Abstract
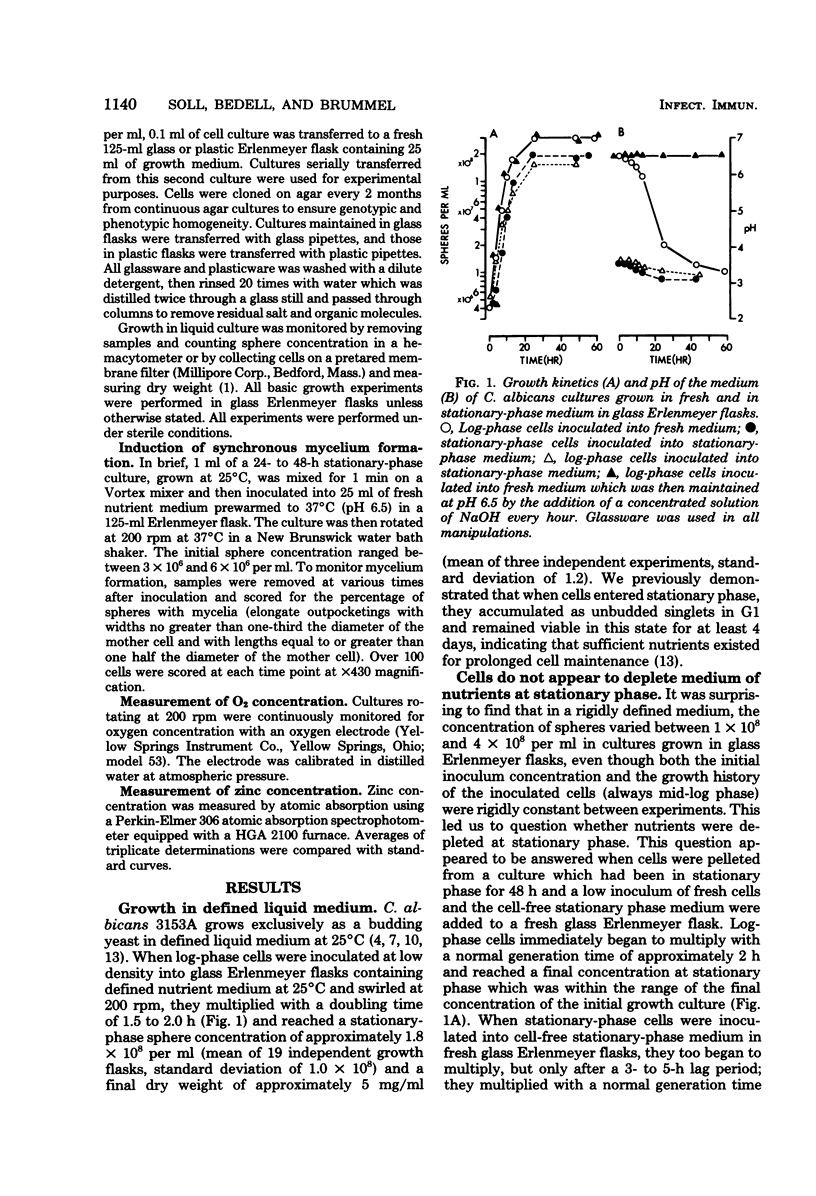
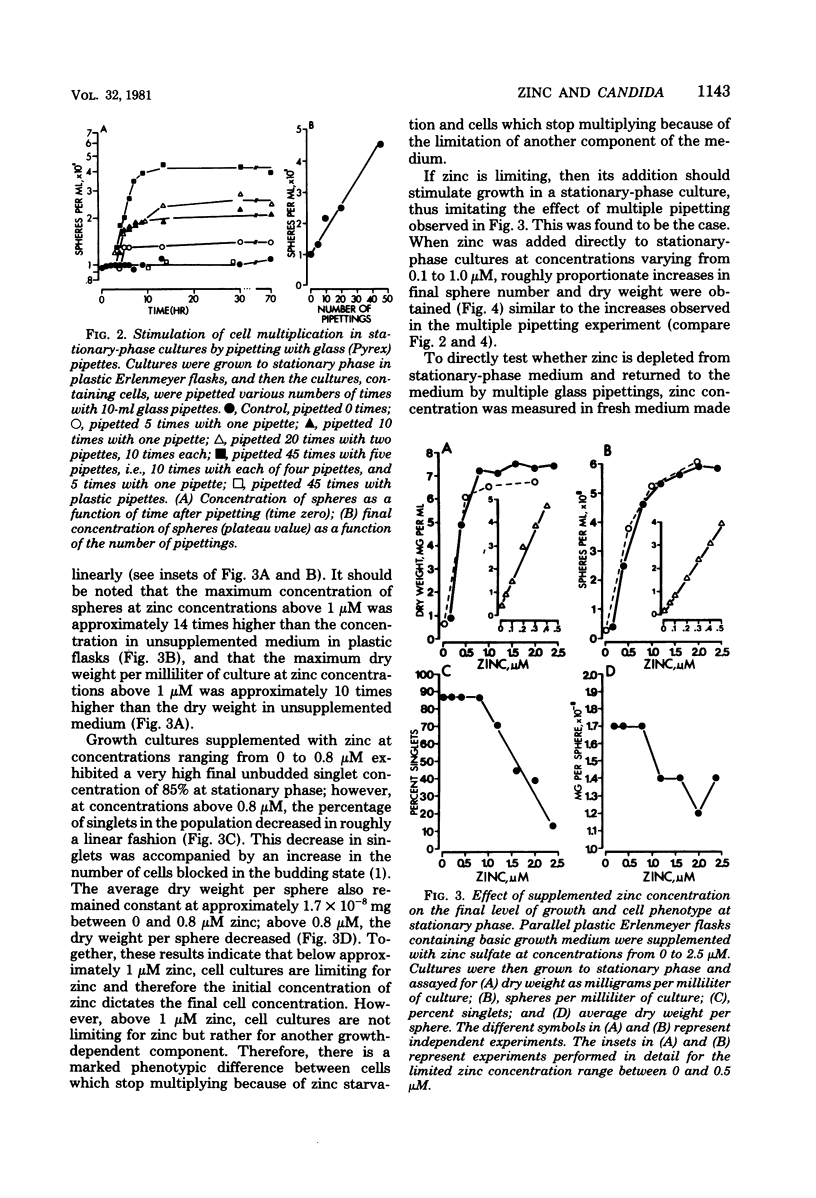
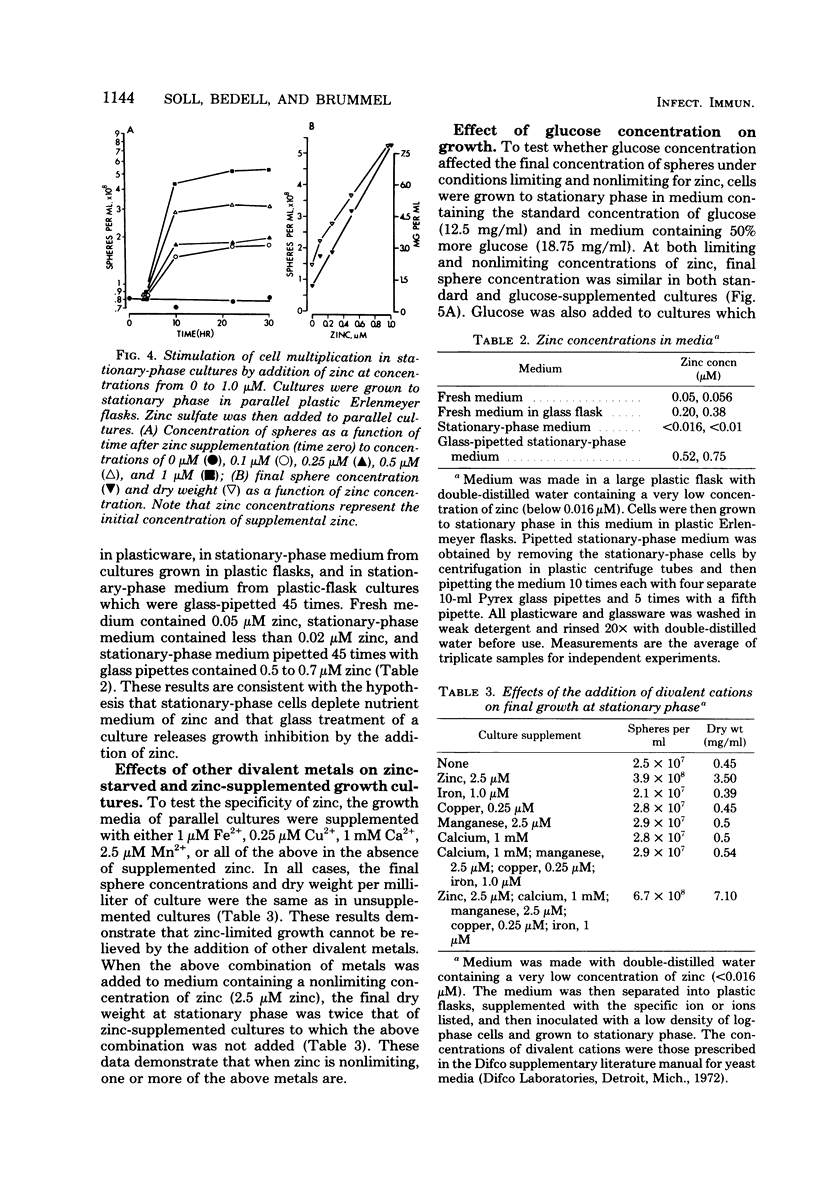
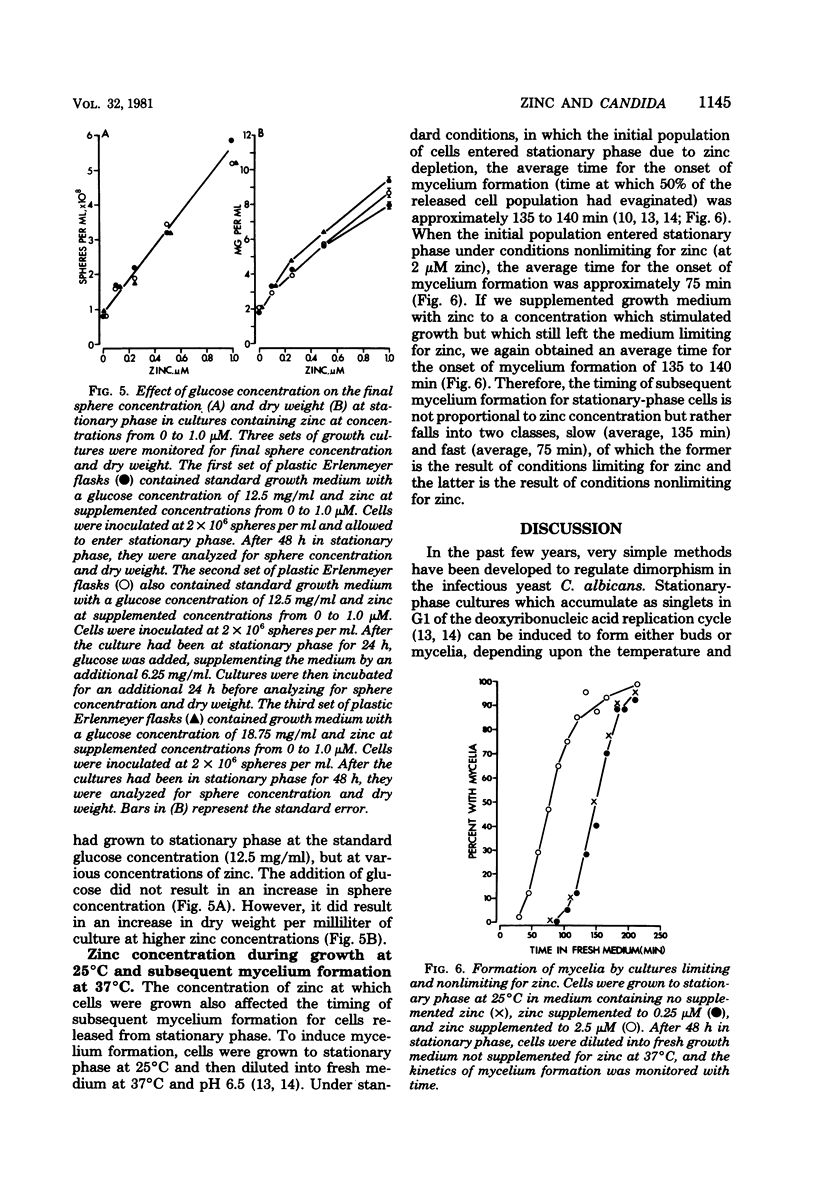
When Candida albicans is grown at 25 degrees C in suspension in defined medium, cells accumulate at stationary phase as singlets in G1 of the deoxyribonucleic acid replication cycle and acquire the capacity to form mycelia. When cells were removed from a stationary-phase culture and a low concentration of fresh cells was inoculated into the cell-free, stationary-phase medium, the fresh cells grew to approximately the same cell density as the original culture. We demonstrated that in the accompanying decrease in pH, nor due to a depletion of O2, an accumulation of CO2, a physical crowding effect, or accumulation of the putative autoinhibitors tryptophol and 2-phenylethyl alcohol. Rather, cells stop multiplying at stationary phase due to the depletion of zinc from the culture medium. The manipulation of cultures with glassware to remove stationary-phase cells and to add fresh cells led to the addition of zinc to the medium and hence a new round of culture growth. The same manipulations with plasticware did not result in zinc supplementation and hence in now new round of culture growth. When cells enter stationary phase in excess zinc, they do not accumulate as singlets; rather, they accumulate as budded cells. When these cells were induced to form mycelia, they did so in half the time it took zinc-starved cells. The usefulness of employing zinc starvation as a method for obtaining a uniform stationary-phase phenotype and for synchronizing induced mycelium or bud formation is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedell G. W., Soll D. R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979 Oct;26(1):348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell W. M., Chaffin W. L. Nutrient-limited yeast growth in Candida albicans: effect on yeast-mycelial transition. Can J Microbiol. 1980 Jan;26(1):102–105. doi: 10.1139/m80-015. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L., Sogin S. J. Germ tube formation from zonal rotor fractions of Candida albicans. J Bacteriol. 1976 May;126(2):771–776. doi: 10.1128/jb.126.2.771-776.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford H. G., Pik J. R., Lawford G. R., Williams T., Kligerman A. Hyperaccumulation of zinc by zinc-depleted Candida utilis grown in chemostat culture. Can J Microbiol. 1980 Jan;26(1):71–76. doi: 10.1139/m80-011. [DOI] [PubMed] [Google Scholar]
- Lawford H. G., Pik J. R., Lawford G. R., Williams T., Kligerman A. Physiology of Candida utilis yeast in zinc-limited chemostat culture. Can J Microbiol. 1980 Jan;26(1):64–70. doi: 10.1139/m80-010. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Lingappa B. T., Prasad M., Lingappa Y., Hunt D. F., Biemann K. Phenethyl alcohol and tryptophol: autoantibiotics produced by the fungus Candida albicans. Science. 1969 Jan 10;163(3863):192–194. doi: 10.1126/science.163.3863.192. [DOI] [PubMed] [Google Scholar]
- Mitchell L. H., Soll D. R. Commitment to germ tube or bud formation during release from stationary phase in Candida albicans. Exp Cell Res. 1979 Apr;120(1):167–179. doi: 10.1016/0014-4827(79)90547-0. [DOI] [PubMed] [Google Scholar]
- Saltarelli C. G. Growth stimulation and inhibition of Candida albicans by metabolic by-products. Mycopathol Mycol Appl. 1973 Sep 28;51(1):53–63. doi: 10.1007/BF02141285. [DOI] [PubMed] [Google Scholar]
- Simonetti N., Strippoli V., Cassone A. Yeast-mycelial conversion induced by N-acetyl-D-glucosamine in Candida albicans. Nature. 1974 Jul 26;250(464):344–346. doi: 10.1038/250344a0. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Stasi M., Bedell G. The regulation of nuclear migration and division during pseudo-mycelium outgrowth in the dimorphic yeast Candida albicans. Exp Cell Res. 1978 Oct 1;116(1):207–215. doi: 10.1016/0014-4827(78)90077-0. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Stasi M., Bedell G. The regulation of nuclear migration and division during pseudo-mycelium outgrowth in the dimorphic yeast Candida albicans. Exp Cell Res. 1978 Oct 1;116(1):207–215. doi: 10.1016/0014-4827(78)90077-0. [DOI] [PubMed] [Google Scholar]



