Abstract
The electronic structure and geometry of redox-active metal cofactors in proteins are tuned by the pattern of hydrogen bonding with the backbone peptide matrix. In this study we developed a method for selective amino acid labeling of a hyper-thermophilic archaeal metalloprotein with engineered Escherichia coli auxotroph strains, and applied this to resolve the hydrogen bond interactions with the reduced Rieske-type [2Fe-2S] cluster by two-dimensional pulsed electron spin resonance (EPR) technique. Because deep electron spin-echo envelope modulation of two histidine 14Nδ ligands of the cluster decreased non-coordinating 15N signal intensities via the cross-suppression effect, an inverse labeling strategy was employed in which 14N amino acid-labeled archaeal Rieske-type ferredoxin samples were examined in an 15N-protein background. This has directly identified Lys45 Nα as providing the major pathway for the transfer of unpaired electron spin density from the reduced cluster by a “through-bond” mechanism. All other backbone peptide nitrogens interact more weakly with the reduced cluster. The extension of this approach will allow visualizing the three-dimensional landscape of preferred pathways for the transfer of unpaired spin density from a paramagnetic metal center onto the protein frame, and will discriminate specific interactions by a “through-bond” mechanism from interactions which are “through-space” in various metalloproteins.
Introduction
Metal-based redox cofactors, such as iron-sulfur clusters, heme, copper, non-heme iron, manganese, and molybdenum ions, are essential to sustain all life forms, and some of them can be finely tuned to catalyze some of the most difficult reactions in biology.1–7 Although these cofactors are often bound to the protein matrix with the (mostly) same ligand set within each class, they can exhibit vastly different redox properties and functions. This demonstrates that accumulative natural mutations of the local non-coordinating residues around the metal binding site can modulate its electronic structure and geometry. Of particular fundamental interest is how the protein matrix achieves the appropriate setting of the metal binding site for the distinctive specificity and reactivity in vivo1–3,8–11
The interactions of the cofactors with the polypeptide matrix can be through covalent bonds (ligation) or hydrogen bonds, or can be due to non-bonding interactions, including, but not exclusively, electrostatic interactions. Among various aspects of metalloprotein structure, reorganization of a metal ligand(s) and/or hydrogen-bonding network around a redox-active site(s) is often utilized in modulating the redox potentials (Em) and functionalities in nature, as in iron-sulfur proteins,2,12–15 cupredoxins,10,16 and cytochromes;11 other strategies in myoglobins and cytochromes include changes in the hydrophobicity11,17,18 and possibly protein-induced distortion of heme-porphyrin structure,19 which can greatly influence on the heme Em and electronic properties (although the solvent effects have shown rather minimal influence in cupredoxins10). The outlook for these structural variations is that subtle interactions outside the primary coordination sphere are exclusively used to attain the required Em and functions in each metalloprotein family. X-ray crystallography is an indispensible tool to define the geometric relationships of residues around a metal cofactor-binding site, but cannot by itself define the strength of the interactions, particularly with putative hydrogen bonds around the transition metal ion sites. A fundamental understanding of the relationship between the structure and redox chemistry of metalloproteins would not only provide deeper insights into the structure and mechanism of proteins, but also facilitate the rational design of proteins with desirable chemical properties for biotechnological and pharmaceutical applications.
Protein-associated iron-sulfur clusters and their derivative cofactors play remarkably versatile roles in vivo.2,9,20,21 Of these, Rieske-type [2Fe-2S](His)2(Cys)2 clusters are involved in various electron transfer reactions such as photosynthesis, aerobic respiration, and biodegradation of various alkene and aromatic compounds.22–25 The Rieske-type clusters exhibit a wide range of the Em relevant to the in vivo functions. The Em’s span from about −150 to −50 mV for a low-potential cluster in archaeal and bacterial Rieske-type ferredoxins, to about +150 to +490 mV for a high-potential cluster in Rieske proteins from quinol-oxidizing cytochrome bc1/b6f complexes.12,23 This demonstrates that accumulative natural mutations of the local non-coordinating residues around the cluster binding site can modulate its electronic structure and geometry. Previous protein structural and electrochemical studies indicate that a lower potential cluster tends to have less extensive hydrogen bonding network around the Rieske-type cluster.12–14,26,27 The (N/O)-H•••S hydrogen bond network around biological iron-sulfur clusters is one of critical factors in modulating their redox properties. For example, a short N-H•••S hydrogen bond would lead to more efficient electron delocalization and stabilization of the negative charge in the reduced, electron-rich state, thereby preferentially increasing the Em of the cluster, whereas the deletion of a direct hydrogen bond to a sulfur ligand can lower the cluster Em by increasing the electron density on this ligand. The theoretical analysis further suggests that the distribution of negatively charged residues around the cluster modulates the pH dependence of cluster Em in the low-potential homologs.28
Pulsed EPR techniques such as electron spin-echo envelope modulation (ESEEM) and electron-nuclear double resonance (ENDOR) probe specific interaction between electron spin of a paramagnetic center and nuclear spin of its protein frame.29–31 In case of the Rieske-type [2Fe-2S](His)2(Cys)2 cluster system, strong antiferromagnetic coupling between the electron spins of the two irons produces an EPR-silent (S=0) ground state in the oxidized Fe3+-Fe3+ form, and a paramagnetic S=1/2 ground state in the reduced Fe3+-Fe2+ form. Direct interaction of the reduced Rieske-type cluster with a particular protein residue by either covalent or hydrogen bonding can result in the distribution of unpaired electron spin density onto the protein matrix. For N-H•••S hydrogen bonds with the cluster, for example, this “through-bond” transfer of unpaired spin density to the nitrogen nucleus via the overlapped electronic orbitals can be probed experimentally by pulsed EPR.32 For this purpose, the two-dimensional, four-pulse ESEEM (also called hyperfine sublevel correlation, HYSCORE) technique has become a powerful tool. Using this technique, one can quantify the nuclear frequencies of interest from electron spin ms=+1/2 and −1/2 manifolds belonging to the same nucleus, such as 1H, 2H, 14N, and 15N, which are resolved as non-diagonal cross-peak coordinates.29,32,33 In conjunction with a protein structure determined by X-ray crystallography, these data can be used to describe local spatial and electronic structures around the cluster which influence the metalloenzyme functionalities and reactivities.
X-band 14N HYSCORE spectra of the reduced Rieske-type clusters are dominated by two histidine Nδ ligands with hyperfine couplings ~4–5 MHz.32 Additionally, one or a few more non-coordinating 14N around the reduced cluster can also produce much less intense lines in the 14N spectra, as a result of the nuclear quadrupole interaction influence.32 The latter features are better pronounced in the (++) quadrant of the spectra of the proteins uniformly labeled with 15N (nuclear spin I=1/2, no quadrupole moment). These signals can potentially provide information about all 15N nuclei involved in the measurable magnetic interactions with the unpaired electron spin of the reduced cluster.33 In initial studies, two resolved splittings of 1.0–1.2 MHz and 0.3–0.5 MHz from non-coodinating 15N cross-features were tentatively assigned to peptide nitrogen(s) (Np) and remote Nε of two histidine imidazole ligands, respectively.33,34 However, the spectral variations of cross-peak intensities and lineshapes of non-coordinating 15N signals in different Rieske-type proteins indicate that such earlier interpretations were oversimplified. The aggregate weakly coupled 15N signals have additional contributions from multiple 15Np nuclei around the reduced cluster with non-equivalent couplings.35 Since these interactions can contribute to tuning the properties of the Rieske-type [2Fe-2S] cluster, further resolution of these interactions has motivated the current study.
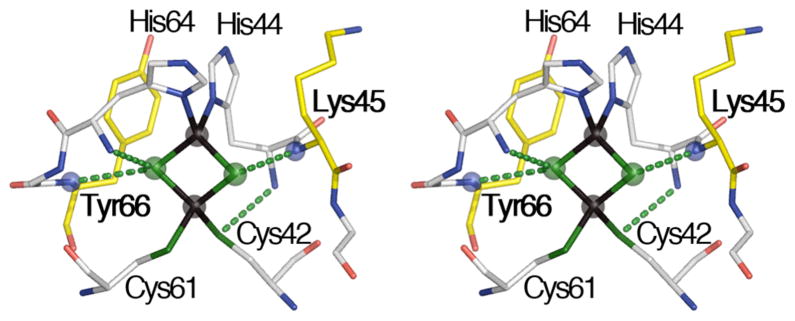
Belonging to the low-potential class of the Rieske-type ferredoxin family, a new tractable model protein of interest is the hyperthermostable archaeal Rieske-type ferredoxin (ARF) from Sulfolobus solataricus strain P1 (Em,7 ~ −60 mV), which is homologous to oxygenase-associated Rieske-type ferredoxins (DDBJ/EMBL/GenBank code AB047031).36 Recombinant ARF has been overproduced in Escherichia coli and can be obtained in appropriate forms for uniformly stable isotope labeling, site-directed ligand mutagenesis, and various spectroscopic analyses.36–38 The dithionite-reduced Rieske-type [2Fe-2S] cluster in ARF is characterized by the anisotropic EPR spectrum, as a result of a rhombic g-tensor (gz,y,x=2.02, 1.90, 1.81).36 Orientation-selective HYSCORE spectra of unlabeled (14N(N/A))32 and uniformly 15N-labeled35 ARF have been analyzed, and the lines from weakly coupled (non-coordinating) 15N of ARF showed lower intensities than those of the corresponding 15N signals of the high-potential Rieske protein homologs. Recent crystal structure of ARF refined to 1.85-Å resolution39 indicates the involvement of four possible amino acid residues in N-H•••S hydrogen bond network with the sulfur atoms of the archaeal Rieske-type [2Fe-2S](His)2(Cys)2 cluster: Lys45 Nα-Sb (N-S distance, 3.28 Å), His64 Nα-Sb (N-S distance, 3.57 Å), His44 Nα-Cys42 Sγ (3.46 Å), and Tyr66 Nα-Sb (3.53 Å) (Fig. 1). The number of possible N-H•••S hydrogen bonds around the cluster is less than those found for the high-potential homologs.39b
Figure 1.
The wall-eye stereoview of the possible hydrogen bond network around the low-potential Rieske-type [2Fe-2S](His)2(Cys)2 cluster (Fe as dark-brown spheres and bridging S (Sb) as green spheres) binding site in the ARF structure.39b Locations of Lys45 and Tyr66 (yellow sticks) and their peptide Nα atoms discussed in the text (blue spheres) are indicated.
In the current work, we demonstrate a strategy utilizing site-specific amino acid labeling and pulsed EPR spectroscopy to compare two predicted N-H•••S hydrogen bonds between backbone peptide nitrogens and the reduced Rieske-type [2Fe-2S] cluster of S. solfataricus ARF. This spectroscopic approach provides additional physicochemical parameters to characterize the through-bond interactions between the cluster and the protein matrix.
Experimental Section
Preparation of new auxotroph strains
Amino acid-selective isotope labeling is an extremely powerful method to elucidate specific contributions of particular residues in the reaction mechanisms and/or folding of a target protein by magnetic resonance and vibrational spectroscopies, often aided by the X-ray crystal structure. One of the most convenient and cost-effective procedures for selective isotope labeling of proteins is to employ amino acid auxotrophic bacteria as the host strains for the overproduction of target proteins. However, no suitable auxotrophic strains are commercially available for high-level expression of the foreign genes coding for metalloenzymes from extremophilic bacteria and archaea, because (i) their high-level expression, e.g., in Escherichia coli, often requires extra copies of tRNA genes for the cognate rare codons and (ii) specific growth conditions must be set for effective overproduction of holoproteins in a form suitable for biophysical studies.33,36
To overcome these problems, Lin et al.40 have reported the construction of a set of cost-effective, high-yield auxotrophs in commonly used E. coli expression strain C43(DE3) (Table 1). Of the amino acids selected for site-specific labeling in this study, L-tyrosine is biosynthesized by the pathways involving transaminases encoded by aspC and tyrB genes in E. coli.41 Although it was possible to delete the aspC gene from the chromosomes of E. coli C43(DE3) and BL21(DE3) strains (Fig. 2, steps 1–4), the previous multiple attempts to replace the cognate tyrB locus of the C43(DE3) strain with an antibiotic resistance cassette have not been successful, for reasons that are not clear.40 In this study we could successfully knock out the both aspC and tyrB genes from the chromosome of E. coli BL21-CodonPlus(DE3)-RIL strain (Stratagene) with a set of polymerase chain reaction (PCR) primers listed in Table S1, and the resulting new BL21(DE3) auxotroph is applicable to L-tyrosine (and probably L-phenylalanine)41 labeling (Table 1, Fig. 2). It should be added that we were not able to delete the tyrB locus in E. coli C43(DE3) strain with these PCR primers (not shown), suggesting chromosomal DNA sequence differences around the tyrB region between the BL21(DE3) and C43(DE3) expression strains.
Table 1.
New E. coli amino acid auxotroph host strains used for overexpression of foreign genes
| Strain | Precursor strain | Genes deleted | Selective amino acid labeling | References |
|---|---|---|---|---|
| ML40K1a | C43(DE3) | ilvE avtA aspC hisG argH metA lysA | Ile, Val, His, Arg, Met, Lys, Leu,b Tyrc | Ref. [40] |
| YM154a,d | C43(DE3) | cysE | Cys | Unpublishedd |
| RF3 | BL21(DE3) | aspC | N.A.e | This work |
| RF4RILa | BL21(DE3) | aspC, tyrB | Tyr, Phe | This work |
These strains can be used for overexpression of foreign genes with rare codons (Fig. 2, step 5).
In the presence of 0.4–1 mM Tyr, tyrB is repressed and Leu is required for growth in minimal medium.40 This strategy can only be applicable for a short-term cultivation but not suitable for a long-term cultivation for heterologous expression of foreign genes.
In the presence of 0.4–1 mM Tyr, tyrB is repressed and Tyr is required for growth in minimal medium.40 This strategy can only be applicable for a short-term cultivation but not suitable for a long-term cultivation for heterologous expression of foreign genes.
C43(DE3) strain YM154 is a new cysteine auxotroph that grows normally in Luria-Bertani medium, but poorly in nonlabeled CHL medium with negligible amount of L-cysteine [to be published].
Not applicable. BL21(DE3) strain RF3 is a precursor without any resistance cassette (Fig. 2, step 4) and was used for construction of the RF4RIL strain.
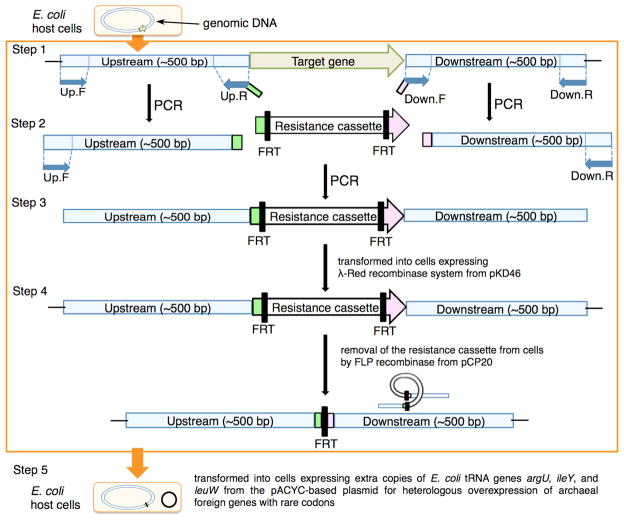
Figure 2.
Schematic procedures used for the deletion of a target chromosomal gene with the λ-Red recombination system42 (steps 1–3).40 In this work, the resistance cassette was removed from the new knock-out strain by FLP recombinase expressed from pCP20 vector43 (step 4),40 and a pACYC-based plasmid harboring tRNA genes (argU, ileY, and leuW) for the E. coli rare codons was subsequently incorporated into the resulting cells (step 5). FRT, FLP recombination target.43
We adapted some of these auxotroph strains by incorporation of a pACYC-based plasmid harboring tRNA genes (argU, ileY, and leuW) for the E. coli rare codons (Agilent Technologies) (step 5 in Fig. 2), and developed heterologous expression procedures suitable for site-specific isotope labeling of archaeal iron-sulfur proteins (see below).
Preparation of site-specifically labeled ARF samples for pulsed EPR
The arf gene coding for the hyperthermostable archaeal Rieske-type [2Fe-2S](His)2(Cys)2 ferredoxin from Sulfolobus solfataricus strain P1 (DSM 1616) has been cloned and sequenced (DDBJ/EMBL/GenBank code AB047031) and heterologously overexpressed in E. coli BL21-CodonPlus(DE3)-RIL strain (Stratagene) using a pET28aARF vector (based on a pET28a His-tag expression vector, Novagen) and purified.36 The structure of the recombinant ARF has been determined and refined at 1.85-Å resolution (to be published),39 and is depicted with PyMOL <http://www.pymol.org> (Fig. 1).
For preparation of the uniformly 15N-labeled ARF sample, the pET28aARF vector36 was transformed into the host strain, E. coli BL21-CodonPlus(DE3)-RIL (Stratagene), and the transformants were grown overnight at 25 °C in 1L culture (in 2L-flask) of the CHL-15N (~97 atm%) medium (Chlorella Industry Co. Ltd., Fukuoka, Japan) containing 50 mg/L kanamycin, 0.2 mM FeCl3, and the recombinant holoprotein was overproduced with 0.25 g/L MgSO4•7 H2O, 0.5 g/L Algal 15N(98.7–99.2%)-Amino Acid Mix (Chlorella Industry Co. Ltd., Fukuoka, Japan) and 1 mM IPTG for ~18–22 h at 25 °C.44 This system was suitable for heterologous overproduction of the uniformly 15N-labeled iron-sulfur holoproteins by employing the combination of a pET28a vector (Novagen) plus E. coli BL21-CodonPlus(DE3)-RIL host strain (Stratagene) system.44 The cells were pelleted at 4 °C by centrifugation, and stored at −80 °C until use.
For preparation of the selectively Lys 15Nα- and Cys 15Nα-labeled ARF samples [on the 14N(N/A)-protein background; see Fig. 3], the E. coli C43(DE3) auxotroph strains ML40K140 and YM154 (Table 1 and Fig. 2, step 5), respectively, were used as parent cells for heterologous overexpression of the S. solfataricus arf gene. Because of the removal of the original resistance cassette during the construction of new auxotroph strains (Fig. 2, step 4), the knock-out of each target chromosomal gene in these host cells was verified by PCR prior to use (Table S1). In addition, our previous heterologous expression strategy developed for site-specific labeling of bacterial [2Fe-2S] ferredoxin40 did not work with this archaeal metalloprotein, for which we found the absolute requirement of a much longer-term cultivation for effective heterologous production of a holoprotein form in E. coli C43(DE3) and BL21(DE3) strains. Therefore, we set the specific growth conditions for effective site-specific labeling of the ARF holoprotein in a form suitable for biophysical studies as described below.
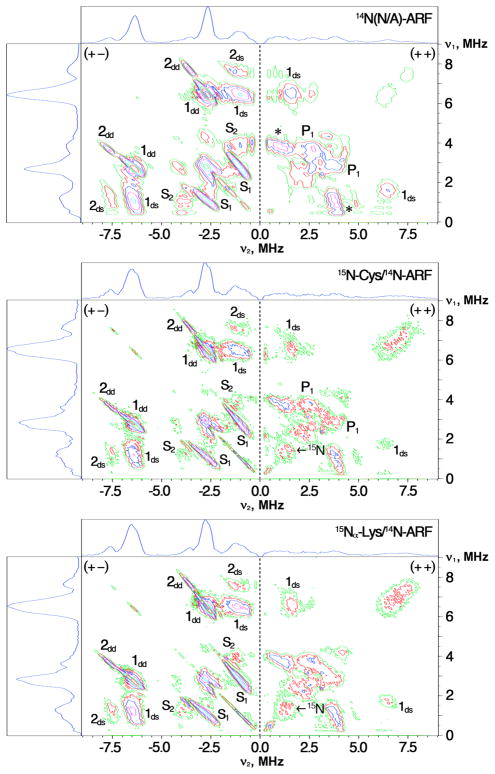
Figure 3.
HYSCORE spectra in contour presentation of the reduced ARF samples measured under the similar conditions at gy=1.90 (363.4 mT for unlabeled (14N(N/A)) ARF (control, top),32 and 363.1 mT for 15N-Cys (middle) and 15Nα-Lys (bottom) labeled ARF samples on the 14N(N/A)-protein background; τ=136 ns; 10 K). Note that the observed 15N signals from the input 15N amino acid are very weak and vague in both 15N-Cys (middle) and 15Nα-Lys (bottom) labeled samples on the 14N(N/A)-protein background, because of the intense signals from the coordinating 14Nδ(His)1 and 14Nδ(His)2 in the Rieske-type protein system32 that overshadowed and cross-suppressed the expected weakly coupled (non-coodinating) 15N cross-peaks (cf., see Figs. 4, 5). 14N nucleus can theoretically produce up to 18 cross-peaks in the HYSCORE spectra including two [dq±, dq∓], eight [dq±, sq(1,2)∓], and eight [sq(1,2)±, sq(1,2)∓] correlations, although only some of possible cross-features are usually observed in the experimental spectra of iron-sulfur proteins.32,44 1dd, 1ds and 2dd, 2ds mark dq–dq and dq–sq transitions for the coordinating 14Nδ(His)1 and 14Nδ(His)2 of ARF, respectively.32 The cross-peaks from 14Nδ(His)1: (1dd), [±6.5, ∓2.7] MHz; (1ds), [±6.5, ∓1.1] MHz and [6.5, 1.6] MHz; (S1) and (S2), sq–sq correlations at [±2.8, ∓1.1] MHz and [±4.0, ∓1.1] MHz, respectively.32 The cross-peaks from 14Nδ(His)2: (2dd), [±7.6, ∓3.5] MHz; (2ds), [±7.6, ∓ 1.2–1.3] MHz.32 Peaks P132: assigned to be Lys45 Nα (Np1) as described in the text. Peaks with asterisks: re-assigned to sq-sq correlations of the coordinating 14Nδ(His) in this work.
The pET28aARF vector36 was transformed into each strain of the resulting auxotrophs, and the transformants were grown overnight at 25 °C in a total of 2 L culture (using two 2 L-flasks, each containing 1L culture medium) of the Luria-Bertani medium containing 25 mg/L kanamycin, 17 mg/L chloramphenicol, and 0.2 mM FeCl3. The cells were harvested at 10 °C by centrifugation, and the resulting cell pellet was subsequently inoculated into a total of 2 L culture (using two 2L-flasks, each containing 1L culture medium) of the freshly prepared, nonlabeled CHL medium (Chlorella Industry Co. Ltd., Fukuoka, Japan) containing 25 mg/L kanamycin, 17 mg/L chloramphenicol, 0.2 mM FeCl3, 0.25 g/L MgSO4•7 H2O, and 0.5 g/L nonlabeled Algal Amino Acid Mix (Chlorella Industry Co. Ltd., Fukuoka, Japan), in the presence of extra unlabeled L-amino acids [0.23 g/L L-isoleucine, 0.25 g/L L-valine, 0.1 g/L L-histidine, 0.4 g/L L-arginine, 0.25 g/L L-methionine, and 0.65 g/L L-glutamic acid for selective Lys 15Nα labeling work; and 0.5 g/L L-alanine, 0.65 g/L L-glutamic acid, 0.55 g/L glycine and 2.1 g/L L-serine, and 0.025–0.05 g/L L-cysteine for selective Cys 15Nα labeling work] (purchased from either Wako Pure Chemicals, Nacalai Tesque, or Sigma Chemicals). The cells were grown in this culture in the presence of 1 mM IPTG for 5 h at 25 °C, and then further allowed to be grown continuously for another 44–50 h at 25 °C in the presence of ~0.05 g/L L-cysteine labeled at the 15Nα position (Cambridge Isotope Laboratories, Inc., Andover, MA) or 0.42 g/L L-lysine labeled at the 15Nα position (Cambridge Isotope Laboratories, Inc., Andover, MA). The cells were pelleted at 4 °C by centrifugation and stored at −80 °C until use.
For preparation of the selectively 14N(N/A) Lys- and 14N(N/A) Tyr-labeled ARF samples [on the 15N-protein background], E. coli auxotrophs C43(DE3) strain ML40K1 and BL21(DE3) strain RF4RIL (Table 1 and Fig. 2, step 5), respectively, were used as parent cells for heterologous overexpression of the S. solfataricus arf gene. The knock-out of each target chromosomal gene was verified by PCR prior to use (Table S1). The pET28aARF vector36 was transformed into each host auxotroph strain, and the transformants were grown overnight at 25 °C in 1 L culture (in 2 L-flask) of the CHL-15N (~97 atm%) medium (Chlorella Industry Co. Ltd., Fukuoka, Japan) containing 25 mg/L kanamycin, 17 mg/L chloramphenicol, 0.2 mM FeCl3, 0.25 g/L MgSO4•7 H2O, and 0.5 g/L Algal 15N(98.7–99.2%)-Amino Acid Mix (Chlorella Industry Co. Ltd., Fukuoka, Japan). The cells were harvested at 10 °C by centrifugation, and the resulting cell pellet was subsequently inoculated into a total of 2 L culture (using two 2L-flasks, each containing 1L culture medium) of the freshly prepared, CHL-15N (~97 atm%) medium (Chlorella Industry Co. Ltd., Fukuoka, Japan) containing 25 mg/L kanamycin, 17 mg/L chloramphenicol, 0.2 mM FeCl3, 0.25 g/L MgSO4•7 H2O, and 0.5 g/L Algal 15N(98.7–99.2%)-Amino Acid Mix (Chlorella Industry Co. Ltd., Fukuoka, Japan). The cells were grown in this culture in the presence of 1 mM IPTG for 5 h at 25 °C, and then further allowed to be grown continuously for another 48–50 h at 25 °C in the presence of ~0.183 g/L unlabeled L-tyrosine (Nacalai Tesque, Japan) or 0.42 g/L unlabeled L-lysine (Sigma Chemicals). The cells were pelleted at 4 °C by centrifugation.
Each ARF holoprotein sample was purified as reported previously for the unlabeled (14N(N/A)) protein,36 and concentrated with Centriprep-10 and Microcon-YM10 apparatus (Amicon) to ~0.5–1 mM in the presence of 1 M NaCl. For high-resolution pulsed EPR analysis, the resulting ARF samples (~0.5–1 mM, ~15–40 μL) were subsequently reduced with sodium dithionite under continuous flow of dry argon gas inside suprasil quartz EPR tubes (Wilmad) prior sealing, rapidly frozen in liquid nitrogen, and shipped in the frozen state in dry ice by international priority delivery service from Tokyo, Japan, to Urbana, U.S.A.
Pulsed EPR related methods
The pulsed EPR experiments were carried out at 10 K using an X-band Bruker ELEXSYS E580 spectrometer equipped with Oxford CF 935 cryostats.32,35,44 The two-dimensional, four-pulse experiment (π/2-τ-π/2-t1-π-t2-π/2-τ-echo, also called HYSCORE) was employed with appropriate phase-cycling schemes to eliminate unwanted features from the experimental echo envelopes. The intensity of the echo after the fourth pulse was measured with t2 and t1 varied and constant τ. The length of a π/2 pulse was nominally 16 ns and a π pulse 32 ns. HYSCORE data were collected in the form of 2D time-domain patterns containing 256×256 points with steps of 20 or 32 ns. Spectral processing of ESEEM patterns, including subtraction of relaxation decay (fitting by polynoms of 3–4 degree), apodization (Hamming window), zero filling, and fast Fourier transformation (FT), was performed using Bruker WIN-EPR software.
Results and Discussion
14,15N HYSCORE analysis of 15N-amino acid labeled ARF on the 14N(N/A)-protein background
For this study we prepared several amino acid specifically nitrogen-labeled ARF samples by using the amino acid auxotroph strains of E. coli C43(DE3)40 and BL21(DE3) derivatives harboring a pACYC-based plasmid harboring tRNA genes (argU, ileY, and leuW) for the E. coli rare codons (Agilent Technologies) (step 5 in Fig. 2), as described under the Experimental Section. These proteins were then subjected to experimentally dissect and identify the non-coordinating residue(s) directly interacting with the reduced cluster by a “through-bond” mechanism.
We initially prepared several different batches of selectively 15N amino acid-labeled ARF samples on the 14N(N/A)-protein background. Such samples had been presumed as suitable for resolution of individual non-coordinating 15Np signals by 15N HYSCORE.40 In the case of Rieske-type proteins, however, none of them unexpectedly showed any recognizable 15N hyperfine splittings (Fig. 3). The resulting spectra were exclusively dominated by 14N features mainly from the two hisidine Nδ ligands, similar to the spectra reported for unlabeled (14N(N/A)) ARF.32 This suggests that deep ESEEM from the two coordinating 14Nδ(His) of the Rieske-type cluster decreases the non-coordinating 15N signal intensities via the cross-suppression mechanism,45 thus leaving only vague 15Np features in the (++) quadrant (Fig. 3).
14,15N HYSCORE analysis of 14N(N/A)-amino acid labeled ARF on the 15N-protein background
To eliminate this problem, we prepared selectively 14N(N/A) L-tyrosine and L-lysine labeled ARF samples on the 15N-protein background, such that the two histidine ligands as well as other non-target amino acid residues around the cluster are exclusively 15N labeled (Fig. 4). L-Tyrosine and L-lysine were selected in the light of putative N-H•••S hydrogen bond network around the Rieske-type cluster in the ARF structure39b (Fig. 1). In both samples, the cross-features produced by different types of 15N could be successfully resolved in the orientation-selected HYSCORE spectra measured at different field positions of the EPR line. In the (+−) quadrant, two pairs of cross-peaks with a contour parallel to the diagonal line are detected, which are attributed to the two histidine nitrogen ligands to the reduced cluster, 15Nδ1 and 15Nδ2. These have hyperfine couplings of the order 6 and 8 MHz, respectively, with predominantly isotropic character. These features are identical to those reported for uniformly 15N-labeled ARF, showing the hyperfine tensors in the axial approximation of a=6.5 and T=1.5 MHz for 15Nδ1 (presumably His44 Nδ), and a=7.9 MHz and T=1.6 MHz for 15Nδ2 (presumably His64 Nδ).35 These tensors are very similar to those reported for other Rieske-type proteins by the orientation-selected 15N Q-band ENDOR.46 These samples do not exhibit any 14Nδ(His) cross-features in the (+−) quadrant, indicating negligible (or limited) scrambling of input 14N(N/A) L-amino acid.
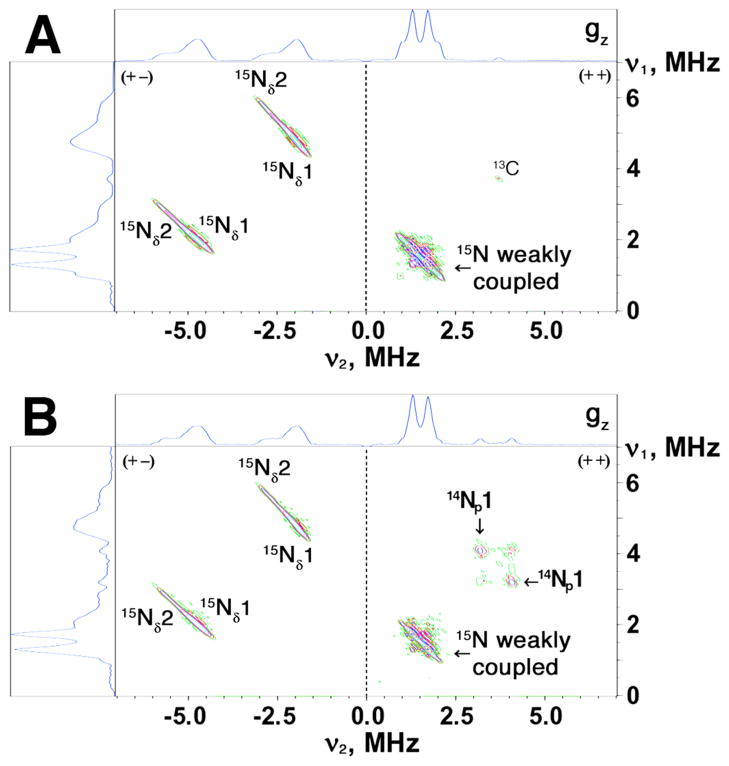
Figure 4.
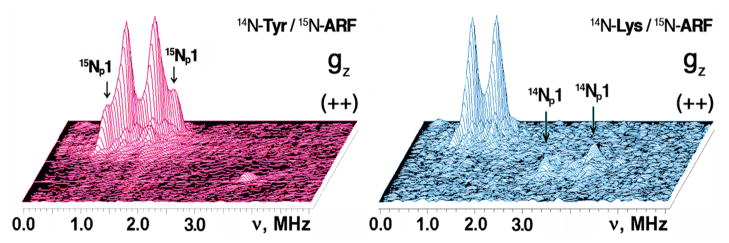
HYSCORE spectra in contour presentation of the reduced Rieske-type cluster in selectively 14N(N/A) L-tyrosine (A) and 14N(N/A) L-lysine (B) labeled ARF on the 15N-protein background, recorded at the gz area (345.0 mT) of the EPR line (time τ=136 ns, 10 K). Because 15N with nuclear spin I=1/2 has only two nuclear frequencies, each 15N may produce only a single pair of the cross-features which are located symmetrically relative to the diagonal line in the (+−) or (++) quadrant of the HYSCORE spectrum (depending on the 15N hyperfine coupling strength).33–35
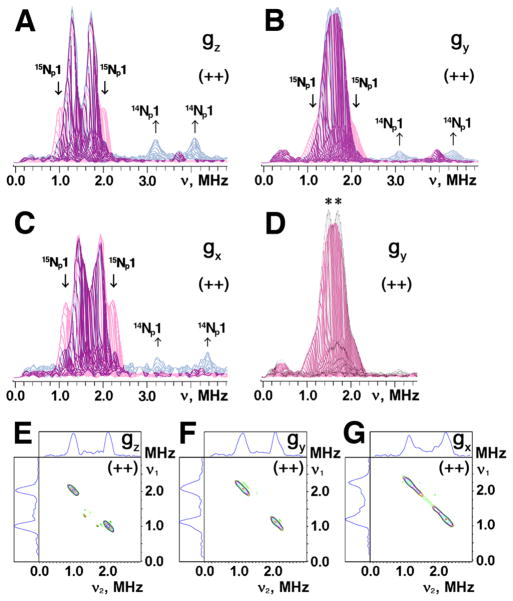
Significant variations in HYSCORE spectra of the two samples are observed in the (++) quadrant (Fig. 5). For 14N L-tyrosine labeled ARF, two superimposed pairs of the cross-features are located symmetrically around the diagonal point with 15N Zeeman frequency, and clearly resolved in the “single-crystal like spectra” recorded at the low- and high-field edges near gz and gx values. These features define the approximate 15N splittings of 1.04 MHz and 0.43 MHz near gz, and 1.12 and 0.49 MHz near gx (Table 2). Similar splittings have been observed in the corresponding uniformly 15N-labeled ARF spectra.35 Simultaneously, the input 14N L-tyrosine gave a narrow, “inverse” 15N splitting of ~0.2 MHz in the 15N spectra recorded near gy (Fig. 6B,D), but did not produce any new 14N lines because the splitting is too small to be observable in the 14N spectra (Fig. 4A).
Figure 5.
3D presentation of the HYSCORE spectra in the (++) quadrant of 14N(N/A) L-tyrosine (left) and 14N(N/A) L-lysine (right) labeled ARF on the 15N-protein background (recorded near gz area at the same conditions as Fig. 4), showing the variation of the 15N cross-feature lineshapes. The similar 15N splittings were also observed at some intermediate positions between the low- and high-field edges (e.g., see Fig. 6), indicating their predominantly isotropic characters. The largest splitting for 15Np1 with the isotropic hyperfine coupling a=1.03 MHz (Table 2), resolved in the 14N L-tyrosine labeled ARF (left), is missing in the 14N L-lysine labeled protein (right).
Table 2.
Hyperfine couplings of weakly coupled 15N nuclei currently resolved in the (++) quadrant of 15N HYSCORE spectra of amino acid-specifically nitrogen labeled ARF samples.
| Samples/assigned peaks | 15Az a (MHz) | 15Ay a (MHz) | 15Ax a (MHz) | 15a b (MHz) | 14Az c (MHz) | 14Ay c (MHz) | 14Ax c (MHz) | 14a b (MHz) | References |
|---|---|---|---|---|---|---|---|---|---|
| 15N-ARF (uniformly labeled) | 1.03 | ~1.22d | 1.1 | ~1.1d | 0.74 | ~0.9d | 0.8 | ~0.8d | Ref. [35] |
| 0.43 | (0.25)e | 0.49 | - | 0.3 | (0.18)e | 0.35 | - | ||
| 14N-Tyr/15N-ARFf | 1.04 | N.R.g | 1.12 | - | 0.74 | N.R.g | 0.80 | - | This work |
| 0.43 | 0.49 | 0.3 | 0.35 | ||||||
| 14N-Lys/15N-ARFh, i | 0.43 | (~0.25)e | 0.49 | - | 0.3 | (~0.18)e | 0.35 | - | This work |
| 15Np1 (Lys45 Nα) peaksj | 1.04 | 0.95 | 1.10 | 1.03 | 0.74 | 0.68 | 0.79 | 0.74 | This work |
HYSCORE spectra recorded at the low- and high-field edges near the maximal and minimal g values give “single-crystal-like” patterns from the reduced Rieske-type cluster, whose gz and gx axes are directed along the external magnetic field. In contrast, the resonance condition at the intermediate gy value is fulfilled by many different, yet well-defined orientations. 15N hyperfine couplings (15Ai (i=z,y,x)) are based on a difference of two cross-peak coordinates described in the first-order by the equation: ν1,2 = |15νN ± 15Ai/2|. The positions of the peak maxima in the (++) quadrant were determined with the accuracy ~0.04 MHz.
Ai = a + Tii (i=z,y,x), where a is an isotropic hyperfine coupling, and Tii is an anisotropic hyperfine tensor component which is a diagonal component of the hyperfine tensor in the g-tensor coordinate system; Txx+Tyy+Tzz=0 due to the tensor properties. Thus, Ax+Ay+Az=3a and a=(Az+Ay+Ax)/3. The variations of the splittings at different gi (i=z,y,x) suggest that the 15T component of an anisotropic tensor (-T,-T,2T) has an order ~0.1–0.15 MHz (or 14T ~ 0.07–0.11 MHz), in agreement with the previous considerations based on the point-dipole approximation model.47 The tensor component would be larger for nitrogen nuclei located around the innermost Fe(III) side and smaller on the outermost Fe(II) side of the reduced [2Fe-2S] cluster. For the isotropic hyperfine couplings 14,15a, only the values for the largest splittings are given in this table.
14N hyperfine couplings, 14Ai (i=z,y,x), were recalculated from the corresponding 15Ai (i=z,y,x) coupling values.
These tentative 14,15Ay and 14,15a values were based on the total width of the 15N HYSCORE spectrum recorded near gy35 and thus probably overestimated.
A very narrow splitting in the (++) quadrant (see Fig. 6B,D).
The 15N cross-peaks resolved in the (++) quadrant: [2.03, 0.99] and [1.73, 1.30] MHz near gz; [2.27, 1.15] and [1.94, 1.45] MHz near gx (see Figs. 5,6).
Not resolved (see Fig. 6B).
The 15N cross-peaks resolved in the (++) quadrant: [1.73, 1.30] MHz near gz, and [1.94, 1.45] MHz near gx.
The 14N cross-peaks (14Np1) resolved in the (++) quadrant in this sample (see Figs. 4B, 6A,C): [4.08, 3.19] MHz near gz and [4.41, 3.34] MHz near gx, which gave the (tentative) 14N hyperfine couplings 14Az of 0.76 MHz near gz and 14Ax of 0.87 MHz near gx, respectively, as estimated using formal expressions for the double-quantum (dq) transitions in the powder-type spectrum, νdq± = 2[(14νN ± 14Ai/2)2 + K2 (3 + η2)]1/2, where K is a nuclear quadrupole coupling constant and η is an asymmetry parameter.
The 15Np1 cross-peaks were well-resolved in the difference HYSCORE spectra in the (++) quadrant (see Fig. 6E–G): [2.03, 0.99] MHz near gz, [2.02, 1.12] MHz near gy, and [2.24, 1.14] MHz near gx.
Figure 6.
Superimposed stacked HYSCORE spectra in the (++) quadrant of 14N(N/A) L-tyrosine (red) and 14N(N/A) L-lysine (blue) labeled ARF on the 15N-protein background (A–C), and their 15N difference (i.e. “14N(N/A) L-tyrosine labeled ARF” minus “14N(N/A) L-lysine labeled ARF”) HYSCORE spectra for 15Np1 cross-peaks in contour presentation (E–G), recorded near the gz (A,E), gy (B,F), and gx (C,G) areas at 10 K. Contribution of 15Np1 (with the isotropic hyperfine coupling a=1.03 MHz, Table 2) and 14Np1 for L-lysine to the nitrogen ESEEM amplitude in the (++) quadrant is evident for 14N L-tyrosine (red) and 14N L-lysine (blue) labeled ARF, respectively, when the stacked spectra (with zero projection angles) were re-scaled and superimposed after normalizing the relative scales of the cross-peak intensities from two Nδ(His) ligands in the (+−) quadrant (A–C).35 Similar approach was used to detect the possible contribution of 15N L-tyrosine (with a narrow splitting of ~0.2 MHz) to the 15N ESEEM amplitude in the (++) quadrant (marked with asterisks in (D)), when the stacked spectra of 14N L-tyrosine (red) and uniformly 15N-labeled35 (gray) ARF (with zero projection angles) were re-scaled and superimposed after normalizing the relative scales of the 15Nδ(His) cross-peak intensities in the (+−) quadrant (D). The same small τ-value (τ=136 ns; slightly exceeding the dead time of the instrument) was chosen for the measurement of these HYSCORE spectra, which allows the preferable observation of the undistorted lineshape of the cross-peaks as well as the minimization of the suppression effect on the ESEEM amplitudes.48 Magnetic field, and microwave frequency, respectively: 345.0 mT (near gz), 9.695 GHz (A,E); 365.5 mT (near gy), 9.695 GHz (B,F); 386.7 mT (near gx), 9.695 GHz (C,G); 363.1 mT (near gy), 9.695 GHz (D).
In stark contrast, the spectra of 14N L-lysine labeled ARF showed simpler cross-features with splittings of 0.42 MHz near gz, and 0.50 MHz near gx (Figs. 5,6 and Table 2). The larger 15N splitting, with the isotropic hyperfine coupling a=1.03 MHz observed in the uniformly 15N-labeled35 and 14N L-tyrosine labeled ARF, is missing (Fig. 6E–G, Table 2). Conversely, the input 14N L-lysine clearly gave 14N cross-peaks in the spectra of 14N L-lysine labeled ARF (Figs. 4,5), at the equivalent positions as previously observed for the peptide nitrogen cross-peaks (P1) in unlabeled (14N(N/A)) ARF spectra.32
Taken together, the results demonstrate that the largest splitting, with an isotropic coupling a=1.03 MHz in the (++) quadrant of the 15N spectra of ARF (Table 2), is predominantly contributed from the single peptide nitrogen Lys45 Nα, which also gives the P1 (Np1) cross-peaks in the corresponding 14N spectra.32 Notably, Lys45 Nα is located near Sb (with the shortest Nα-Sb distance of 3.28 Å), in a reasonable orientation for an Nα-H•••Sb hydrogen bond (Fig. 1). Thus, Lys45 Nα provides the major pathway for unpaired spin density transfer from the reduced cluster via overlapped orbitals, i.e., by a “through-bond” mechanism. A similar conclusion was reached from a more qualitative theoretical analysis of a high-potential Rieske protein domain from Rhodobacter sphaeroides.34 Thus, this particular chemical bond interaction affecting the ground-state electronic structure of the reduced cluster is probably conserved among the Rieske-type protein scaffolds, and is not linked specifically to differences in cluster Em. On the other hand, although Tyr66 Nα is located also in the range of a possible hydrogen bond distance from the cluster (with a slightly longer Nα-Sb distance of 3.53 Å, Fig. 1), the interaction with Tyr66 Nα appears to be predominantly an electron-nuclear dipole interaction (i.e. mainly by a “through-space” effect), and may be much weaker. Thus, although Lys45 Nα and Tyr66 Nα exhibit a near pseudo-two fold symmetry relative to the cluster plane, the unpaired electron spin density distribution over the polypeptide frame around the reduced cluster is highly asymmetric. The reason for this extreme asymmetry is not apparent and will require computational approaches to clarify.
Conclusions
The electronic structure and geometry of clusters in the Rieske-type proteins are fine-tuned by the pattern of hydrogen bonding with the backbone peptide matrix. The current work has demonstrated that the interaction between the Rieske-type [2Fe-2S] cluster with Lys45 Nα is dominant in ARF, but there are other interactions indicated with non-coordinating residues. Further resolution of individual non-coordinating backbone peptide 15N signals with splittings in the range ~0.4–0.5 MHz or less (Fig. 5B) will require an extension of the current labeling strategy, with the systematic double labeling of 15N histidine plus 15N amino acid with an 14N(N/A)-protein background. This will bypass the cross-suppression45 of the non-coordinating 15N signals by the otherwise predominant 14Nδ cross-peaks from two histidine ligands in the Rieske-type cluster system (see Fig. 3). By defining which interactions result in the transfer of unpaired electron spin density to the protein frame quantitatively with reference to the atomic coordinates, the application of the experimental approach described in this work provides physico-chemical parameters to characterize the through-bond interactions with the protein matrix which modulate the electronic structure, geometry, and reactivity of protein-associated, paramagnetic iron-sulfur cluster systems.2,3,8,9
It should be noted that chemical and biological activity of metalloenzymes depends upon the association of the metallo-cofactor and the protein matrix moiety. The present strategy of applying 2D pulsed EPR techniques in conjunction with selective isotope labeling should be applicable to probe the primary coordination sphere and the outer sphere through-bond and other interactions with paramagnetic active site metal centers in various metalloproteins and model peptides, which are usually obscured by multiple weak electron-nuclear interactions32 and/or can be cross-suppressed by strongly coupled histidine imidazole 14N ligands when exist.45 This will not only help to reveal hidden structural features and possibly dynamics of active site residues during catalysis that may be inaccessible from other techniques, but also can contribute to develop and verify strategies for designing and engineering new metalloenzymes for biotechnological and pharmaceutical applications.
Supplementary Material
Acknowledgments
This investigation was supported in part by the International Collaborations in Chemistry Grant from JSPS (T.I.) and NSF (CHE-1026541 to S.A.D.), the JSPS Grant-in-aid 24659202 (T.I.), the DE-FG02-87ER13716 (R.B.G.) and DE-FG02-08ER15960 (S.A.D.) Grants from US DOE, NIH & NIGMS Roadmap Initiative (R01GM075937), and NIH grant GM062954 (S.A.D.).
Footnotes
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supporting Information. Table S1. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Holm RH, Kennepohl P, Solomon EI. Chem Rev. 1996;96:2239. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]
- 2.Beinert H, Holm RH, Münck E. Science. 1997;277:653. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- 3.Solomon EI, Xie X, Dey A. Chem Soc Rev. 2008;37:623. doi: 10.1039/b714577m. [DOI] [PubMed] [Google Scholar]
- 4.Rees DC. Annu Rev Biochem. 2002;71:221. doi: 10.1146/annurev.biochem.71.110601.135406. [DOI] [PubMed] [Google Scholar]
- 5.Thauer RK, Kaster AK, Goenrich M, Schick M, Hiromoto T, Shima S. Annu Rev Biochem. 2010;79:507. doi: 10.1146/annurev.biochem.030508.152103. [DOI] [PubMed] [Google Scholar]
- 6.Peters JW, Broderick JB. Annu Rev Biochem. 2012;81:429. doi: 10.1146/annurev-biochem-052610-094911. [DOI] [PubMed] [Google Scholar]
- 7.Hosler JP, Ferguson-Miller S, Mills DA. Annu Rev Biochem. 2006;75:165. doi: 10.1146/annurev.biochem.75.062003.101730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin IJ, Gebel EB, Machonkin TE, Westler WM, Markley JL. Proc Natl Acad Sci USA. 2005;102:14581. doi: 10.1073/pnas.0505521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machonkin TE, Westler WM, Markley JL. Inorg Chem. 2005;44:779. doi: 10.1021/ic048624j. [DOI] [PubMed] [Google Scholar]
- 10.Marshall NM, Garner DK, Wilson TD, Gao YG, Robinson H, Nilges MJ, Lu Y. Nature. 2009;462:113. doi: 10.1038/nature08551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolla A, Blanchard L, Guerlesquin F, Bruschi M. Biochimie. 1994;76:471. doi: 10.1016/0300-9084(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 12.Zu Y, Couture MMJ, Kolling DRJ, Crofts AR, Eltis LD, Fee JA, Hirst J. Biochemistry. 2003;42:12400. doi: 10.1021/bi0350957. [DOI] [PubMed] [Google Scholar]
- 13.Hunsicker-Wang LM, Heine A, Chen Y, Luna EP, Todaro T, Zhang YM, Williams PA, McRee DE, Hirst J, Stout CD, Fee JA. Biochemistry. 2003;42:7303. doi: 10.1021/bi0342719. [DOI] [PubMed] [Google Scholar]
- 14.Brown EN, Friemann R, Karlsson A, Parales JV, Couture MM, Eltis LD, Ramaswamy S. J Biol Inorg Chem. 2008;13:1301. doi: 10.1007/s00775-008-0413-4. [DOI] [PubMed] [Google Scholar]
- 15.Zuris JA, Halim DA, Conian AR, Abresch EC, Nechushtai R, Paddock ML, Jennings PA. J Am Chem Soc. 2010;132:13120. doi: 10.1021/ja103920k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanagisawa S, Banfield MJ, Dennison C. Biochemistry. 2006;45:8812. doi: 10.1021/bi0606851. [DOI] [PubMed] [Google Scholar]
- 17.Varadarajan R, Zewert TE, Gray HB, Boxer SG. Science. 1989;243:69. doi: 10.1126/science.2563171. [DOI] [PubMed] [Google Scholar]
- 18.Shifman JM, Gibney BR, Sharp RE, Dutton PL. Biochemistry. 2000;39:14813. doi: 10.1021/bi000927b. [DOI] [PubMed] [Google Scholar]
- 19.Olea C, Jr, Kuriyan J, Marletta MA. J Am Chem Soc. 2010;132:12794. doi: 10.1021/ja106252b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DC, Dean DR, Smith AD, Johnson MK. Annu Rev Biochem. 2005;74:247. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 21.Fontecave M. Nat Chem Biol. 2006;2:171. doi: 10.1038/nchembio0406-171. [DOI] [PubMed] [Google Scholar]
- 22.Mason JR, Cammack R. Annu Rev Microbiol. 1992;46:277. doi: 10.1146/annurev.mi.46.100192.001425. [DOI] [PubMed] [Google Scholar]
- 23.Link TA. Adv Inorg Chem. 1999;47:83. [Google Scholar]
- 24.Berry EA, Guergova-Kuras M, Huang LS, Crofts AR. Annu Rev Biochem. 2000;69:1005. doi: 10.1146/annurev.biochem.69.1.1005. [DOI] [PubMed] [Google Scholar]
- 25.Cramer WA, Zhang H, Yan J, Kurisu G, Smith JL. Annu Rev Biochem. 2006;75:769. doi: 10.1146/annurev.biochem.75.103004.142756. [DOI] [PubMed] [Google Scholar]
- 26.Iwata S, Saynovits M, Link TA, Michel H. Structure. 1996;4:567. doi: 10.1016/s0969-2126(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 27.Colbert CL, Couture MMJ, Eltis LD, Bolin J. Structure. 2000;8:1267. doi: 10.1016/s0969-2126(00)00536-0. [DOI] [PubMed] [Google Scholar]
- 28.Klingen AR, Ullmann GM. Biochemistry. 2004;43:12383. doi: 10.1021/bi0488606. [DOI] [PubMed] [Google Scholar]
- 29.Dikanov SA. In: New Advances in Analytical Chemistry. Attaur-Rahman, editor. Gordon and Breach; Amsterdam: 2000. p. 523. [Google Scholar]
- 30.Prisner T, Rohrer M, MacMillan F. Annu Rev Phys Chem. 2001;52:279. doi: 10.1146/annurev.physchem.52.1.279. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman BM. Proc Natl Acad Sci USA. 2003;100:3575. doi: 10.1073/pnas.0636464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dikanov SA, Shubin AA, Kounosu A, Iwasaki T, Samoilova RI. J Biol Inorg Chem. 2004;9:753. doi: 10.1007/s00775-004-0571-y. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki T, Kounosu A, Uzawa T, Samoilova RI, Dikanov SA. J Am Chem Soc. 2004;126:13902. doi: 10.1021/ja045898x. [DOI] [PubMed] [Google Scholar]
- 34.Dikanov SA, Kolling DRJ, Endeward B, Samoilova RI, Prisner TF, Nair SK, Crofts AR. J Biol Chem. 2006;281:27416. doi: 10.1074/jbc.M604103200. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki T, Samoilova RI, Kounosu A, Dikanov SA. FEBS Lett. 2009;583:3467. doi: 10.1016/j.febslet.2009.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kounosu A, Li Z, Cosper NJ, Shokes JE, Scott RA, Imai T, Urushiyama A, Iwasaki T. J Biol Chem. 2004;279:12519. doi: 10.1074/jbc.M305923200. [DOI] [PubMed] [Google Scholar]
- 37.Iwasaki T, Kounosu A, Tao Y, Li Z, Shokes JE, Cosper NJ, Imai T, Urushiyama A, Scott RA. J Biol Chem. 2005;280:9129. doi: 10.1074/jbc.M414051200. [DOI] [PubMed] [Google Scholar]
- 38.Iwasaki T, Kounosu A, Kolling DRJ, Crofts AR, Dikanov SA, Jin A, Imai T, Urushiyama A. J Am Chem Soc. 2004;126:4788. doi: 10.1021/ja031976p. [DOI] [PubMed] [Google Scholar]
- 39.(a) Kounosu A, Hasegwa K, Iwasaki T, Kumasaka T. Acta Cryst Sect F. 2010;66:842. doi: 10.1107/S1744309110019263. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hasegawa K, Urushiyama A, Miyajima-Nakano Y, Baldansuren A, Dikanov SA, Iwasaki T, Kumasaka T. in preparation for submission. [Google Scholar]
- 40.Lin MT, Sperling LJ, Frericks Schmidt HL, Tang M, Samoilova RI, Kumasaka T, Iwasaki T, Dikanov SA, Rienstra CM, Gennis RB. Methods. 2011;55:370. doi: 10.1016/j.ymeth.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waugh DS. J Biomol NMR. 1996;8:184. doi: 10.1007/BF00211164. [DOI] [PubMed] [Google Scholar]
- 42.Datsenko KA, Wanner BL. Proc Natl Acad Sci USA. 2000;97:6640. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherepanov PP, Wackernagel W. Gene. 1995;158:9. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 44.Iwasaki T, Samoilova RI, Kounosu A, Ohmori D, Dikanov SA. J Am Chem Soc. 2009;131:13659. doi: 10.1021/ja903228w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoll S, Calle G, Mitrikas G, Schweiger A. J Magn Reson. 2005;177:93. doi: 10.1016/j.jmr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Gurbiel RJ, Doan PE, Gassner GT, Macke TJ, Case DA, Ohnishi T, Fee JA, Ballou DP, Hoffman BM. Biochemistry. 1996;35:7834. doi: 10.1021/bi960380u. [DOI] [PubMed] [Google Scholar]
- 47.Dikanov SA, Samoilova RI, Kappl R, Crofts AR, Hüttermann J. Phys Chem Chem Phys. 2009;11:6807. doi: 10.1039/b904597j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dikanov SA, Tyryshkin AM, Bowman MK. J Magn Reson. 2000;144:228. doi: 10.1006/jmre.2000.2055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.