women and all partnerships of less than six months may be a simple way to increase partner testing.
While the model we developed here may be useful in an STI clinic setting, its generalizability to other settings where partner notification may be implemented may be limited. Partners of index patients seeking treatment for STIs might be more motivated to respond because of the potential for STI treatment. However, STI diagnosis was not an important predictor in the final model and was not included in the risk score. Future validation in a variety of HIV counseling and testing settings where partner notification is implemented will help refine the algorithm’s usefulness.
The small number of events limited our ability to examine additional potential predictors. Also, the ability of a model to predict future events in new populations is reduced when too many parameters are used to estimate for the amount of information in the data. Internal validation using bootstrapping was performed, however the precision and optimism of the model performance may be exaggerated by the over fitting of the model. The model should be refined, including expanding the predictors examined, in future, larger study populations to improve its usefulness as a clinical screening tool. However, very little is currently known about partner testing in sub-Saharan Africa and these characteristics will continue to be refined in practice as partner notification is implemented in the region.
HIV partner notification is new to sub-Saharan Africa. Understanding index and partner responses and who to target with different strategies will guide efficient and effective implementation. All partners are at high risk for HIV infection and contact with a provider greatly increases the probability of partners receiving testing. A simple, easy to calculate risk score may be a useful tool for partner notification programs to target provider referral and increase the yield of partner notification efforts while controlling implementation costs.
Acknowledgments
This research was supported by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program #P30 AI50410, NIH grant 1F30MH085431, NIH grant 5R01AI83059 and NIH grant T32 GM008719.
References
- 1.Bunnell R, Opio A, Musinguzi J, et al. HIV transmission risk behavior among HIV-infected adults in Uganda: results of a nationally representative survey. Aids. 2008 Mar 12;22(5):617–24. doi: 10.1097/QAD.0b013e3282f56b53. [DOI] [PubMed] [Google Scholar]
- 2.Malawi Demographic and Health Survey 2004. Calverton, Maryland: National Statistical Office (Malawi) and ORC Macro; 2005. [Google Scholar]
- 3.Malamba SS, Mermin JH, Bunnell R, et al. Couples at risk: HIV-1 concordance and discordance among sexual partners receiving voluntary counseling and testing in Uganda. J Acquir Immune Defic Syndr. 2005 Aug 15;39(5):576–80. [PubMed] [Google Scholar]
- 4.Guthrie BL, de Bruyn G, Farquhar C. HIV-1-discordant couples in sub-Saharan Africa: explanations and implications for high rates of discordancy. Curr HIV Res. 2007 Jul;5(4):416–29. doi: 10.2174/157016207781023992. [DOI] [PubMed] [Google Scholar]
- 5.Mathews C, Coetzee N, Zwarenstein M, et al. A systematic review of strategies for partner notification for sexually transmitted diseases, including HIV/AIDS. Int J STD AIDS. 2002 May;13(5):285–300. doi: 10.1258/0956462021925081. [DOI] [PubMed] [Google Scholar]
- 6.Recently diagnosed sexually HIV-infected patients: seroconversion interval, partner notification period and a high yield of HIV diagnoses among partners. Qjm. 2001 Jul;94(7):379–90. doi: 10.1093/qjmed/94.7.379. [DOI] [PubMed] [Google Scholar]
- 7.Harry TC, Sillis M. Outcome of partner notification of HIV infection in a provincial clinic in East Anglia, UK. Int J STD AIDS. 2008 Jan;19(1):53–4. doi: 10.1258/ijsa.2007.007070. [DOI] [PubMed] [Google Scholar]
- 8.Marcus JL, Bernstein KT, Klausner JD. Updated outcomes of partner notification for human immunodeficiency virus, San Francisco, 2004–2008. Aids. 2009 May 15;23(8):1024–6. doi: 10.1097/QAD.0b013e32832921a7. [DOI] [PubMed] [Google Scholar]
- 9.Mir N, Scoular A, Lee K, et al. Partner notification in HIV-1 infection: a population based evaluation of process and outcomes in Scotland. Sex Transm Infect. 2001 Jun;77(3):187–9. doi: 10.1136/sti.77.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown LB, Miler WC, Kamanga G, et al. HIV Partner Notification Is Effective and Feasible in Sub-Saharan Africa: Opportunities for HIV Treatment and Prevention. Journal of Acquired Immune Deficiency Syndromes. 2011;56(5):437–42. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000 Jun;56(2):645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 12.McClamroch KJ, Kaufman JS, Behets FM. A formal decision analysis identifies an optimal treatment strategy in a resource-poor setting. J Clin Epidemiol. 2008 Aug;61(8):776–87. doi: 10.1016/j.jclinepi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Steyerberg EW, Bleeker SE, Moll HA, Grobbee DE, Moons KG. Internal and external validation of predictive models: a simulation study of bias and precision in small samples. J Clin Epidemiol. 2003 May;56(5):441–7. doi: 10.1016/s0895-4356(03)00047-7. [DOI] [PubMed] [Google Scholar]
- 14.Tih PM, Forgwei G, Welty T, Welty S, Harrington C, editors. Integrated HIV contact tracing and partner notification in Cameroon: a feasible HIV infection risk reduction intervention for resource-poor settings. 5th IAS Conference on HIV Pathogenesis and Treatment; 2009; Cape Town. [Google Scholar]
- 15.Boileau C, Bruneau J, Al-Nachawati H, Lamothe F, Vincelette J. A prognostic model for HIV seroconversion among injection drug users as a tool for stratification in clinical trials. J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):489–95. doi: 10.1097/01.qai.0000153424.56379.61. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Branson B, Ballenger A, Peterman TA. Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients. Sex Transm Dis. 1998 Nov;25(10):539–43. doi: 10.1097/00007435-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Menza TW, Hughes JP, Celum CL, Golden MR. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009 Sep;36(9):547–55. doi: 10.1097/OLQ.0b013e3181a9cc41. [DOI] [PMC free article] [PubMed] [Google Scholar]