Abstract
The induction of a high-affinity state of the CO2-concentration mechanism was investigated in two cyanobacterial species, Synechococcus sp. strain PCC7002 and Synechococcus sp. strain PCC7942. Cells grown at high CO2 concentrations were resuspended in low-CO2 buffer and illuminated in the presence of carbonic anhydrase for 4 to 10 min until the inorganic C compensation point was reached. Thereafter, more than 95% of a high-affinity CO2-concentration mechanism was induced in both species. Mass-spectrometric analysis of CO2 and HCO3− fluxes indicated that only the affinity of HCO3− transport increased during the fast-induction period, whereas maximum transport activities were not affected. The kinetic characteristics of CO2 uptake remained unchanged. Fast induction of high-affinity HCO3− transport was not inhibited by chloramphenicol, cantharidin, or okadaic acid. In contrast, fast induction of high-affinity HCO3− transport did not occur in the presence of K252a, staurosporine, or genistein, which are known inhibitors of protein kinases. These results show that induction of high-affinity HCO3− transport can occur within minutes of exposure to low-inorganic-C conditions and that fast induction may involve posttranslational phosphorylation of existing proteins rather than de novo synthesis of new protein components.
Cyanobacteria possess a CCM (Badger and Price, 1992; Kaplan et al., 1994) that functions to elevate CO2 levels around Rubisco. This CCM is absolutely essential in cyanobacteria for the performance of efficient photosynthesis in their aquatic environment. A functionally active CCM in cyanobacteria consists of two functional components: (a) a Ci-transport system that actively acquires Ci from the surrounding medium and accumulates HCO3− in the cell cytosol; and (b) the carboxysome, which provides a compartment in which Rubisco and CA are specifically co-localized, allowing the cytosolic HCO3− to be converted to CO2, leading to the localized elevation of CO2 in the vicinity of Rubisco (Badger and Price, 1992; Kaplan et al., 1994).
The efficiency of the CCM changes in response to the environmental conditions, especially the availability of Ci (Mayo et al., 1986; Badger and Gallagher, 1987). When cyanobacteria are grown at high CO2 they develop a CCM with a reduced affinity for external Ci and a diminished capacity to accumulate internal Ci. When cells that have been cultivated at high CO2 are transferred to limiting CO2 conditions (e.g. 20 μL L−1), a high photosynthetic affinity for external Ci is induced (Badger and Price, 1992; Kaplan et al., 1994). The induction process at low Ci is known to include both an increase in the number of carboxysomes and carboxysomal CA activity and an induction of a high-affinity Ci transport system (Badger and Price, 1992; Kaplan et al., 1994). Evidence indicates that both HCO3− and CO2 simultaneously serve as substrates for both low- and high-affinity Ci transport systems (Miller and Canvin, 1985; Badger et al., 1994; Yu et al., 1994a, 1994b; Sültemeyer et al., 1995, 1997a, 1997b; Salon et al., 1996; Tyrrell et al., 1996).
Yu et al. (1994a) investigated the induction time course of the kinetic changes of the CO2 and HCO3− transport during transfer from air levels of CO2 to 20 μL CO2 L−1. When rapidly sparged with ambient air, air-grown cells show physiological characteristics similar to those of high-Ci cells (Mayo et al., 1986; Badger and Gallagher, 1987; Yu et al., 1994a). Within 24 h of transfer to limiting Ci, the values for K1/2(CO2) and K1/2(HCO3−) were reduced by about 3- and 15-fold, respectively, and the induction was close to completion after 4 h. Similar time courses for induction of a high-affinity CCM are also reported for eukaryotic green algae (Palmqvist et al., 1988; Sültemeyer et al., 1991; Matsuda and Colman, 1995).
The molecular genetic and biochemical basis of the processes involved in induction are poorly understood. The presence of light seems to be an absolute requirement for complete induction (Badger and Price, 1992; Kaplan et al., 1994), and some evidence indicates involvement of de novo protein synthesis during the induction process. Such evidence includes: an increase in the abundance of carboxysomes per cell by severalfold in cells grown under Ci limitation compared with those grown under high-Ci conditions (Turpin et al., 1984; McKay et al., 1993); an increase in activities of carboxysomal and membrane-bound CA by up to 10-fold during growth at low Ci (Badger and Price, 1989; Price et al., 1992); and an increase in a 42-kD polypeptide that has been shown to accumulate significantly within the plasma membrane fraction in cyanobacteria upon transfer to low-CO2 conditions (Omata and Ogawa, 1985; Omata et al., 1990). Unfortunately, specific inactivation of the structural gene (cmpA) coding for this protein by interposon mutagenesis did not affect the ability of the cells to acclimate to 300 μL L−1 CO2 (Omata et al., 1990), indicating that the 42-kD polypeptide may not be directly involved in the adaptation of plasma membrane Ci transport processes to air levels of CO2. In addition to these observed protein increases, the induction of a high-affinity state over a period of hours rather than minutes is most consistent with protein-synthesis processes resulting in the appearance of new polypeptide components.
High- and low-Ci-adapted cells of cyanobacteria are able to transport both CO2 and HCO3− species during steady-state photosynthesis with more or less similar maximum rates (Yu et al., 1994a, 1994b; Sültemeyer et al., 1995, 1997a, 1997b). The main difference in transport characteristics between high- and low-Ci cells appears to be in the affinity for substrate rather than maximum activities, because the K1/2 values for CO2 and HCO3− decrease by more than 1 order of magnitude (Yu et al., 1994a; Sültemeyer et al., 1995, 1997a, 1997b). It was therefore suggested that the increase in transport efficiency was caused by a qualitative change in the transport system rather than a quantitative increase in transport components (Yu et al., 1994a; Sültemeyer et al., 1995). In considering the induction process, it is therefore interesting to notice that changes in the pattern of phosphorylation of polypeptides with changes in the efficiency of Ci transport have been reported (Bloye et al., 1992). A correlation has been found between the appearance of phosphorylated proteins and the loss of efficiency of Ci transport, either by transferring low-Ci cells to high-Ci conditions or by switching to a medium containing Glc (Bloye et al., 1992), indicating that the affinity of Ci transport may be under the control of a protein kinase/phosphatase regulation.
This paper explores further the nature of the changes that occur to cyanobacteria when they are adapted to growth at limiting Ci. In contrast to previous research, we find that induction of a high-affinity state can be very rapid, occurring within minutes, which raises questions about the primary involvement of de novo protein synthesis. The results show that it is HCO3− and not CO2 transport that is rapidly induced, and that the induction processes may be primarily mediated by posttranslational modification to existing protein components.
MATERIALS AND METHODS
Growth of Cyanobacteria
The marine unicellular cyanobacteria Synechococcus sp. strain PCC7002 (formerly Agmenellum quadruplicatum PR-6) was a kind gift of Professor D.A. Bryant (Pennsylvania State University, University Park). This species and the freshwater species Synechococcus sp. strain PCC7942 (R2) were grown under continuous photoautotrophic conditions (200–300 μmol photons m−2 s−1) as described previously (Yu et al., 1994; Sültemeyer et al., 1995). Cultures were vigorously sparged with air enriched with 2% CO2 (20,000 μL of CO2 L−1; high-Ci cells) or air containing 20 μL of CO2 L−1 (low-Ci cells). For MS measurements, the cells were collected by centrifugation (4000g for 10 min) and washed twice with fresh CO2-free assay buffer (growth medium enriched with 50 mm bis-tris-propane, pH 8.0 and 8.2 for the 7942 and 7002 strains, respectively). The pellet was resuspended in the same buffer to a final Chl content of 200 to 500 μg mL−1 and kept in the dark until used for the experiments (usually not longer than 30 min). Chl was extracted in methanol and the content determined according to the method of Porra et al. (1989). CO2-free assay medium was prepared by continuously sparging the buffer with CO2-free air, which had been passed through a carbosorb column (Dräger, Leipzig, Germany), for a period of several days. After this time the total Ci in the medium was less than 30 μm.
Fast Induction of High-Affinity HCO3− Transport
All experiments were performed in a thermostated reaction cuvette that was connected to a mass spectrometer via a semipermeable membrane inlet system (Fock and Sültemeyer, 1989; Sültemeyer et al., 1995). Four milliliters of fresh CO2-free assay buffer (see above) was pipetted into the chamber and the temperature was adjusted to 30°C (strain 7942) or 33°C (strain 7002). To obtain “fast-induced” cells, aliquots from concentrated high-Ci cells of Synechococcus sp. strain 7002 or Synechococcus sp. strain 7942 were injected into CO2-free assay medium in the cuvette to yield a final Chl content of 10 μg mL−1. The chamber was then closed with a plexiglass stopper and the cells were kept in the dark for up to 3 min to allow for temperature equilibration. When necessary, CA (100 Wilbur-Anderson units mL−1) or chloramphenicol (200 μg mL−1) was added during the dark period. After 2 to 3 min of darkness, the light (750 μmol photons m−2 s−1) was switched on and the cells were allowed to consume the remaining Ci in the cuvette. Changes in the concentration of CO2 (m/z = 44) and O2 (m/z = 32) were simultaneously followed by MS (see Fig. 1). The initial Ci concentration before the onset of illumination was between 50 and 100 μm in all experiments, depending on the amount of Ci that was carried over from the concentrated cell suspensions. After 4 to 10 min in the light, the cells were darkened again for 2 to 3 min. During this second dark period the cells were prepared for measurements of O2 evolution, CO2 uptake, and HCO3− transport.
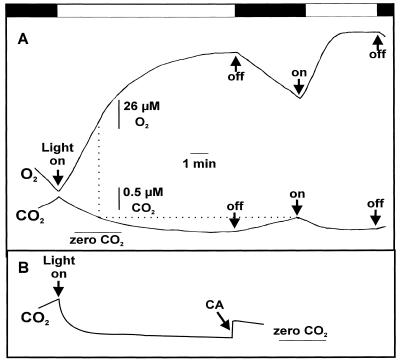
Figure 1.
A, Time course of O2 evolution and Ci depletion in high-Ci cells of Synechococcus sp. strain PCC7002. O2 and CO2 exchange were measured simultaneously by MS. The reaction chamber was filled with 4 mL of assay medium containing CA (100 units mL−1). Cell density was adjusted to 10 μg of Chl mL−1. Light (750 μmol photons m−2 s−1) was turned on and off as indicated by arrows. The [Ci] at the start of the first light period and at the onset of the second was 92 and 52 μm, respectively. The [O2] at the beginning and end of the experiment was 18 and 28%, respectively. The rate of O2 evolution at the end of illumination was 8 μmol mg−1 Chl h−1. B, Uptake of CO2 in illuminated high-Ci cells of Synechococcus sp. strain PCC7002. CA was omitted from the start of the experiment but added after 10 min of illumination. Initial [Ci] was 104 and 56 μm at the beginning and after addition of CA, respectively. The rate of O2 evolution at the end of illumination was 33 μmol mg−1 Chl h−1.
Flux Measurements of O2 Evolution, CO2 Uptake, and HCO3− Transport
In the second dark period an aliquot of fast-induced cells containing 8 to 16 μg of Chl mL−1 was removed, centrifuged, resuspended in 4 mL of CO2-free assay buffer, and placed into the reaction chamber attached to the mass spectrometer. Rates of O2 evolution, CO2 uptake, and HCO3− transport were determined during steady-state photosynthesis using a MS disequilibrium technique (Badger et al., 1994). Application of this method is only possible if extracellular CA activity is absent, because the method relies on the occurrence of only the relatively slow and uncatalyzed spontaneous hydration and dehydration of CO2 and HCO3−, respectively. Therefore, after dilution of the fast-induced cells we added 50 μm AZA to the suspension. Separate measurements of 18O exchange from doubly labeled CO2 (13C18O2; Badger and Price, 1989) have shown that this amount of AZA was sufficient enough to inhibit all remaining external CA activity (data not shown).
From the changes in the CO2 and O2 concentrations, the fluxes of Ci uptake (e.g. gross CO2 and HCO3− uptake) were calculated using the previously published formulas (Badger et al., 1994). The equations also permit the estimation of the actual CO2 and HCO3− concentrations at the time the measurements are made. The pseudo-first-order rate constant k2 (HCO3− to CO2) was obtained by injection of a known amount of HCO3− into the reaction chamber under experimental conditions (Sültemeyer et al., 1995). The initial slope of CO2 evolution from HCO3− was a direct measure for k2, and was determined as 4.89 × 10−1 min−1. Using the separately measured HCO3−:CO2 ratio of 41 (at pH 8.0 and 30°C) and 78 (at pH 8.2 and 33°C), the k1 rate constants were calculated as 3.05 and 3.82 min−1, respectively. Initial rates of CO2 uptake were obtained from the slope of the CO2 uptake traces within the first 10 to 15 s of illumination according to the method of Sültemeyer et al. (1997a, 1997b). Comparative analysis of Ci fluxes revealed that 50 μm AZA had no effect on the kinetics of CO2 and HCO3− uptake in high- or low-Ci cells (data not shown).
Chemicals
CA (EC 4.2.1.1) from bovine erythrocytes was purchased from Sigma (C-3934). Cantharidin, okadaic acid, K252a, staurosporine, and genistein were from Biomol (Hamburg, Germany).
RESULTS
Fast Induction of High-Ci Cells
Our first indications that acclimation to low-Ci conditions could occur within a few minutes was obtained from experiments similar to those shown in Figure 1. In Figure 1A, the experimental protocol for the fast induction of high-affinity cells from cyanobacteria is shown. The assay mixture contained Chl (10 μg mL−1), CA (100 Wilbur-Anderson units mL−1), and a low initial Ci concentration (50–100 μm). After a short time in the dark, the light was turned on and the cells were allowed to deplete external Ci to the compensation point. O2 evolution was initially high but declined as Ci became limiting. After about 10 min the Ci compensation point was reached, as indicated by no further changes in the O2 and CO2 concentrations. Under these conditions, the total Ci concentration in the medium was almost 0. After reaching the Ci compensation point, the cell suspension was darkened again for up to 3 min. This second dark period was applied to provide the cells with Ci that was released mainly by respiratory processes.
In the second light period, O2 evolution and Ci uptake were much faster compared with the rates that could be calculated from the initial Ci depletion trace, although the external Ci concentration was approximately one-half of the concentration at the beginning of the first light period. Consistent with an enhanced ability to use limiting Ci concentrations in this second light period is the observation that the Ci compensation was reached more quickly in the second light period and that Ci limitation of rate was not reached until just before running out of external Ci (Fig. 1A). It is noteworthy that the second dark period is not an absolute requirement for obtaining fast-induced cells. After 10 min of continuous light, the addition of small amounts of Ci to the cell suspension in the light revealed a similar enhancement of photosynthetic efficiency (data not shown). From experiments such as that shown in Figure 1, it is clear that exposure of cells to very low Ci for only 10 min is sufficient time to allow high-Ci cells of Synechococcus sp. strain PCC7002 to significantly acclimate and use limiting Ci more efficiently (Fig. 1A).
Figure 1B shows the CO2 exchange of a similar experiment except that CA was initially excluded. Clearly, CO2 is rapidly taken up in the light, which is consistent with previous observations (Badger and Andrews, 1982; Miller et al., 1988, 1991; Badger et al., 1994; Sültemeyer et al., 1995, 1997b). After about 10 min the CO2 concentration was almost 0; however, the addition of CA in the light caused a sudden increase in the CO2 concentration, indicating that a considerable amount of HCO3− (approximately 56 μm) was still present. These measurements show that CA is necessary to enable high-Ci cells to rapidly deplete Ci to the compensation point so that rapid-induction experiments at very low Ci can be conducted.
Effect of Fast Induction on Photosynthetic Affinity
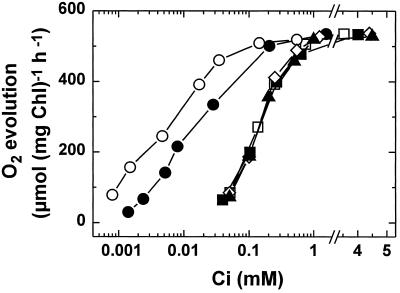
The extent of acclimation during the fast-induction period shown in Figure 1 is examined further in Table I and Figure 2. Synechococcus sp. strain PCC7002 cells that were continuously grown on 2% CO2 (high-Ci cells) or 20 μL of CO2 L−1 (low-Ci cells) exhibited similar maximum rates of O2 evolution but had vastly different affinities for external Ci. High-Ci cells had a K1/2(Ci) of 161 μm, whereas low-Ci cells showed a value of 13 μm (Fig. 2; Table I), indicating that the latter are more than 10 times more effective at using small amounts of external Ci. Compared with these two acclimation states, fast-induced cells showed a decline in K1/2(Ci) to 20 μm, indicating that about 95% of the high-affinity state seen with low-Ci cells had been induced within 10 min of exposure to very low Ci (Fig. 2; Table I). The ability of high-Ci cells to rapidly induce a high-affinity state within the 10-min period is facilitated by the addition of active extracellular CA. In the absence of CA, high-Ci cells were not able to induce a high-affinity state, with these cells showing almost the same K1/2(Ci) as high-Ci cells (167 μm) (Fig. 2; Table I). Similarly, when CA was inhibited with 50 μm AZA at the start of the fast-induction experiment the photosynthetic affinity for Ci remained almost unchanged (Fig. 2).
Table I.
Vmax and K1/2(Ci) of photosynthetic O2 evolution in fast-induced and noninduced cells of two cyanobacterial species
| Kinetic Parameters | High-Ci Cells | Low-Ci Cells | Fast-Induced Cells
|
|
|---|---|---|---|---|
| +CA | −CA | |||
| PCC7002 | ||||
| Vmax (μmol mg−1 Chl h−1) | 535 ± 35 | 519 ± 45 | 536 ± 32 | 534 ± 24 |
| K1/2(Ci) (μm) | 161 ± 18 | 13 ± 2 | 20 ± 2 | 167 ± 22 |
| PCC7942 | ||||
| Vmax (μmol mg−1 Chl h−1) | 205 ± 20 | 298 ± 31 | 218 ± 26 | 222 ± 17 |
| K1/2(Ci) (μm) | 253 ± 28 | 14 ± 4 | 24 ± 6 | 265 ± 22 |
High- and low-Ci cells of the marine cyanobacterium Synechococcus sp. strain PCC7002 and the freshwater Synechococcus sp. strain PCC7942 were grown continuously on 2% CO2 or 20 μL of CO2 L−1, respectively. Fast-induced cells were obtained from high-Ci cells, which were allowed to run out of external Ci for 10 min either in the presence or absence of CA (100 Wilbur-Anderson units mL−1). The data were obtained from experiments similar to those shown in Figure 1 and they represent the mean ± sd from three to four independent experiments.
Figure 2.
Rate of O2 evolution during steady-state photosynthesis in relation to external Ci in Synechococcus sp. strain PCC7002. ○, Cells grown continuously on 20 μL of CO2 L−1 (low-Ci cells); □, cells grown continuously on 2% CO2 (high-Ci cells); •, high-Ci cells subjected to the fast-induction protocol for 10 min in the presence of CA; ▪, high-Ci cells subjected to the fast-induction protocol for 10 min in the absence of CA; ▴, high-Ci cells subjected to the fast-induction protocol for 10 min in the presence of CA and 50 μm AZA; ♦, high-Ci cells that were not fast induced but tested in the presence of CA. Fast-induced cells were obtained in experiments similar to those shown in Figure 1. After the 10-min illumination period (750 μmol photons m−2 s−1) an aliquot of the cells containing 10 μg of Chl was centrifuged and resuspended in 4 mL of assay buffer to a final [Chl] of 2.5 μg mL−1. To fully inhibit CA activity, 50 μm AZA was added and the Ci-dependent O2 evolution was followed.
The data in Table I also indicate that fast induction of a high-affinity state is not restricted to one particular species of cyanobacteria. When the freshwater cyanobacterium Synechococcus sp. strain PCC7942 was fast induced for 10 min, the K1/2(Ci) decreased from 253 μm (high-Ci cells) to 24 μm. In comparison, the K1/2(Ci) for low-Ci cells was 14 μm (Table I). Again, the presence of CA was important for fast induction, as shown in high-Ci cells from Synechococcus sp. strain PCC7942. Without the addition of external CA, cells were unable to induce a high-affinity state within the 10-min time period (Table I).
CO2 and HCO3− Uptake under Steady-State Photosynthesis
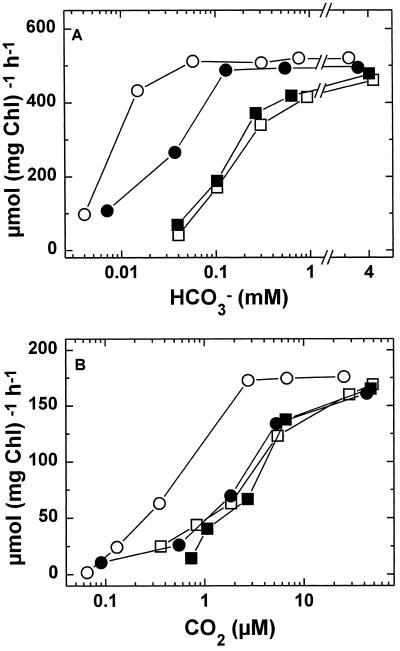
It is known that both CO2 and HCO3− serve as substrates for the transport systems in cyanobacteria (Badger and Andrews, 1982; Miller and Canvin, 1985; Yu et al., 1994a, 1994b; Sültemeyer et al., 1995, 1997a, 1997b; Salon et al., 1996; Tyrrell et al., 1996). To determine how various components of Ci transport are affected after fast induction we analyzed CO2 and HCO3− uptake in fast-induced cells from Synechococcus sp. strain PCC7002 and compared the kinetics with those of high- and low-Ci cells (Fig. 3; Table II). Regardless of the CO2 concentration during growth and induction treatment, all types of cells exhibited similar maximum transport activities during steady-state photosynthesis; maximum rates varied from 461 to 521 μmol mg−1 Chl h−1 for HCO3− transport, and from 161 to 176 μmol mg−1 Chl h−1 for CO2 uptake.
Figure 3.
Rates of HCO3− (A) and CO2 transport (B) during steady-state photosynthesis in Synechococcus sp. strain PCC7002. ○, Cells grown continuously on 20 μL of CO2 L−1 (low-Ci cells); □, cells grown continuously on 2% CO2 (high-Ci cells); •, high-Ci cells subjected to the fast-induction protocol for 4 min in the presence of CA (100 units mL−1); ▪, high-Ci cells subjected to the fast-induction protocol for 4 min in the absence of CA. Fast-induced cells were obtained after depletion of Ci in experiments similar to those shown in Figure 1. After Ci was depleted, an aliquot of cells containing 10 μg of Chl was centrifuged and resuspended in 4 mL of fresh CO2-free assay buffer to a final [Chl] of 2.5 μg mL−1. To fully inhibit CA activity, 50 μm AZA was added and HCO3− and CO2 uptake were measured mass spectrometrically in relation to their respective substrates (see Methods).
Table II.
Summary of kinetic parameters of HCO3− and CO2 transport in fast-induced and noninduced cyanobacterial cells
| Kinetic Parameters | High-Ci Cells | Low-Ci Cells | Fast-Induced Cells
|
|
|---|---|---|---|---|
| +CA | −CA | |||
| PCC7002 | ||||
| HCO3− transport | ||||
| Vmax (μmol mg−1 Chl h−1) | 461 ± 35 | 521 ± 49 | 495 ± 45 | 479 ± 48 |
| K1/2(HCO3−) (μm) | 167 ± 22 | 9.0 ± 3.2 | 23.5 ± 3.0 | 153 ± 14 |
| CO2 transport | ||||
| Vmax (μmol mg−1 Chl h−1) | 167 ± 11 | 176 ± 14 | 161 ± 10 | 165 ± 14 |
| K1/2(CO2) (μm) | 2.8 ± 0.3 | 0.5 ± 0.1 | 3.8 ± 0.4 | 3.5 ± 0.5 |
| PCC7942 | ||||
| HCO3−-transport | ||||
| Vmax (μmol mg−1 Chl h−1) | 206 ± 25 | 230 ± 19 | 248 ± 10 | n.d.a |
| K1/2(HCO3−) (μm) | 248 ± 21 | 9.0 ± 0.7 | 15.5 ± 3.8 | n.d. |
| CO2 transport | ||||
| Vmax (μmol mg−1 Chl h−1) | 116 ± 11 | 103 ± 14 | 133 ± 14 | n.d. |
| K1/2(CO2) (μm) | 3.6 ± 0.9 | 0.5 ± 0.1 | 3.4 ± 0.4 | n.d. |
High- and low-Ci cells of Synechococcus sp. strain PCC7002 and Synechococcus sp. strain PCC7942 were grown continuously on 2% CO2 and 20 μL of CO2 L−1. Fast-induced cells were obtained from high-Ci cells, which were allowed to run out of Ci for 4 min (strain PCC7002) and 10 min (strain PCC7942). When indicated, CA (100 Wilbur-Anderson units mL−1) was included during the Ci-depletion period. The data were obtained from experiments similar to those shown in Figure 3 and represent the mean ± sd from three to four independent experiments.
n.d, Not determined.
The major differences between high- and low-Ci cells are the affinities with which they transport HCO3− and CO2. Although the K1/2(HCO3−) and K1/2(CO2) were 167 and 3.8 μm in high-Ci cells, respectively, the corresponding values for low-Ci cells were 9.0 and 0.5 μm (Fig. 3; Table II). After a very short induction period of only 4 min, the K1/2(HCO3−) decreased to 27.5 μm in fast-induced cells (Fig. 3; Table II). If CA was omitted during the 4-min depletion of Ci, there was almost no change in the affinity of HCO3− transport for its substrate. After the same 4-min period of induction, the kinetics of CO2 uptake were only slightly affected, regardless of the presence or absence of CA (Fig. 3; Table II).
Similar results were obtained with the freshwater cyanobacterium Synechococcus sp. strain PCC7942 (Table II). Within 10 min of fast induction in the presence of CA, the K1/2(HCO3−) of HCO3− transport decreased from 248 μm (high-Ci cells) to 15.5 μm (fast-induced cells), and was approaching the K1/2(HCO3−) value of low-Ci cells (9 μm HCO3−). No dramatic changes in the maximum activities of HCO3− uptake were found regardless of the treatment (Table II). Again, the affinity of CO2 uptake remained unchanged after the short induction period, but was about 7-fold higher in low-Ci than in high-Ci cells (Table II).
Initial Rates of CO2 Uptake
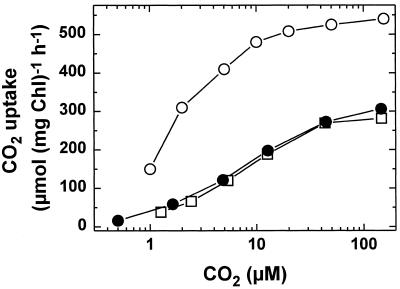
Kinetic analysis of CO2 and HCO3− transport in fast-induced cells (Fig. 3; Table II) indicates that high-affinity CO2 transport is not rapidly induced during initial exposure to low Ci. To further investigate whether any changes occurred to CO2 uptake processes we measured CO2 uptake under nonsteady-state photosynthetic conditions. Nonsteady-state CO2 uptake occurs during the initial phase of illumination in the absence of net O2 evolution and even in the presence of inhibitors that block photosynthetic electron flow (Badger and Andrews, 1982; Miller et al., 1991; Sültemeyer et al., 1991, 1997b). To investigate the effect of fast induction on initial CO2 transport we measured the rates of this process at various CO2 concentrations (Fig. 4). In all cases the initial rates of CO2 transport were strongly dependent on the CO2 concentration in the assay medium.
Figure 4.
Initial rates of CO2 uptake in relation to the CO2 concentration in the external medium in Synechococcus sp. strain PCC7002. ○, Cells grown continuously on 20 μL of CO2 L−1 (low-Ci cells); □, cells grown continuously on 2% CO2 (high-Ci cells); •, high-Ci cells subjected to the fast-induction protocol for 4 min in the presence of CA (100 units mL−1). Thereafter, an aliquot of cells containing 10 to 20 μg of Chl was centrifuged and resuspended in 4 mL of fresh CO2-free assay buffer to yield a final [Chl] of 2.5 to 5 μg mL−1. To fully inhibit CA activity 50 μm AZA was added and initial CO2 uptake was measured mass spectrometrically.
The maximum activities of initial CO2 uptake in high-Ci cells and fast-induced cells reached about 280 and 307 μmol mg−1 Chl h−1, respectively. When the cells were continuously grown on 20 μL of CO2 L−1 the maximum rate of initial CO2 transport increased to 533 μmol mg−1 Chl h−1. In addition to the quantitative differences in the rates of initial CO2 uptake, the apparent affinities of CO2 transport for its substrate increased in low-Ci cells, indicated by a decrease in K1/2(CO2) from 8.5 μm in high-Ci cells to 1.6 μm in low-Ci cells. Similar changes in the maximum activity and the CO2 affinity of CO2 transport have previously been reported after acclimation to low CO2 (Sültemeyer et al., 1997a, 1997b). In contrast, 4-min fast-induced cells exhibited a K1/2(CO2) of 8.1 μm CO2, which was not further improved even after a prolonged induction period of 15 min (data not shown).
Mediation of Fast Induction by Protein Synthesis or Protein Modification
The ability of the high-Ci cells to rapidly develop high-affinity HCO3− transport within 4 min may indicate that protein synthesis is not involved in this fast-acclimation process. To test this hypothesis the 4-min fast-induction experiment (Fig. 3) was repeated with high-Ci cells to which chloramphenicol (200 μg mL−1) was added during the first dark period (Fig. 5). After fast induction in the presence of chloramphenicol, the cells showed a K1/2(HCO3−) for net O2 evolution and HCO3− transport of 27.5 and 21.0 μm, respectively (Fig. 5), both being much lower than for the corresponding high-Ci cells (Table II). As expected, the affinity for CO2 uptake remained unaffected by chloramphenicol (data not shown). However, it is noteworthy that the maximum rates of O2 evolution and HCO3− and CO2 transport were inhibited by about 20% by chloramphenicol treatment (Fig. 5).
Figure 5.
The effect of chloramphenicol on the kinetic characteristics of substrate-dependent O2 evolution (▪) and HCO3− transport (▴) after a 4-min fast-induction period. High-Ci cells from Synechococcus sp. strain PCC7002 (10 μg of Chl mL−1) were allowed to run out of Ci for 4 min in the presence of CA (100 units mL−1) and chloramphenicol (200 μg mL−1). During the subsequent dark period of 2 to 3 min, the cells were diluted to a [Chl] of 2.5 μg mL−1 with fresh CO2-free medium containing the same amount of chloramphenicol. AZA (50 μg mL−1) was added to inhibit the remaining CA activity.
Because the rapid induction of high-affinity HCO3− transport was insensitive to chloramphenicol, it is reasonable to conclude that its regulation does not depend on de novo synthesis of proteins. Among cyanobacteria, posttranslational modifications of proteins by phosphorylation/dephosphorylation are common (Mann, 1994). In addition to His protein kinases, which are part of the two-component regulatory systems, eukaryote-like Ser/Thr protein kinases have been found in cyanobacteria (Mann, 1994; Kaneko et al., 1996). A Ser/Thr protein kinase from a gram-negative bacterium, Mycobacterium tuberculosis, was inhibited by staurosporine (Peirs et al., 1997), which is thought to act on the ATP-binding site of the catalytic domain common to eukaryotic and prokaryotic Ser/Thr protein kinases (Meggio et al., 1995). In addition, indications for a protein Tyr phosphorylation have been reported (McCartney et al., 1997), and a low-molecular-weight protein Tyr phosphatase has been identified based on sequence similarities to eukaryotic protein phosphatases (Kaneko et al., 1996).
To test the possibility that a protein phosphatase or a kinase may be involved in the induction process, we added cantharidin or okadaic acid, which are known specific inhibitors for Ser/Thr phosphatases of type 1 and 2A (MacKintosh and MacKintosh, 1994). As shown in Table III, the presence of either of these inhibitors had almost no effect on the rapid induction of high-affinity O2 evolution and HCO3− transport. However, when high-Ci cells were fast induced in the presence of K252a or staurosporine, which are widely used inhibitors for eukaryotic Ser/Thr protein kinases (MacKintosh and MacKintosh, 1994), no high-affinity O2 evolution or HCO3− transport was induced (Table III). Even genistein, which inhibits some Tyr kinases (MacKintosh and MacKintosh, 1994), caused almost complete repression of the fast-inducible high-affinity state (Table III). Unlike chloramphenicol, treatment of the cells with these inhibitors had basically no effect on the maximum rates of O2 evolution or HCO3− transport (data not shown).
Table III.
Protein kinase inhibitors prevent fast induction of high- affinity HCO3− transport
| Treatment |
K1/2
|
||
|---|---|---|---|
| Total Ci | HCO3− | CO2 | |
| μm | |||
| Control (no additions) | 28 ± 3.7 | 17 ± 2.5 | 3.5 ± 0.8 |
| Cantharidin (18 μm) | 35 ± 4.1 | 19 ± 1.1 | 5.0 ± 1.2 |
| Okadaic acid (2 μm) | 29 ± 1.4 | 14 ± 0.9 | 4.2 ± 0.5 |
| K252a (1 μm) | 202 ± 32 | 168 ± 28 | 4.8 ± 0.7 |
| Staurosporine (0.1 μm) | 153 ± 28 | 142 ± 18 | 4.4 ± 0.3 |
| Genistein (10 μm) | 167 ± 25 | 122 ± 21 | 4.9 ± 0.4 |
The effect of inhibitors of protein phosphatases (cantharidin, okadaic acid) and kinases (K252a, staurosporine, genistein) on the K1/2 values for O2 evolution, HCO3− transport, and CO2 uptake after fast induction in Synechococcus sp. strain PCC7002. Fast-induced cells were obtained from high-Ci cells (10 μg mL−1), which were allowed to run out of Ci for 4 to 8 min in the presence of CA (100 Wilbur-Anderson units mL−1) and the desired inhibitor. During the second dark period, an aliquot of the cells (8–12 μg of Chl) was centrifuged and resuspended in 4 mL of CO2-free assay buffer to enable mass spectrometric flux measurements. The data represent the mean ± sd from three independent experiments.
DISCUSSION
Fast Induction of High-Affinity HCO3− Transport
We have described an experimental system that allows cyanobacterial cells to significantly induce a high-affinity state of the CCM by more than 95% within 4 to 10 min of exposure to very low Ci levels (Figs. 1 and 2; Table I). Kinetic analysis of these fast-induced cells shows that it is the affinity of HCO3− transport that is dramatically increased during this period rather than any change in the CO2-transport kinetics of the cells (Fig. 3; Table II). The rapid nature of the acclimation and the fact that fast induction of high-affinity HCO3− transport is not inhibited by chloramphenicol (Fig. 5), but is inhibited by protein kinase inhibitors such as K252a, staurosporine, and genistein, indicates that posttranslational modification rather than de novo protein synthesis mediates these dramatic kinetic changes to HCO3− transport. To our knowledge, this is the first report of fast modulation of Ci transport that is potentially mediated by phosphorylation of a protein component upon transfer of cells to limiting Ci concentrations.
Previous data have indicated that the high-affinity state of the CCM is induced during a 4- to 6-h acclimation period at low [Ci]. This induction has been closely correlated with a simultaneous increase in the efficiencies of both CO2 and HCO3− transport and with an enhanced capacity to accumulate Ci (Omata and Ogawa, 1985; Badger and Price, 1992; Kaplan et al., 1994; Yu et al., 1994a, 1994b; Sültemeyer et al., 1995, 1997a, 1997b). Therefore, we were surprised to see that only the affinity of HCO3− transport was dramatically increased within 4 to 10 min of fast induction (Table II; Fig. 3), whereas no significant changes in the kinetics of CO2 uptake occurred, regardless of whether CO2 transport was observed under steady-state (Fig. 3; Table II) or nonsteady-state conditions (Fig. 4). Apparently, a longer adaptation period is required to induce the high-affinity CO2 transport system, suggesting that induction of the high-affinity CO2 and HCO3− transporters is regulated differently. A similar conclusion has been previously reported (Yu et al., 1994a).
The Requirement for External CA
Our experimental system for fast induction relies on the cells being rapidly exposed to very low initial Ci concentrations (Figs. 1 and 2). To achieve this, the cells must photosynthesize in the presence of CA, which allows the Ci compensation point to be obtained within minutes of illumination in a low-Ci reaction buffer. The lower the initial [Ci] the faster the Ci compensation point is reached and the more rapidly kinetic changes for HCO3− transport are observed. With an initial [Ci] of around 50 μm the cells run out of external Ci within 4 min (Figs. 3–5), whereas it took about 10 min when the initial [Ci] was 100 μm (Figs. 1 and 2).
The presence of active external CA was found to be an absolute requirement for fast induction, because without the enzyme neither the photosynthetic affinity nor the affinity of HCO3− transport increased within these short time periods (Figs. 1–3; Tables I and II). The fact that the photosynthetic affinity for Ci remained unchanged when CA was inhibited by AZA (Fig. 2) indicates that the catalytic properties of CA rather than the physical presence of a foreign protein is important for fast induction. The requirement for CA can be simply interpreted as allowing both CO2 and HCO3− transport processes to contribute to Ci uptake at low Ci and alkaline pH (8.0–8.2), rather than being chiefly dependent on CO2 transport, as occurs under conditions of slow interconversion between CO2 and HCO3− when CA is absent (Price and Badger, 1989). Consumption of external Ci by both transport systems thus allows the compensation [Ci] (close to 0) to be reached within 4 to 10 min of illumination (Fig. 1A). This was not achieved in the absence of CA (Fig. 1B).
The Trigger for Induction
Although it is still unclear what external factor primarily triggers the fast induction of high-affinity HCO3− transport, we can draw some clues from the experiments shown in Figure 1. First, because the CO2 concentration decreased to almost 0 in both experiments, but fast induction was observed only when total Ci was close to 0 in the presence of CA (Figs. 1A and 2), it seems unlikely that the CO2 concentration in the external medium is the primary signal to which the cells respond. Previous experiments have hinted at a similar conclusion (Mayo et al., 1986; Badger and Gallagher, 1987). Second, it has been suggested that the CO2:O2 ratio in the medium regulates the induction of the high-affinity CCM (Kaplan et al., 1994), indicating that a metabolite in the photorespiratory cycle might be involved in the signal transduction pathway. However, in the presence of CA (Fig. 1A), the O2 concentration during the fast-induction procedure increased from 18 to 28% and similar changes in O2 content were observed when CA was omitted (Fig. 1B, and data not shown). Because in both experiments the CO2 concentration was close to 0, a similar CO2:O2 ratio in the different assays and, consequently, a similar degree of induction would be expected if the CO2:O2 ratio were responsible for induction. This was clearly not the case (Figs. 1 and 2) and, therefore, we conclude that the CO2:O2 ratio is not crucial for fast induction.
The major difference between the two experiments shown in Figure 1 is the minimum HCO3− concentration in the medium after 10 min of illumination. Because fast induction of a high-photosynthetic affinity for Ci occurred only when total Ci and, therefore, HCO3− was almost 0 (Fig. 1A), it seems reasonable to assume that HCO3− is involved as a primary signal. This conclusion is consistent with previous suggestions for cyanobacteria (Mayo et al., 1986; Badger and Gallagher, 1987) and green algae (Matsuda and Colman, 1995). So far, however, the threshold concentration for HCO3− below which the high-affinity state of the CCM is induced is unknown, as is what components HCO3− is interacting with to initiate the induction changes.
Induction in Synechococcus sp. Strain PCC7002 versus PCC7942
Most of the data presented in this paper have been obtained with the marine cyanobacterium Synechococcus sp. strain PCC7002, but the ability for fast induction of the high-affinity CCM appears not to be restricted to one particular species. A comparison of the fast-induction characteristics between the marine species and the freshwater species, Synechococcus sp. strain PCC7942, revealed a high degree of similarity: almost the same time period for fast induction, a similar net increase in photosynthetic affinity for Ci (Table I), and fast induction being specific to high-affinity HCO3− transport without changing the kinetics of the CO2-uptake system (Table II). These similarities in the induction characteristics between two species acclimated to different habitats may be indicative of a common regulatory pathway of fast-inducible, high-affinity HCO3− transport among cyanobacteria. This possibility needs further investigation.
Posttranslational Modification of an Existing Protein
Several lines of evidence indicate that de novo protein synthesis is not responsible for the rapid change in the kinetic characteristics of photosynthetic O2 evolution and HCO3− transport. Within 4 min of illumination more than 90% of the high-affinity HCO3− uptake system was induced compared with low-Ci cells (Fig. 3; Table II). At a concentration of 200 μg mL−1, chloramphenicol did not inhibit fast induction of high-affinity HCO3− transport (Fig. 5). The observation that the maximum rate of O2 evolution was reduced by about 20% in the presence of the drug (Fig. 5) indicates that it had entered the cells and, therefore, should have affected protein synthesis. It is interesting to note in this context that we added a four-times-higher concentration of chloramphenicol than other authors used to inhibit protein synthesis in a short time in intact cells of Anacystis nidulans (Herrero et al., 1984).
Inhibitors of protein kinases, but not protein phosphatases, completely prevented fast induction of high-affinity HCO3− transport (Table III). The effects of these inhibitors on cyanobacterial protein kinases have not been investigated in detail. However, there are eight putative, eukaryotic-like protein kinases represented in the genome database from Synechocystis sp. strain PCC6803 (Kaneko et al., 1996). Three of these have both the ATP-binding and catalytic signatures for Ser/Thr protein kinases present as perfect matches to the Prosite signatures (namely SLR0599, SLR0152, and SLL0776), whereas one has only the conserved catalytic site (SLL1575), and another possesses only the ATP-binding site (SLR1697).
Molecular studies indicate that inhibitors such as K252a and staurosporine bind competitively at the ATP site (Meggio et al., 1995), and it is therefore likely that they act similarly against cyanobacterial homologs of protein kinases. In fact, staurosporine has already been demonstrated to inhibit a protein kinase from the gram-negative bacterium M. tuberculosis (Peirs et al., 1997). Taken together, our results are consistent with the view that a constitutive low-affinity HCO3− transporter (present in high-Ci cells) is posttranslationally modified into a high-affinity HCO3− uptake system, and that this step may involve phosphorylation of a polypeptide(s) by a protein kinase(s).
There is mounting evidence for the occurrence of protein phosphorylation in cyanobacteria (Bloye et al., 1992; Mann, 1994). For instance, Bloye et al. (1992) reported a significant increase in the extent of phosphorylation of several polypeptides during 1 to 2 h when low-Ci cells of Synechocystis sp. strain PCC6803 were shifted to high-Ci conditions or supplied with Glc, which causes a decline in the ability to accumulate Ci. This indicates that a protein kinase is involved in the down-regulation of the efficiency of the CCM. At this stage we have no explanation for the apparent differences between their results and ours, except that different time scales were used in the different experiments and different processes were being examined. However, in this context it is interesting to note that Goosney and Miller (1997) were unable to repeat the work of Bloye et al. (1992) with respect to the repression of HCO3− uptake by growth of Synechocystis sp. strain PCC6803 on Glc. Current work in our laboratory is aimed at identifying the polypeptide(s) that is phosphorylated during the fast-induction period.
Short-Term and Long-Term Acclimation at Low Ci
In this paper we have reported on a short-term (4–10 min) acclimation to limiting Ci in two cyanobacterial species, in which the properties of HCO3− transport in particular appear modified by posttranslational phosphorylation. This does not exclude the involvement of de novo protein synthesis during long-term acclimation to limiting Ci. Increasing numbers of carboxysomes as well as the proteins located within these structures, such as Rubisco and CA, are almost certainly changed via protein biosynthesis (Price et al., 1992; McKay et al., 1993), as is the case for plasma membrane proteins such as the 42-kD polypeptide (Omata and Ogawa, 1985). The slower induction of high-affinity CO2 transport may indicate that this is also achieved via protein synthesis, but this remains to be determined. As a consequence, the pathway for full induction of the high-affinity CCM may proceed in two steps: the first step may consist of posttranslational modification of a constitutive HCO3− transporter to provide a rapid response to limiting Ci concentrations, and the second would require a longer time period and depend on protein biosynthesis to allow full optimization of the acclimation response.
ACKNOWLEDGMENT
We thank Dr. Uwe Conrath (Fachbereich Biologie, Universität Kaiserslautern, Kaiserslautern, Germany) for providing us with the inhibitors for protein phosphatases and kinases.
Abbreviations:
- AZA
acetazolamide
- CA
carbonic anhydrase
- CCM
CO2-concentrating mechanism
- Chl
chlorophyll
- Ci
inorganic carbon
- K1/2(HCO3−)
K1/2(CO2), concentration of HCO3− and CO2, respectively, required for half-maximal activity
Footnotes
This work was supported by a research fellowship (to D.S.) from the Deutsche Forschungsgemeinschaft.
LITERATURE CITED
- Badger MR, Andrews TJ. Photosynthesis and inorganic carbon usage by the marine cyanobacterium, Synechococcus. Plant Physiol. 1982;70:517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Gallagher A. Adaptation of photosynthetic CO2 and HCO3− accumulation by the cyanobacterium Synechococcus PCC 6301 to growth at different inorganic carbon concentrations. Aust J Plant Physiol. 1987;14:189–201. [Google Scholar]
- Badger MR, Palmqvist K, Yu J-W. Measurement of CO2 and HCO3− fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Physiol Plant. 1994;90:529–536. [Google Scholar]
- Badger MR, Price GD. Carbonic anhydrase activity associated with the cyanobacterium Synechococcus PCC7942. Plant Physiol. 1989;89:51–60. doi: 10.1104/pp.89.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Price GD. The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiol Plant. 1992;84:606–615. [Google Scholar]
- Bloye SA, Silman NJ, Mann NH, Carr NG. Bicarbonate concentration by Synechococcus PCC6803. Modulation of protein phosphorylation and inorganic carbon transport by glucose. Plant Physiol. 1992;99:601–606. doi: 10.1104/pp.99.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fock HP, Sültemeyer D. O2 evolution and uptake measurements in plant cells by mass spectrometry. In: Linskens HF, Jackson JF, editors. Modern Methods of Plant Analysis. New Series, Vol 9: Gases in Plant and Microbial Cells. Berlin: Springer-Verlag; 1989. pp. 3–18. [Google Scholar]
- Goosney DA, Miller AG. High rates of O2 photoreduction by the unicellular cyanobacterium Synechocystis PCC 6803 as determined by the quenching of chlorophyll fluorescence. Can J Bot. 1997;75:394–401. [Google Scholar]
- Herrero A, Flores E, Guerrero MG. Regulation of the nitrate reductase level in Anacystis nidulans: activity decay under nitrogen stress. Arch Biochem Biophys. 1984;234:454–459. doi: 10.1016/0003-9861(84)90292-3. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S and others. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Research. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- Kaplan A, Schwarz R, Lieman-Hurwitz J, Ronen-Tarazi M, Reinhold L. Physiological and molecular studies on the response of cyanobacteria to changes in the ambient inorganic carbon concentration. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 469–485. [Google Scholar]
- MacKintosh C, MacKintosh RW. Inhibitors of protein kinases and phosphatases. Trends Biochem Sci. 1994;19:444–448. doi: 10.1016/0968-0004(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Mann NH. Protein phosphorylation in cyanobacteria. Microbiology. 1994;140:3207–3215. doi: 10.1099/13500872-140-12-3207. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Colman B. Induction of CO2 and bicarbonate transport in the green alga Chlorella ellipsoidea. I. Time course of induction of the two systems. Plant Physiol. 1995;108:247–252. doi: 10.1104/pp.108.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo WP, Williams TG, Birch DG, Turpin DH. Photosynthetic adaptation by Synechococcus leopoliensis in response to exogenous dissolved inorganic carbon. Plant Physiol. 1986;80:1038–1040. doi: 10.1104/pp.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney B, Howell D, Kennelly PJ, Potts M. Protein tyrosine phosphorylation in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179:2314–2318. doi: 10.1128/jb.179.7.2314-2318.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay RML, Gibbs SP, Espie GS. Effect of dissolved inorganic carbon on the expression of carboxysomes, localisation of Rubisco and the mode of inorganic carbon transport in cells of the cyanobacterium Synechococcus UTEX 625. Arch Microbiol. 1993;159:21–29. [Google Scholar]
- Meggio F, Donella Deana A, Ruzzene M, Brunati AM, Cesaro L, Guerra B, Meyer T, Mett H, Fabbro D, Furet P and others. Different susceptibility of protein kinases to staurosporine inhibition: kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur J Biochem. 1995;234:317–322. doi: 10.1111/j.1432-1033.1995.317_c.x. [DOI] [PubMed] [Google Scholar]
- Miller AG, Canvin DT. Distinction between HCO3− and CO2-dependent photosynthesis in the cyanobacterium Synechococcus leopoliensis based on the selective response of HCO3− transport to Na+ FEBS Lett. 1985;187:29–32. [Google Scholar]
- Miller AG, Espie GS, Canvin DT. Active transport of CO2 by the cyanobacterium Synechococcus UTEX 625. Measurements by mass spectrometer. Plant Physiol. 1988;86:677–683. doi: 10.1104/pp.86.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AG, Espie GS, Canvin DT. Active CO2 transport in cyanobacteria. Can J Bot. 1991;69:925–935. [Google Scholar]
- Omata T, Carlson TJ, Ogawa T, Pierce J. Sequencing and modification of the gene encoding the 42-kilodalton protein in the cytoplasmic membrane of Synechococcus PCC 7942. Plant Physiol. 1990;93:305–311. doi: 10.1104/pp.93.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T, Ogawa T. Changes in the polypeptide composition of the cytoplasmic membrane in the cyanobacterium Anacystis nidulans during adaptation to low CO2 conditions. Plant Cell Physiol. 1985;26:1075–1081. [Google Scholar]
- Palmqvist K, Sjöberg S, Samuelsson G. Induction of inorganic carbon accumulation in the unicellular green alga Scenedesmus obliquus and Chlamydomonas reinhardtii. Plant Physiol. 1988;87:437–442. doi: 10.1104/pp.87.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs P, de Witt L, Braibant M, Huygen K, Content J. A serine/threonine protein kinase from Mycobacterium tuberculosis. Eur J Biochem. 1997;244:604–612. doi: 10.1111/j.1432-1033.1997.00604.x. [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- Price GD, Badger MR. Ethoxyzolamide inhibition of CO2 uptake in the cyanobacterium Synechococcus PCC7942 without apparent inhibition of internal carbonic anhydrase activity. Plant Physiol. 1989;89:37–43. doi: 10.1104/pp.89.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Coleman JR, Badger MR. Association of carbonic anhydrase activity with carboxysomes isolated from the cyanobacterium Synechococcus PCC7942. Plant Physiol. 1992;100:784–793. doi: 10.1104/pp.100.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salon C, Mir NA, Canvin DT. Influx and efflux of inorganic carbon in Synechococcus UTEX 625. Plant Cell Environ. 1996;19:247–259. [Google Scholar]
- Sültemeyer D, Klughammer B, Ludwig M, Badger MR, Price GD. Random insertional mutagenesis used in the generation of mutants of the marine cyanobacterium Synechococcus sp. strain PCC7002 with an impaired CO2 concentrating mechanism. Aust J Plant Physiol. 1997a;24:317–327. [Google Scholar]
- Sültemeyer D, Price GD, Bryant DA, Badger MR. PsaE- and ndhF-mediated electron transport pathways affect bicarbonate transport rather than carbon dioxide uptake during steady-state photosynthesis in the marine cyanobacterium Synechococcus sp. PCC7002. Planta. 1997b;201:36–42. [Google Scholar]
- Sültemeyer D, Price GD, Yu J-W, Badger MR. Characterisation of carbon dioxide and bicarbonate transport during steady-state photosynthesis in the marine cyanobacterium Synechococcus strain PCC7002. Planta. 1995;197:597–607. [Google Scholar]
- Sültemeyer DF, Fock HP, Canvin DT. Active uptake of inorganic carbon by Chlamydomonas: evidence for a simultaneous transport of HCO3− and CO2 and characterisation of active transport. Can J Bot. 1991;69:995–1002. [Google Scholar]
- Turpin DH, Miller AG, Canvin DT. Carboxysome content of Synechococcus leopoliensis (cyanophyta) in response to inorganic carbon. J Phycol. 1984;20:249–253. [Google Scholar]
- Tyrrell PN, Kandasamy RA, Crotty CM, Espie GS. Ethoxyzolamide differentially inhibits CO2 uptake and Na+-independent and Na+-dependent HCO3− uptake in the cyanobacterium Synechococcus sp. UTEX 625. Plant Physiol. 1996;112:79–88. doi: 10.1104/pp.112.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J-W, Price GD, Badger MR. Characterisation of CO2 and HCO3− uptake during steady-state photosynthesis in the cyanobacterium Synechococcus strain PCC7942. Aust J Plant Physiol. 1994a;21:185–195. [Google Scholar]
- Yu J-W, Price GD, Badger MR. A mutant isolated from the cyanobacterium Synechococcus PCC7942 is unable to adapt to low inorganic carbon conditions. Plant Physiol. 1994b;104:605–611. doi: 10.1104/pp.104.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]