Abstract
Asthma is a chronic inflammatory disease of the lungs. Both the number of cases and severity of asthma have been increasing without a clear explanation. Recent evidence suggests that obesity, which has also been increasing alarmingly, may worsen or precipitate asthma, but there is little evidence of how obesity may contribute to lung inflammation. We propose that mast cells are involved in both asthma and obesity by being the target and source of adipocytokines, “alarmins” such as interleukin-9 (IL-9) and interleukin-33 (IL-33), and stress molecules including corticotropin-releasing hormone (CRH) and neurotensin (NT), secreted in response to the metabolic burden. In particular, CRH and NT have synergistic effects on mast cell secretion of vascular endothelial growth factor (VEGF). IL-33 augments VEGF release induced by substance P (SP) and tumor necrosis factor (TNF) release induced by NT. Both IL-9 and IL-33 also promote lung mast cell infiltration and augment allergic inflammation. These molecules are also expressed in human mast cells leading to autocrine effects. Obese patients are also less sensitive to glucocorticoids and bronchodilators. Development of effective mast cell inhibitors may be a novel approach for the management of both asthma and obesity. Certain flavonoid combinations may be a promising new treatment approach.
Keywords: adipocytokines, asthma, cytokines, inflammation, obesity, treatment
Introduction
The prevalence of both asthma [1] and obesity [2] has been increasing steadily over the last 20 years. A meta-analysis of prospective epidemiological studies indicated that obesity and asthma co-exist in many patients [3]. Moreover, triggers of severe asthma are still not well understood [4,5]. Obesity has been linked to inflammation [6,7]. White adipose tissue (WAT) has been implicated in several pathophysiologic mechanisms: (a) metabolism of fatty acids, (b) production of adipocytokines including C-reactive protein (CRP), IL-6, IL-9, IL-18 [8] (c) synthesis of angiotensinogen, adiponectin, resistin and leptin [9], as well as (d) insulin resistance. Macrophages [10] and mast cells [11] are increased in obese WAT compared to lean tissue. Saturated fatty acids can stimulate toll-like receptors (TLRs) [12] and lead to cardiometabolic deregulation [13].
Obesity is a major risk factor for type 2 diabetes, possibly due to the inflammatory response that could alter adipose tissue function [7], thus leading to insulin resistance [14], and worsen asthma control [5,15–18]. One study concluded that the association between obesity and asthma (atopic and non-atopic) was independent of insulin resistance and socio-demographic factors [19]. Adipocytokines have been associated with allergic inflammation and mast cells [4,20,21]. Mast cells are involved in asthma pathogenesis [22,23] and in the metabolic syndrome [23,24]. Advanced glycation end products (AGEs) that accumulate in diabetes and obesity can also activate mast cells [25].
Obesity as an inflammatory state
Obesity is now considered a chronic inflammatory state involving cytokine release from adipocytes [6,26]. WAT secretes a number of hormones, such as adiponectin and leptin. Leptin is mainly secreted by adipocytes, is markedly increased in obesity, it regulates body weight but also regulates various immune and inflammatory processes [27].
One study showed that leptin levels in children correlate positively with the basal metabolic index (BMI), airway reactivity and total immunoglobulin E (IgE) [28], as well as with exercise-induced bronchoconstriction [29]. Higher serum leptin was reported in children with asthma, but did not have a direct effect on the airways [30]. In fact, letpin was independent of obesity in mice [31]. One review of such studies concluded that leptin does not have a significant direct role in the association between obesity and asthma [32]. Instead, leptin may be affecting lung function through mast cells, especially since both leptin and leptin receptors are expressed by human mast cells [33].
Decreased adiponectin release from adipocytes, observed in obesity, is associated with insulin resistance and hyperinsulinemia [34]. Weight loss and adipocyte mass reduction result in a decrease in pro-inflammatory adipokine production and an increase in circulating adiponectin.
Visfatin is an insulin-mimicking adipokine and is significantly increased in subjects diagnosed with obesity, type 2 diabetes, and the metabolic syndrome [35]. A strong correlation was found between visfatin and tumor necrosis factor-alpha (TNF-α) expression in adipose tissue and peripheral blood mononuclear cells [36].
Resistin is an adipocytokine with a controversial role in the pathogenesis of obesity- mediated insulin resistance and type 2 diabetes, but acts like a pro-inflammatory cytokine [37] and also leads to secretion of other pro-inflammatory cytokines [38]. Patients with asthma were found to have higher levels of resistin, and resistin levels were increased with disease severity [39]. Adipose tissue of obese individuals also releases IL-6 and Regulated on Activation Normal T Cell Expressed and Secreted (RANTES) [40], which is a potent mast cell chemoattractant [41].
Obesity and asthma
Numerous studies suggest that obesity and adipocytokines [32,42] are risk factors for asthma [3,43,44]. Obese patients appear to have abnormal levels of serum and airway adipocytokines [45]. In contrast to leptin, adiponectin has anti-inflammatory properties. In children, adiponectin negatively correlates with BMI [46], and lower adiponectin levels in cord blood are associated with increased risk of developing wheezing disorders within the first two years of life [47]. It was also shown that adiponectin levels in asthmatic children negatively correlate with exercise-induced bronchoconstriction [29]. A recent study also reported that childhood obesity is associated with higher risk of asthma control and severity [48]. Interestingly, a recent study of 3 year old children with asthma reported a positive relationship between obesity and asthma, but only in boys and not in girls [49]. In contrast, a study of 411 adults demonstrated, after adjusting for body mass, that high serum adiponectin was strongly associated with asthma in men than in women [50]. Nevertheless, a recent study of 1,450 women reported that low adiponectin levels at year = 15 predicted significantly higher risk for asthma at year = 20, especially in smokers [51].
Obesity also worsens airway inflammation [52] as obese asthma patients are in need of increased inhaled steroids to achieve good asthma control [48]. Overweight children had increased hospital admissions for asthma [53] and required longer and higher doses of steroids to recover than those of normal weight [54]. There was also a correlation between obesity, asthma severity and exacerbations, as well as increased serum IgE [55].
Westernized diet, such as low antioxidant intake and high saturated fat intake, contributes to an elevated inflammatory state in asthma due to activation of the innate immune response [56]. Levels of 8-isoprostane and other markers of oxidative stress are increased both in the blood [57] and the lungs [58] of obese versus lean patients with asthma. Moreover, oxidized lipoproteins can activate mast cells [59].
Mast cells and lung inflammation
Mast cells are necessary for the development of allergic reactions [60], through crosslinking of their surface high affinity receptors for IgE (FcεRI) leading to degranulation and the release of vasoactive, pro-inflammatory and nociceptive mediators, such as arachidonic acid metabolites, histamine, cytokines and proteolytic enzymes [60,61]. Many of these molecules are known to participate in asthma [62].
Mast cells can also be activated by various immune and environmental triggers. These include proteases, such as chymase and tryptase, stem cell factor (SCF) and TNF, as well as TLR ligands [63,64] and immunoglobulin light chains that have been implicated in allergic asthma [65].
IL-33 [66] induces mast cell production of IL-13 [67] and promotes mast cell survival [67]. Mast cells have been considered “sensors of cell injury” through IL-33 [68]. IL-33 is produced by mast cells and regulates airway allergic inflammation [69]. Moreover, IL-33 connects mast cells, dendritic cells and Th2 cells in an animal model of asthma [70]. In addition to IL-33, IL-9 is involved in allergic inflammation [71] and permits antigen-induced mast cell infiltration of the lungs [72].
Many triggers may participate in lung inflammation together with neuropeptides secreted locally that stimulate mast cells. For instance, we showed that corticotropin-releasing hormone (CRH) and NT act synergistically to increase vascular permeability [73]. We further showed that IL-33 augments human mast cell release of vascular endothelial growth factor (VEGF) in response to substance P (SP) [74].
Mast cells are adjacent to blood vessels in the lamina propria of airway mucosa [20]. In patients with asthma, mast cells also migrate into airway epithelium [62,75] and airway smooth muscle [76]. This anatomical proximity to key structures involved in asthma and in vitro evidence for direct interaction between mast cells and airway smooth muscle cells [77], suggest that mast cells play a significant role in the pathophysiology of asthma [75,78] through the release of multiple mediators in response to both immunoglobulin E (IgE) [20] and monomeric IgE [79].
After activation, mast cells secrete histamine, prostaglandin D2 (PGD2) and leukotriene C4 (LTC4), which induce bronchoconstriction, mucus secretion and mucosal edema, thus contributing to the acute symptoms observed in asthma [75,78,80]. Mast cells also secrete IL-4, IL-5, IL-6, IL-8 and TNF-α, which increase airway smooth muscle (ASM) hyperresponsiveness, induce IgE synthesis, and recruit other immune cells, including T cells and eosinophils. In fact, mast cells are the only cell type that secrete preformed TNF [66] and can deliver it to the lymph nodes further stimulating the immune response [81], especially by stimulating T cells through TNF [82,83]. When TNF is administered by inhalation to humans, it induces both bronchial hyperresponsiveness (BHR) and sputum neutrophilia in normal subjects. TNF also exacerbates BHR in patients with asthma [84].
Moreover, mast cells counteract Treg cell suppression and promote the development of T17 cells involved in autoimmune diseases [85]. In fact, mast cells can synthesize IL-17 [86]. Mast cell-derived transforming growth factor-beta1 (TGF-β1) also induced the development of T17 cells [87]. Mast cells can also induce TGFβ1 expression in SMC via release of tryptase, resulting in differentiation of SMC into a more contractile phenotype [88].
Lung mast cell participation in asthma may not involve exocytosis of granular content typical of allergic or anaphylactic reactions. As a result, histologic studies are not likely to show evidence of mast cell activation. For instance, the ultrastructural appearance of activated mast cells often indicates a process of piecemeal degranulation and was associated with selective release of mast cell mediators [89]. In particular, IL-1 induced release of IL-6 [90]. CRH stimulated selective release of VEGF, which is also pro-inflammatory and vasodilatory [91]. LPS induced release of TNF [92] without degranulation. Viral double-stranded RNA stimulated toll- like receptor-3 (TLR-3) to induce selective release of IL-13 [63]. Moreover, TLR-4 regulated allergic airway inflammation through mast cell activation [93]. TLRs are increasingly invoked in the development of airway inflammation [94], through regulation of mast cell function [94].
Proteases derived from isolated human lung mast cells stimulated by IgE/anti-IgE increased Chemokine C-C Motif Ligand 8 (CCL8) and fibronectin production from cultured airway SMC [95]. Moreover, alveolar mast cells had higher FcεRI expression in patients with mild allergic asthma than in allergic rhinitis implying that mast cells in asthma may present with a unique phenotype thus making them even more relevant as targets for novel treatments [96].
Stress, mast cells and asthma
There is considerable evidence that stress worsens allergic diseases in general [97–99], as well as asthma [100,101]. The effect of stress may be mediated through activation of mast cells [97]. Mast cells infiltrated bronchial smooth muscle (BSM) in asthmatics and human lung mast cells adhered to BSM cells through type I collagen CD51 and CD44 [102].
Stress typically results in secretion of CRH from the hypothalamus and activates the hypothalamic-pituitary-adrenal (HPA) axis. However, CRH is also released outside the CNS where it has pro-inflammatory effects, through mast cell activation [103,104]. Moreover, human mast cells express mRNA and functional CRHR-1 [91], activation of which induces selective release of VEGF [91]. Stress induces local release of CRH in the skin and stimulates skin mast cells leading to increased skin vascular permeability [73]. Mast cells can also release large amounts of CRH [105].
CRH secreted from mast cells can decrease the ability of Treg cells to produce the immunosuppressant IL-10, thus further increasing inflammation [106]. It is of interest that human adipose tissue expresses CRHR-1 and CRHR-2, as well as the CRH related peptides urocortin and stresscopin [107], implying that CRH could affect adipose tissue both directly and indirectly, through mast cells. A recent paper reported that prenatal stress was associated with increased cord blood IgE, and this correlation was stronger between a mother with a history of atopy and an offspring sensitive to dust mites [108].
Mast cells and obesity
We propose that mast cells are involved in both asthma and obesity by being the target and source of adipocytokines, “alarmins” such as IL-9 and IL-33, and stress molecules including CRH and NT, secreted in response to the metabolic burden. Such molecules may act through mast cells, especially since they, as well as CRH and IL-33, are also expressed in human mast cells leading to autocrine effects (Fig. 1).
Figure 1.
Diagrammatic representation of the proposed interactions among adipocyte-derived molecules, mast cells and their pro-inflammatory mediators in the pathogenesis of asthma and obesity.
Mast cells are often found within WAT tissue (Fig. 2). In fact, WAT from obese humans and mice contain more mast cells than WAT from their lean counterparts [22]. These mast cells also contribute to diet-induced obesity by producing inflammatory mediators such as IL-6, interferon-gamma (IFN-γ), TNF-α, IL-1β and CCL2[22]. KitW-sh/W-sh mast cell deficient mice fed a high fat and carbohydrate rich Western diet for 12 weeks gained significantly less body weight than wild-type (WT) congenic controls and had reduced serum and WAT levels of inflammatory cytokines, chemokines and proteases [22]. Kit W-sh/W-sh mast cell deficient mice or those mice receiving the rodent mast cell stabilizer, disodium cromoglycate (cromolyn), also had significantly lower concentrations of serum leptin than WT controls [22]. Administration of leptin during allergen challenge of sensitized mice augmented allergen-induced airway hyperresponsiveness (AHR), even though it did not affect eosinophil influx or Th2 cytokine expression [31].
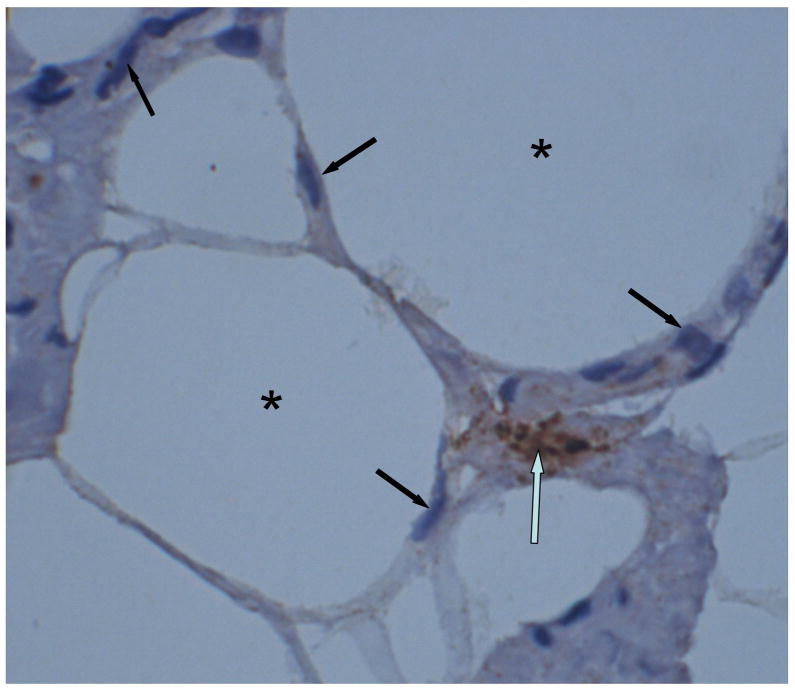
Figure 2.
Light photomicrograph of human abdominal fat (obtained during plastic surgery of a 37 year old Caucasian female) showing adipocytes (asterisk), lymphocytes (dark arrow) and a tryptase-positive mast cell (open arrow).
Clinical relevance and proposed treatment approaches
Current asthma treatment modalities are not as effective in the obese patient with asthma [15,16,109,110]. This may be due to the fact that asthma associated with obesity may be of late onset, may involve non-eosinophilic inflammatory cells, or other mast cell-dependent processes.
Inhibition of mast cell activation and/or secretion would certainly be desirable on many levels, since mast cells appear to be involved both in obesity and asthma, as well as serve as a link between these two diseases (Table 1). Unfortunately, there is no effective mast cell inhibitor clinically available. Cromolyn or the histamine-1 receptor antagonist ketotifen (administered intraperitoneally) reduced body weight and glucose intolerance in mice [22], but are ineffective in asthma. Moreover, cromolyn inhibits histamine secretion from rodent mast cells, but is a weak inhibitor of human mast cells [111,112].
Table 1.
Summary of clinical relevance
|
Inhaled corticosteroids are heavily used for asthma, but mostly in order to reduce inflammation rather than inhibit mast cell activation. A recent paper reported that inhaled corticosteroids reduced the number of bronchial epithelial and smooth muscle mast cells, but not subepithelial mast cells [113]. The anti-IgE humanized antibody omalizumab is also frequently used for severe asthma, but is characterized by 33% non-responders [114]. Reduction of the IgE appears to reduce bronchial inflammation and airway remodeling [115]. Nevertheless, both corticosteroids and omalizumab have the potential of serious side effects, including infections. A new approach involved aggregation of the FcεRI with the low affinity IgG receptor (FcγRIIb) by a novel bispheric fusion protein that led to more effective allergic basophil inhibition of cytokine release in vitro than omalizumab [116].
Certain natural flavonoids [117], such as quercetin and luteolin, posses potent anti-oxidant, anti-inflammatory and mast cell blocking actions [118,119] making them potential candidates for prophylactic treatment of asthma and the metabolic syndrome. In fact, aerosolized quercetin was used in experimental murine allergic asthma [120]. Moreover quercetin mimics the action of glucagon-like peptide-1, a promising treatment candidate for type 2 diabetes [121]. Luteolin, the flavone of the flavonoid quercetin, can inhibit human mast cells and mast cell dependent T cell activation [83], as well as adipocyte-dependent activation of macrophages [122]. Luteolin also improves insulin sensitivity of the endothelium [123].
Unfortunately, flavonoids are poorly absorbed orally in powder form (less than 10%) and are rapidly metabolized [117]. Better formulations using liposomal or phosphatidyl choline-carrier luteolin could provide increased delivery and oral absorption.
Acknowledgments
Aspects of our work discussed here were supported in part by US National Institutes of Health (NIH) grants: AR 47652; NS 66205; and NS 71361 to TCT. Thanks are due to Smaro Panagiotidou for help with the word processing and Alexandra Miniati for help with the light micrograph.
Footnotes
Conflict of interest
There is no conflict of interest.
Dr. Theoharides is the recipient of US Patents No. 6,624,148; 7,115,278; and 7,759,307, covering the use of flavonoids in the treatment of CAD, which were assigned to Theta Biomedical Consulting and Development Co. Inc. (Brookline, MA).
Author Contributions
Dr. Theoharides wrote the manuscript and the rest of the authors contributed to it by providing bibliographic information, graphics and corrections. Each author listed on the manuscript has seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript.
Reference List
- 1.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165–1174. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee AB, Zhang Z. Allergic asthma: influence of genetic and environmental factors. J Biol Chem. 2011;286:32883–32889. doi: 10.1074/jbc.R110.197046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanez P, Wenzel SE, Anderson GP, Anto JM, Bel EH, Boulet LP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007;119:1337–1348. doi: 10.1016/j.jaci.2006.11.702. [DOI] [PubMed] [Google Scholar]
- 6.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 8.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 10.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theoharides TC, Makris M, Kalogeromitros D. Allergic inflammation and adipocytokines. Int J Immunopathol Pharmacol. 2008;21:1–4. doi: 10.1177/039463200802100101. [DOI] [PubMed] [Google Scholar]
- 12.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 14.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saint-Pierre P, Bourdin A, Chanez P, Daures JP, Godard P. Are overweight asthmatics more difficult to control? Allergy. 2006;61:79–84. doi: 10.1111/j.1398-9995.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 16.Lavoie KL, Bacon SL, Labrecque M, Cartier A, Ditto B. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respir Med. 2006;100:648–657. doi: 10.1016/j.rmed.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varraso R, Siroux V, Maccario J, Pin I, Kauffmann F. Asthma severity is associated with body mass index and early menarche in women. Am J Respir Crit Care Med. 2005;171:334–339. doi: 10.1164/rccm.200405-674OC. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Xiao L, Knowles SB. Obesity, insulin resistance and the prevalence of atopy and asthma in US adults. Allergy. 2010;65:1455–1463. doi: 10.1111/j.1398-9995.2010.02402.x. [DOI] [PubMed] [Google Scholar]
- 20.Bradding P, Walls AF, Holgate ST. The role of the mast cells in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 21.Moiseeva EP, Bradding P. Mast cells in lung inflammation. Adv Exp Med Biol. 2011;716:235–269. doi: 10.1007/978-1-4419-9533-9_13. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theoharides TC, Sismanopoulos N, Delivanis DA, Zhang B, Hatziagelaki EE, Kalogeromitros D. Mast cells squeeze the heart and stretch the gird: Their role in atherosclerosis and obesity. Trends Pharmacol Sci. 2011;32:534–542. doi: 10.1016/j.tips.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Shi GP. Mast cells and metabolic syndrome. Biochim Biophys Acta. 2012;1822:14–20. doi: 10.1016/j.bbadis.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sick E, Brehin S, Andre P, Coupin G, Landry Y, Takeda K, et al. Advanced glycation end products (AGEs) activate mast cells. Br J Pharmacol. 2010;161:442–455. doi: 10.1111/j.1476-5381.2010.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 27.Lago R, Gomez R, Lago F, Gomez-Reino J, Gualillo O. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252:139–145. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Mai XM, Bottcher MF, Leijon I. Leptin and asthma in overweight children at 12 years of age. Pediatr Allergy Immunol. 2004;15:523–530. doi: 10.1111/j.1399-3038.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 29.Baek HS, Kim YD, Shin JH, Kim JH, Oh JW, Lee HB. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011;107:14–21. doi: 10.1016/j.anai.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114:254–259. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 31.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Jartti T, Saarikoski L, Jartti L, Lisinen I, Jula A, Huupponen R, et al. Obesity, adipokines and asthma. Allergy. 2009;64:770–777. doi: 10.1111/j.1398-9995.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 33.Taildeman J, Perez-Novo CA, Rottiers I, Ferdinande L, Waeytens A, De CV, et al. Human mast cells express leptin and leptin receptors. Histochem Cell Biol. 2009;131:703–711. doi: 10.1007/s00418-009-0575-3. [DOI] [PubMed] [Google Scholar]
- 34.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 35.Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27:515–527. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 36.Dedoussis GV, Kapiri A, Samara A, Dimitriadis D, Lambert D, Pfister M, et al. Visfatin: the link between inflammation and childhood obesity. Diabetes Care. 2009;32:e71. doi: 10.2337/dc08-2304. [DOI] [PubMed] [Google Scholar]
- 37.Nagaev I, Bokarewa M, Tarkowski A, Smith U. Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes. PloS One. 2006;1:e31. doi: 10.1371/journal.pone.0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 39.Larochelle J, Freiler J, Dice J, Hagan L. Plasma resistin levels in asthmatics as a marker of disease state. J Asthma. 2007;44:509–513. doi: 10.1080/02770900701495785. [DOI] [PubMed] [Google Scholar]
- 40.Matter CM, Handschin C. RANTES (regulated on activation, normal T cell expressed and secreted), inflammation, obesity, and the metabolic syndrome. Circulation. 2007;115:946–948. doi: 10.1161/CIRCULATIONAHA.106.685230. [DOI] [PubMed] [Google Scholar]
- 41.Conti P, Pang X, Boucher W, Letourneau R, Reale M, Barbacane RC, et al. Impact of Rantes and MCP-1 chemokines on in vivo basophilic mast cell recruitment in rat skin injection model and their role in modifying the protein and mRNA levels for histidine decarboxylase. Blood. 1997;89:4120–4127. [PubMed] [Google Scholar]
- 42.Lugogo NL, Bappanad D, Kraft M. Obesity, metabolic dysregulation and oxidative stress in asthma. Biochim Biophys Acta. 2011;1810:1120–1126. doi: 10.1016/j.bbagen.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol. 2005;115:925–927. doi: 10.1016/j.jaci.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 44.Stenius-Aarniala B, Poussa T, Kvarnstrom J, Gronlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320:827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holguin F, Rojas M, Brown LA, Fitzpatrick AM. Airway and plasma leptin and adiponectin in lean and obese asthmatics and controls. J Asthma. 2011;48:217–223. doi: 10.3109/02770903.2011.555033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2009;20:81–88. doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 47.Rothenbacher D, Weyermann M, Fantuzzi G, Brenner H. Adipokines in cord blood and risk of wheezing disorders within the first two years of life. Clin Exp Allergy. 2007;37:1143–1149. doi: 10.1111/j.1365-2222.2007.02759.x. [DOI] [PubMed] [Google Scholar]
- 48.Quinto KB, Zuraw BL, Poon KY, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol. 2011;128:964–969. doi: 10.1016/j.jaci.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 49.Suglia SF, Chambers EC, Rosario A, Duarte CS. Asthma and obesity in three-year-old urban children: role of sex and home environment 1. J Pediatr. 2011;159:14–20. doi: 10.1016/j.jpeds.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sood A, Dominic E, Qualls C, Steffes MW, Thyagarajan B, Smith LJ, et al. Serum Adiponectin is Associated with Adverse Outcomes of Asthma in Men but Not in Women. Front Pharmacol. 2011;2:55. doi: 10.3389/fphar.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sood A, Qualls C, Schuyler M, Thyagarajan B, Steffes MW, Smith LJ, et al. Low Serum Adiponectin Predicts Future Risk for Asthma in Women. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201110-1767OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM, Williamson A, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178:469–475. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 53.Carroll CL, Stoltz P, Raykov N, Smith SR, Zucker AR. Childhood overweight increases hospital admission rates for asthma. Pediatrics. 2007;120:734–740. doi: 10.1542/peds.2007-0409. [DOI] [PubMed] [Google Scholar]
- 54.Carroll CL, Bhandari A, Zucker AR, Schramm CM. Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr Crit Care Med. 2006;7:527–531. doi: 10.1097/01.PCC.0000243749.14555.E8. [DOI] [PubMed] [Google Scholar]
- 55.Fitzpatrick S, Joks R, Silverberg JI. Obesity is associated with increased asthma severity and exacerbations, and increased serum immunoglobulin E in inner-city adults. Clin Exp Allergy. 2012;42:747–759. doi: 10.1111/j.1365-2222.2011.03863.x. [DOI] [PubMed] [Google Scholar]
- 56.Wood LG, Gibson PG. Dietary factors lead to innate immune activation in asthma. Pharmacol Ther. 2009;123:37–53. doi: 10.1016/j.pharmthera.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M, et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelley J, Hemontolor G, Younis W, Li C, Krishnaswamy G, Chi DS. Mast cell activation by lipoproteins. Methods Mol Biol. 2006;315:341–348. doi: 10.1385/1-59259-967-2:341. [DOI] [PubMed] [Google Scholar]
- 60.Theoharides TC, Kalogeromitros D. The critical role of mast cell in allergy and inflammation. Ann NY Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- 61.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, et al. Mast cells and inflammation. Biochim Biophys Acta. 2010;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyce JA. The role of mast cells in asthma. Prostaglandins Leukot Essent Fatty Acids. 2003;69:195–205. doi: 10.1016/s0952-3278(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 63.Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 64.McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003;170:1625–1629. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 65.Kraneveld AD, Kool M, van Houwelingen AH, Roholl P, Solomon A, Postma DS, et al. Elicitation of allergic asthma by immunoglobulin free light chains. Proc Natl Acad Sci U S A. 2005;102:1578–1583. doi: 10.1073/pnas.0406808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moulin D, Donze O, Talabot-Ayer D, Mezin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 68.Enoksson M, Lyberg K, Moller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol. 2011;186:2523–2528. doi: 10.4049/jimmunol.1003383. [DOI] [PubMed] [Google Scholar]
- 69.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS ONE. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eiwegger T, Akdis CA. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur J Immunol. 2011;41:1535–1538. doi: 10.1002/eji.201141668. [DOI] [PubMed] [Google Scholar]
- 71.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 72.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011;183:865–875. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Theoharides TC. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA. 2006;103:7759–7764. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balzar S, Fajt ML, Comhair SA, Erzurum SC, Bleecker E, Busse WW, et al. Mast cell phenotype, location, and activation in severe asthma: data from the severe asthma research program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 77.Girodet PO, Ozier A, Bara I, Tunon de Lara JM, Marthan R, Berger P. Airway remodeling in asthma: new mechanisms and potential for pharmacological intervention. Pharmacol Ther. 2011;130:325–337. doi: 10.1016/j.pharmthera.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Bingham CO, III, Austen KF. Mast-cell responses in the development of asthma. J Allergy Clin Immunol. 2000;105:S527–S534. doi: 10.1016/s0091-6749(00)90056-3. [DOI] [PubMed] [Google Scholar]
- 79.Cruse G, Kaur D, Yang W, Duffy SM, Brightling CE, Bradding P. Activation of human lung mast cells by monomeric immunoglobulin E. Eur Respir J. 2005;25:858–863. doi: 10.1183/09031936.05.00091704. [DOI] [PubMed] [Google Scholar]
- 80.Bradding P. The role of the mast cell in asthma: a reassessment. Curr Opin Allergy Clin Immunol. 2003;3:45–50. doi: 10.1097/00130832-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Kunder CA, St John AL, Li G, Leong KW, Berwin B, Staats HF, et al. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med. 2009;206:2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, et al. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell dependent stimulation of Jurkat T cells. Br J Pharmacol. 2008;155:1076–1084. doi: 10.1038/bjp.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas PS, Heywood G. Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax. 2002;57:774–778. doi: 10.1136/thorax.57.9.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, et al. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–2648. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 86.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hollins F, Kaur D, Yang W, Cruse G, Saunders R, Sutcliffe A, et al. Human airway smooth muscle promotes human lung mast cell survival, proliferation, and constitutive activation: cooperative roles for CADM1, stem cell factor, and IL-6. J Immunol. 2008;181:2772–2780. doi: 10.4049/jimmunol.181.4.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woodman L, Siddiqui S, Cruse G, Sutcliffe A, Saunders R, Kaur D, et al. Mast cells promote airway smooth muscle cell differentiation via autocrine up-regulation of TGF-beta 1. J Immunol. 2008;181:5001–5007. doi: 10.4049/jimmunol.181.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 90.Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher W, et al. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003;171:4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- 91.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 92.Varadaradjalou S, Feger F, Thieblemont N, Hamouda NB, Pleau JM, Dy M, et al. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003;33:899–906. doi: 10.1002/eji.200323830. [DOI] [PubMed] [Google Scholar]
- 93.Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, et al. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci U S A. 2006;103:2286–2291. doi: 10.1073/pnas.0510685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen K, Xiang Y, Yao X, Liu Y, Gong W, Yoshimura T, et al. The active contribution of Toll-like receptors to allergic airway inflammation. Int Immunopharmacol. 2011;11:1391–1398. doi: 10.1016/j.intimp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alkhouri H, Hollins F, Moir LM, Brightling CE, Armour CL, Hughes JM. Human lung mast cells modulate the functions of airway smooth muscle cells in asthma. Allergy. 2011;66:1231–1241. doi: 10.1111/j.1398-9995.2011.02616.x. [DOI] [PubMed] [Google Scholar]
- 96.Andersson CK, Tufvesson E, Aronsson D, Bergqvist A, Mori M, Bjermer L, et al. Alveolar mast cells shift to an FcepsilonRI-expressing phenotype in mild atopic asthma: a novel feature in allergic asthma pathology. Allergy. 2011;66:1590–1597. doi: 10.1111/j.1398-9995.2011.02723.x. [DOI] [PubMed] [Google Scholar]
- 97.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 98.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5:23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. 2008;70:102–116. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- 100.Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21:993–999. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Theoharides TC, Enakuua S, Sismanopoulos N, Papadimas E, Angelidou A, Alysandratos K. Stress contributes to asthma worsening through mast cell activation. Annals of Allergy, Asthma and Immunology. 2012;109:14–19. doi: 10.1016/j.anai.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 102.Girodet PO, Ozier A, Trian T, Begueret H, Ousova O, Vernejoux JM, et al. Mast cell adhesion to bronchial smooth muscle in asthma specifically depends on CD51 and CD44 variant 6. Allergy. 2010;65:1004–1012. doi: 10.1111/j.1398-9995.2009.02308.x. [DOI] [PubMed] [Google Scholar]
- 103.Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its pro-inflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 104.Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J Clin Endocrinol Metab. 2003;88:5427–5432. doi: 10.1210/jc.2003-030377. [DOI] [PubMed] [Google Scholar]
- 105.Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 106.Oh SH, Park CO, Wu WH, Kim JY, Jin S, Byamba D, et al. Corticotropin-releasing hormone downregulates IL-10 production by adaptive forkhead box protein 3-negative regulatory T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129:151–159. doi: 10.1016/j.jaci.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 107.Seres J, Bornstein SR, Seres P, Willenberg HS, Schulte KM, Scherbaum WA, et al. Corticotropin-releasing hormone system in human adipose tissue. J Clin Endocrinol Metab. 2004;89:965–970. doi: 10.1210/jc.2003-031299. [DOI] [PubMed] [Google Scholar]
- 108.Peters JL, Cohen S, Staudenmayer J, Hosen J, Platts-Mills TA, Wright RJ. Prenatal negative life events increases cord blood IgE: interactions with dust mite allergen and maternal atopy. Allergy. 2012;67:545–551. doi: 10.1111/j.1398-9995.2012.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101:2240–2247. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 110.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 111.Vieira Dos SR, Magerl M, Martus P, Zuberbier T, Church MK, Escribano L, et al. Topical sodium cromoglicate relieves allergen- and histamine-induced dermal pruritus. Br J Dermatol. 2010;162:674–676. doi: 10.1111/j.1365-2133.2009.09516.x. [DOI] [PubMed] [Google Scholar]
- 112.Weng Z, Zhang B, Asadi S, Sismanopoulos N, Butcher A, Fu X, et al. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PloS One. 2012 doi: 10.1371/journal.pone.0033805. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.James A, Gyllfors P, Henriksson E, Dahlen SE, Adner M, Nilsson G, et al. Corticosteroid treatment selectively decreases mast cells in the smooth muscle and epithelium of asthmatic bronchi. Allergy. 2012;67:958–961. doi: 10.1111/j.1398-9995.2012.02836.x. [DOI] [PubMed] [Google Scholar]
- 114.Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66:671–678. doi: 10.1111/j.1398-9995.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 115.Rabe KF, Calhoun WJ, Smith N, Jimenez P. Can anti-IgE therapy prevent airway remodeling in allergic asthma? Allergy. 2011;66:1142–1151. doi: 10.1111/j.1398-9995.2011.02617.x. [DOI] [PubMed] [Google Scholar]
- 116.Eggel A, Buschor P, Baumann MJ, Amstutz P, Stadler BM, Vogel M. Inhibition of ongoing allergic reactions using a novel anti-IgE DARPin-Fc fusion protein. Allergy. 2011;66:961–968. doi: 10.1111/j.1398-9995.2011.02546.x. [DOI] [PubMed] [Google Scholar]
- 117.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 118.Kimata M, Schchijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 1999;30:501–508. doi: 10.1046/j.1365-2222.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- 119.Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N, et al. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rogerio AP, Kanashiro A, Fontanari C, da Silva EV, Lucisano-Valim YM, Soares EG, et al. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007;56:402–408. doi: 10.1007/s00011-007-7005-6. [DOI] [PubMed] [Google Scholar]
- 121.Wootten D, Simms J, Koole C, Woodman OL, Summers RJ, Christopoulos A, et al. Modulation of the glucagon-like peptide-1 receptor signaling by naturally occurring and synthetic flavonoids. J Pharmacol Exp Ther. 2011;336:540–550. doi: 10.1124/jpet.110.176362. [DOI] [PubMed] [Google Scholar]
- 122.Ando C, Takahashi N, Hirai S, Nishimura K, Lin S, Uemura T, et al. Luteolin, a food-derived flavonoid, suppresses adipocyte-dependent activation of macrophages by inhibiting JNK activation. FEBS Lett. 2009;583:3649–3654. doi: 10.1016/j.febslet.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 123.Deqiu Z, Kang L, Jiali Y, Baolin L, Gaolin L. Luteolin inhibits inflammatory response and improves insulin sensitivity in the endothelium. Biochimie. 2011;93:506–512. doi: 10.1016/j.biochi.2010.11.002. [DOI] [PubMed] [Google Scholar]