Abstract
Background
Determining the underlying causes of racial disparities in sexually transmitted infections (STIs) is important. In the USA, rates of the most common STIs range from 5 to 20 times higher for African–Americans compared to Caucasians, and the health consequences of STIs can be serious. Residential racial segregation results in very different contexts for individuals and may be an important determinant of sexual risk. The purpose of this study was to examine how segregation and race interact to impact the age trajectory of sexual risk behaviours.
Methods
Using 11 years of data from the National Longitudinal Survey of Youth 1997 (1997–2007) and 2000 Census data, the authors performed three-level hierarchical linear regression to examine the associations between hypersegregation, race and a sexual risk behaviour index among black and white non-Hispanic adolescents as they transition to adulthood.
Results
Through most of the teenage years, African–Americans are at higher sexual risk than Caucasians. However, by age 19, Caucasians are at higher risk. Hypersegregation was not associated with increased sexual risk index score on average and did not impact the trajectory of the race–sexual risk association.
Conclusions
The authors did not find any evidence that hypersegregation was associated with the sex risk index or that it modified the race–sex risk association as individuals got older. Future studies should examine whether segregation is associated with other causes of STI/HIV acquisition risk, such as sexual network patterns.
BACKGROUND
Sexual activity among adolescents in the USA is common, with about half of all high school students reporting ever having sexual intercourse.1 Using national survey data, Abma et al2 documented low rates of condom use among sexually active adolescents, with about half of males and two-thirds of females reporting not always using a condom. Additionally, while adolescents have low rates of concurrent partnerships, they tend to have multiple partners in the context of short-term, serial monogamous sexual relationships.3,4 Together with high prevalence of sexually transmitted infections (STIs),5,6 sexual activity among adolescents can have serious and costly consequences.5,7
Racial disparities in STIs, especially among adolescents, are well documented.5,8 However, reasons for these disparities are not clear. Using condoms and having only one partner can reduce one's risk of contracting STIs9,10; however, studies have shown that differences in these behaviours cannot explain the racial disparities in STI risk11,12 and that African–Americans are at higher risk of STIs even when their behaviours are low risk.11
Structural factors, such as residential racial segregation, may underlie the disparities in sexual risk. Residential racial segregation (herein, segregation) describes the separation of racial groups in the urban environment. In the USA, African–Americans remain more segregated than any other racial/ethnic group and are more likely to experience high segregation across multiple dimensions of segregation simultaneously.13,14
While growing literature has suggested that segregation may be a fundamental factor underlying the black–white disparity in sexual risk, the empirical evidence is just emerging. Our group has found evidence that African–Americans living in segregated areas are at higher risk for gonorrhoea than African–Americans living in non-segregated areas.15 Additionally, our group found that segregation may help to explain the racial disparity in age at adolescent sexual initiation.16 However, the mechanisms are not clear. It is posited that segregation increases STI risk in two distinct ways.
First, segregation might lead to increased rates of STIs among African–Americans by impacting the sexual network. Individuals tend to select sexual partners in their residential area.17 It then follows that segregation of blacks fosters racial assortativity or the tendency of individuals of one race to select sexual partners among members of the same race. Additionally, segregation may lead to denser sexual networks so that sexual contacts are more interconnected in a given area.17–19
Second, segregation may create or foster environments that are conducive to sexual risk behaviours and increased STI risk. Segregation restricts the socioeconomic opportunities of residents living in the segregated communities, leading to fewer resources and increased social problems. These characteristics are associated with increased STI risk.20 Additionally, segregated areas are often characterised by disordered neighbourhoods (eg, abandoned buildings, vandalism). A disordered neighbourhood may suggest that objectionable and risky behaviours are acceptable21,22 and, as a result, become normative.21 Living in disordered neigh-bourhoods is associated with increased STI risk,21 as well as increased risk behaviours.23,24
To begin to understand how segregation impacts STI risk, the primary aim of this study was to examine longitudinally whether segregation was associated with sexual risk behaviours among black and white non-Hispanic adolescents as they transitioned to adulthood in the USA. Prior to examining this, we also described the age trajectory of sexual risk behaviours and the racial differences in this trajectory in this population.
METHODS
Person-level data
Person-level data came from the National Longitudinal Survey of Youth 1997 (NLSY97), a nationally representative longitudinal survey of 8984 youths aged 12–16 years on 31 December 1996. Participants were interviewed on a yearly basis with baseline interviews completed in 1997 and 1998 and continuing to the present. Further details have been described elsewhere.25 Eleven rounds of data were used for this analysis (1997–2007). Retention rates were >80%.25
Because black–white segregation was assessed, the sample was limited to non-Hispanic blacks and whites (n=6746 in 121 metropolitan areas (MAs)) and to those living in Census-defined MAs (n=5176 in 120 MAs), the level at which segregation is defined. Segregation measures can be unreliable in small MAs so the sample was further limited to those living in MAs with a total population >100 000 and a black population >5000 (n=4794 individuals in 110 MAs).26 Those who did not have at least one valid outcome measure over 11 years of follow-up were excluded (n=198). Finally, MAs where three or fewer individuals were represented in the sample were excluded (n=13 individuals in five MAs). The final sample was 4583 individuals in 105 MAs. There were on average 43.65 individuals per MA (range 4–181).
Outcome measure: sexual risk behaviour index
To assess sexual risk at each survey year, an index that combines assessments of sexual behaviours since the date of last interview (or past year for baseline) was used: 0= abstinent, 1= always uses a condom, 2= monogamous and has sex without a condom, 3= non-monogamous and has sex without a condom sometimes and 4= non-monogamous and always has sex without a condom.27 Sexual behaviour questions were asked to individuals aged 14 years or older and refer to experiences with individuals of the opposite sex.
Primary person-level exposure: race/ethnicity
Race and ethnicity were self-reported and categorised into non-Hispanic white and non-Hispanic black.
Person-level baseline covariates
Socio-demographic person-level covariates were assessed at baseline and included the following predictors of sexual risk.
Individual characteristics
Sex of participant was self-reported.
Family characteristics
To account for family socioeconomic status, gross household income in the last year was reported by the parent, maternal education and paternal education were dichotomised into less than high school or high school or more, and number of rooms per person in the household was calculated. To assess family structure, measures indicating whether the participant lived with both biological parents at age 2, whether he/she lived in a single-parent home at baseline and number of children who lived with the participant at baseline were included.
Geographic characteristics
Based on participant's baseline address, it was determined whether they lived in a census-defined urban area (urban vs rural or unknown). It was determined in what part of the MA (in a central city, outside a central city, unknown) and in what census region of residence (northeast, central, west or south) they resided.
As suggested by the NLSY97 technical report, a variable indicating whether the participant was part of the oversample of African–Americans and Hispanics was included in all analyses.25
MA data
Values for segregation indices were obtained from the U.S. Census Bureau Housing and Household Economic Statistics Division, which used U.S. Census Bureau 2000 data and 1999 MA definitions to calculate these indices.28 Other MA-level socio-demographic measures were obtained from the U.S. Census Bureau 2000 Census Summary 1 and 3 Files.
Primary MA-level exposure: hypersegregation
Broadly, residential racial segregation is measured by assessing the distribution of African–Americans compared with Caucasians across neighbourhoods (eg, Census tracts) within larger MAs that represent housing and labour markets (eg, Census-defined Metropolitan Statistical Areas).29 It is represented by five distinct dimensions—exposure, concentration, centralisation, clustering and unevenness.30 Indices assessing each have been described in detail elsewhere and have been used by segregation demographers to operationalise each dimension.13,15,28,30,31 Briefly, exposure, the extent of contact that African–Americans have with other African–Americans in their neighbourhood, was operationalised using the isolation index. Concentration, the density of African–Americans in a neighbourhood, was measured using the relative concentration index. Centralisation, the extent to which black neighbourhoods are located around an urban centre, was assessed using the absolute centralisation index. Clustering, the degree to which black neighbourhoods are contiguous within a MA, was measured using the spatial proximity index. Finally, unevenness, the extent to which the proportion of African–Americans in each neighbourhood differs from the proportion of African–Americans in the MA as a whole, was measured by the dissimilarity index.28 Higher scores indicate higher black–white segregation, with scores above 0.60 considered highly segregated.26,31
Because simultaneous high segregation across multiple dimensions of segregation is thought to multiply the negative impact of segregation, we examined the effect of hyper-segregation on sexual risk. Hypersegregation has been operationalised by segregation demographers as being highly segregated on four or five of these dimensions.26,31
MA-level covariates
Population size (log), population density (people per square mile) and racial composition (proportion black) were included as MA-level covariates.14,32 A socioeconomic position index was used to measure overall MA-level socioeconomic status, summing standardised scores for multiple MA-level socioeconomic measures (eg, per cent unemployed, per cent in poverty, per cent with less than high school education).33 Higher scores represent higher socioeconomic disadvantage.
Statistical analysis
MA of residence of survey respondents at baseline was provided by NLSY97 and defined according to 1999 Census demarcations of Metropolitan Statistical Areas. MA-level data and person-level survey data were then linked based on these MAs.
Several person-level covariates had missing data (25.3% for gross household income, 20.0% for paternal education, 11.1% for biological parents in the home, 7.0% for maternal education, 1.6% for rooms per people in home, 0.2% for single-parent household). We performed multiple imputation using an iterative Markov Chain Monte Carlo procedure.34,35
The sexual risk behaviour index scores were temporally arranged by age at time of interview (range 14–27).27 Age at interview was then centred at 14 and used as the measure of time since we expected that sexual risk would change as a function of age rather than time on study or calendar year. Each participant contributed data at a specific age if he/she was in the NLSY at that age (regardless of NLSY round). As a result, some individuals could not contribute to certain measurement occasions if they were not in the study at that age, reflected in the varied sample sizes at each age (table 1). This approach assumes that all baseline age cohorts can be pooled together and considered as a single cohort and that those who do not provide data for a given age can be adequately represented by those who do.36
Table 1.
Distribution of participants for sexual risk index scores and mean and median scores, by age
| Age | No. of participants | Mean score (SD), range 0–4 | Median |
|---|---|---|---|
| 14 | 57 | 0.19 (0.58) | 0 |
| 15 | 790 | 0.32 (0.80) | 0 |
| 16 | 1565 | 0.53 (0.95) | 0 |
| 17 | 2339 | 0.78 (1.07) | 0 |
| 18 | 2714 | 0.99 (1.14) | 1 |
| 19 | 2982 | 1.15 (1.17) | 1 |
| 20 | 2503 | 1.21 (1.18) | 1 |
| 21 | 2080 | 1.22 (1.22) | 1 |
| 22 | 1870 | 1.23 (1.20) | 1 |
| 23 | 1966 | 1.34 (1.18) | 1 |
| 24 | 1613 | 1.46 (1.17) | 2 |
| 25 | 1364 | 1.57 (1.17) | 2 |
| 26 | 1023 | 1.68 (1.09) | 2 |
| 27 | 448 | 1.50 (1.07) | 2 |
Sexual risk index is defined as: 0= abstinent, 1= always uses a condom, 2= monogamous and has unprotected sex, 3= non-monogamous and has unprotected sex sometimes and 4= non-monogamous and always has unprotected sex. Questions refer to practices since the date of last interview (or past year for baseline) and refer specifically to experiences with individuals of the opposite sex.
Three-level hierarchical linear regression models were created to account for dependence of measurement occasions (defined by age) within individuals and individuals within MAs. Random intercept terms were specified at the individual and MA levels. Age was included as a covariate in the random intercepts model to assess the trajectories in the sexual risk behaviour index as participants aged. Based on preliminary analyses, age with both linear and quadratic terms was modelled. Race and race by age interactions were added, allowing the effect of race on sexual risk to differ as age increased. The baseline person-level and MA-level measures were then included as time-unvarying covariates. Next, the main effect of hypersegregation was tested. To examine whether hypersegregation modified the race–sexual risk association, race-by-hypersegregation interaction terms were included. Estimation was done using maximum likelihood. All analyses were conducted using SAS V.9.2.
This study was approved by the Yale University Human Subjects Committee.
RESULTS
Sample characteristics
With the exception of age at baseline and sex, African–Americans were at significantly higher risk than Caucasians across all person-level covariates in both hypersegregated and non-hypersegregated areas (table 2). Additionally, of the 105 MAs included, 17 (16.2%) were hypersegregated; in this sample, 1388 (30.3%) individuals lived in hypersegregated MAs.
Table 2.
Baseline individual, family, contextual and MA characteristics, overall and by hypersegregation, stratified by race
| Hypersegregated, N = 1388 |
Not hypersegregated, N = 3195 |
||||||
|---|---|---|---|---|---|---|---|
| Overall, N = 4583* | Black non-Hispanic, N = 647 | White non-Hispanic, N = 741 | p Value† | Black non-Hispanic, N = 1183 | White non-Hispanic, N = 2012 | p Value† | |
| NLSY person-level measures | |||||||
| Age at baseline, mean (SD) | 14.3 (1.5) | 14.3 (1.5) | 14.3 (1.5) | 0.617 | 14.3 (1.5) | 14.2 (1.5) | 0.097 |
| Sex, n (%) | 0.091 | 0.297 | |||||
| Male | 2331 (50.9) | 319 (49.3) | 399 (53.8) | 583 (49.3) | 1030 (51.2) | ||
| Female | 2252 (49.1) | 328 (50.7) | 342 (46.2) | 600 (50.7) | 982 (48.8) | ||
| Paternal education, n (%) | 0.012 | <0.0001 | |||||
| High school or more | 3112 (84.8) | 337 (83.0) | 589 (88.4) | 638 (78.2) | 1548 (87.0) | ||
| Less than high school | 556 (15.2) | 69 (17.0) | 77 (11.6) | 178 (21.8) | 232 (13.0) | ||
| Maternal education, n (%) | <0.0001 | <0.0001 | |||||
| High school or more | 3591 (84.3) | 450 (76.9) | 635 (89.9) | 800 (75.6) | 1706 (89.3) | ||
| Less than high school | 669 (15.7) | 135 (23.1) | 71 (10.1) | 258 (24.4) | 205 (10.7) | ||
| Household income in $10 000, mean (SD) | 5.3 (4.7) | 3.2 (3.1) | 7.6 (6.0) | <0.0001 | 2.9 (2.5) | 6.2 (4.6) | <0.0001 |
| Rooms per people in household, mean (SD) | 1.6 (0.6) | 1.5 (0.7) | 1.8 (0.6) | <0.0001 | 1.4 (0.6) | 1.7 (0.6) | <0.0001 |
| Both biological parents in home (age 2), n (%) | <0.0001 | <0.0001 | |||||
| Yes | 1738 (42.3) | 104 (18.8) | 418 (62.6) | 193 (18.6) | 1023 (56.4) | ||
| No | 2336 (57.3) | 450 (81.2) | 250 (37.4) | 845 (81.4) | 791 (43.6) | ||
| Single-parent household, n (%) | <0.0001 | <0.0001 | |||||
| Yes | 1530 (33.5) | 326 (50.5) | 152 (20.5) | 606 (51.5) | 446 (22.2) | ||
| No | 3043 (66.5) | 320 (49.5) | 588 (79.5) | 571 (48.5) | 1564 (77.8) | ||
| Number of children in home, n (%) | 0.0001 | <0.0001 | |||||
| 2 or fewer | 2829 (61.7) | 355 (54.9) | 482 (65.1) | 654 (55.3) | 1338 (66.5) | ||
| More than 2 | 1754 (38.3) | 292 (45.1) | 259 (34.9) | 529 (44.7) | 674 (33.5) | ||
| Residential location, n (%) | <0.0001 | <0.0001 | |||||
| Urban | 3586 (78.3) | 622 (96.1) | 593 (80.0) | 957 (80.9) | 1414 (70.3) | ||
| Not urban | 997 (21.7) | 25 (3.9) | 148 (20.0) | 226 (19.1) | 598 (29.7) | ||
| Location of residence in MA, n (%) | <0.0001 | <0.0001 | |||||
| Inside central city | 1678 (36.6) | 446 (68.9) | 107 (14.4) | 647 (54.7) | 478 (23.8) | ||
| Outside central city | 2842 (62.0) | 198 (30.6) | 630 (85.0) | 511 (43.2) | 1503 (74.7) | ||
| Unknown | 63 (1.4) | 3 (0.5) | 4 (0.5) | 25 (2.1) | 31 (1.5) | ||
| Census region of residence, n (%) | <0.0001 | <0.0001 | |||||
| Northeast | 919 (20.0) | 228 (35.2) | 218 (29.4) | 98 (8.3) | 375 (18.6) | ||
| North Central | 1195 (26.1) | 198 (30.6) | 315 (42.5) | 195 (16.5) | 487 (24.2) | ||
| South | 1912 (41.7) | 192 (29.7) | 183 (24.7) | 784 (66.3) | 753 (37.4) | ||
| West | 557 (12.2) | 29 (4.5) | 25 (3.4) | 106 (9.0) | 397 (19.7) | ||
| MA-level measures | |||||||
| Log population size, mean (SD) | 14.2 (1.2) | 15.2 (0.9) | 13.7 (1.0) | ||||
| Population density (people/square mile), mean (SD) | 962.7 (1603.6) | 1906.1 (2350.3) | 552.8 (857.6) | ||||
| Proportion black non-Hispanic, mean (SD) | 0.16 (0.10) | 0.21 (0.05) | 0.14 (0.11) | ||||
| Socioeconomic position index, mean (SD) | –1.4 (3.3) | –2.1 (2.7) | –1.1 (3.6) | ||||
N's vary due to missing values.
p Values are based on χ2 test for NLSY categorical variables and t test for NLSY continuous variables.
MA, metropolitan area; NLSY, National Longitudinal Survey of Youth.
Sexual risk differences
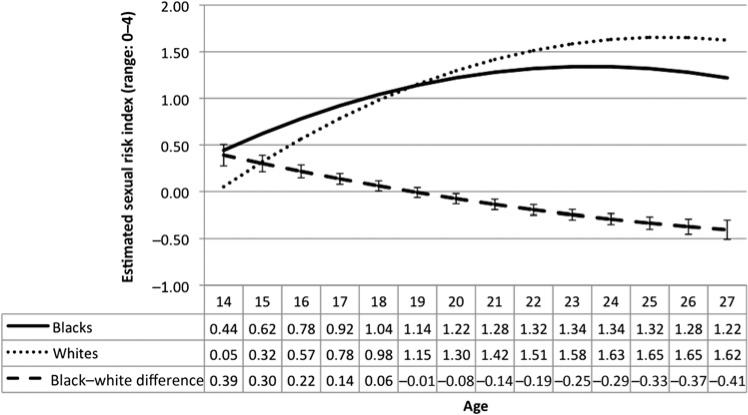
Mean sexual risk was lowest at age 14 and highest at age 26 (table 1). On average, sex risk behaviour index scores increased as adolescents became older and then began to level off and decreased when they reached their mid-20s (figure 1). This trajectory was quadratic (p for age2 ≤0.0001) (data not shown). On average, African–Americans were at lower sexual risk (Est.= –0.07, 95% CI –0.12 to –0.02, p=0.005) (table 3, model 1). However, the trajectory differed by race (p for joint effect of race × age interactions <0.0001) (table 3, model 2). Through most of the teenage years, African–Americans were at higher sexual risk than Caucasians. However, by age 19, Caucasians were at higher risk (figure 1). Risk increased for African–Americans and Caucasians over time; however, the risk for whites increased faster than for African–Americans, resulting in an eventual reversal of the initial blackewhit–disparity (figure 1 and table 3, model 2). After inclusion of covariates, the black–white disparity in sexual risk scores at age 14 was reduced by 33%, but the racial differences in the trajectories of sexual risk scores over time did not change (table 3, models 2 and 3).
Figure 1.
Unadjusted sex risk scores from age 14 to 27, by race, National Longitudinal Survey of Youth, 1997–2007. Error bars represent 95% CIs around the black–white difference in means at each age.
Table 3.
Predictors of trajectories of sexual risk in multiple hierarchical linear regressions, NLSY, 1997–2007*
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age and race models | Estimate | 95% CI | p Value | Estimate | 95% CI | p Value | Estimate | 95% CI | p Value |
| Main effects | |||||||||
| Black race | –0.07 | –0.12 to –0.02 | 0.005 | 0.39 | 0.28 to 0.51 | <0.0001 | 0.28 | 0.16 to 0.41 | <0.0001 |
| Age | 0.25 | 0.23 to 0.26 | <0.0001 | 0.28 | 0.26 to 0.30 | <0.0001 | 0.28 | 0.26 to 0.30 | <0.0001 |
| Interactions | |||||||||
| Black race × age | – | – | – | –0.99 | –0.13 to –0.06 | <0.0001 | –0.09 | –0.13 to –0.06 | <0.0001 |
| Age2 | –0.011 | –0.013 to –0.010 | <0.0001 | –0.012 | –0.014 to –0.011 | <0.0001 | –0.012 | –0.014 to –0.011 | <0.0001 |
| Black race × age2 | – | – | – | 0.002 | 0.000 to 0.005 | 0.059 | 0.002 | –0.0001 to 0.005 | 0.061 |
| Model 4 |
Model 5 |
Model 6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypersegregation models | Estimate | 95% CI | p Value | Estimate | 95% CI | p Value | Estimate | 95% CI | p Value |
| Main effects | |||||||||
| Black race | 0.28 | 0.16 to 0.41 | <0.0001 | 0.24 | 0.09 to 0.39 | 0.002 | 0.27 | 0.13 to 0.40 | <0.0001 |
| Age | 0.28 | 0.26 to 0.30 | <0.0001 | 0.29 | 0.26 to 0.31 | <0.0001 | 0.28 | 0.26 to 0.30 | <0.0001 |
| Hypersegregation | 0.02 | –0.05 to 0.09 | 0.642 | 0.06 | –0.11 to 0.22 | 0.493 | –0.01 | –0.10 to 0.08 | 0.815 |
| Interactions | |||||||||
| Black race × age | –0.09 | –0.13 to –0.06 | <0.0001 | –0.08 | –0.12 to –0.04 | 0.0001 | –0.09 | –0.13 to –0.06 | <0.0001 |
| Age2 | –0.012 | –0.014 to –0.011 | <0.0001 | –0.013 | –0.014 to –0.011 | <0.0001 | –0.012 | –0.014 to –0.011 | <0.0001 |
| Black race × age2 | 0.002 | –0.0001 to 0.005 | 0.062 | 0.002 | –0.001 to 0.005 | 0.302 | 0.002 | –0.0001 to 0.005 | 0.062 |
| Hypersegregation × black race | 0.12 | –0.13 to 0.37 | 0.350 | 0.06 | –0.05 to 0.17 | 0.267 | |||
| Hypersegregation × age | –0.02 | –0.07 to 0.03 | 0.408 | ||||||
| Hypersegregation × black race × age | –0.02 | –0.10 to 0.05 | 0.515 | ||||||
| Hypersegregation × age2 | 0.001 | –0.002 to 0.004 | 0.503 | ||||||
| Hypersegregation × black race × age2 | 0.002 | –0.003 to 0.007 | 0.459 | ||||||
| Joint test of significance | |||||||||
| Hypersegregation grouped variables | 0.085 | 0.287 | |||||||
Age is centred at 14.
Models 1 and 2 only included the variables shown (unadjusted) and models 3, 4, 5 and 6 included all covariates (adjusted).
NLSY, National Longitudinal Survey of Youth.
Hypersegregation was not associated with increased sexual risk index score on average (Est.=0.02, 95% CI –0.05 to 0.09, p=0.642)(table 3, Model 4). Additionally, inclusion of hypersegregation did not impact the black–white disparity in sexual risk (table 3, Models 3 and 4).
Hypersegregation did not modify the trajectory of the association between race and sexual risk behaviour index scores over adolescence and into young adulthood (p value for joint effect of hypersegregation × race × age and hypersegregation × race × age2 interactions =0.521) (result not shown). Additionally, holding the effect of age constant, the race–sexual risk association was not modified by hypersegregation (Est.=0.06, 95% CI –0.05 to 0.17, p=0.267) (table 3, model 6) nor was hypersegregation associated with sexual risk overall (p value for joint effect of hypersegregation grouped variables =0.287) (table 3, model 6).
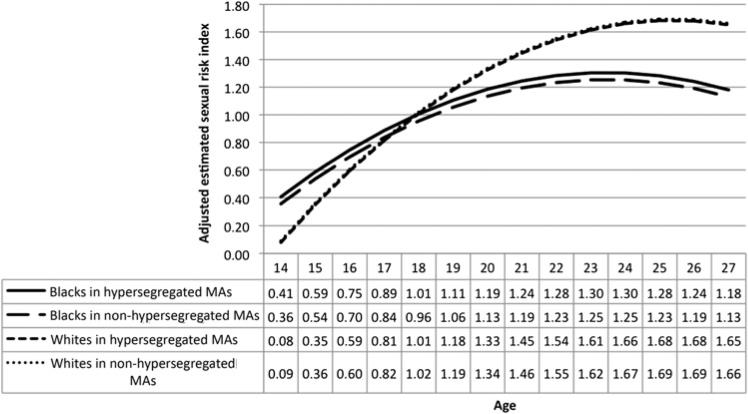
In analyses stratified by race, hypersegregation was not associated with sexual risk (among African–Americans, Est. = 0.07, 95% CI –0.04 to 0.17, p=0.225; among Caucasians, Est. =–0.05, 95% CI –0.15 to 0.05, p=0.324) (figure 2).
Figure 2.
Adjusted sex risk scores from age 14 to 27, by race and hypersegregation, National Longitudinal Survey of Youth, 1997–2007. Based on table 3, model 6. Adjusted for sample type, sex, paternal education, maternal education, household income, rooms per people in household, both biological parents in home (age 2), single-parent household, number of children in home, residential location, location of residence in metropolitan area (MA), census region of residence, log population size in MA, population density (people per square mile) in MA, proportion black non-Hispanic in MA, socioeconomic position index of MA. All covariates were centred at their grand mean so that the scores are predicted scores for the ‘average’ person.
DISCUSSION
STIs among adolescents and young adults place a substantial burden on this population. Black adolescents have a nearly 10-fold increased risk of contracting chlamydia, the most common STI, and a 20-fold increased risk of contracting gonorrhoea, the second most common STI, compared with white adolescents.37 While there are multitude of factors that likely put individuals at increased risk for STIs, sexual activity, inconsistent condom use and multiple partners are established behavioural risk factors. Results from this study found that at younger ages, African–Americans were at increased sexual risk compared with Caucasians but that this disparity reversed by late adolescence. This same race and age pattern of sexual risk has been found previously among adolescents and young adults.38 The higher risk at younger ages for African–Americans was likely strongly influenced by African–Americans initiating sex at younger ages compared to Cauasians.11
There was no evidence that hypersegregation was associated with the sex risk behaviour index or that it modified the race–sex risk association as individuals got older. Prior studies have shown that segregation is associated with earlier age at sexual debut and higher risk of STIs, both of which have established racial disparities (eg, African–Americans have more STIs than Caucasians).15,16 This might indicate that for outcomes and behaviours that African–Americans are at higher risk than Caucasians, segregation can help explain the disparity. However, for behaviours that are not characterised by large racial disparities (such as those examined in the current study—condom use and multiple partners), segregation may not be an important risk factor. In fact, Lang et al21 found that neighbourhood disorder was associated with STI acquisition but that it was not associated with inconsistent condom use or multiple partners and that inconsistent condom use was not associated with STI acquisition. This lends support to the hypothesis that segregation might impact risk in large part through its effect on sexual network characteristics, aspects that condom use and number of partners do not fully capture.
Because individuals, particularly adolescents, tend to select sexual partners in the area in which they live, segregation likely impacts partner choice.17 Additionally, evidence suggests that African–Americans are more racially assortative than other racial groups,39 resulting in infections remaining in the subpopulation. Additionally, segregation may lead to denser sexual networks so that sexual contacts are more interconnected in a given area. Evidence also suggests that African–Americans are also more dissortative in their partner choice.17,39 Specifically, low-risk African–Americans (one partner in the past year) are five times more likely to have a partner who is at high risk (four or more partners in the past year) as compared with Caucasians.39 Together, this may help explain why infection is more widespread in this subpopulation and why even low-risk African–Americans are at higher risk of STIs than Cauasians.11
Strengths and limitations
Due to the original survey sampling and the exclusion criteria, the sample may not be representative of all MAs in the USA. Additionally, NLSY97 survey data are self-reported and may be subject to misclassification error. However, computer-assisted personal interviews were used to collect data on sexual behaviours to reduce bias. Survey questions related to sexual intercourse referred to sex with a person of the opposite sex; as a result, sexual intercourse with a same sex partner is impossible to determine and could not be considered. The sexual risk score, while previously used for this data set, has not been validated. By structuring the data by age at time of interview, we cannot rule out age by cohort effects.36 However, since the largest age difference possible in a given year is <5 years and we have no reason to believe that any societal changes took place that would impact sex risk over this short period of time, it is unlikely that this would have a major effect on the conclusions.
The sexual risk score is a five-group ordinal variable. While statistical techniques exist to model ordinal outcomes with hierarchical data, these models would not converge due to the statistically complex nature of three-level hierarchical models with ordinal outcomes. Therefore, while it did not meet the normality assumption, it was modelled as continuous. Notably, it has been shown that treating non-normal ordinal outcomes as continuous most often results in smaller SEs. In this analysis, larger SEs would only strengthen our findings that segregation is not associated with sexual risk scores.
We used MA measures of segregation, rather than more local measures. This could have attenuated any effect if the MA level is too large to meaningfully classify exposure for capturing differences in adolescent sexual risk. However, while more local environments (eg, the neighbourhood) are certainly important to understand health, the social processes that impact health often operate across larger areas. Rather than looking at individual neighbourhoods, it is important to examine how the distribution of these neighbourhoods in larger areas (ie, MAs) impacts health. MAs approximate housing and labour markets. In MAs, there is a separation between the central city and more suburban areas, a major factor in residential racial segregation.40 The impact of segregation is hypothesised to not just impact residents of a few neighbourhoods but to impact minorities across the MA as a whole.41
We were able to examine the potential impact of a number of possible limitations. First, use of the sexual risk index may hide differences for the individual behaviours (eg, condom use); however, a post-hoc analysis examining the association of segregation with proportion of sex acts where a condom was used did not result in qualitatively different findings. Second, the hypersegregation measure could be misclassified due to the operationalisation of it as the baseline place of residence of respondents. Few studies have examined the effect of segregation longitudinally; however, using the baseline place of residence is supported in neighbourhood effects research in general.23 Additionally, evidence suggests that individuals do not move from high segregation areas to low segregation areas or vice versa.42 Third, some variables had substantial missing values, and multiple imputation may not reduce bias; however, our findings were robust to changes in assumptions in that the main findings did not meaningfully change when performing complete case analysis or when dropping those variables with large number of missing values. Finally, due to our modelling techniques and the complex relationships between variables over time, the analysis might be subject to type 2 error because some of these variables may be mediators and on the causal pathway between segregation and sexual risk. However, even in unadjusted models, hypersegregation was not associated with the sexual risk behaviour index and did not impact the race–sexual risk association trajectory.
This study also has a number of strengths. First, it addressed an important public health problem, identified as a priority by the federal government. The FY2012 Trans-National Institutes of Health plan for HIV-related research identifies the importance of exploring the effects of ‘residential segregation... upon HIV transmission among racial and ethnic populations across the lifespan’.43 Examining the effect of segregation on sexual risk behaviours as adolescents transition into adulthood is an important step. Additionally, a hierarchical, repeated measures design was used to validly examine the association between a contextual variable on individual outcomes over time in a large national survey.
Conclusions
Emerging evidence suggests that residential racial segregation is related to racial disparities in STI and early sexual initiation, but the mechanisms are still unclear. The current study suggests that segregation may not be resulting in differences in sexual behaviours particularly among sexually experienced individuals. Instead, future studies should examine whether segregation is associated with sexual network patterns, whether it can help explain the racial disparities in sexual network patterns and whether sexual networks can mediate the relationship between segregation and STIs. Additionally, future studies might examine whether segregation is associated with behaviours that have been more consistently shown to explain disparities in STI risk, including concurrency and sex with casual and high-risk partners.
What is already known on this subject.
▶ Sexual behaviours put individuals at risk of STIs; however, these behaviours do not fully explain the large racial disparities in STIs among adolescents.
▶ Residential racial segregation has been shown to be associated with gonorrhoea rates among African–American.
What this study adds.
▶ Segregation is not associated with a sexual risk behaviour index.
▶ Rather than through behaviour, segregation may impact STI risk through another mechanism, such as sexual networks.
Acknowledgments
Funding This work was supported by the National Institute of Mental Health at the National Institutes of Health (T32MH020031 and P30MH062294). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Contributors Each of the authors contributed substantively to the development, writing and editing of this manuscript. KBB was primarily responsible for the analysis of the data.
Competing interests None.
Ethics approval Ethics approval was provided by the Yale University Human Subjects Committee.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Eaton DK, Lowry R, Brener ND, et al. Trends in human immunodeficiency virus- and sexually transmitted disease-related risk behaviors among U.S. high school students, 1991-2009. Am J Prev Med. 2011;40:427–33. doi: 10.1016/j.amepre.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Abma JC, Martinez G, Mosher W, et al. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23. 2004;24:1–48. [PubMed] [Google Scholar]
- 3.Ott MA, Katschke A, Tu W, et al. Longitudinal associations among relationship factors, partner change, and sexually transmitted infection acquisition in adolescent women. Sex Transm Dis. 2011;38:153–7. doi: 10.1097/OLQ.0b013e3181f2e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley SS, Borawski EA, Flocke SA, et al. The role of sequential and concurrent sexual relationships in the risk of sexually transmitted diseases among adolescents. J Adolesc Health. 2003;32:296–305. doi: 10.1016/s1054-139x(02)00710-3. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Sexually Transmitted Disease Surveillance 2009. U.S. Department of Health and Human Services; Atlanta, GA: 2010. [Google Scholar]
- 6.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for HIV, STD and TB Prevention (NCHSTP), Division of STD/HIV Prevention Sexually Transmitted Disease Morbidity for Selected STDs by Age, Race/Ethnicity and Gender 1996-2008. CDC WONDER On-line Database. 2009.
- 7.Chesson HW, Blandford JM, Gift TL, et al. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health. 2004;36:11–19. doi: 10.1363/psrh.36.11.04. [DOI] [PubMed] [Google Scholar]
- 8.Harris K, Gordon-Larsen P, Chantala K, et al. Longitudinal trends in race/ethnic disparities in leading health indicators from adolescence to young adulthood. Arch Pediatr Adolesc Med. 2006;160:74–81. doi: 10.1001/archpedi.160.1.74. [DOI] [PubMed] [Google Scholar]
- 9.Gallo MF, Steiner MJ, Warner L, et al. Self-reported condom use is associated with reduced risk of chlamydia, gonorrhea, and trichomoniasis. Sex Transm Dis. 2007;34:829–33. doi: 10.1097/OLQ.0b013e318073bd71. [DOI] [PubMed] [Google Scholar]
- 10.DiClemente RJ, Crosby RA, Wingood GM, et al. Reducing risk exposures to zero and not having multiple partners: findings that inform evidence-based practices designed to prevent STD acquisition. Int J STD AIDS. 2005;16:816–18. doi: 10.1258/095646205774988037. [DOI] [PubMed] [Google Scholar]
- 11.Hallfors DD, Iritani BJ, Miller WC, et al. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health. 2007;97:125–32. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellen JM, Aral SO, Madger LS. Do differences in sexual behaviors account for the racial/ethnic differences in adolescents’ self-reported history of a sexually transmitted disease? Sex Transm Dis. 1998;25:125–9. doi: 10.1097/00007435-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Massey D, Denton N. Hypersegregation in U.S. metropolitan areas: black and Hispanic segregation along five dimensions. Demography. 1989;26:373–91. [PubMed] [Google Scholar]
- 14.Wilkes R, Iceland J. Hypersegregation in the twenty-first century. Demography. 2004;41:23–36. doi: 10.1353/dem.2004.0009. [DOI] [PubMed] [Google Scholar]
- 15.Biello KB, Kershaw T, Nelson R, et al. Residential racial segregation and rates of gonorrhea in the United States, 2003-2007. Am J Public Health. 2012;102:1370–7. doi: 10.2105/AJPH.2011.300516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biello KB, Ickovics J, Niccolai L, et al. Racial differences in age at sexual initiation: can residential racial segregation explain the Black-White disparity among adolescents in the United States? 2011 Under Review.
- 17.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191(Suppl 1):S115–22. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 18.Hogben M, Leichliter JS. Social determinants and sexually transmitted disease disparities. Sex Transm Dis. 2008;35:S13–18. doi: 10.1097/OLQ.0b013e31818d3cad. [DOI] [PubMed] [Google Scholar]
- 19.Aral SO. Sexual network patterns as determinants of STD rates: paradigm shift in the behavioral epidemiology of STDs made visible. Sex Transm Dis. 1999;26:262–4. doi: 10.1097/00007435-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Chesson HW, Sternberg M, Leichliter JS, et al. The distribution of chlamydia, gonorrhoea and syphilis cases across states and counties in the USA, 2007. Sex Transm Infect. 2010;86:iii52–7. doi: 10.1136/sti.2009.040873. [DOI] [PubMed] [Google Scholar]
- 21.Lang DL, Salazar LF, Crosby RA, et al. Neighborhood environment, sexual risk behaviors and acquisition of sexually transmitted infections among adolescents diagnosed with psychological disorders. Am J Community Psychol. 2010;46:303–11. doi: 10.1007/s10464-010-9352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keizer K, Lindenberg S, Steg L. The spreading of disorder. Science. 2008;322:1681–5. doi: 10.1126/science.1161405. [DOI] [PubMed] [Google Scholar]
- 23.Browning C, Leventhal T, Brooks-Gunn J. Neighborhood context and racial differences in early adolescent sexual activity. Demography. 2004;41:697–720. doi: 10.1353/dem.2004.0029. [DOI] [PubMed] [Google Scholar]
- 24.Latkin CA, Curry AD, Hua W, et al. Direct and indirect associations of neighborhood disorder with drug use and high-risk sexual partners. Am J Prev Med. 2007;32:S234–41. doi: 10.1016/j.amepre.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Center for Human Resource Research . NLSY97 User's Guide. The Ohio State University; Centre for Human Resource Research; 2009. [Google Scholar]
- 26.Osypuk T, Acevedo-Garcia D. Are racial disparities in preterm birth larger in hypersegregated areas? Am J Epidemiol. 2008;167:1295–304. doi: 10.1093/aje/kwn043. [DOI] [PubMed] [Google Scholar]
- 27.Murphy D, Brecht M, Herbeck D, et al. Trajectories of HIV risk behavior from age 15 to 25 in the national longitudinal survey of youth sample. J Youth Adolesc. 2009;38:1226–39. doi: 10.1007/s10964-008-9323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iceland J, Weinberg D, Steinmetz E. U.S. Census Bureau, Series CENSR-3, Racial and Ethnic Residential Segregation in the United States: 1980-2000. US Government Printing Office; Washington, DC: 2002. [Google Scholar]
- 29.White K, Borrell LN. Racial/ethnic residential segregation: framing the context of health risk and health disparities. Health Place. 2011;17:438–48. doi: 10.1016/j.healthplace.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massey DS, Denton NA. The dimensions of residential segregation. Soc Forces. 1988;67:281–315. [Google Scholar]
- 31.Massey DS, White MJ, Phua V-C. The dimensions of segregation revisited. Sociol Methods Res. 1996;25:172–206. [Google Scholar]
- 32.Iceland J. Beyond black and white: metropolitan residential segregation in multi-ethnic America. Soc Sci Res. 2004;33:248–71. [Google Scholar]
- 33.Krieger N, Chen J, Waterman P, et al. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the public health disparities geocoding project. Am J Public Health. 2003;93:1655–71. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Wiley; Hoboken, NJ: 2002. [Google Scholar]
- 35.Davey A, Shanahan MJ, Schafer JL. Correcting for selective nonresponse in the National Longitudinal Survey of Youth using multiple imputation. J Hum Resour. 2001;36:500–19. [Google Scholar]
- 36.Nonnemaker JM, Morgan-Lopez AA, Pais JM, et al. Youth BMI trajectories: evidence from the NLSY97. Obesity (Silver Spring) 2009;17:1274–80. doi: 10.1038/oby.2009.5. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention . Sexually Transmitted Disease Surveillance, 2008. U.S. Department of Health and Human Services; Atlanta, GA: 2009. [Google Scholar]
- 38.Fergus S, Zimmerman MA, Caldwell CH. Growth trajectories of sexual risk behavior in adolescence and young adulthood. Am J Public Health. 2007;97:1096–101. doi: 10.2105/AJPH.2005.074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26:250–61. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Acevedo-Garcia D, Osypuk TL. Residential segregation and health—the complexity of modeling separate social contexts. Am J Epidemiol. 2008;168:1255–8. doi: 10.1093/aje/kwn290. [DOI] [PubMed] [Google Scholar]
- 41.Kramer MR, Cooper HL, Drews-Botsch CD, et al. Do measures matter? Comparing surface-density-derived and census-tract-derived measures of racial residential segregation. Int J Health Geogr. 2010;9:29. doi: 10.1186/1476-072X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharkey P. The intergenerational transmission of context. Am J Sociol. 2008;113:931. [Google Scholar]
- 43.Whitescarver J. Trans-NIH Plan for HIV-Related Research. National Institutes of Health; Bethesda, MD: 2011. [Google Scholar]