Abstract
While the eukaryotic genome is the same throughout all somatic cells in an organism, there are specific structures and functions that discern one type of cell from another. These differences are due to the cell's unique gene expression patterns that are determined during cellular differentiation. Interestingly, these cell-specific gene expression patterns can be affected by an organism's environment throughout its lifetime leading to phenotypical changes that have the potential of altering risk of some diseases. Both cell-specific gene expression signatures and environment mediated changes in expression patterns can be explained by a complex network of modifications to the DNA, histone proteins and degree of DNA packaging called epigenetic marks. Several areas of research have formed to study these epigenetic modifications, including DNA methylation, histone modifications, chromatin remodeling and microRNA (miRNA). The original definition of epigenetics incorporates inheritable but reversible phenomena that affect gene expression without altering base pairs. Even though not all of the above listed epigenetic traits have demonstrated heritability, they can all alter gene transcription without modification to the underlying genetic sequence. Because these epigenetic patterns can also be affected by an organism's environment, they serve as an important bridge between life experiences and phenotypes. Epigenetic patterns may change throughout ones lifespan, by an early life experience, environmental exposure or nutritional status. Epigenetic signatures influenced by the environment may determine our appearance, behavior, stress response, disease susceptibility, and even longevity. The interaction between types of epigenetic modifications in response to environmental factors and how environmental cues affect epigenetic patterns will further elucidate how gene transcription can be affectively altered.
Keywords: epigenetics, DNA methylation, histone modification, chromatin remodeling, microRNA, nutrition, environment
1. Introduction
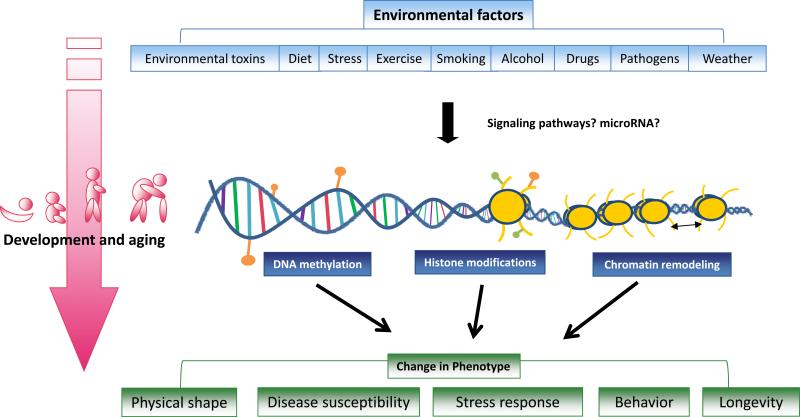
Epigenetics is the field of study surrounding stable alterations to the DNA and histone proteins that alter gene expression (Jaenisch and Bird, 2003). Epigenetic modifications are responsible for tightly regulated tissue and cell-type specific gene expression patterns. Aberrations in these expression patterns can give rise to certain diseases, most notably some types of cancer (Esteller, 2008). Interestingly, certain environmental factors can influence the expression of genes within a cell without mutations to the genome, but instead through modifying epigenetic marks. These environmental changes to the epigenome are so robust that even monozygotic twins can be identified by analyzing their unique epigenetic patterns (Fraga et al., 2005). Changes in epigenetic patterns can result in an alteration of gene expression, which in itself can have many downstream effects including changes in disease risk, stress response and metabolism (Lillycrop et al., 2005; Liu et al., 1997; McGowan et al., 2009). Epigenetics is the broad term used to describe a variety of reversible modifications to the genome that are meiotically and mitotically heritable, although the requirement for a modification to heritable has been contested. Epigenetic programming may begin while the fetus is developing in the uterus. Maternal environmental exposures during gestation are one important area of epigenetic research, but the gametes of the offspring might also be affected by those environmental factors while in the uterus, leading some to believe that our grandmother's environment might be affecting our own gene transcription through epigenetic mechanisms (Cropley et al., 2006). Furthermore, it is suggested that preconceptional paternal exposure to environmental factors can determine the offspring's phenotype epigenetically (Puri et al., 2010). Several changes to the genome fall into the category of epigenetics, including DNA methylation, histone modifications, chromatin remodeling and miRNA, although it is still debated if miRNA can be categorized as an epigenetic phenomenon. In this review we included miRNA, because of the ability miRNA has in affecting epigenetic phenomena, as well as the ability of epigenetic phenomena to change expression of miRNA. Taken together, these epigenetic mechanisms can provide the link between environmental factors and phenotypical changes during the whole lifetime of an organism (Figure 1).
Figure 1.
Epigenetic mechanisms provide the link between environmental factors and phenotypical changes during the whole lifetime.
2. DNA Methylation
2.1 Functions of DNA methylation
DNA methylation is perhaps the most well studied epigenetic mark. Methylation of the 5’ position of a cytosine within the genome occurs by the enzymatic family of DNA methyltransferases (DNMTs), thereby forming 5-methylcytosine (5-mC). S-adenosylmethionine (SAM), a modified amino acid produced in the one carbon metabolism pathway, donates the methyl group in this reaction (Niculescu and Zeisel, 2002). 5-methylcytosine, often times called the “fifth-base”, is present in an estimated 4-6% of the cytosine bases within a human genome, depending on cell type (Lister and Ecker, 2009; Lister et al., 2009). Most of DNA methylation occurs within CpG dinucleotides, although methylation outside of the CpG context has been reported in human DNA in recent years (Lister et al., 2009; Yan et al., 2011). The human genome contains about 30 million CpG dinucleotides that exist in a methylated or unmethylated state (Cocozza et al., 2011). Dense repeats of CpG nucleotides are called CpG islands and occur throughout the genome. Methylation of CpG islands located in the promoter region of a gene is usually inversely associated with transcription of that gene due to binding of methyl-CpG binding proteins, which recruit proteins to the promoter of the gene thereby blocking transcription (Meehan et al., 1992; Valinluck et al., 2004). The methylation status of intragenic regions or regions within a gene-body is also important in regulation of transcription, although the mechanism of action is not as well established (Shenker and Flanagan, 2011). Abnormally increased or loss of DNA methylation has been indicated as an important mechanism by which aberrant gene transcription occurs in diseases such as cancer.
2.2 DNA methylation through the lifespan
Upon fertilization, DNA methylation signatures of the genomes of both the oocytes and the sperm are erased, and a series of de novo lineage specific patterns of methylation is completed (Hajkova et al., 2002). It is during this time of re-programming that each gene will gain a specific DNA methylation pattern (Hajkova et al., 2002). Additionally, the process of X-chromosome inactivation will occur in female embryos at this time, leaving only one copy of each X-linked gene to be expressed (Allen et al., 1992). While historically X-chromosome inactivation in female development was thought to be random across alleles so that the ratio of maternal and paternal X-chromosome linked genes expressed is equal, recent research suggests that this ratio can deviate from equal inactivation, an incidence called skewed X-chromosome inactivation (Minks et al., 2008). One such study found skewed X-chromosome inactivation develops with age, beginning as early as the age of 10 years (Wong et al., 2011). This research may shed more light on the understanding of sex differences in diseases.
While changes in global DNA methylation seem to occur naturally in aging, aberrations in methylation have also been established in cancerous cells. Global hypomethylation with site-specific increases in methylation is an epigenetic pattern that is associated both with age and cancer (Liu et al., 2003). While age-associated changes in DNA methylation may be a natural pattern of aging, specific regions of methylation changes have been associated with decreased organ function, memory, bone density and other age-related health problems (Lepeule et al., 2012; Liu et al., 2011). The future of studying epigenetics in aging may provide a key link in methods to prevent these age-related health problems.
2.3 Maintenance and de novo DNA methylation
DNA methylation patterns are passed on from the parental strand of DNA to the daughter cells during cellular replication. DNA methyltransferase 1, or DNMT1, is the enzyme that tends to keep the methylation mark in the nascent DNA during mitosis at cytosines that were methylated on the parental strand, called maintenance methylation. Maintenance methylation ensures that programmed DNA methylation patterns remain through cellular generations. Contrarily, DNMT3A and DNMT3B are referred to as the de novo methyltransferases because of their ability to methylate both unmodified cytosines and hemimethylated cytosines with similar efficiency, producing new DNA methylation marks (Gowher and Jeltsch, 2001). These de novo DNMTs primarily establish methylation patterns in early development as well as methylate maternally imprinted genes in oocytes (Gowher and Jeltsch, 2001; Hata et al., 2002). De novo methylation can also occur in differentiated somatic cells, albeit at a slow rate. It has been suggested that DNMT3a and DNMT3b may be the methyltransferases that are responsible for methylation of cytosines in non-CpG contexts, although this area of research is still developing (Aoki et al., 2001; Gowher and Jeltsch, 2001).
2.4 Localization of DNA methylation by the DNA sequence
While understanding how methylation is incorporated and maintained in the genome is one important area of epigenetic research, another is exploring the causes of where methyl groups are added throughout the genome. One potential determinant of DNA methylation is the genetic code itself. An association between single nucleotide polymorphisms (SNPs) and changes in DNA methylation patterns in cis surrounding the SNP was made in early 2011 (Bell et al., 2011). Following this discovery, Lienert et al. investigated the role of the DNA sequence in DNA methylation in vitro by randomly inserting ~1,000 bp fragments of gene promoters into the genomes of mouse embryonic cells. They subsequently tracked the methylation of the DNA both in the original and the transplanted region, and found that the degree of methylation was the same regardless of location within the genome (Lienert et al., 2011). This interesting experiment provided novel insight that DNA methylation is dependent on the sequence in cis, that is, the genetic code of a promoter determines the methylation of surrounding cytosines on the same strand of DNA. Furthermore, the methylation patterns of the randomly inserted promoters maintained their expected methylation patterns throughout differentiation of the stem cells, further demonstrating the impact of the genetic sequence on methylation status.
While the local base composition of a promoter seems to determine methylation, this is not the only determining factor. Factor binding sites along the genome may also impact DNA methylation patterns. For example, a mutation within a factor-binding site limits the maintenance of methylation patterns in the surrounding region of DNA (Lienert et al., 2011). Further research is needed to determine the degree by which the underlying genetic sequence establishes DNA methylation patterns, and the impact exposure to certain environmental factors may have on altering these patterns.
3. DNA Hydroxymethylation
3.1 Hydroxymethylation as an intermediate in demethylation
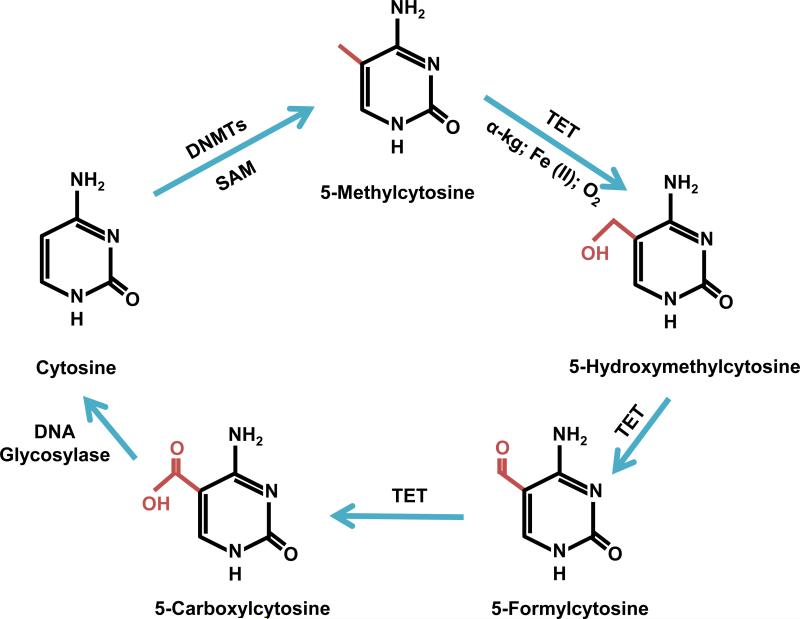
Recently a new epigenetic mark called 5-hydroxymethylcytosine has been proposed as a player in the removal of methyl groups from cytosine bases. Hydroxymethylcytosine (or 5-hmC) has been referred to as the “sixth-base” because of its potential regulatory role in gene transcription similar to 5-mC (Münzel et al., 2011). Less than 1% of cytosines are hydroxylated in mammalian DNA, and the level varies by tissue with the central nervous system having the highest amount (Globisch et al., 2010). Embryonic stem cells also have high levels of 5-hmC, but these levels decrease during differentiation (Globisch et al., 2010; Tahiliani et al., 2009). The most commonly regarded function of 5-hmC is as an intermediate in the removal of the methyl group from 5-mC, returning the cytosine to its unmodified form. The mechanism for the removal of methyl groups from cytosine residues is a growing topic in current epigenetic research. Several mechanisms have been proposed, including passive demethylation where the methyl marks are simply not replaced in the daughter cell after cell division resulting in an unmodified cytosine. Other proposed mechanisms of active demethylation involves the active removal of methyl groups by DNA glycosylase, deamination of the cytosine forming a thymine base followed by removal of that thymine by DNA repair machinery, or the oxidation of 5-mC to 5-hmC and several other intermediates before the return of an unmodified cytosine (Globisch et al., 2010; Jurkowski and Jeltsch, 2011). The latter hypothesis has garnered much attention in the research community, and has been supported by the discovery of the proposed intermediates (5-formylcytosine and 5-carboxylcytosine) of this oxidation process to be present in genomic mouse DNA (Ito et al., 2011). Interestingly, the same family of enzymes responsible for the conversion of methylcytosine to hydroxymethylcytosine may also convert methylcytosine to 5-formylcytosine and 5-carboxylcytosine, which are then excised by a DNA glycosylase (Figure 2) (He et al., 2011; Ito et al., 2011; Maiti and Drohat, 2011). These enzymes are called TET enzymes, discussed below.
Figure 2.
Proposed mechanism of active demethylation through hydroxymethylation by TET
3.2 Functions of the TET enzymes
The TET (ten-eleven translocation) family of proteins are responsible for the hydroxylation of 5-mC to 5-hmC in an iron (II), α-ketoglutarate and divalent oxygen dependent fashion (Ito et al., 2010; Tahiliani et al., 2009). TET1 is the form of the enzyme that is mainly expressed in embryonic stem cells, whereas TET2 and TET3 are more common throughout all other tissues (Tahiliani et al., 2009). Knockout of TET1 in embryonic stem cells leads to global increases in DNA methylation with a correlating decrease in hydroxymethylation, suggesting a role for TET1 in removal of DNA methyl groups (Dawlaty et al., 2011; Ficz et al., 2011).
Hydroxymethylation of a gene has been associated with changes in transcription of that gene, but it is still unclear whether that transcriptional regulation is due to the 5-hmC mark itself or by the binding of the TET enzyme to the DNA. One key study that indicts TET as playing some part in transcriptional regulation found the enzymatic activity of TET was indispensable for the transcriptional regulation to occur (Zhang et al., 2010). In mouse embryonic stem cells TET1 binds mostly to regions of the DNA that are gene-rich, primarily at transcription start sites (TSS) of genes (Williams et al., 2011). Additionally TET1 target genes are associated with intermediate and high densities of CpG sites in the promoters and TSS (Williams et al., 2011; Xu et al., 2011b). One proposed function of TET1 at these locations might be to prevent any unwanted DNA methylation by preventing the binding of DNMTs, interfering with the addition of methyl groups.
Additionally, TET1 may repress transcription of some of its target genes in a manner separate from the removal of DNA methylation by recruiting Polycomb repressor complex 2 (PRC2). The TET1 protein binds to many Polycomb group target genes and recruits PRC2 to the promoters resulting in changes in chromatin structure and gene transcription (Williams et al., 2011; Wu et al., 2011). Polycomb group proteins will be discussed in further detail in section 5.
3.3 Hydroxymethylation as a transcriptional regulator
Hydroxymethylation of cytosines within a gene promoter or CpG island is associated with an increase in transcription of that gene, the opposite pattern that is described for methylation of cytosines (Ficz et al., 2011). It has therefore been proposed that hydroxymethylation may in itself act as a regulator of gene transcription. The mechanism by which hydroxymethylation may cause a change in gene transcription is through the release of methyl-binding proteins from the DNA. Most of these 5-mC-binding proteins will no longer bind to DNA once the methylcytosine has been hydroxylated. These proteins then dissociate from the DNA, perhaps changing the transcriptional status of that gene (Jin et al., 2010; Valinluck et al., 2004). The longevity and heritability of 5-hmC has not been elucidated, but for the amount of time this epigenetic mark is present in the DNA gene transcription may be affected.
4. Histone Modifications
4.1 Compacting the DNA
DNA is compacted by tightly weaving approximately 147 base pairs around proteins called histones, forming a DNA-protein complex called a nucleosome. Each nucleosome consists of an octamer of two copies of four core histones: H2A, H2B, H3 and H4. Various post-translational modifications to the N-terminal histone tails can occur in mammalian cells, including histone acetylation, methylation, phosphorylation, ubiquitination, ADP-ribosylation and biotinylation. These modifications result in a dynamic chromatin environment, where the epigenetic regulation of gene transcription is altered by histone modifications. Compared to DNA methylation, which is stably inherited between cell divisions, it is still unclear whether histone modifications can be reproduced following DNA replication and transmitted from one cell generation to the next. Recent research has however postulated several models for the heritability of chromatin structure (Margueron and Reinberg, 2010; Xu et al., 2011a).
4.2 Common modifications to histone tails
Histone modifications can act by either altering the ionic charge of the histone tail or by functioning as a binding platform for other proteins. Histone acetyltransferases (HATs) are the class of enzymes that transfer acetyl groups onto the ε amino group of a lysine residue within the histone tail. Through this action the positive charge of the lysine is neutralized and the interaction between the histone tail and DNA is weakened (Allfrey et al., 1964). In contrast to HATs, histone deacetylases (HDACs) remove acetyl groups from lysines and restore the positive charge on the histone tail. HATs are generally classified as transcriptional activators, whereas HDACs are transcriptional repressors (Shahbazian and Grunstein, 2007). Histone kinases and phosphatases add or remove phosphate groups from the hydroxyl group of serines, threonines and tyrosines on the histone tails. Like acetylation, phosphorylation of the histone tails alters the charge and ionic properties of the histone, resulting in a changed structure of the local chromatin environment (Xhemalce et al., 2011).
Unlike the acetylation and phosphorylation of histone tails, methylation does not function by changing the ionic charge of the protein (Bannister and Kouzarides, 2011). Methylation of a lysine within the histone tail occurs via a histone lysine methyltransferase (HKMT). Likewise, methylation of arginine residues within the histone tail is catalyzed by the protein arginine methyltransferase (PRMT) family. Both of these protein methyltransferases use SAM as the methyl donor, similar to the DNMTs discussed in Section 2.1 above. Methylation of histone tails will affect gene transcription through the recruitment of chromatin factors. For example, histone 3 lysine 4 trimethylation is associated with active gene transcription, and is recognized by specific protein domains (Bannister and Kouzarides, 2011). One such domain is the tandem chromodomains within CHD1, which is an ATP-dependent chromatin remodeling protein (Sims et al., 2005). When both of the tandem chromodomains within CHD1 bind histone 3 lysine 4 trimethylation, factors mediating post-transcriptional initiation events are recruited, including components of the spliceosome (Sims III et al., 2007). Other proteins that recognize and bind the histone 3 lysine 4 trimethylation mark are proteins containing the plant homeodomain (PHD) fingers in the ING family, and the tandem Tudor domains within JMJD2A (Bannister and Kouzarides, 2011; Huang et al., 2006; Shi et al., 2006; Wysocka et al., 2006). It is through this specific recruitment of proteins that histone modifications can participate in the transcription of genes.
5. Chromatin Remodeling
5.1 Mechanisms for chromatin remodeling
Regulation of gene transcription can also occur at the chromatin level. Through tightly packing the flexible euchromatin into nucleosome-rich heterochromatin, transcription factors and RNA polymerases can no longer transcribe regions of DNA and that gene becomes silenced. This compacted chromatin will form both to stabilize the genome (at the centromere and telomeres of a chromosome for example) and to prevent transcription of a specific target gene. The mechanism by which chromatin are remodeled to either silence or activate a gene is either through an ATP-dependent protein complex, or through combined covalent modifications of histone tails by histone modifying proteins like the Polycomb group proteins.
5.2 ATP-dependent chromatin remodeling
The SWI/SNF (SWItch/Sucrose NonFermentable) complex is a common ATP-dependent chromatin remodeling protein complex in eukaryotes. This remodeling complex binds to the nucleosome and disconnects the DNA from the histones, creating a transient DNA loop (Havas et al., 2000). This DNA loop will then travel around the nucleosome until it reaches the exit side, resulting in repositioning of that nucleosome (Tang et al., 2010). As the nucleosome is repositioned, it moves closer to its neighboring nucleosome, and the distance between nucleosomes is shortened (Dechassa et al., 2010). It has been posited that the SWI/SNF complex disassembles the nucleosome by removing the histone proteins and transferring those proteins to nearby free DNA (Bruno et al., 2003). This process may be dependent on dinucleosomes being present, as in vitro studies using mononucleosomes result in negative findings (Dechassa et al., 2010). After the nucleosome has been repositioned, transcription of targeted genes can be increased or decreased depending on whether the gene is located in the open chromatin or compacted chromatin region.
5.3 Polycomb proteins and their function
Polycomb group (PcG) proteins are also involved in gene silencing through chromatin remodeling. PcG proteins repress transcription by maintaining a heterochromatin state by means of particular histone modifications and DNA methylation. This is accomplished by the formation of multiprotein complexes, termed Polycomb repressor complexes (PRC). The composition of a PRC varies between organisms, cell types, developmental stage and even in some disease states (Otte and Kwaks, 2003). These complexes are recruited to regions of the DNA where they regulate gene transcription through histone modifications. For example, in humans the EED-EZH2 complex, a component of PRC2, tri-methylates histone 3 at lysine 27, which then facilitates binding of PRC1. This is not always the case however, as PRC1 can also be recruited in a PRC2 independent manner, demonstrating the versatility and complexity of transcription regulation (Cao et al., 2002; Kuzmichev et al., 2002; Schoeftner et al., 2006). Recruitment of PRC1 can then lead to transcriptional repression through the mono-ubiquitination of histone 2A lysine 119. The addition of the ubiquitin mark ultimately stops gene transcription by inhibiting elongation of mRNA by RNA polymerase (Zhou et al., 2008). Through this multi-step protein recruitment pathway, gene expression can be affectively regulated, illustrating another important epigenetic mechanism.
5.4 Interaction of ncRNAs and PRC
Recent research has found a role of non-coding RNAs (ncRNA) in interacting with PcG protein complexes to bring the PRC to their target DNA. Kanhere and colleagues revealed that even when a Polycomb target gene was not being actively expressed, short non-coding RNAs about 50-200 nucleotides in length were transcribed from the 5’ end of the gene. These short ncRNAs would then form stem-loop structures that interacted with PRC2 through the SUZ12 protein, which in turn caused gene repression (Kanhere et al., 2010). Furthermore, evidence for large intergenic noncoding RNAs (lincRNAs) in the silencing of genes through PRC2 has also been established. In a study analyzing lincRNAs in the human genome, it was found that approximately 20% were bound by PRC2. Additionally, inhibition of those PRC2-bound lincRNAs led to changes in expression of genes that would normally be silenced by PRC2 (Khalil et al., 2009).
The interaction between ncRNAs and PRC2 also comes to light in the silencing of the extra X-chromosome in females. Xist is a ncRNA that is expressed on the inactive X-chromosome in females and plays an important part in the inactivation of the X-chromosome through PRC2 recruitment. A 1.6 kb region of ncRNA within Xist called RepA targets PRC2 and brings it to the X-chromosome (Zhao et al., 2008). This ncRNA seems to be required for the initiation and spreading of X-chromosome inactivation, but not for the maintenance of the silencing. Taken together, different forms of ncRNAs seen to contribute to the regulation of gene transcription via interactions with Polycomb repressor complexes.
6. MicroRNAs
6.1 Mechanism of action
A relatively new area of epigenetic research focuses around a form of small non-coding RNAs that are usually about 20 to 30 nucleotides in length called miRNAs. Unlike the ncRNAs discussed in section 5.4 above, the primary mechanism by which miRNAs act to post-transcriptionally silence target genes is by recognizing target sequences in the mRNA by Watson-Crick base pairing of nucleotides 2 through 8 in the miRNA, called the seed region. The miRNA can inhibit translation of mRNA either by directly binding the target sequence in the 3’ UTR, forming double-stranded mRNA which is degraded, or through the formation of the RNA-induced silencing complex (RISC) (Bartel, 2004). Some miRNAs reside within introns of protein-coding genes and are transcribed along with the primary transcript. Other miRNAs are transcribed from their own gene, the primary transcript being called a pri-miRNA which is then processed by cellular machinery into mature miRNA(Winter et al., 2009). A single miRNA may have multiple target mRNA; likewise each mRNA may also be regulated by several miRNAs.
6.2 Influencing miRNA
Certain environmental factors can alter the expression of genes through expression of new or removal of old miRNAs (Carthew and Sontheimer, 2009). There is some evidence of feedback between miRNA expression and DNA methylation and histone modifications (Barski et al., 2009; Sato et al., 2011). For example, DNA methylation and histone modifications can regulate the transcription of miRNAs, and some miRNAs can alter the expression of DNMT3A and DNMT3B, and Polycomb group genes (Fabbri et al., 2007; Juan et al., 2009). Additionally, aberrant miRNA expression caused by changes in DNA methylation has been discovered in cancer cells (Bandres et al., 2009; Furuta et al., 2010; Kozaki et al., 2008).
Besides endogenous sources of miRNA regulating protein expression, there is some evidence that exogenous miRNA may have an effect on our cells. In an interesting recently published study, the consumption of exogenous miRNA altered protein translation of target mRNA in mammals. Zhang et al. demonstrated that exogenous miRNAs that were consumed from rice can pass through the mammalian GI tract where they travel through the sera in microvesicles and are delivered to organs (Zhang et al., 2011). Furthermore, these miRNAs bound to their target mRNA sequences where they exerted their downstream effects of diminished protein expression. One such miRNA found abundantly in rice, called miR168a, was able to bind to human and mouse low-density lipoprotein receptor adapter protein 1 (LDLRAP1) mRNA and inhibit its translation in murine liver. Because LDLRAP1 is involved in removing LDL from the plasma, an increase in exogenous miR168a elevated plasma LDL-cholesterol levels (Zhang et al., 2011). This study provides a novel role of miRNAs as the bridge between our diet and gene expression through post-transcriptional silencing of mRNA translation, and gives evidence of a new mechanism by which our environment may alter our phenotype.
7. Environmental Influences and Epigenetics
7.1 Nutritional epigenetics
Certain dietary bioactive food components can change gene expression via alterations in DNA methylation and histone modifications, a field of study that may one day lead to the development of the “epigenetic diet” (Hardy and Tollefsbol, 2011; Park et al., 2011). The availability of the universal methyl donor, SAM, is determined by one-carbon metabolism, a pathway involving vitamins B6, B12, folate, betaine and choline along with the amino acids methionine, cysteine, serine and glycine. When a component of one-carbon metabolism is missing, in B-vitamin deficiency for example, DNA methylation and histone modifications are altered (Niculescu and Zeisel, 2002). Chronic alcohol consumption can also change epigenetic patterns through both the wastage of methionine and choline as well as modifying B-vitamin availability, thus reducing the amount of SAM available for methylation reactions (Mason and Choi, 2005).
While perturbations in the one-carbon metabolism pathway are one way nutrition can have an impact on epigenetic patterns, other bioactive nutrients can have an affect through different pathways. For example, epigallocatechin-3-gallate (EGCG), the primary polyphenol found in green tea, can reduce global DNA methylation in cancer cell lines through the competitive inhibition of DNMT (Fang et al., 2003). The addition of EGCG to cancer cell lines reverses the repression of some tumor suppressor genes, including p16, O-6-methylguanine-DNA methyltransferase (MGMT) and reversion-inducing-cysteine-rich protein with kazal motifs (RECK) (Fang et al., 2003; Kato et al., 2008). In 2011, Wang et al. found EGCG to have the ability to increase miR-210, a miRNA that stabilizes the hypoxia response protein hypoxia-inducible factor 1-alpha (HIF-1α), as well as decrease cancer cell proliferation (Wang et al., 2011). This provides another mechanism through which EGCG demonstrates anticancer activity. In recent years many other nutrients have demonstrated epigenetic capabilities. Selenium is a mineral found in grains and vegetables grown in selenium rich soil and can also alter DNA methylation. A reduction in selenium results in a decrease in global DNA methylation, as well as a decrease in the expression of DNMT1 in prostate and colon cancer cell lines as well as rat liver and colon tissue (Davis et al., 2000; Xiang et al., 2008). The red carotenoid lycopene potentially has demethylating capabilities in a breast cancer cell culture line, although these results have been questioned and further validation is needed (King-Batoon et al., 2008; Liu and Erdman, 2011).
Sulforaphane is a bioactive food component found in broccoli sprouts and possesses HDAC inhibition activity in cancer cells. Other bioactive food components that have garnered interest in the nutritional epigenetics field are butyrate, resveratrol, genistein and curcumin (Chung, 2010; King-Batoon et al., 2008; Zhang and Chen, 2011). The research into the mechanisms of action for these molecules is ongoing, and sure to bring new insight into the field. Modifying the epigenome through the diet may one day provide means of early disease intervention or prevention through the diet.
7.2 Maternal diet
Maternal transmission of epigenetic traits may be altered by nutrients, affecting the offspring's phenotype or disease risk. Maternal intake of B-vitamins is associated with changes in risk of both colon and breast cancer in the offspring. Specifically, loss of imprinting of the insulin-like growth factor 2 gene (Igf2) has been implicated in colorectal cancer in both rodents and humans (Cui et al., 2003; Sakatani et al., 2005). Periconceptional maternal supplementation of folic acid is associated with a higher degree of methylation of Igf2 in the offspring (Steegers-Theunissen et al., 2009). Moreover, maternal supplementation of choline, another nutritional factor involved in one-carbon metabolism, may influence histone modifications in the liver and brain of the offspring in rats (Davison et al., 2009). Both histone 3 lysine 9 dimethylation and histone 3 lysine 27 trimethylation are increased in the fetal tissue when the mother has increased choline intake, potentially due to an increase in histone methyltransferase expression (Davison et al., 2009).
Maternal protein-restriction in rats seems to epigenetically program metabolism in the offspring. In pups whose mothers were fed a diet low in protein, reduced methylation and increased expression of peroxisome proliferator-activated receptor α (PPARα) in the liver has been demonstrated. A similar trend is seen for the glucocorticoid receptor gene, but these effects are lost in the offspring of mothers fed a protein-restricted diet supplemented with folate. This not only demonstrates the influence of the maternal diet on the pup's fat and carbohydrate metabolism, but also that the effect most likely occurred through altered one-carbon metabolism (Lillycrop et al., 2005). More recently, low and high protein maternal diets in pigs were shown to effect global DNA methylation in the newborn offspring through changes in Dnmt1, Dnmt2 and Dnmt3 expression in both the liver and skeletal muscle (Altmann et al., 2012).
7.3 Early life experiences
A rather interesting area of research in epigenetics is the influence of one's environment during childhood on central nervous system and stress response in later life. For example, when comparing the epigenetic patterns of the hippocampus of suicide victims with a history of childhood abuse to suicide victims without a history of abuse there is an increase in methylation of the promoter of the nuclear receptor subfamily 3 (NR3C1) gene (McGowan et al., 2009). NR3C1 is the gene encoding the neuron-specific glucocorticoid receptor, which when stimulated inhibits the hypothalamic-pituitary-adrenal (HPA) stress response (De Kloet et al., 2005). These same results were seen in a much earlier study in rats, where pups that were raised with less licking and grooming, and less arched-back nursing also had an altered stress response (Liu et al., 1997). Social stress in young mice has been linked to epigenetic changes in the hippocampus. In a previous study, mice that were exposed to social defeat stress caused by the introduction of a highly aggressive mouse develop long-term depression-like symptoms (Tsankova et al., 2006). Interestingly, both male and female offspring whose father had been exposed to social defeat stress also exhibited depressive and anxiety like behaviors, along with increased plasma corticosterone concentrations, potentially caused through epigenetic inheritance (Dietz and Nestler, 2012). Molecularly, mice that are subjected to social defeat stress have a decrease in expression of brain-derived neurotrophic factor (BDNF) transcripts, as well as an increase in methylation at histone 3 lysine 27 corresponding to the promoter regions of the transcripts (Tsankova et al., 2006). Decreased transcription of two splice variants of BDNF and corresponding increases in methylation of the promoter regions is also associated with maternal neglect in rodents (Roth et al., 2009). These studies suggest that early life experiences lead to molecular changes in the brain's stress response through alterations in epigenetic patterns.
7.4 Epigenetics and Aging
Epigenetic signatures tend to change naturally as we age. In general, there is a genomic decrease in DNA methylation, with a paradoxical hypermethylation of certain gene promoters throughout the genome (Bjornsson et al., 2008; Calvanese et al., 2009). While these patterns have been demonstrated in multiple organisms and tissues, it is still unclear whether the changes in epigenetic patterns are programmed or stochastic (Gravina and Vijg, 2010). Interestingly, the same DNA methylation patterns that are seen with aging appear in cancer development, where tumor suppressor genes are suppressed by hypermethylation of their promoter (Esteller, 2008). Changes in histone modifications also seem to occur with age. It has been demonstrated that in vitro senescence is associated with a decrease in the repressive histone mark histone 3 lysine 27 trimethylation as well as an increase in methylation of histone 4 lysine 20, which is indicative of a heterochromatic region, thus repression of gene transcription (Bracken et al., 2007; Sarg et al., 2002). These alterations to epigenetic patterns may contribute to age-associated diseases, like cancer, or to the physiological process of aging itself. With better understanding of the specific changes in epigenetic marks that occur with aging a proper dietary or pharmaceutical intervention may be proposed.
8. Conclusion and Future Perspectives
The field of epigenetics is rapidly evolving to contain many types of modifications to the genome that do not alter the genetic sequence itself. As research continues, the mechanism of action for each of these epigenetic modifications is further elucidated. An interesting aspect of epigenetics is the interaction between the genome and the environment, a research area that is bettering our understanding of how the environment shapes our own phenotype along with the phenotype of our offspring, and potentially even lasting into the following generation. Nutritional epigenetics may provide a novel way to naturally modulate epigenetic traits that are seen in the progression of particular diseases, such as cancer. Future research is still needed to better discriminate a “healthy” epigenetic pattern from those related to disease state, which may aid the medical field in making earlier and more specific disease diagnoses and prognoses (Chu et al., 2009; Dworkin et al., 2009; Levenson and Melnikov, 2012; Lorincz, 2011).
Epigenetic research does not come without its limitations. Because epigenetic traits are both tissue and cell-type specific, and most studies have been conducted in a single tissue or type of cell line, it is difficult to extrapolate results across an entire organism. Furthermore, studies in model organisms typically have the advantage of comparing inbred animals that are genetically similar. Because the underlying genetic sequence may impact the epigenetic pattern, the environmental impact on modifying these patterns is also under genetic influence. Conversely epigenetic studies in humans must tease out differences in epigenetic patterns that are truly due to the environment and not genetic factors (Feil and Fraga, 2012). Compared to animal models, humans are exposed to a much higher variety of environmental factors that may also interact with genes. Thus it is not an easy task for researchers to infer the epigenetic effects of particular environmental exposure in humans.
The timing of an intervention may be important in the alteration of epigenetic patterns, be it a bioactive food component, hormone or pharmaceutical. For example, exposure to a nutrient prenatally may affect the epigenome differently than exposure in adolescence or adulthood. Likewise, the efficacy of an epigenetic intervention to prevent a disease or delay aging might depend on the timing of the intervention. Currently, inhibitors of DNMT or HDAC are being developed and used in cancer treatment to restore normal expression of tumor-suppressor genes. In the future, an epigenetic intervention may be extended to a secondary prevention or to treatment of diseases other than cancer. Through epigenetic research the scientific community can develop a better understanding of mammalian inheritance, changes in disease risk and progression, and the degree of impact certain environmental exposures may have on both our own, and our children's phenotype.
Acknowledgement
This material is based upon work supported by the U.S. Department of Agriculture, under agreement No. 51000-074-01S. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture. This project has been supported in part by the National Institute of Health Grants R01 AG025834 (SWC). Authors do not have any conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RC, Zoghbi H, Moseley A, Rosenblatt H, Belmont J. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. American journal of human genetics. 1992;51(6):1229. [PMC free article] [PubMed] [Google Scholar]
- Allfrey V, Faulkner R, Mirsky A. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1964;51(5):786. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann S, Murani E, Schwerin M, Metges CC, Wimmers K, Ponsuksili S. Maternal dietary protein restriction and excess affects offspring gene expression and methylation of non-SMC subunits of condensin I in liver and skeletal muscle. Epigenetics. 2012;7(3):0–-1. doi: 10.4161/epi.7.3.19183. [DOI] [PubMed] [Google Scholar]
- Aoki A, Suetake I, Miyagawa J, Fujio T, Chijiwa T, Sasaki H, Tajima S. Enzymatic properties of de novo-type mouse DNA (cytosine-5) methyltransferases. Nucleic Acids Research. 2001;29(17):3506–3512. doi: 10.1093/nar/29.17.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandres E, Agirre X, Bitarte N, Ramirez N, Zarate R, Roman- Gomez J, Prosper F, Garcia- Foncillas J. Epigenetic regulation of microRNA expression in colorectal cancer. International journal of cancer. 2009;125(11):2737–2743. doi: 10.1002/ijc.24638. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Research. 2011;21(3):381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Jothi R, Cuddapah S, Cui K, Roh TY, Schones DE, Zhao K. Chromatin poises miRNA-and protein-coding genes for expression. Genome research. 2009;19(10):1742–1751. doi: 10.1101/gr.090951.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bell JT, Pai AA, Pickrell JK, Gaffney DJ, Pique-Regi R, Degner JF, Gilad Y, Pritchard JK. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome biology. 2011;12(1):R10. doi: 10.1186/gb-2011-12-1-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, Yu W, Rongione MA, Ekström TJ, Harris TB. Intra-individual change over time in DNA methylation with familial clustering. JAMA: the journal of the American Medical Association. 2008;299(24):2877. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Mönch K, Minucci S, Porse BT, Marine JC. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes & development. 2007;21(5):525. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M, Flaus A, Stockdale C, Rencurel C, Ferreira H, Owen-Hughes T. Histone H2A/H2B dimer exchange by ATP-dependent chromatin remodeling activities. Molecular cell. 2003;12(6):1599–1606. doi: 10.1016/s1097-2765(03)00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvanese V, Lara E, Kahn A, Fraga MF. The role of epigenetics in aging and age-related diseases. Ageing research reviews. 2009;8(4):268–276. doi: 10.1016/j.arr.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298(5595):1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Burke B, Bunce K, Surti U, Allen Hogge W, Peters DG. A microarray- based approach for the identification of epigenetic biomarkers for the noninvasive diagnosis of fetal disease. Prenatal diagnosis. 2009;29(11):1020–1030. doi: 10.1002/pd.2335. [DOI] [PubMed] [Google Scholar]
- Chung IRS. Dietary Polyphenols, Deacetylases and Chromatin Remodeling in Inflammation. World Review of Nutrition and Dietetics. 2010;101:84–94. doi: 10.1159/000314513. [DOI] [PubMed] [Google Scholar]
- Cocozza S, Akhtar MM, Miele G, Monticelli A. CpG Islands Undermethylation in Human Genomic Regions under Selective Pressure. PloS one. 2011;6(8):e23156. doi: 10.1371/journal.pone.0023156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley JE, Suter CM, Beckman KB, Martin DIK. Germ-line epigenetic modification of the murine Avy allele by nutritional supplementation. Proceedings of the National Academy of Sciences. 2006;103(46):17308–17312. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR, Feinberg AP. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299(5613):1753. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- Davis CD, Uthus EO, Finley JW. Dietary selenium and arsenic affect DNA methylation in vitro in Caco-2 cells and in vivo in rat liver and colon. The Journal of nutrition. 2000;130(12):2903–2909. doi: 10.1093/jn/130.12.2903. [DOI] [PubMed] [Google Scholar]
- Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. Journal of Biological Chemistry. 2009;284(4):1982–1989. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawlaty MM, Ganz K, Powell BE, Hu YC, Markoulaki S, Cheng AW, Gao Q, Kim J, Choi SW, Page DC. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell stem cell. 2011;9(2):166–175. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dechassa ML, Sabri A, Pondugula S, Kassabov SR, Chatterjee N, Kladde MP, Bartholomew B. SWI/SNF has intrinsic nucleosome disassembly activity that is dependent on adjacent nucleosomes. Molecular cell. 2010;38(4):590–602. doi: 10.1016/j.molcel.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Nestler EJ. From Father to Offspring: Paternal Transmission of Depressive-Like Behaviors. Neuropsychopharmacology. 2012;37(1):311–312. doi: 10.1038/npp.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin AM, Huang THM, Toland AE. Epigenetic alterations in the breast: Implications for breast cancer detection, prognosis and treatment. Elsevier. 2009:165–171. doi: 10.1016/j.semcancer.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Epigenetics in cancer. New England Journal of Medicine. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proceedings of the National Academy of Sciences. 2007;104(40):15805. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (–)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer research. 2003;63(22):7563. [PubMed] [Google Scholar]
- Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nature Reviews Genetics. 2012 doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Ficz G, Branco MR, Seisenberger S, Santos F, Krueger F, Hore TA, Marques CJ, Andrews S, Reik W. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473(7347):398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, Cigudosa JC, Urioste M, Benitez J. Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10604. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Kozaki K, Tanaka S, Arii S, Imoto I, Inazawa J. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2010;31(5):766–776. doi: 10.1093/carcin/bgp250. [DOI] [PubMed] [Google Scholar]
- Globisch D, Münzel M, Müller M, Michalakis S, Wagner M, Koch S, Brückl T, Biel M, Carell T. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS One. 2010;5(12):e15367. doi: 10.1371/journal.pone.0015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowher H, Jeltsch A. Enzymatic properties of recombinant Dnmt3a DNA methyltransferase from mouse: the enzyme modifies DNA in a non-processive manner and also methylates non-CpA sites1. Journal of Molecular Biology. 2001;309(5):1201–1208. doi: 10.1006/jmbi.2001.4710. [DOI] [PubMed] [Google Scholar]
- Gravina S, Vijg J. Epigenetic factors in aging and longevity. Pflügers Archiv European Journal of Physiology. 2010;459(2):247–258. doi: 10.1007/s00424-009-0730-7. [DOI] [PubMed] [Google Scholar]
- Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J, Surani MA. Epigenetic reprogramming in mouse primordial germ cells. Mechanisms of development. 2002;117(1):15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011;3(4):503–518. doi: 10.2217/epi.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002;129(8):1983–1993. doi: 10.1242/dev.129.8.1983. [DOI] [PubMed] [Google Scholar]
- Havas K, Flaus A, Phelan M, Kingston R, Wade PA, Lilley DMJ, Owen-Hughes T. Generation of superhelical torsion by ATP-dependent chromatin remodeling activities. Cell. 2000;103(7):1133–1142. doi: 10.1016/s0092-8674(00)00215-4. [DOI] [PubMed] [Google Scholar]
- He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333(6047):1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Fang J, Bedford MT, Zhang Y, Xu RM. Recognition of histone H3 lysine-4 methylation by the double tudor domain of JMJD2A. Science. 2006;312(5774):748. doi: 10.1126/science.1125162. [DOI] [PubMed] [Google Scholar]
- Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010 doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Shen L, Dai Q, Wu S, Collins L, Swenberg J, He C, Zhang Y. Tet Proteins Can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science (New York, NY) 2011 doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jin SG, Kadam S, Pfeifer GP. Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine. Nucleic acids research. 2010;38(11):e125. doi: 10.1093/nar/gkq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan AH, Kumar RM, Marx JG, Young RA, Sartorelli V. Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells. Molecular cell. 2009;36(1):61–74. doi: 10.1016/j.molcel.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowski TP, Jeltsch A. Burning off DNA Methylation: New Evidence for Oxygen- Dependent DNA Demethylation. ChemBioChem. 2011 doi: 10.1002/cbic.201100549. [DOI] [PubMed] [Google Scholar]
- Kanhere A, Viiri K, Araújo CC, Rasaiyaah J, Bouwman RD, Whyte WA, Pereira CF, Brookes E, Walker K, Bell GW. Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Molecular cell. 2010;38(5):675–688. doi: 10.1016/j.molcel.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Long N, Makita H, Toida M, Yamashita T, Hatakeyama D, Hara A, Mori H, Shibata T. Effects of green tea polyphenol on methylation status of RECK gene and cancer cell invasion in oral squamous cell carcinoma cells. British journal of cancer. 2008;99(4):647–654. doi: 10.1038/sj.bjc.6604521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, Van Oudenaarden A. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proceedings of the National Academy of Sciences. 2009;106(28):11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King- Batoon A, Leszczynska JM, Klein CB. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environmental and molecular mutagenesis. 2008;49(1):36–45. doi: 10.1002/em.20363. [DOI] [PubMed] [Google Scholar]
- Kozaki K, Imoto I, Mogi S, Omura K, Inazawa J. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer research. 2008;68(7):2094. doi: 10.1158/0008-5472.CAN-07-5194. [DOI] [PubMed] [Google Scholar]
- Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes & development. 2002;16(22):2893. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepeule J, Baccarelli A, Tarantini L, Motta V, Cantone L, Litonjua AA, Sparrow D, Vokonas PS, Schwartz J. Gene promoter methylation is associated with lung function in the elderly: The Normative Aging Study. Epigenetics. 2012;7(3):0–-1. doi: 10.4161/epi.7.3.19216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson VV, Melnikov AA. DNA Methylation as Clinically Useful Biomarkers—Light at the End of the Tunnel. Pharmaceuticals. 2012;5(1):94–113. doi: 10.3390/ph5010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienert F, Wirbelauer C, Som I, Dean A, Mohn F, Schübeler D. Identification of genetic elements that autonomously determine DNA methylation states. Nature Genetics. 2011;43(11):1091–1097. doi: 10.1038/ng.946. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. The Journal of nutrition. 2005;135(6):1382. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- Lister R, Ecker JR. Finding the fifth base: genome-wide sequencing of cytosine methylation. Genome research. 2009;19(6):959–966. doi: 10.1101/gr.083451.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462(7271):315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AG, Erdman JW. Lycopene and apo-10'-lycopenal do not alter DNA methylation of GSTP1 in LNCaP cells. Biochemical and Biophysical Research Communications. 2011 doi: 10.1016/j.bbrc.2011.07.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu L, van Groen T, Kadish I, Li Y, Wang D, James SR, Karpf AR, Tollefsbol TO. Insufficient DNA methylation affects healthy aging and promotes age-related health problems. Clinical Epigenetics. 2011:1–12. doi: 10.1007/s13148-011-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wylie RC, Andrews LG, Tollefsbol TO. Aging, cancer and nutrition: the DNA methylation connection. Mechanisms of ageing and development. 2003;124(10-12):989–998. doi: 10.1016/j.mad.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Lorincz AT. The promise and the problems of epigenetic biomarkers in cancer. Expert opinion on medical diagnostics. 2011;5(5):375–379. doi: 10.1517/17530059.2011.590129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti A, Drohat AC. Thymine DNA Glycosylase Can Rapidly Excise 5-Formylcytosine and 5-Carboxylcytosine. Journal of Biological Chemistry. 2011;286(41):35334–35338. doi: 10.1074/jbc.C111.284620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nature Reviews Genetics. 2010;11(4):285–296. doi: 10.1038/nrg2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Choi SW. Effects of alcohol on folate metabolism: implications for carcinogenesis. Alcohol. 2005;35(3):235–241. doi: 10.1016/j.alcohol.2005.03.012. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan R, Lewis JD, Bird AP. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Research. 1992;20(19):5085–5092. doi: 10.1093/nar/20.19.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minks J, Robinson WP, Brown CJ. A skewed view of X chromosome inactivation. Journal of Clinical Investigation. 2008;118(1):20–22. doi: 10.1172/JCI34470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzel M, Globisch D, Carell T. 5- Hydroxymethylcytosine, the Sixth Base of the Genome. Angewandte Chemie International Edition. 2011;50(29):6460–6468. doi: 10.1002/anie.201101547. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. The Journal of nutrition. 2002;132(8):2333S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- Otte AP, Kwaks THJ. Gene repression by Polycomb group protein complexes: a distinct complex for every occasion? Current opinion in genetics & development. 2003;13(5):448–454. doi: 10.1016/s0959-437x(03)00108-4. [DOI] [PubMed] [Google Scholar]
- Park L, Friso S, Choi S. Nutritional influences on epigenetics and age-related disease. The Proceedings of the Nutrition Society. 2011:1. doi: 10.1017/S0029665111003302. [DOI] [PubMed] [Google Scholar]
- Puri D, Dhawan J, Mishra RK. The paternal hidden agenda: epigenetic inheritance through sperm chromatin. Epigenetics. 2010;5(5):386–391. doi: 10.4161/epi.5.5.12005. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting Epigenetic Influence of Early-Life Adversity on the BDNF Gene. Biological Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, de Boom Witzel S, Okano H, Ko MSH, Ohlsson R, Longo DL, Feinberg AP. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307(5717):1976. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- Sarg B, Koutzamani E, Helliger W, Rundquist I, Lindner HH. Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. Journal of Biological Chemistry. 2002;277(42):39195–39201. doi: 10.1074/jbc.M205166200. [DOI] [PubMed] [Google Scholar]
- Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS Journal. 2011;278(10):1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- Schoeftner S, Sengupta AK, Kubicek S, Mechtler K, Spahn L, Koseki H, Jenuwein T, Wutz A. Recruitment of PRC1 function at the initiation of X inactivation independent of PRC2 and silencing. The EMBO journal. 2006;25(13):3110–3122. doi: 10.1038/sj.emboj.7601187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- Shenker N, Flanagan J. Intragenic DNA methylation: implications of this epigenetic mechanism for cancer research. British journal of cancer. 2011 doi: 10.1038/bjc.2011.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, Carney D, Peña P, Lan F, Kaadige MR. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature. 2006;442(7098):96–99. doi: 10.1038/nature04835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RJ, III, Millhouse S, Chen CF, Lewis BA, Erdjument-Bromage H, Tempst P, Manley JL, Reinberg D. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Molecular cell. 2007;28(4):665–676. doi: 10.1016/j.molcel.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RJ, Chen CF, Santos-Rosa H, Kouzarides T, Patel SS, Reinberg D. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. Journal of Biological Chemistry. 2005;280(51):41789. doi: 10.1074/jbc.C500395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, Slagboom PE, Heijmans BT. Periconceptional maternal folic acid use of 400 µg per day is related to increased methylation of the IGF2 gene in the very young child. PloS one. 2009;4(11):e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Nogales E, Ciferri C. Structure and function of SWI/SNF chromatin remodeling complexes and mechanistic implications for transcription. Progress in biophysics and molecular biology. 2010;102(2-3):122–128. doi: 10.1016/j.pbiomolbio.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature neuroscience. 2006;9(4):519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Valinluck V, Tsai HH, Rogstad DK, Burdzy A, Bird A, Sowers LC. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2). Nucleic Acids Research. 2004;32(14):4100–4108. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PAC, Rappsilber J, Helin K. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473(7347):343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nature cell biology. 2009;11(3):228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- Wong CCY, Caspi A, Williams B, Houts R, Craig IW, Mill J. A Longitudinal Twin Study of Skewed X Chromosome-Inactivation. PloS one. 2011;6(3):e17873. doi: 10.1371/journal.pone.0017873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, D'Alessio AC, Ito S, Xia K, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y. Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature. 2011;473(7347):389–393. doi: 10.1038/nature09934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442(7098):86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- Xhemalce B, Dawson MA, Bannister AJ. Histone modifications. Encyclopedia of Molecular Cell Biology and Molecular Medicine. 2011 [Google Scholar]
- Xiang N, Zhao R, Song G, Zhong W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis. 2008;29(11):2175. doi: 10.1093/carcin/bgn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wang W, Chen S, Zhu B. A model for mitotic inheritance of histone lysine methylation. EMBO reports. 2011a doi: 10.1038/embor.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wu F, Tan L, Kong L, Xiong L, Deng J, Barbera AJ, Zheng L, Zhang H, Huang S. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Molecular cell. 2011b doi: 10.1016/j.molcel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zierath JR, Barrès R. Evidence for non-CpG methylation in mammals. Experimental Cell Research. 2011 doi: 10.1016/j.yexcr.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang X, Clark E, Mulcahey M, Huang S, Shi YG. TET1 is a DNA-binding protein that modulates DNA methylation and gene transcription via hydroxylation of 5-methylcytosine. Cell Research. 2010 doi: 10.1038/cr.2010.156. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y, Li J, Bian Z, Liang X, Cai X. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Research. 2011 doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen H. Genistein, an epigenome modifier during cancer prevention. Epigenetics: official journal of the DNA Methylation Society. 2011;6(7) doi: 10.4161/epi.6.7.16315. [DOI] [PubMed] [Google Scholar]
- Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322(5902):750. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhu P, Wang J, Pascual G, Ohgi KA, Lozach J, Glass CK, Rosenfeld MG. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Molecular cell. 2008;29(1):69–80. doi: 10.1016/j.molcel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]