Abstract
Wolbachia, an endosymbiont present in filarial nematodes, have been implicated in a variety of roles, including the worm development and survival. Elucidation of the role of Wolbachia in filarial nematode biology and pathogenesis has become the focus of many studies and its contribution to parasite survival or immune response is still unclear. Recombinant Wolbachia HSP60 decreases T cell activation and lymphoproliferation in filarial infected people compared to endemic controls as observed by the assessment of T cell activation markers and cytokine responses in the peripheral blood mononuclear cells. Reduced T cell activation may be linked to T regulatory cell activity since it is associated with increased expression of CTLA4 and CD25 on CD4+ T cells in filarial infected group upon stimulation with recombinant Wolbachia HSP60. In addition, elevated interleukin-10 and TGF-β cytokines corroborate the reduced CD4+ T cell activation and interferon-γ observed upon recombinant Wolbachia HSP60 stimulation in filarial patients. Hence, these findings indicate that Wolbachia HSP60 may also contribute to the immune modulation seen in filarial patients.
Keywords: Wolbachia, Immune modulation, Filaria, Brugia malayi, Cytokines, T cells
1. Introduction
Human lymphatic filariasis (LF) is a debilitating parasitic infection caused by the nematode worms, Brugia malayi and Wuchereria bancrofti. The clinical manifestations are varied and range from the clinically asymptomatic microfilaremic infection to chronic pathology. Typically, individuals with active filarial infections have compromised antigen-specific T cell responses. Most evident is the lack of in vitro proliferation and diminished IFN-gamma (IFN-γ) responses to antigen challenge [1]. Further, it has been demonstrated that in active infections, this immune downregulation extends to bystander and exogenously administered vaccine-derived antigens [2]. Studies [3] with live L3 and mf suggested an impairment of both Th1 and Th2 cytokines in patients (IFN-γ, TNF-α, IL-4, IL-5, and IL-10). Therefore, the pattern of immune suppression in filarial infections does not map neatly across a Th1 versus Th2 profile, suggesting a role for regulatory cells and cytokines. The hyporesponsiveness to antigen-specific and polyclonal stimuli in chronic helminth infections seems to be associated with immunosuppressive cytokines like IL-10 and TGF-β that are secreted by antigen presenting cells and regulatory T cells (Treg cells) [4].
Wolbachia is a gram-negative endosymbiont bacterium of filarial nematodes, which is essential for worm fertility, survival and also contributes to the pathogenesis of filarial disease [5–7]. The severe systemic inflammatory reactions following chemotherapy are at least partially attributed to the release of Wolbachia into the circulation following worm death [8,9]. Studies on animals infected with B. malayi have shown a direct relationship between immune responses originated against Wolbachia and the development of filarial pathology [10]. Wolbachia surface protein (WSP), one of the most abundantly expressed proteins of the endosymbiont has been shown to stimulate innate immune responses through TLR2 and TLR4 in humans with Onchocerciasis [7]. In contrast, Turner et al. had shown that constant exposure to Wolbachia would drive macrophage tolerance in vitro and in vivo through TLR2 and MyD88 thereby contributing to the dysregulated and tolerized immunological phenotype observed in filarial infections [11].
Since the death and disintegration of worms are thought to expose the endosymbiont and its product into the host immune system, the possibility that other Wolbachia antigens play a role in immune regulation necessitates further investigation. In this perspective, Wolbachia heat shock protein-60 (HSP60) was shown to evoke the IgG1 antibody response in chronic filarial patients [12] compared to normals (putatively immune) by serological studies. HSP60 functions as a key signal to the immune system where its expression is upregulated under inflammation and HSP60 reactive T and B cells were demonstrated in almost all inflammatory diseases. It is well known that heat shock proteins (HSPs), highly conserved across species, induce pro-inflammatory cytokines from innate immune cells. However, HSP60 can also inhibit the secretion of pro-inflammatory cytokines from activated T cells via TLR signaling [13]. More recently, it has been shown that human HSP60 downregulates immune function by the activation of CD4+ CD25+ regulatory T cells (Tregs) [14]. In this study, we evaluated a role for recombinant Wolbachia HSP60 in modulating the immune response in filarial patients by examining the lymphocyte proliferation, T cell activation and cytokine response in filarial patients.
2. Materials and methods
2.1. Study population
Ten asymptomatic amicrofilaremic endemic normals (Uninfected) and fifteen individuals with active infection and/or lymphatic pathology (infected) were included in this study. Standardized histories were obtained and physical examinations were done on all the participant residents during epidemiological surveys in and around Chennai, India, an area endemic for W. bancrofti infection. Patients were recruited through the Filariasis Control Unit under the Directorate of Public Health (Chennai, India) after obtaining informed consent with protocols approved by the Institutional Review Board of Anna University (Chennai). All the individuals were screened for the presence of circulating filarial antigens by Og4C3 mAb ELISA, a marker of W. bancrofti infection and adult worm burden [15], (TropBio, Townsville, QLD, Australia) (Table 1).
Table 1.
Characteristics of the study population. Demographic details of the individuals included in the study.
| Group | Male/female | Median age | Range (years) | Clinical manifestations | Treatment | mf Load (mf/ml) | CFA Og4C3 (IU) |
|---|---|---|---|---|---|---|---|
| Uninfected (10) | 5/5 | 23 | 22–30 | None | None | None | None |
| Infected (15) | 7/8 | 30 | 12–40 | Grade II–IV lymph edema | DEC/heat therapy | 50–2000 | 2000–30,000 |
2.2. Antigens and mitogens
B. malayi adult worm antigen (BmA) was prepared as a saline extract as described previously [16]. Mitogen PHA (Phytohemagglutinin) was used as a positive control at a concentration of 10 μg/ml (Sigma Chemical Co; St. Louis, MO). Recombinant HSP60 (rHSP60) was expressed and purified as described previously [12]. In brief, Wolbachia HSP60 gene was PCR amplified from B. malayi genomic DNA and cloned in pRSET-A vector for expression of the recombinant protein. Recombinant plasmid was then transformed into BL21 (DE3) Escherichia coli host, and expression was induced using 1 mM IPTG, followed by purification using immobilized metal affinity chromatography. Assessment of endotoxin contamination was done using a Limulus amoebocyte lysate assay, which showed <1 pg of LPS/10 μg of protein.
2.3. Isolation and stimulation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated from the study population by density gradient centrifugation with Ficoll–Hypaque (Pancoll, Pan Biotech, GmBH). Cells were washed in RPMI 1640 medium (Pan Biotech, GmBH, HEPES (25 mM), and 80 mg gentamicin per liter of RPMI 1640) for 10 min at 1200 rpm and resuspended in the medium supplemented with 10% fetal calf serum (PAN BIOTECH, GmBH). Finally, PBMCs (0.2 × 106/well) were cultured in 96-well round-bottom tissue culture plates (Becton Dickinson, Franklin, NJ, U.S.A.) at 37 °C in a humidified 5% CO2 incubator and stimulated with 10 μg/ml of PHA (Sigma) or 10 μg/ml of soluble crude extract of BmA. Dose response study was carried out with normal healthy volunteers to determine the optimum proliferative dose of rHSP60, which was found to be 10 μg/ml. Antigen stimulated PBMCs were cultured for 96 h and PBMCs stimulated with PHA were cultured for 48 h in the presence or absence of polymyxin-B sulfate (10 μg/ml). The proliferation was quantified by [H3] thymidine (Amersham Life Science, United Kingdom) incorporation during the last 16 h of incubation. Viability of the cells was tested by trypan blue exclusion. All experiments were performed in triplicates.
2.4. Whole blood flow cytometry
Eight milliliters of heparinized whole blood was diluted twice with RPMI 1640 medium. Four milliliters of this diluted blood was seeded into 6 well tissue culture plates (Becton Dickinson, Franklin, NJ, U.S.A.) and cultured for 24 h at 37°C in a humidified atmosphere of 5% CO2 in the presence or absence of stimulation with 10 μg/ml of rHSP60, PHA and BmA. After 24 h, cells were scraped and washed with 1:10 diluted BD FACS lysing solution (BD Biosciences, San Jose, CA) in 1× PBS. Subsequently, the cells were fixed in 4% paraformaldehyde and permeabilized in PBS/0.1% saponin (J.T. Baker) for the detection of costimulatory molecules and cytokines. Staining was done with previously titrated volumes of PerCP (CD4) and PE conjugated (CD69, CD127, CD62L, CTLA4 and CD25) antibodies and incubated at 4 °C for 1 h. All the antibodies used in the study were purchased from eBiosciences. The cells were subsequently washed, and fluorescence was measured on a FACSCalibur (BD Biosciences, San Jose, CA) using 50,000 gated lymphocytes. Analysis was performed using Flow JO software (Tree Star) and the data or the stimulated conditions was expressed as the percentage of CD4+ T cells expressing a particular marker upon stimulations.
2.5. Cultures for real-time PCR
PBMCs (1 × 106 cells/ml) were cultured in cRPMI 1640 medium supplemented with 10% fetal calf serum in 24 well tissue culture plates (Becton Dickinson, Franklin, NJ, U.S.A.) for 24 h in the presence or absence of stimulation with 10 μg/ml of PHA, BmA and rHSP60. The cultures were harvested by centrifugation, following which, the supernatants were stored at −20 °C for the estimation of cytokines and the pellet was used for RNA extraction.
2.6. RNA extraction
PBMCs were lysed and the total RNA was extracted according to the manufacturer’s protocol (RNeasy Mini kit; Qiagen). RNA was dissolved in 20 μl of RNase free water.
2.7. cDNA synthesis
Reverse transcription of RNA was performed in a final volume of 40 μl containing 0.25 mM mix of the four deoxy-nucleotide triphosphates (dATP, dGTP, dTTP, and dCTP) (New England Biolabs, MA, U.S.A.); 1× reverse transcriptase buffer (50 mM Tris–HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2); 8 mM DTT; 20 U RNase inhibitor (Gibco BRL); and 200 U of MMLV-reverse transcriptase (New England Biolabs, MA, U.S.A.) followed by incubation of the tubes at 37 °C for 60 min. The reverse transcription reaction was stopped by heating the tubes at 90 °C for 5 min. The cDNAs were snap-chilled in ice for 5–10 min and stored at −20 °C until use.
2.8. Real-time RT-PCR
Real-time quantitative RT-PCR was performed in an ABI 7500 sequence detection system (Applied Biosystems) using TaqMan Assays on Demand reagents for IL-10 (Hs0017 4086_m1), TGF-β (Hs99999918_m1), IFN-γ (Hs001741 43_m1) and an endogenous 18S (4319413E) ribosomal RNA control. The end point used in real-time PCR quantification is CT that is the threshold cycle during the exponential phase of amplification, according to the manufacturer’s protocol. Quantification of gene expression was performed using the comparative CT method (Sequence Detector User Bulletin 2, Applied Biosystems) and reported as the fold change relative to the house keeping gene. To calculate the fold change, the CT of the house keeping gene (18S rRNA) was subtracted from the CT of the target gene to yield the ΔCT. Change in the expression of the target gene as a result of antigenic exposure was expressed as 2−ΔΔCT, where ΔΔCT = ΔCT of stimulated −ΔCT of unstimulated. Along with fold change, basal level expression of the same genes was also assessed.
2.9. ELISA for IL-10 and TGF-β
The levels of cytokines IL-10 and TGF-β in the culture supernatants were measured by ELISA. IL-10 was determined by Bio plex multiplex cytokine assay system (Bio rad, Hercules, CA) and TGF-β by conventional ELISA following manufacturer’s protocol (R&D, Minneapolis, MN). The lowest detection limits for the IL-10 and TGF-β were 53.2 pg/ml and 7.8 pg/ml respectively.
2.10. Statistical analysis
All statistics were performed using GraphPad Prism version 5 for Windows (GraphPad Software, Inc., San Diego, CA). Comparisons were made using the Mann–Whitney U test (for unpaired data); * denotes p < 0.05.
3. Results
3.1. Expression of activation markers on CD4+ T cells in response to rHSP60
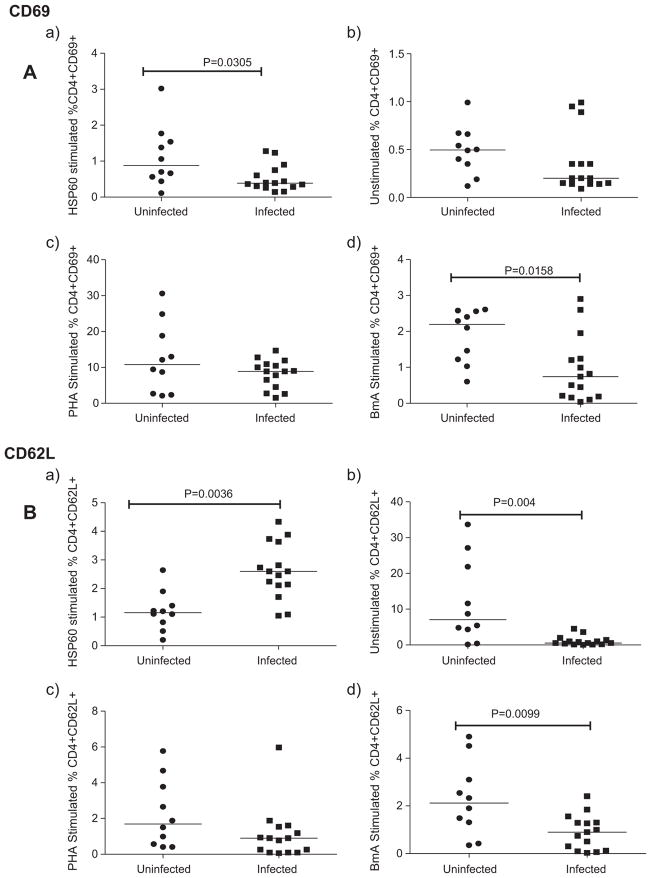
To examine the activation profile of T cells in filarial individuals upon stimulation with rHSP60, we determined the expression of activation markers – CD69, CD127 and CD62L on CD4+ T cells by whole blood flow cytometry. CD69 (very early activation antigen), a C-type lectin gets upregulated rapidly on activated T cells, while the expression of CD127 (IL-7 receptor α) and CD62L (L-selectin) is downregulated.
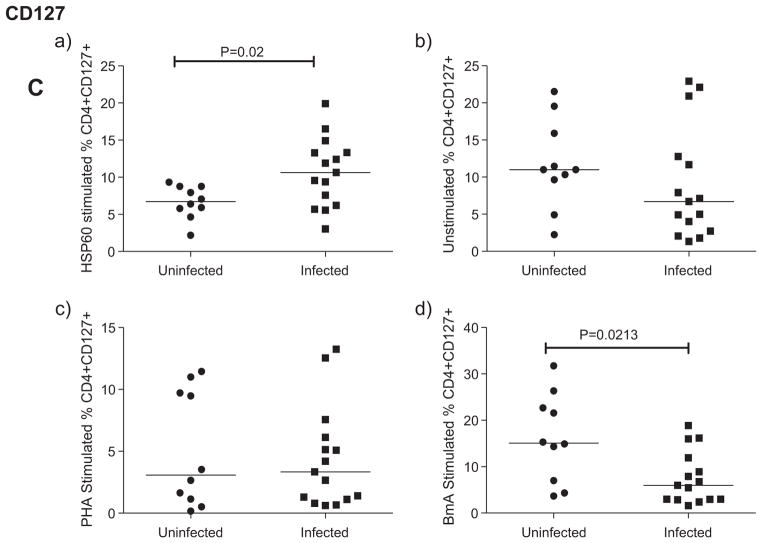
In response to Wolbachia rHSP60, CD69 expression was significantly reduced in infected patients compared to uninfected patients (Fig. 1A(a)) while the expression of CD62L was significantly increased in the infected compared to the uninfected group of individuals (Fig. 1B(a)). Similarly, the CD127expression was also upregulated in filarial patients upon stimulation with rHSP60 (p < 0.05) (Fig. 1C(a)).
Fig. 1.
rHSP60 reduced the T cell activation in filarial patients. Expression of T cell activation markers (CD69(A), CD62L(B) and CD127(C)) on CD4+ cells analyzed by flow cytometry after stimulation of whole blood with HSP60(a), BmA(b) and PHA(c) in uninfected (n = 10) and infected (n = 15) group of individuals. Each dot represents the percentage of expression of these markers and horizontal bars indicate geometric mean.
On the contrary, the crude filarial antigen BmA, greatly diminished the expression of CD69, CD127 and CD62L in filarial infected compared to uninfected individuals (p > 0.05) (Fig. 1d). Spontaneous CD62L expression was lower in infected individuals compared to the uninfected (p < 0.05) (Fig. 1B(b)), while the spontaneous expression of CD127 (Fig. 1C(b)) and CD69 did not show any significant difference among the groups (Fig. 1A(b)).
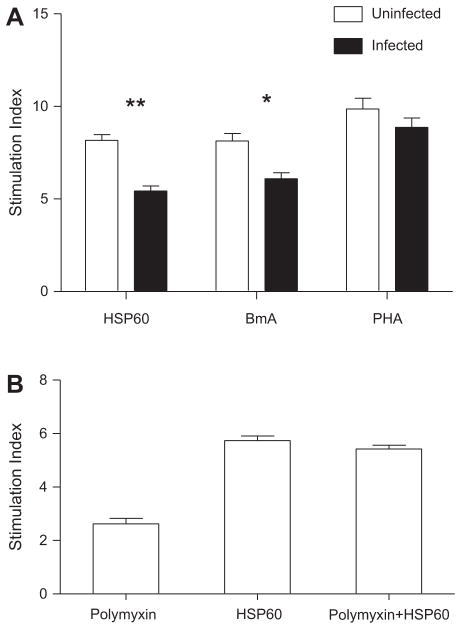
3.2. Suppressed lymphocyte proliferation in filarial patients stimulated by rHSP60
The findings from analysis on T cell activation were corroborated by the lymphocyte proliferation assay, where a significantly lower lymphocyte proliferation was observed in filarial patients when stimulated with rHSP60 (Fig. 2A). BmA significantly decreased the proliferation in the infected compared to the uninfected individuals (p < 0.05) as reported previously [17]. The proliferative responses to PHA were found to exhibit no significant differences among the groups (Fig. 2A).
Fig. 2.
A: Lymphocyte proliferation is reduced with rHSP60 in infected patients. Lymphocyte proliferation assay was carried out with PBMCs from uninfected (n = 10) and infected group (n = 15) of patients, stimulated with HSP60 and BmA (96 h) along with PHA (48 h). The results are expressed as stimulation index and the horizontal bar denotes the geometric mean of all the individuals in each group. B: Lymphoproliferative response to rHSP60 is not mediated by contaminating endotoxin. Shown is the geometric mean stimulation index for PBMCs from uninfected (n = 5) individuals following stimulation with rHSP60 in the presence or absence of polymyxin B sulfate. Error bars indicate standard deviations.
Similar lymphoproliferation observed upon stimulation of uninfected PBMC with rHSP60 (10 μg/ml) in the presence and absence of polymyxin-B sulfate (Fig. 2B) indicated that the contaminating LPS did not contribute to the proliferative responses and the observed immune regulatory effect of rHSP60 is not due to LPS contamination. Endotoxin activity of each rHSP60 preparation was measured by Limulus amoebocyte lysate assay beforehand to ensure that the preparation is essentially free of LPS contamination. This is important, since some studies suggest that the reported cytokine effects of rHSP60 may be attributed to the contaminating LPS, on account of failure to use highly purified LPS free HSP60 [18,19].
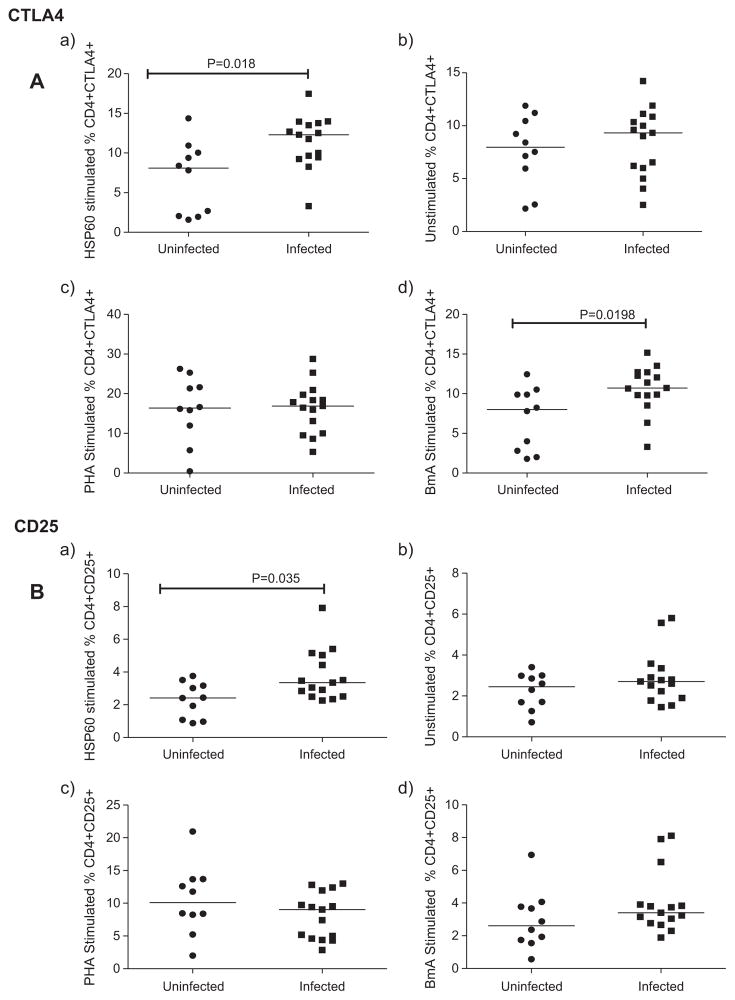
3.3. Augmented expression of CTLA4 and CD25 on CD4+ lymphocytes in filarial patients upon rHSP60 stimulation
The importance of CD4+ CD25+ CTLA4+ Tregs in immune suppression was exposed by the reversal of antigen-specific hyporesponsiveness leading to parasite clearance, upon depletion of Tregs [20]. We examined the role of CD4+ T cells expressing either CTLA4 or CD25 in filarial infections. In response to rHSP60, the expression of CTLA4, a receptor for B-7 molecules (CD80 and CD86) that is expressed on activated CD4+ and CD8+T cells, was upregulated in infected patients compared to uninfected individuals (p ≤ 0.05) (Fig. 3A(a)). Similarly, CD25 expression on CD4+ lymphocytes was also elevated upon stimulation with rHSP60 in infected compared to uninfected groups (p < 0.05) (Fig. 3B(a)). Although BmA significantly induced the expression of CTLA4 in infected patients, it did not exhibit any difference in the expression of CD25 among the groups (Fig. 3d). PHA induced expression of CTLA4 and CD25 did not show any difference among the groups (Fig. 3c). Moreover, in unstimulated controls, spontaneous expression of CTLA4 and CD25 was similar in both the groups (Fig. 3b).
Fig. 3.
rHSP60 augmented the expression of CTLA4 and CD25 in filarial patients. Expression of CTLA4 and CD25 on CD4+ cells analyzed by flow cytometry after stimulation of whole blood with rHSP60, BmA and PHA in uninfected (n = 10) and infected (n = 15) group of individuals. Each dot represents the percentage of expression of these markers and horizontal bars indicate geometric mean.
3.4. rHSP60 reduced the expression of IFN-γ in filarial patients
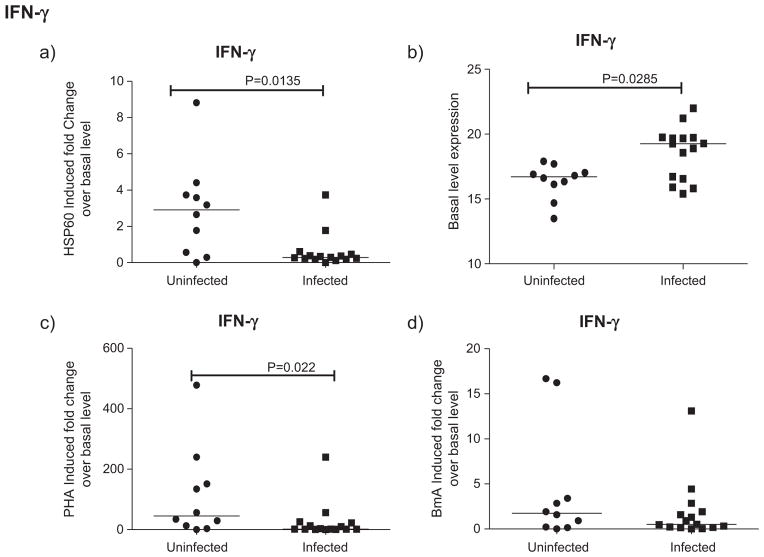
rHSP60 stimulated IFN-γ expression was reduced in infected patients compared to uninfected controls (p < 0.05) (Fig. 4a) unlike BmA, where such a change was not noticed (Fig. 4d). In contrast, the spontaneous IFN-γ expression levels were higher in infected than uninfected individuals (p < 0.05) (Fig. 4b). PHA induced IFN-γ expression was also reduced in infected patients compared to uninfected controls (p < 0.05) (Fig. 4c).
Fig. 4.
Downregulated expression of IFN-γ in rHSP60 stimulated filarial patients. IFN-γ gene expression by Real-time PCR analysis in PBMCs of uninfected (n = 10) and infected (n = 15) group of patients after 24 h stimulation with HSP60, BmA and PHA is depicted as the fold change over basal level. Horizontal bars indicate geometric mean.
3.5. rHSP60 augmented release of IL-10 and TGF-β in filarial patients
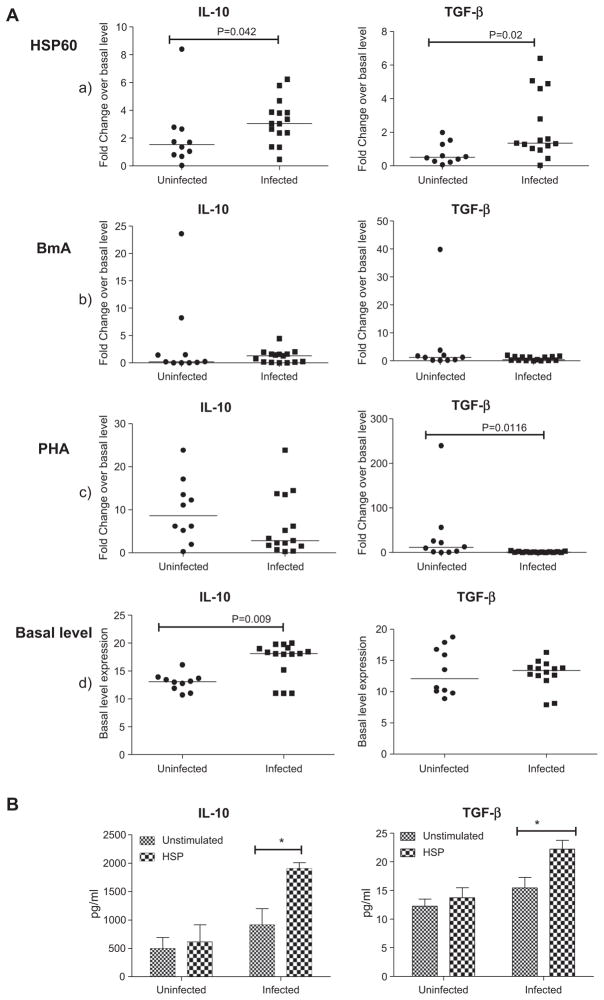
Based on the induced expression of CTLA4 and CD25 in filarial patients upon stimulation with rHSP60, the expression profile of suppressor cytokines (IL-10 and TGF-β) was determined by real-time PCR. rHSP60 significantly upregulated the mRNA expression of suppressor cytokines, IL-10 and TGF-β in infected compared to uninfected group of individuals (p < 0.05) (Fig. 5A(a)). However, BmA did not induce any significant change in TGF-β and IL-10 levels among the groups (Fig. 5A(b)). Although PHA significantly down-regulated the expression of TGF-β in infected patients, it did not exhibit any difference in the expression of IL-10 among the groups (Fig. 5A(c)). Spontaneous IL-10 mRNA expression was however, elevated in the infected groups (p < 0.05), which supports the previous findings [21], while spontaneous TGF-β expression did not show any significant change among the groups (Fig. 5A(d)).
Fig. 5.
A: rHSP60 induced mRNA expression of suppressor cytokines in filarial patients. IL-10 and TGF-β gene expression in PBMCs of uninfected (n = 10) and infected (n = 15) group of patients after 24 h stimulation with HSP60, BmA and PHA by Real-time PCR analysis is depicted as the fold change over basal level. Horizontal bars indicate geometric mean. B: rHSP60 induced IL-10 & TGF-β proteins in the culture supernatants of filarial patients. Release of IL-10 and TGF-β protein levels in the culture supernatants of PBMCs after 24 h stimulation with HSP60 was measured by ELISA. Each vertical bar represents the mean value of uninfected (n = 10) and infected (n = 15) samples.
The levels of suppressor cytokines IL-10 and TGF-β were elevated in the rHSP60 stimulated culture supernatants of filarial patients compared to the normal group of individuals as determined by ELISA (Fig. 5B).
4. Discussion
The role of Wolbachia in the pathogenesis of filarial infection and disease is a matter of intense investigation. However, while elucidation of the role of Wolbachia in filarial nematode biology and pathogenesis has become the focus of many research investigations, its contribution to parasite survival or immune response is still unclear. Previous studies by our group and others have determined the antibody response against Wolbachia antigens in the serum samples of filaria infected individuals [12,22,23]. Recently, Wolbachia lipoproteins have been implicated as the prime candidate ligands for the activation of TLR2/6 dependent innate and adaptive inflammation associated with filarial pathogenesis [24]. In this regard, functional characterization of endosymbiont antigens is imperative in understanding the elements of filarial pathogenesis.
In the present study, we have examined the role of recombinant Wolbachia heat shock protein 60 (rHSP60) in modulating the host immune responses by the analysis of lymphoproliferation, cytokine responses and T cell activation in filarial infected and uninfected individuals. The possibility of cross reactivity of Wolbachia HSP60 with HSPs of different origin can be excluded by the fact that putative amino acid sequence alignment of the HSP60 gene from nematode and other bacteria causing secondary infections in chronic pathology subjects, failed to show significant homology with HSP60 of the B. malayi Wolbachia endosymbiont (Data not shown).
rHSP60 induced T cell activation was assessed by evaluating the expression of T cell activation markers like CD69, CD127 and CD62L. Decreased CD69 along with elevated expressions of CD127 and CD62L in filarial infected patients when compared to uninfected endemic controls suggests reduced T cell activation in the former compared to the latter upon stimulation with rHSP60. Similar observations were reported earlier in different studies, with respect to the expression of these activation markers [25–27]. Reduced lymphocyte proliferation in filarial infected compared to the uninfected group of individuals upon rHSP60 stimulation supports the lesser T cell activation in the former. Bacterial HSPs, particularly HSP60 and HSP70, are shown to be capable of inducing antibody production and T cell activation in many infectious diseases [28].
Upregulated expression of CTLA4 and CD25 upon rHSP60 stimulation in the infected people may be attributed to Tregs supported by studies that show the role of CD4+ CD25+ CTLA4+ Tregs in the inhibition of protective immunity to filarial parasites in vivo [20]. The potent inhibitory properties of CTLA4 make it a prime candidate for mediating filarial immune suppression either acting directly through CD4+ CD25+ CTLA4+ Tregs or forming an independent suppressive mechanism that may act in conjunction with CD4+ Treg cells [29,30]. Moreover, elevated IL-10 and TGF-β levels corroborate the reduced CD4+ T cell activation and IFN-γ in filarial patients. Therefore, rHSP60 induced expression of CTLA4 and CD25 together with elevated IL-10 and TGF-β in patients proposes that Wolbachia HSP60 modulates the immune responses by reducing T cell activation in filarial patients. This impairment may be attributed to Treg activity and needs further assessment by determining the expression of Foxp3 and distinguishing CD25hi from CD25lo populations since the latter represents effector T cell activation. In experimental disease models, HSPs can prevent or arrest inflammatory damage, and in initial clinical trials in patients with chronic inflammatory disease, HSP-derived peptides have been shown to promote the production of anti-inflammatory cytokines, indicating immunoregulatory potential of HSPs [31]. In addition, mycobacterial HSP70 induced suppression of inflammation and tissue damage in another experimental disease through the antigen-specific IL-10 production has been reported [32].
BmA was the control in the present study, as this is being extensively used as a parasite antigen material in similar studies elsewhere [3,33,34]. BmA induced reduced lymphoproliferation and elevated CTLA4 expression in infected group supports diminished T cell reactivity and T cell anergy as mentioned previously [35,36]. On the contrary, reduced CD62L expression in the infected compared to the uninfected group of individuals indicates T cell activation in these individuals. This difference may be attributed to the heterogeneous nature of the crude parasite antigen and endorses the usage of recombinant proteins for such investigations.
Thus, Wolbachia HSP60 may contribute to the hyporesponsiveness observed in the human host, supported by the studies that showed a repeated exposure of macrophages to Wolbachia antigens induces tolerance in vitro and in vivo [11]. Moreover, Wolbachia surface protein (WSP) that readily comes in contact with the host immune system upon its release from filarial worm had also been shown to suppress the T cell activation in filarial patients when compared to endemic normals (unpublished). Chronic immune activation results in general hyporesponsiveness and anergy of the host lymphocytes, thereby contributing to the incapacity of these individuals to cope with infections [4,37].
In conclusion, our data indicate that Wolbachia HSP60 regulates immune activation that may mediate an additional level of immune tolerance along with nematode products in filarial patients, who are chronically exposed to filarial/endosymbiont antigens. Future studies should address in more detail the complex system of regulatory events that occurs upon Wolbachia exposure to immune cells, which can include downregulation of receptors and blockade of signaling pathways.
Acknowledgments
The authors wish to thank the Department of Public Health and Preventive Medicine, Government of Tamil Nadu, Chennai, India, for their help with blood samples from the filarial patients. C.S. is a recipient of a Senior Research Fellowship award by the Council of Scientific and Industrial Research (CSIR), New Delhi, India. This work was supported by grants from the Department of Science and Technology, Government of India, New Delhi and received partial support from the National Institutes of Health through NIAID/DIR/ICER program.
References
- 1.Maizels RM, Sartono E, Kurniawan A, Partono F, Selkirk ME, Yazdanbakhsh M. Cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol Today. 1995;11:50–56. doi: 10.1016/0169-4758(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 2.Suba N, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun. 2004;72:2598–2604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 4.Doetze A, Satoguina J, Burchard G, Rau T, Loliger C, Fleischer B, Hoerauf A. Antigen specific cellular hyporesponsiveness in chronic human helminth infection is mediated by Th-3/Tr-1 type cytokines IL-10 and transforming growth factor-β, but not by a Th-1 to Th-2 shift. Int Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MJ, Hoerauf A. Wolbachia bacteria of filarial nematodes. Parasitol Today. 1999;15:437–442. doi: 10.1016/s0169-4758(99)01533-1. [DOI] [PubMed] [Google Scholar]
- 6.Bandi C, Trees AJ, Brattig NW. Wolbachia in filarial nematodes: evolutionary aspects and implications for the pathogenesis and treatment of filarial diseases. Vet Parasitol. 2001;98:215–238. doi: 10.1016/s0304-4017(01)00432-0. [DOI] [PubMed] [Google Scholar]
- 7.Brattig NW, Bazzocchi C, Kirschning CJ, Reiling N, Buttner DW, Ceciliani F, Geisinger F, Hochrein H, Ernst M, Wagner H, Bandi C, Hoerauf A. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J Immunol. 2004;173:437–445. doi: 10.4049/jimmunol.173.1.437. [DOI] [PubMed] [Google Scholar]
- 8.Haarbrink M, Abadi GK, Buurman WA, Dentener MA, Terhill AJ, Yazdanbakhsh M. Strong association of interleukin-6 and lipopolysaccharide-binding protein with severity of adverse reactions after diethylcarbamazine treatment of microfilaremic patients. J Infect Dis. 2000;182:564–569. doi: 10.1086/315735. [DOI] [PubMed] [Google Scholar]
- 9.Cross HF, Haarbrink M, Egerton G, Yazdanbkhsh M, Taylor MJ. Severe reactions to filarial chemotherapy and the release of endosymbiotic Wolbachia into blood. Lancet. 2001;358:1873–1875. doi: 10.1016/S0140-6736(01)06899-4. [DOI] [PubMed] [Google Scholar]
- 10.Punkosdy GA, Dennis VA, Lasater BL, Tzertzinis G, Foster JM, Lammie PJ. Detection of serum IgG antibodies specific for Wolbachia surface protein in rhesus monkeys infected with Brugia malayi. J Infect Dis. 2001;184:385–389. doi: 10.1086/322023. [DOI] [PubMed] [Google Scholar]
- 11.Turner JD, Langley RS, Johnston KL, Egerton G, Wanji S, Taylor MJ. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J Immunol. 2006;177:1240–1249. doi: 10.4049/jimmunol.177.2.1240. [DOI] [PubMed] [Google Scholar]
- 12.Suba N, Shiny C, Taylor MJ, Narayanan RB. Brugia malayi Wolbachia hsp60 IgG antibody and isotype reactivity in different clinical groups infected or exposed to human bancroftian lymphatic filariasis. Exp Parasitol. 2007;116:291–295. doi: 10.1016/j.exppara.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Zanin-Zhorov A, Bruck R, Tal G, Oren S, Aeed H, Hershkoviz R, Cohen IR, Lider O. Heat shock protein 60 inhibits Th1-mediated hepatitis model via innate regulation of Th1/Th2 transcription factors and cytokines. J Immunol. 2005;174:3227–3236. doi: 10.4049/jimmunol.174.6.3227. [DOI] [PubMed] [Google Scholar]
- 14.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4 CD25 regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Chanteau S, Moulia-Pelat JP, Glaziou NL. Og4C3 circulating antigen: a marker of infection and adult worm burden in Wuchereria bancrofti filariasis. J Infect Dis. 1994;170:247–250. doi: 10.1093/infdis/170.1.247. [DOI] [PubMed] [Google Scholar]
- 16.Hussain R, Hamilton RG, Kumaraswami V, Adkinson NF, Jr, Ottesen EA. IgE responses in human filariasis. I. Quantitation of filaria-specific IgE. J Immunol. 1981;127:1623–1629. [PubMed] [Google Scholar]
- 17.King CL, Kumaraswami V, Poindexter RW, Kumari S, Jayaraman K, Alling DW, Ottesen EA, Nutman TB. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J Clin Invest. 1992;89:1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao B, Tsan MF. Endotoxin contamination in recombinant human Hsp70 preparation is responsible for the induction of TNF release by murine macrophages. J Biol Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 19.Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004;286:C739–C744. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- 20.Taylor MD, Harris A, Babayan SA, Bain O, Culshaw A, Allen JE, Maizels RM. CTLA4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol. 2007;179:4626–4634. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- 21.Mahanty S, Mollis SN, Ravichandran M, Abrams JS, Kumaraswami V, Jayaraman K, Ottesen EA, Nutman TB. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J Infect Dis. 1996;173:769–773. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- 22.Shiny C, Krushna NSA, Archana B, Farzana B, Narayanan RB. Serum antibody responses to Wolbachia surface protein in patients with human lymphatic filariasis. Microbiol Immunol. 2009;53:685–693. doi: 10.1111/j.1348-0421.2009.00172.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoerauf A, Volkmann L, Hamelmann C, Adjei O, Buttner DW. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet. 2000;355:1242–1243. doi: 10.1016/S0140-6736(00)02095-X. [DOI] [PubMed] [Google Scholar]
- 24.Turner JD, Langley RS, Johnston KL, Gentil K, Ford L, Wu B, Graham M, Sharpley F, Stalko B, Pearlman E, Taylor MJ. Wolbachia lipoprotein stimulates innate and adaptive immunity through toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J Biol Chem. 2009;284:22364–22378. doi: 10.1074/jbc.M901528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Hengel RL, Thaker V, Pavlick MV, Metcalf JA, Dennis G, Jr, Yang J, Lempicki RA, Sereti I, Lane HC. Cutting edge: L-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J Immunol. 2003;170:28–32. doi: 10.4049/jimmunol.170.1.28. [DOI] [PubMed] [Google Scholar]
- 27.Kiazyk SA, Fowke KR. Loss of CD127 expression links immune activation and CD4(+) T cell loss in HIV infection. Trends Microbiol. 2008;16:567–573. doi: 10.1016/j.tim.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Zugel U, Kaufmann SHE. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggena MP, Walker LS, Nagabhushanam V, Barron L, Chodos A, Abbas AK. Cooperative roles of CTLA4 and regulatory T cells in tolerance to an islet cell antigen. J Exp Med. 2004;199:1725–1730. doi: 10.1084/jem.20040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coenen JJ, Koenen HJ, van Rijssen E, Boon L, Joosten I, Hilbrands LB. CTLA4 engagement and regulatory CD4+ CD25+ T cells independently control CD8-mediated responses under costimulation blockade. J Immunol. 2006;176:5240–5246. doi: 10.4049/jimmunol.176.9.5240. [DOI] [PubMed] [Google Scholar]
- 31.Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 32.Wieten L, Berlo SE, Ten Brink CB, van Kooten PJ, Singh M, van der Zee R, Glant TT, Broere F, van Eden W. IL-10 is critically involved in mycobacterial HSP70 induced suppression of proteoglycan-induced arthritis. Plos One. 2009;4:e4186. doi: 10.1371/journal.pone.0004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravichandran M, Mahanty S, Kumaraswami V, Nutman TB, Jayaraman K. Elevated IL-10 mRNA expression and downregulation of Th1-type cytokines in microfilaremic individuals with Wuchereria bancrofti infection. Parasite Immunol. 1997;19:69–77. doi: 10.1046/j.1365-3024.1997.d01-185.x. [DOI] [PubMed] [Google Scholar]
- 34.Sasisekhar B, Aparna M, Augustin DJ, Kaliraj P, Kar SK, Nutman TB, Narayanan RB. Diminished monocyte function in microfilaremic patients with lymphatic filariasis and its relationship to altered lymphoproliferative responses. Infect Immun. 2005;73:3385–3393. doi: 10.1128/IAI.73.6.3385-3393.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steel C, Nutman TB. CTLA4 in filarial infections: implications for a role in diminished T cell reactivity. J Immunol. 2003;170:1930–1938. doi: 10.4049/jimmunol.170.4.1930. [DOI] [PubMed] [Google Scholar]
- 36.Sansom DM, Walker LS. The role of CD28 and CTLA4 in regulatory T cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 37.Borkow G, Leng Q, Weisman Z, Stein M, Galai N, Kalinkovich A, Bentwich Z. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. 2000;106:1053. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]