Abstract
BACKGROUND
Stimulating the glycineB binding site on the N-methyl-D-aspartate receptor (NMDAR) has been proposed as a novel mechanism for modulating behavioral effects of ethanol (EtOH) that are mediated via the NMDAR, including acute intoxication. Here, we pharmacologically interrogated this hypothesis in mice.
METHODS
Effects of systemic injection of the glycineB agonist, D-serine, the GlyT-1 glycine transporter inhibitor, ALX-5407, and the glycineB antagonist, L-701,324, were tested for effects on EtOH-induced ataxia, hypothermia, loss of righting reflex duration (LORR) in C57BL/6J (B6) and 129S1/SvImJ (S1) inbred mice. Effects of the glycineB partial agonist, D-cycloserine, the GlyT-1 inhibitor, NFPS, and the glycineB antagonist, DCKA, on EtOH-induced LORR duration were also tested. Interaction effects on EtOH-induced LORR duration were examined via combined treatment with D-serine and ALX-5407, D-serine and MK-801, D-serine and L-701,324, as well as L-701,324 and ALX-5407, in B6 mice, as D-serine in GluN2A and PSD-95 KO mice. The effect of dietary depletion of Magnesium (Mg), an element which interacts the glycineB site, was also tested.
RESULTS
Neither D-serine, D-cycloserine, ALX-5407, nor NFPS significantly affected EtOH intoxication on any of the measures or strains studied. L-701,324, but not DCKA, dose-dependently potentiated the ataxia-inducing effects of EtOH and increased EtOH-induced (but not pentobarbital-induced) LORR duration. D-serine did not have interactive effects on EtOH-induced LORR duration when combined with ALX-5407. The EtOH-potentiating effects of L-701,324, but not MK-801, on LORR duration were prevented by D-serine, but not ALX-5407. Mg depletion potentiated LORR duration in B6 mice and was lethal in a large proportion of S1 mice.
CONCLUSIONS
GlycineB site activation failed to produce the hypothesized reduction in EtOH intoxication across a range of measures and genetic strains, but blockade of the glycineB site potentiated EtOH intoxication. These data suggest endogenous activity at the glycineB opposes EtOH intoxication, but it may be difficult to pharmacologically augment this action, at least in non-dependent subjects, perhaps due to physiological saturation of the glycineB site.
Keywords: alcohol, D-serine, glutamate, Zinc, Magnesium
INTRODUCTION
The N-methyl-D-aspartate ionotropic glutamate receptor (NMDAR) is a major pharmacological target for ethanol (EtOH). At behaviorally intoxicating doses, EtOH acts as an allosteric inhibitor of NMDARs in vitro, probably via direct receptor occupancy and actions on gating, and receptor phosphorylation (Lovinger et al., 1989). In vivo treatment with NMDAR antagonists mimic the subjective feelings of intoxication in humans, and substitute for the discriminative stimulus effects and exacerbate certain acute intoxicating effects of EtOH in rodents (for review, see Gass and Olive, 2008). These effects indicate profound functional interactions between EtOH and the NMDAR and suggest that pharmacological manipulation of the NMDAR could provide a potent means to modulate intoxication, with potential therapeutic implications (Krupitsky et al., 2007).
While directly stimulating NMDARs risks excitotoxicity (Lipton, 2007), there are various modulatory sites on the receptor complex that could produce more selective effects and may be more amenable to therapeutic targeting. In this context, there is a site on the NR1 subunit of the NMDAR that recognizes the amino acids glycine and D-serine (Johnson and Ascher, 1987). This site is referred to as the glycineB or strychnine-insensitive site, to distinguish it from the strychnine-sensitive glycine receptor, which has ~100-fold lesser affinity for D-serine/glycine and a pattern of distribution in the brain that, unlike glycineB, does not overlap with NMDAR distribution.
Glycine or D-serine binding at the glycineB site is obligatory for the NMDAR to be activated by glutamate (Clements and Westbrook, 1991). GlycineB binding has also been shown to allosterically modulate NMDAR function (Parsons et al., 1998), for example by enhancing affinity and efficacy of glutamate’s actions at the receptor (Fadda et al., 1988) and retarding receptor desensitization (Vyklicky et al., 1990). This profile of action has led to the suggestion that, by promoting NMDAR function, activating the glycineB site could oppose EtOH’s NMDAR-mediated intoxicating effects (Olive et al., 2005; Vengeliene et al., 2008a).
A growing number of studies have now shown that treatment with drugs acting on the glycineB site can modify behavioral responses to EtOH and voluntary EtOH drinking. For example, systemic administration of the GlyT-1 inhibitor Org25935 decreased voluntary and deprivation-driven EtOH drinking in rats (Molander et al., 2007; Vengeliene et al., 2010). Systemic treatment with the glycineB partial agonist, D-cycloserine, blocked the anxiolytic-like effects of EtOH in rats tested on the elevated plus-maze (Moraes Ferreira and Morato, 1997) and promoted tolerance to EtOH’s ataxia-inducing effects in the rat tilt-plane test (Khanna et al., 1993; Khanna et al., 1995), but did not block the discriminative stimulus effects of EtOH in rats (Bienkowski et al., 1997). D-cycloserine treatment has also been shown to facilitate extinction of a conditioned alcohol-seeking behavior in rats (Vengeliene et al., 2008b), and the reconditioning, but not acquisition or extinction, of EtOH conditioned place preference (CPP) in mice (Groblewski et al., 2009). Another glycineB partial agonist, 1-aminocyclopropanecarboxylic acid (ACPC), reduced EtOH drinking in a rat limited access procedure (Stromberg et al., 1999). Finally, the glycineB antagonist L-701,324 substituted for EtOH (Bienkowski et al., 1998; Kotlinska and Liljequist, 1997), attenuated EtOH-withdrawal (Kotlinska, 2001b; Kotlinska and Liljequist, 1996), reduced cue and deprivation-induced EtOH drinking (Backstrom and Hyytia, 2004; Vengeliene et al., 2005), and in combination with MK-801, but not alone, prevented the acquisition of EtOH CPP in rats (Biala and Kotlinska, 1999). Collectively, this prior set of findings in rodents indicates complex effects of glycineB site stimulation and blockade on EtOH-related behaviors and drinking. How these various effects might translate to humans has not yet been well studied, although a recent study found that orally administered D-cycloserine failed to alter EtOH’s stimulatory, sedative or euphoric effects in a cohort of healthy individuals (Trevisan et al., 2008).
The aim of the current study was to further examine the role of the glycineB site in modulating EtOH behaviors by examining the effects of various pharmacological manipulations of the glycineB site on measures of EtOH intoxication in mice. We began by assessing the effects of direct stimulation of the site by D-serine (or D-cycloserine) treatment, indirect stimulation of the site via blocking the glycine transporter type 1 (GlyT-1) with ALX-5407 or NFPS, or blocking the site with L-701,324 or DCKA. Drug effects were examined in two genetically inbred mouse strains, C57BL/6J and 129S1/SvImJ, that we have previously shown to differ in baseline sensitivity to the intoxicating effects of EtOH and therefore represent differing baseline trait levels of sensitivity to EtOH intoxication (Chen and Holmes, 2009; Chesler et al., 2012) and in EtOH drinking (the present study). Next, to further parse their mechanisms of action on EtOH intoxication, we tested, in C57BL/6J mice, for interactions between glycineB site agents and between glycineB site drugs and the NMDAR blocker MK-801, and assessed whether the effects of D-serine were altered in knockout (KO) mice lacking either the GluN2A subunit or the NMDAR scaffold protein, post-synaptic density 95 (PSD-95). Lastly, we examined the effects of depleting endogenous Magnesium (Mg), a modulator of the NMDAR that behaviorally interacts with the glycineB site (reviewed in Szewczyk et al., 2008).
MATERIALS AND METHODS
Subjects
With the exception of the strain comparison and KO experiments, subjects were male C57BL/6J (hereafter abbreviated B6) and 129S1/SvImJ (hereafter abbreviated S1) mice obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6J was chosen because of its common use in studies of alcohol-related behavior including salient studies from our laboratory on the effects of glutamate receptor manipulations on EtOH behaviors (Boyce-Rustay and Holmes, 2005; Boyce-Rustay and Holmes, 2006a; Palachick et al., 2008). 129S1/SvImJ was chosen based on our previous demonstration that this strain shows relatively high sensitivity to EtOH-induced LORR duration (Chen and Holmes, 2009), as well as this strain’s frequent use as a genetic background for mutants and inclusion as ‘group A’ priority strains in the Mouse Phenome Project, an international effort to provide the biomedical research community with phenotypic data on the most commonly used mouse strains (www.jax.org/phenome). Mice were housed 2/cage in a temperature and humidity controlled vivarium under a 12 h light/dark cycle (lights on 0600 h) with ad libitum access to food and water. All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee and strictly followed the NIH guidelines ‘Using Animals in Intramural Research.’
Confirmation of basal strain differences in basal EtOH intoxication and EtOH drinking
Many of our experiments analyzing the effects glycineB-acting drugs involved both the B6 and S1 inbred strains. Within the context of the current study, we sought to replicate our earlier finding (Chen and Holmes, 2009; Chesler et al., 2012) that these strains differ in sensitivity to EtOH intoxication by comparing B6 and S1 mice for EtOH-induced induced ataxia (using the accelerating rotarod, as previously described Hefner and Holmes, 2007), hypothermia (as previously described Boyce-Rustay et al., 2006) and LORR duration (as previously described Daws et al., 2006). We employed these 3 behavioral assays based on their common use in the literature, particularly in studies using mice as a model species (Crabbe, 2007). In addition, in order to determine whether differences in intoxication sensitivity to EtOH challenge extended to differences in voluntary EtOH drinking, a separate cohort of B6 and S1 mice were tested for 2-bottle EtOH-drinking (as previously described Camp et al., 2011). For details of behavioral procedures, see Supplemental Information.
Stimulation and blockade of glycineB
Effects of the glycineB agonist D-serine (and partial agonist D-cycloserine)
D-serine is a selective and potent glycineB agonist. To test the effects of D-serine treatment on EtOH intoxication, D-serine (Sigma-Aldrich, St. Louis, MO) was dissolved in a saline (0.9% NaCl) solution and injected subcutaneously (s.c.) 30 min before EtOH challenge. B6 and S1 mice were tested for EtOH-induced ataxia (using the accelerating rotarod, as previously described Hefner and Holmes, 2007), hypothermia (as previously described Boyce-Rustay et al., 2006) and LORR duration (as previously described Daws et al., 2006). Doses were 0, 300, 600, and 900 mg/kg and chosen based on previous behavioral studies in mice (Labrie and Roder, 2010).
Given our negative data with D-serine (see Results), two additional experiments were conducted. We have previously found that certain clinically relevant drugs (e.g., topiramate) alter EtOH-induced intoxication in a strain-dependent manner (Chen and Holmes, 2009). Therefore, to extend our analysis of D-serine’s effects, we examined the effects of 900 mg/kg D-serine on EtOH-induced LORR duration in the DBA/2J and BALB/cJ, and for comparison, B6, strains (obtained from The Jackson Laboratory and housed as above).
Next we tested the effects of the partial agonist D-cycloserine in B6 and S1. D-cycloserine (Sigma-Aldrich, St. Louis, MO) was dissolved in a saline (0.9% NaCl) solution and injected i.p. 30 min before EtOH challenge. Mice were tested for EtOH-induced LORR duration. Doses were 0, 3, 15, or 30 mg//kg, and chosen based on previous behavioral effects in mice (Hefner et al., 2008). To extend these data, the effects of 30 mg/kg DCS were also tested in DBA/2J mice.
Effects of the GlyT-1 inhibitors ALX-5407 and NFPS
Extracellular glycine levels are tightly regulated by GlyT-1 (Kinney et al., 2003). ALX-5407 ((R)-N-[3-(4′-fluorophenyl)-3(4′-phenylphenoxy)propyl]sarcosine hydrochloride) is a GlyT-1 inhibitor that has been shown to increase extracellular glycine levels in forebrain (Atkinson et al., 2001) and potentiate NMDAR function via activation of the glycineB site (Chen et al., 2003). To test for effects of boosting endogenous glycine levels on EtOH intoxication, ALX-5407 (Sigma-Aldrich, St. Louis, MO) was dissolved in 80% saline, 10% DMSO, 10% Tween80 and injected i.p. 90 min before EtOH challenge. B6 and S1 mice were tested for EtOH-induced ataxia, hypothermia and LORR duration. Doses were 0, 0.5, 1.0, and 5.0 mg/kg, chosen based on previous behavioral studies in mice (Labrie and Roder, 2010).
We next tested another selective GlyT-1 inhibitor, NFPS (N-[3-(4′-Fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine), that also increases forebrain extracellular glycine levels and potentiates NMDAR-mediated currents (Atkinson et al., 2001). NFPS (Tocris, Ellisville, MO) was dissolved in 90% saline, 10% DMSO and injected i.p. 60 min before EtOH challenge. B6 mice (only) were tested for EtOH-induced LORR duration (only). Doses were 0, 1, 3, 10 mg/kg and chosen based on previous behavioral studies (Kinney et al., 2003).
Effects of the glycineB site antagonists L-701,324 and DCKA
We next tested the consequences of blocking the glycineB site for EtOH intoxication. The glycineB antagonist L-701,324 (7-chloro-4-hydroxy-3(3-phenoxy)phenylquinoline-2-(H)-one) (Sigma-Aldrich, St. Louis, MO) was dissolved in 98% saline, 2% Tween80 and injected i.p. 30 min before EtOH challenge. B6 and S1 mice were tested for EtOH-induced ataxia, hypothermia and LORR duration. Doses were 0, 3, 6, and 9 mg/kg, chosen based on previous behavioral studies in mice (Backstrom and Hyytia, 2006). Due to the possibility of a ‘ceiling effect’ of high basal sensitivity to EtOH in S1 mice precluding detection of a potentiating effect of L-701,324, another cohort of S1 mice was tested in the same manner but with a 3.0 g/kg dose of EtOH.
To determine whether L-701,324 potentiation of EtOH-induced LORR duration was specific to EtOH, we tested another sedative/hypnotic, pentobarbital. A naïve cohort of B6 mice was tested for effects of L-701,324 on pentobarbital-induced LORR duration. Mice were injected with 0 or 9 mg/kg L-701,324 followed, 30 min later, by 30 mg/kg pentobarbital (dose based on previous studies: (Boyce-Rustay et al., 2007; Boyce-Rustay et al., 2008; Hefner and Holmes, 2007) and immediately placed into the supine position in a ‘V’-shaped chamber and assessed for LORR duration.
To extend the analysis of L-701,324 effects on EtOH-induced LORR, we tested another glycineB site antagonist, DCKA (5,7-dichlorokynurenic). DCKA (Ascent Scientific, Princeton, New Jersey) was dissolved in 90% saline, 10% DMSO and injected i.p. 60 min before EtOH challenge. B6 and S1 mice were tested for EtOH-induced LORR duration (only). Doses were 0, 25, 50, 100 mg/kg and chosen based on previous behavioral studies in rats (Kotlinska, 2001a).
Interactions between glycineB-acting drugs
Effects of D-serine and ALX-5407
This experiment tested effects of a combination of direct activation of glycineB, via D-serine treatment, and increasing endogenous glycine levels, via ALX-5407 treatment, on EtOH intoxication. Mice were injected with vehicle or 5 mg/kg ALX-5407 and then, 60 min later, vehicle or 900 mg/kg D-serine. Thirty min later, mice were challenged with 3.5 g/kg EtOH and tested for LORR duration. This and the following drug interaction experiments were limited to the B6 strain.
Effects of D-serine and MK-801
Blockade of the NMDAR with, for example, the uncompetitive NMDAR antagonist MK-801 [(+)-5-methyl-10,11-dihydro-SH-dibenzo[a,d]cyclohepten-5,10-imine maleate] potently potentiates EtOH-induced LORR duration (Palachick et al., 2008). As glycineB stimulates the NMDAR, we tested whether treatment with D-serine would oppose the EtOH-potentiating effects of MK-801. Mice were injected with vehicle or 900 mg/kg D-serine and then immediately injected with vehicle or 0.2 mg/kg MK-801 (an effective potentiating dose, e.g., Palachick et al., 2008). Thirty min later, mice were challenged with 3.5 g/kg EtOH and tested for LORR duration.
Effects of D-serine and L-701,324
Here we sought to confirm that D-serine and L-701,324 were acting at the same site by testing whether treatment with one drug nullified the effects of the other. Mice were injected with vehicle or 9 mg/kg L-701,324 and immediately injected with vehicle or 900 mg/kg D-serine. Thirty min later, mice were challenged with 3.5 g/kg EtOH and tested for LORR duration.
Effects of ALX-5407 and L-701,324
This experiment was designed to confirm that the effects of ALX-5407 were mediated via the actions at glycineB - by antagonizing those effects with L-701,324. Mice were injected with vehicle or 5 mg/kg ALX-5407 and then, 60 min later, vehicle or 9 mg/kg L-701,324. Thirty min later, mice were challenged with 3.5 g/kg EtOH and tested for LORR duration.
To confirm that these drugs altered the pharmacodynamic properties of EtOH, rather than EtOH pharmacokinetics, mice were sacrificed on recovery of the LORR, via cervical dislocation and rapid decapitation, to obtain trunk blood for analysis of blood EtOH concentrations (BECs). BECs were measured using the Analox AM1 Alcohol Analyzer (Analox Instruments USA Inc, Lunenburg, MA).
Effects of D-serine in GluN2A and PSD-95 KO mice
To compliment our pharmacological interactions experiments, we tested whether the effects of D-serine on EtOH intoxication were altered in KO mice with constitute deletion of either GluN2A or PSD-95. GluN2A KO mice were generated as previously described and backcrossed onto a C57BL/6J background for the current study (for details see Boyce-Rustay and Holmes, 2006b). KO and wild-type (WT) littermates were bred, in-house, from heterozygous parents to avoid parental genotype effects (Crusio et al., 2009). PSD-95 KO mice were generated as previously described and backcrossed onto a C57BL/6J background (for details see Camp et al., 2011). KO and WT littermates were bred from heterozygous parents at The Jackson Laboratory and shipped to the NIH for testing. For both KO lines, adult males and females were used. Mice were injected with vehicle or 900 mg/kg D-serine and 30 min later challenged with 3.5 g/kg EtOH and tested for LORR duration.
Effects of Mg depletion
To examine the effects of depleting the NMDAR and glycineB interacting element, Mg (Szewczyk et al., 2008), B6 and S1 mice were fed a Mg-deficient (Zeigler Bros, Inc, Gardners, PA, USA) diet (or constitutional equivalent control diet (Zeigler Bros, Inc), as previously described for Zn (Whittle et al., 2010). Three weeks after the start of feeding, mice were tested in the 3-test EtOH-challenge battery described above. Mice remained on the diet through the completion of testing (i.e., 5 weeks at the time of LORR).
Statistical analysis
Strain differences in EtOH intoxication and drinking, effects of L-701,324 on pentobarbital-induced LORR duration, and effects of Mg depletion ataxia, hypothermia and the LORR were all analyzed via t-tests. Change in latency to fall from the rotarod over training trials was analyzed using repeated measures analysis of variance (ANOVA). The effects of drug treatment on ataxia, hypothermia and the LORR were analyzed using ANOVA, followed by Fisher’s LSD post hoc tests. For the 3-strain D-serine experiment, the effects of strain and drug were analyzed using 2-factor ANOVA. The threshold for statistical significance was p<.05.
RESULTS
Confirmation of basal strain differences in basal EtOH intoxication and EtOH drinking
There was a significant increase in latency to fall across rotarod training trials in B6 and S1 mice (data not shown). Injection of 2.0 g/kg EtOH produced statistically similar ataxia, as measured by negative delta latency to fall, in B6 and S1 mice (B6=54.0 ±11.3, S1=88.7 ±24.0 sec, n=10). Following challenge with 3.5 g/kg EtOH, S1 mice showed a significantly greater hypothermic response than B6 mice (B6=2.69 ±0.32, S1=4.99 ±0.40 °C, n=8–10, t(16)=4.51, p<.01). A 3.5 g/kg EtOH injection produced a LORR response that was significantly longer in duration in S1 than B6 mice (B6=112.2 ±14.7, S1=150.7 ±10.7 min, n=8, t(18)=2.11, p<.05).
Averaged over a 4-day period, B6 consumed significantly more of (B6=4.2 ±0.8, S1=1.0 ±0.2 g/kg/day, n=8, t(14)=4.05, p<.01) and had a significantly greater preference for (B6=47.5 ±9.7, S1=8.8 ±1.8 %, n=8, t(14)=3.93, p<.01) an EtOH containing fluid over water than did S1.
Stimulation and blockade of glycineB
Effects of the glycineB agonist D-serine and partial agonist D-cycloserine
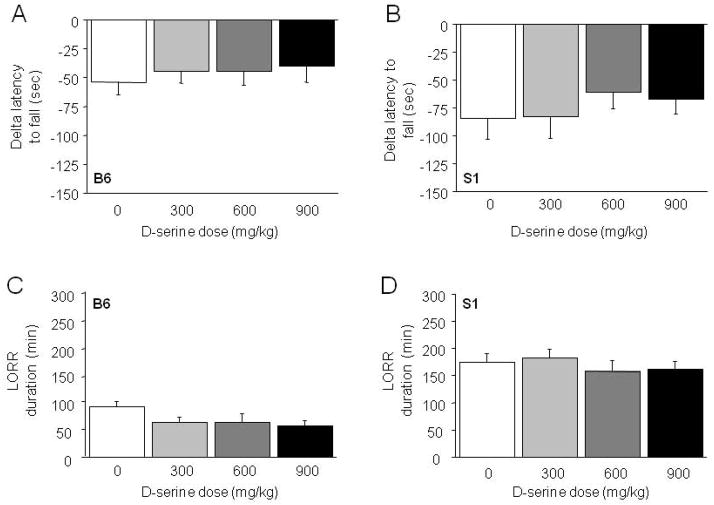
There was a significant increase in latency to fall across rotarod training trials in B6 and S1 mice for this and all experiments. These data will not be described further in the Results (for all data, see Supplemental Figure S1). Injection of 2.0 g/kg EtOH produced ataxia, as measured by negative delta latency to fall, but this was not affected by D-serine pre-treatment in B6 (Figure 1A) or S1 (Figure 1B) mice.
Figure 1. Effects of the glycineB agonist D-serine on EtOH-induced ataxia and LORR duration.
Treatment with D-serine did not alter EtOH-induced ataxia on the accelerating rotarod test in B6 (A) or S1 (B) mice (n=7–9/dose/strain), or EtOH-induced LORR duration in B6 (C) or S1 (D) mice (n=7–8/dose/strain). Data are means ±SEM.
Challenge with 3.5 g/kg EtOH produced a marked hypothermic response in both B6 and S1 mice, but again this effect was not significantly altered by D-serine pre-treatment, although a trend for a lesser response was evident in S1 mice treated with the highest, 900 mg/kg, dose (Figure S2).
On a third measure of EtOH intoxication, 3.5 g/kg EtOH produced a marked sedative/hypnotic response, but pre-treatment with D-serine did not alter this in either B6 (Figure 1C) or S1 (Figure 1D) mice.
Comparing the effects of D-serine in the B6, DBA/2J and BALB/cJ strains revealed no effects of the drug on EtOH-induced LORR duration in any strain, although there was a main effect of strain, driven by relatively longer LORR duration in DBA/2J (F2,58=8.79, p<.01) (Table 1). The glycineB partial agonist, D-cycloserine, failed to affect EtOH-induced LORR duration in B6 or S1 mice (Table 2). The highest dose also failed to affect EtOH-induced LORR duration in DBA/2J mice (vehicle=62.4 ±6.8 min, 30 mg/kg=84.4 ±10.8 min, n=7–8 per dose).
Table 1. Effects of the glycineB agonist D-serine on EtOH-induced LORR duration in B6, DBA/2J or BALB/cJ mice.
Treatment with 900 mg/kg D-serine did not affect EtOH-induced LORR duration in any of 3 strains tested.
| Dose of D-serine (mg/kg) | ||
|---|---|---|
| 0 | 900 | |
| B6 | 56.5 ±8.2 | 58.7 ±10.0 |
| DBA/2J | 103.8 ±15.5 | 85.1 ±10.0 |
| BALB/cJ | 66.1 ±11.6 | 58.6 ±7.0 |
Data are means ±SEM LORR duration (min). n=9–13 per strain, per dose.
Table 2. Effects of the glycineB partial agonist D-cycloserine on EtOH-induced LORR duration in B6 and S1 mice.
Treatment with D-cycloserine did not affect EtOH-induced LORR duration in either B6 or S1 mice.
| Dose of D-cycloserine (mg/kg) | ||||
|---|---|---|---|---|
| 0 | 3 | 15 | 30 | |
| B6 | 83.9 ±15.5 | 91.6 ±18.0 | 80.1 ±12.7 | 86.0 ±9.3 |
| S1 | 144.6 ± 17.8 | 145.8 ± 10.4 | 143.7 ± 13.4 | 162.7 ± 14.4 |
Data are means ±SEM LORR duration (min). n=5–9 per strain, per dose.
Effects of the GlyT-1 inhibitors ALX-5407 and NFPS
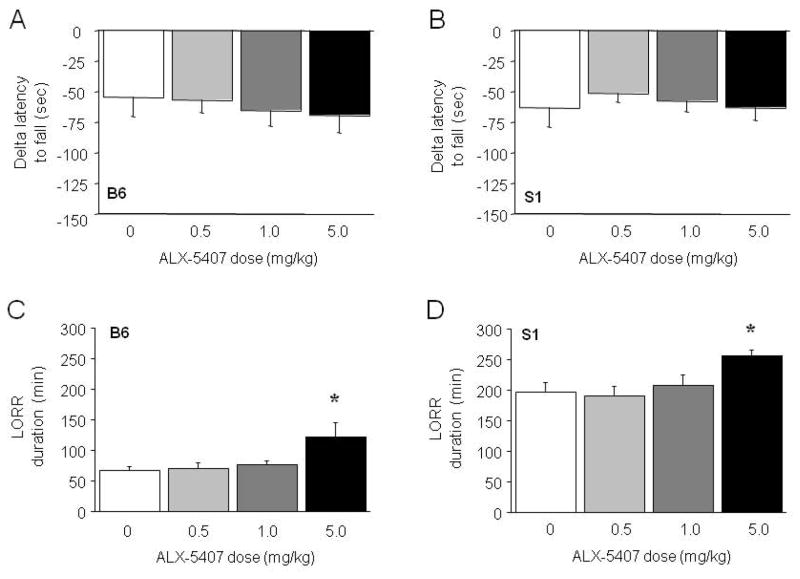
EtOH-induced ataxia and hypothermia were unaffected by pre-treatment with ALX-5407 in either B6 or S1 mice (Figure 2A,B, Figure S2). ALX-5407 pre-treatment significantly affected LORR duration in B6 mice (F3,33=3.68, p<.05) and S1 mice (F3,39=3.19, p<.05). Post hoc tests showed that there was a longer LORR duration after pre-treatment with the highest (5 mg/kg) dose tested, relative to vehicle, in B6 mice (Figure 2C) and S1 mice (Figure 2D).
Figure 2. Effects of the GlyT-1 inhibitor ALX-5407 on EtOH-induced ataxia and LORR duration.
Treatment with ALX-5407 did not alter EtOH-induced rotarod ataxia in B6 (A) or S1 (B) mice (n=7–11/dose/strain). The highest dose of ALX-5407 potentiated (3.5 g/kg) EtOH-induced LORR duration in B6 (C) and S1 (D) mice (n=8–14/dose/strain). Data are means ±SEM. *p<.05 vs. 0 mg/kg
NFPS pre-treatment did not alter EtOH-induced LORR duration in B6 mice as indicated by a main ANOVA effect (vehicle=95.7 ±10.0 min, 1 mg/kg=142.5 ±8.7, 3 mg/kg=122.0 ±20.5 min, 10 mg/kg=98.8 ±17.3 min, n=6 per dose).
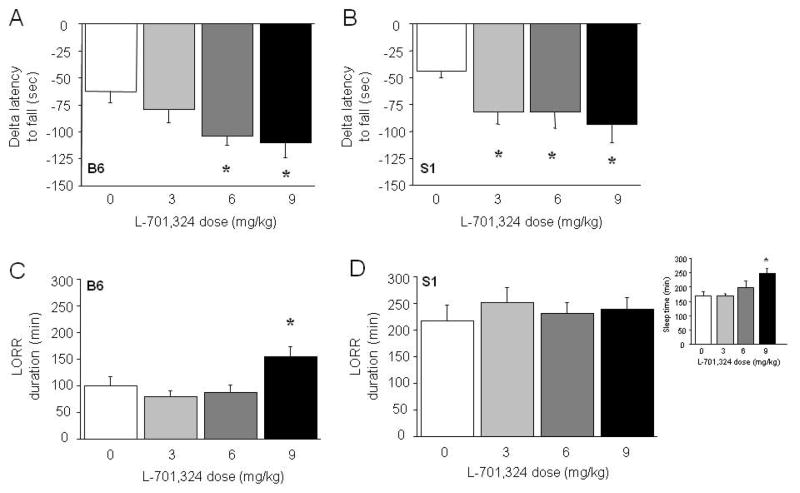
Effects of the glycine site antagonist L-701,324
Pre-treatment with L-701,324 had a significant effect on ataxia in B6 mice (F3,28=2.86, p<.05) and a borderline significant effect on this measure in S1 mice (F3,28=2.86, p=.0547). Planned post hoc tests showed that the 6 and 9 mg/kg doses of L-701,324 significantly potentiated EtOH-induced ataxia in B6 mice (Figure 3A), and all 3 doses did so in S1 mice (Figure 3B). L-701,324 pre-treatment did not affect EtOH-induced hypothermia (Figure S2), but had a significant effect on LORR duration (F3,27=4.77, p<.01). Post hoc tests showed that there was a longer LORR duration after pre-treatment with the highest (9 mg/kg) dose tested, relative to vehicle, in B6 mice (Figure 3C). Although this effect was not seen in S1, this could have been due to a ‘ceiling’ effect due to the high baseline response in this strain (Figure 3D). Indeed, when we tested a separate cohort of S1 treated with a lower (3 g/kg) EtOH dose, S1 had significantly longer LORR duration after treatment with the highest (9 mg/kg) dose, relative to vehicle (F3,32=5.60, p<.05) (Figure 3D, inset).
Figure 3. Effects of the glycineB antagonist L-701,324 on EtOH-induced ataxia and LORR duration.
L-701,324 dose-dependently potentiated the rotarod ataxic effects of EtOH in B6 (A) and S1 (B) mice (n=7–9/dose/strain). The highest dose of L-701,324 potentiated (3.5 g/kg) EtOH-induced LORR duration in B6 (C) but S1 (D) mice (n=7–8/dose/strain). At a lower (3.0 g/kg) EtOH dose, the highest dose of L-701,324 potentiated EtOH-induced LORR duration in S1 (inset) mice (n=7–12/dose). Data are means ±SEM. *p<.05 vs. 0 mg/kg
L-701,324 pre-treatment did not affect pentobarbital-induced LORR duration in B6 mice (Table 3).
Table 3. Effects of the glycineB antagonist L-701,324 on pentobarbital-induced LORR duration in B6 mice.
Treatment with L-701,324 did not affect pentobarbital-induced LORR duration in B6 mice.
| Dose of L-701,324 (mg/kg) | ||
|---|---|---|
| 0 | 9 | |
| LORR duration | 28.6 ±1.0 | 30.0 ±2.8 |
Data are means ±SEM LORR duration (min). n=10 per dose.
DCKA pre-treatment did not affect EtOH-induced LORR duration in either B6 or S1 mice (Table 4).
Table 4. Effects of the glycineB antagonist DCKA on EtOH-induced LORR duration in B6 and S1 mice.
Treatment with DCKA did not affect EtOH-induced LORR duration in B6 or S1 mice.
| Dose of DCKA (mg/kg) | ||||
|---|---|---|---|---|
| 0 | 25 | 50 | 100 | |
| B6 | 107.5 ±20.0 | 104.0 ±12.7 | 103.5 ±13.6 | 128.9 ±15.8 |
| S1 | 167.5 ±9.7 | 167.0 ±12.2 | 175.2 ±16.2 | 166.9 ±31.5 |
Data are means ±SEM LORR duration (min). n=7–13 per strain per dose.
Interactions between glycineB-acting drugs
Effects of D-serine and ALX-5407
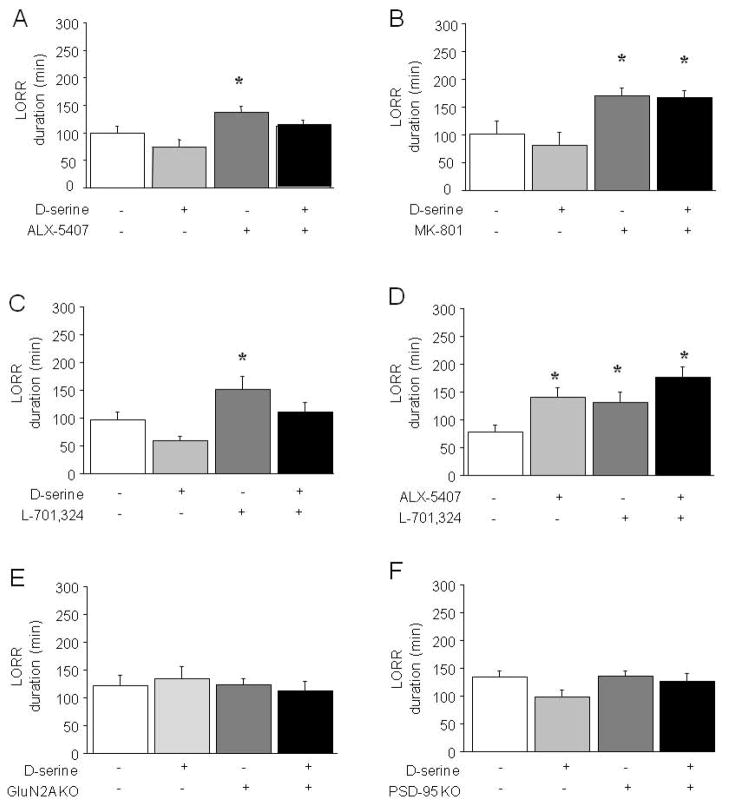
There was a significant effect of drug treatment on LORR duration in B6 mice (F3,33=4.66, p<.01). Post hoc tests showed that ALX-5407 alone increased LORR duration, relative to vehicle, and that combined ALX-5407 + D-serine treatment, or D-serine alone, did not increase LORR duration relative to vehicle (Figure 4A).
Figure 4. Effects of D-serine, MK-801, ALX-5407 and L-701,324 combinations on EtOH-induced LORR duration in B6 mice, and effects of D-serine in GluN2A and PSD-95 KO mice.
(A) ALX-5407 administered alone potentiated EtOH-induced LORR duration in B6 mice and D-serine blocked this effect (n=9–14/treatment). (B) The NMDAR antagonist MK-801 potentiated EtOH-induced LORR duration in B6 mice irrespective of co-treatment with D-serine, and D-serine alone had no effect (n=8/treatment). (C) L-701,324 potentiated EtOH-induced LORR duration in B6 mice and D-serine blocked this effect (n=8–10/treatment). (D) L-701,324, ALX-5407 and their co-treatment potentiated EtOH-induced LORR duration in B6 mice (n=14–16/treatment). (E) D-serine did not alter EtOH-induced LORR duration in GluN2A KO mice or WT controls (n=12–14/treatment). (F) D-serine did not alter EtOH-induced LORR duration in PSD-95 KO mice or WT controls (n=8–21/treatment). Data are means ±SEM. *p<.05 vs. vehicle/vehicle
Effects of D-serine and MK-801
There was a significant effect of treatment on LORR duration in B6 mice (F3,28=5.03, p<.01). Post hoc tests showed that MK-801 increased LORR duration relative to vehicle, irrespective of D-serine co-treatment (Figure 4B).
Effects of D-serine and L-701,324
There was a significant effect of treatment on LORR duration in B6 mice (F3,42=3.84, p<.05). Post hoc tests showed that L-701,324 treatment increased LORR duration as compared to vehicle, and that D-serine blocked this effect (Figure 4C).
Effects of ALX-5407 and L-701,324
There was a significant effect of drug treatment on LORR duration in B6 mice (F3,57=5.14, p<.01). Post hoc tests revealed that treatment with ALX-5407 alone, L-701,324 alone, and in combination increased LORR duration relative to vehicle (Figure 4D).
There was a significant effect of drug treatment on BECs at recovery of the LORR in this experiment (F3,44=2.86, p<.05). Post hoc tests revealed that on recovery, mice treated with L-701,324 alone or the combination of L-701,324 and ALX-5407, but not ALX-5407 alone, had significantly lower BECs than vehicle treated controls (vehicle=350.1 ±9.2 mg/dL, L-701,324=310.5 ±18.6, ALX-5407=330.4 ±11.6, L-701,324+ALX-5407=298.5 ±12.3, n=12 per treatment).
Effects of D-serine in GluN2A KO and PSD-95 KO mice
There was no significant effect of drug treatment or genotype (or interaction between the two) on LORR duration in either the GluN2A KO (Figure 4E) or PSD-95 KO (Figure 4F) mutant lines.
Effects of Mg depletion
B6 and S1 mice fed a Mg deficient diet had lower plasma levels of Mg than mice fed a control diet (control diet=1.00 ±0.1, Mg deficient diet=0.65 ±0.1, n=4–8, Fisher’s test=p<.05). B6 and S1 mice showed improved rotarod performance with training that was unaffected by Mg depletion (data not shown). In B6 mice, there was no effect of diet on EtOH-induced ataxia or EtOH-induced hypothermia (Table 5), but there was a significant effect of diet on EtOH-induced LORR duration (due to higher scores in Mg deficient mice than non-depleted controls) (t(25)=2.10, p<.05) (Table 5). There were not effects of diet on any measure in S1 mice (Figure S3D-F), with the caveat that approximately 75% of the S1 sample died during the course of treatment and testing, apparently due to seizures.
Table 5. Effects of Mg depletion on EtOH-related behaviors.
In B6 mice, dietary depletion of Mg significantly increased EtOH-induced LORR duration relative to control diet, but did not significantly affect EtOH-induced ataxia or EtOH-induced hypothermia (n=13–15/treatment). In S1 mice, dietary depletion of Mg did not significantly affect any measure of EtOH intoxication, but approximately 75% of S1 mice died as a result of Mg treatment (n=4–16/treatment).
| B6 | S1 | |||
|---|---|---|---|---|
| Control diet | Mg deficient diet | Control diet | Mg deficient diet | |
| Ataxia | 69.6 ±7.0 | 58.5 ±11.3 | 66.1 ±6.1 | 39.3 ±16.1 |
| Hypothermia | 2.8 ±0.3 | 2.5 ±0.4 | 3.9 ±0.2 | 3.7 ±0.3 |
| LORR duration | 66.9 ±10.2 | * 97.4 ±9.5 | 179.8 ±9.8 | 126.0 ±35.4 |
Data are Means ±SEM.
p<.05 vs. Control diet
DISCUSSION
There has been increasing interest in targeting the glycineB site to facilitate NMDAR function as a therapeutic approach for neuropsychiatric disorders in which the NMDAR is implicated, including anxiety disorders (Richardson et al., 2004), schizophrenia (Labrie and Roder, 2010) and alcoholism (Olive et al., 2005; Vengeliene et al., 2008a). The aim of the current study was to investigate the effects of glycineB site modulation on measures of acute EtOH intoxication in mice. Results showed that directly activating the glycineB site did not alter various measures of EtOH intoxication, while blocking the glycineB site potentiated intoxication. The GlyT-1 blockers ALX-5407 and NFPS also, in this case unexpectedly, potentiated EtOH intoxication. Drug interaction experiments demonstrated that the combination of D-serine activation and GlyT-1 blockade was still not sufficient to alter intoxication, while D-serine opposed the effects of the glycineB antagonist, but not the GlyT-1 blocker or a NMDAR antagonist.
The main hypothesis underlying the current experiments was that, by virtue of allosteric enhancement of NMDAR function (Parsons et al., 1998), pharmacologically stimulating the glycineB site could counter intoxicating effects of EtOH that are attributable, in part, to inhibition of NMDAR function. However, we found no clear evidence of reductions in three measures of intoxication (ataxia, hypothermia, LORR duration) following systemic treatment with D-serine at dose-ranges previously shown to alter other behaviors in mice (reviewed in Labrie and Roder, 2010). Negative findings for D-serine were replicated in two inbred mouse strains, B6 and S1. These strains differ in sensitivity to the sedative/hypnotic effects of EtOH, with S1 being relatively more sensitive, as shown previously (Chen and Holmes, 2009; Chesler et al., 2012) and replicated here. The use of these two strains thereby widens the range of basal scores and reduces the likelihood that the inability of D-serine to reduce intoxication was a false negative due to a ‘floor effect.’ Indeed, we confirmed the absence of D-serine effects on one measure, EtOH-induced LORR duration, in two other inbred strains, DBA/2J and BALB/cJ. Finally, we also found null effects of the glycineB partial agonist, DCS, EtOH-induced LORR duration in B6, S1 and DBA/2J.
Our interaction experiments indicated that combined treatment with D-serine and the GlyT-1 inhibitor ALX-540, expected to activate the glycineB site more than either drug alone, was still insufficient to affect intoxication. These experiments also showed that clear effects of D-serine were not unmasked by gene KO of either the modulatory NMDAR subunit, GluN2A, or the NMDAR scaffold protein, PSD-95, both of which we have previously shown to play a role in certain measures of EtOH intoxication (Boyce-Rustay and Holmes, 2006a; Camp et al., 2011), albeit at lower doses than tested here. Finally, D-serine was unable to oppose potentiation of EtOH-induced LORR duration by the NMDAR antagonist MK-801. Taken together, these data demonstrate a failure of glycineB stimulation to lessen the intoxicating effects of EtOH across a range of measures, strains and experimental conditions. On the other hand, glycineB stimulation was not wholly without effects. D-serine treatment blocked the ability of both the glycineB antagonist L-701,324 and the GlyT-1 inhibitor ALX-5407 to potentiate EtOH-induced LORR. This indicates that D-serine was not inert under our experimental conditions.
Although ALX-5407 is expected to boost extracellular serine/glycine levels (Atkinson et al., 2001), the highest dose of this drug (as well as at least one dose of another GlyT-1 inhibitor, NFPS) paradoxically potentiated EtOH’s sedative/hypnotic effects when given alone. One explanation for these effects is that they stemmed from actions extraneous to the glycineB site, most notably at strychnine-sensitive glycine receptors. The failure of the glycineB antagonism to prevent the effects of ALX-5407 would be consistent with such an ‘off-target’ action. However, we also found that BECs at recovery of the LORR were no different between vehicle- and ALX-5407-treated mice, despite the latter group having a significantly longer LORR duration. BECs are expected to be lesser the longer the LORR duration, as EtOH is cleared from the blood (a negative relationship that we observed for L-701,324 treatment). The fact that such a relationship was not found, leaves open the possibility that ALX-5407 potentiated LORR duration by interfering with the pharmacokinetics of EtOH, rather than by altering brain NMDAR function. Thus, EtOH-related effects produced by systemically blocking GlyT-1, at least with the compounds tested here remain to be clarified by additional studies (e.g., involving intracranial infusions).
Our largely negative findings with glycineB stimulations on acute EtOH sensitivity do not rule out the possibility that there would different effects of these same manipulations on other EtOH-related behaviors. Indeed, previous studies have shown that the glycineB partial agonists DCS or the ACPC GlyT-1 inhibitor Org 25935 significantly alters EtOH-induced anxiolytic-like effects and ataxia-tolerance, basal or deprivation-induced EtOH and certain conditioned responses to EtOH (Groblewski et al., 2009; Khanna et al., 1993; Khanna et al., 1995; Molander et al., 2007; Moraes Ferreira and Morato, 1997; Stromberg et al., 1999; Vengeliene et al., 2008b). These earlier findings, together with the current data, suggest a tentative conclusion that glycineB agonists and partial agonists can modify specific EtOH-related behaviors, but do not have clear effects on acute intoxication. This conclusion would be consistent with a recent clinical study showing that orally administered DCS was mildly sedative and memory impairing when given alone, but did not alter EtOH’s stimulatory, sedative or euphoric effects (Trevisan et al., 2008). An important caveat to both this finding and our study in mice is that non-alcoholic/non-dependent subjects were examined. An important question for future studies will be whether a different pattern of results emerges in alcoholic dependent subjects.
While we did not observe reductions in EtOH intoxication with glycineB agonists/partial agonists, treatment with the glycineB antagonist, L-701,324, potentiated both EtOH-induced ataxia and LORR duration at the highest dose (9 mg/kg) tested in B6 and (with a lower EtOH dose) S1 mice. A similar EtOH-potentiating profile is seen with blockade of the NMDAR itself, as shown here and in previous studies utilizing NMDAR blockers (e.g., Chen and Holmes, 2009; Meyer and Phillips, 2003). Because the action of glycine at the glycineB site is necessary for NMDAR activation, glycineB antagonism with L-701,324 could mimic this effect of NMDAR blockers by effectively attenuating NMDAR function. Generally in support of this scheme are electrophysiological studies showing that, for example, glycine opposes EtOH inhibition of NMDAR currents (e.g., Peoples and Stewart, 2000; Popp et al., 1999; Woodward et al., 1991). A general qualification here is that interpretation of the EtOH-potentiating effect of a glycineB or NMDAR antagonist is that it is not possible to dissociate an increase in ‘sensitivity’ from a reduction in acute functional tolerance from measurement of LORR duration.
Interestingly, L-701,324’s effects in B6 mice were blocked by co-treatment with D-serine, but not ALX-5407, possibly indicating an action of L-701,324 at the glycineB site that is ‘competed out’ by the direct agonist. Our observation of EtOH-potentiating effects of L-701,324 extend earlier evidence that the drug attenuates deprivation-induced EtOH drinking and CPP in rats (Biala and Kotlinska, 1999; Vengeliene et al., 2005). This also helps frame the negative effects of D-serine and DCS in the current study by demonstrating that pharmacological modulation of the glycineB site can alter acute intoxication on the measures employed herein. It is not, however, clear why glycineB drugs were able to modulate intoxication in one direction but not the other. One possibility is that under conditions of EtOH intoxication, glycine is at levels that saturate the glycineB site. This would render further stimulation ineffective but account for the ability of the antagonist to augment intoxication (i.e., via blockade glycine-mediated enhancement of NMDAR function). However, this scheme remains speculative in the absence of additional evidence, such as direct measurement of glycine levels. It may also be incongruent with the null effects of DCS, because at site-saturating glycine levels, DCS acts more like a glycineB antagonist in terms of reducing NMDAR function (Emmett et al., 1991; Hood et al., 1989; Watson et al., 1990) and would, therefore, be expected to mimic the effects of L-701,324. Clearly, further studies are needed to fully elucidate these actions.
Additional work will also be needed to clarify the potential functional link between Mg on glycine/D-serine modulation of EtOH sensitivity. Mg blocks the NMDAR channel to inhibit receptor function and, as such, a reduction in endogenous levels of brain Mg might be predicted to mimic the effects of stimulating glycineB site. Our preliminary analysis showed that Mg depletion potentiated LORR duration in B6 mice, providing some of the first evidence to our knowledge that depleting Mg affects sensitivity to EtOH intoxication. Testing for similar effects in S1 mice was compromised by the lethal effects of Mg depletion in this strain, apparently (from informal observations) from seizures. This is in and of itself an interesting observation given hypomagnesemia is found in epileptic patients (Dharnidharka and Carney, 2005). Previous work has shown D-serine treatment can reverse antidepressant-related effects produced by Mg (reviewed in Szewczyk et al., 2008). This indicates functional interactions between these two regulators of NMDAR function and suggests that studies testing the interrelationship between Mg and the glycineB site in mediating EtOH intoxication may be informative.
In conclusion, the results of the current study do not show a major modulatory effect of drugs stimulating the glycineB co-agonist site on measures of acute EtOH intoxication in non-dependent mice. On the other hand, glycineB blockade was sufficient to potentiate EtOH intoxication, suggesting a functional contribution of endogenous actions at the site. Depletion of either Zn or Mg, elements that interact with glycineB and the NMDAR, did not alter basal sensitivity EtOH intoxication or the (negative) effects of glycineB stimulation. These preclinical data are in line with preliminary clinical evidence that D-cycloserine did not alter EtOH’s subjective effects in non-alcoholic volunteers (Trevisan et al., 2008) and, together, do not support glycineB as a mechanism for modulating intoxication, at least in non-dependent subjects. On the other hand, these negative effects on acute intoxication suggest that treatment with these drugs for other neuropsychiatric disorders, such as schizophrenia and anxiety (Davis et al., 2006; Labrie and Roder, 2010), may not be contra-indicated with alcohol drinking.
Supplementary Material
Acknowledgments
We are very grateful to Claudia Schmuckermair and Nicolas Singewald for performing the Mg plasma measurements and to Adron Harris for comments on an earlier version of the manuscript.
Support: Research supported by the National Institute of Alcohol Abuse and Alcoholism Intramural Research Program (Z01-AA000411). SGNG was supported by the Wellcome Trust Genes to Cognition Programme Grant and European Union 7th Framework Programme under grant agreements n° HEALTH-F2-2009-242167 and HEALTH-F2-2009-241498 (“SynSYS” and “EUROSPIN” projects).
References
- Atkinson BN, Bell SC, De Vivo M, Kowalski LR, Lechner SM, Ognyanov VI, Tham CS, Tsai C, Jia J, Ashton D, Klitenick MA. ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol Pharmacol. 2001;60:1414–1420. doi: 10.1124/mol.60.6.1414. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Biala G, Kotlinska J. Blockade of the acquisition of ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol. 1999;34:175–182. doi: 10.1093/alcalc/34.2.175. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Danysz W, Kostowski W. Study on the role of glycine, strychnine-insensitive receptors (glycineB sites) in the discriminative stimulus effects of ethanol in the rat. Alcohol. 1998;15:87–91. doi: 10.1016/s0741-8329(97)00103-1. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Stefanski R, Kostowski W. Discriminative stimulus effects of ethanol: lack of antagonism with N-methyl-D-aspartate and D-cycloserine. Alcohol. 1997;14:345–350. doi: 10.1016/s0741-8329(96)00181-4. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Functional roles of NMDA receptor NR2A and NR2B subunits in the acute intoxicating effects of ethanol in mice. Synapse. 2005;56:222–225. doi: 10.1002/syn.20143. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 2006a;187:455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006b;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Palachick B, Hefner K, Chen YC, Karlsson RM, Millstein RA, Harvey-White J, Holmes A. Desipramine potentiation of the acute depressant effects of ethanol: modulation by alpha2-adrenoreceptors and stress. Neuropharmacology. 2008;55:803–811. doi: 10.1016/j.neuropharm.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Camp MC, Feyder M, Ihne J, Palachick B, Hurd B, Karlsson RM, Noronha B, Chen YC, Coba MP, Grant SG, Holmes A. A novel role for PSD-95 in mediating ethanol intoxication, drinking and place preference. Addict Biol. 2011;16:428–439. doi: 10.1111/j.1369-1600.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Muhlhauser M, Yang CR. Glycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol. 2003;89:691–703. doi: 10.1152/jn.00680.2002. [DOI] [PubMed] [Google Scholar]
- Chen YC, Holmes A. Effects of topiramate and other anti-glutamatergic drugs on the acute intoxicating actions of ethanol in mice: modulation by genetic strain and stress. Neuropsychopharmacology. 2009;34:1454–1466. doi: 10.1038/npp.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Plitt A, Fisher D, Hurd B, Lederle L, Bubier JA, Kiselycznyk C, Holmes A. Quantitative trait loci for sensitivity to ethanol intoxication in a C57BL/6J x 129S1/SvImJ inbred mouse cross. Mamm Genome. 2012 Feb 28; doi: 10.1007/s00335-012-9394-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Current Protocols in Neuroscience. Wiley; 2007. Overview of Mouse Assays of Ethanol Intoxication. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharnidharka VR, Carney PR. Isolated idiopathic hypomagnesemia presenting as aphasia and seizures. Pediatr Neurol. 2005;33:61–65. doi: 10.1016/j.pediatrneurol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Emmett MR, Mick SJ, Cler JA, Rao TS, Iyengar S, Wood PL. Actions of D-cycloserine at the N-methyl-D-aspartate-associated glycine receptor site in vivo. Neuropharmacology. 1991;30:1167–1171. doi: 10.1016/0028-3908(91)90161-4. [DOI] [PubMed] [Google Scholar]
- Fadda E, Danysz W, Wroblewski JT, Costa E. Glycine and D-serine increase the affinity of N-methyl-D-aspartate sensitive glutamate binding sites in rat brain synaptic membranes. Neuropharmacology. 1988;27:1183–1185. doi: 10.1016/0028-3908(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Lattal KM, Cunningham CL. Effects of D-cycloserine on extinction and reconditioning of ethanol-seeking behavior in mice. Alcohol Clin Exp Res. 2009;33:772–782. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood WF, Compton RP, Monahan JB. D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Shah G, Chau A. Effect of D-cycloserine on rapid tolerance to ethanol. Pharmacol Biochem Behav. 1993;45:983–986. doi: 10.1016/0091-3057(93)90152-j. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Morato GS, Chau A, Shah G. D-cycloserine enhances rapid tolerance to ethanol motor incoordination. Pharmacol Biochem Behav. 1995;52:609–614. doi: 10.1016/0091-3057(95)00149-q. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Sur C, Burno M, Mallorga PJ, Williams JB, Figueroa DJ, Wittmann M, Lemaire W, Conn PJ. The glycine transporter type 1 inhibitor N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine potentiates NMDA receptor-mediated responses in vivo and produces an antipsychotic profile in rodent behavior. J Neurosci. 2003;23:7586–7591. doi: 10.1523/JNEUROSCI.23-20-07586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinska J. Attenuation of morphine dependence and withdrawal by glycine B site antagonists in rats. Pharmacol Biochem Behav. 2001a;68:157–161. doi: 10.1016/s0091-3057(00)00443-3. [DOI] [PubMed] [Google Scholar]
- Kotlinska J. NMDA antagonists inhibit the development of ethanol dependence in rats. Pol J Pharmacol. 2001b;53:47–50. [PubMed] [Google Scholar]
- Kotlinska J, Liljequist S. Oral administration of glycine and polyamine receptor antagonists blocks ethanol withdrawal seizures. Psychopharmacology (Berl) 1996;127:238–244. [PubMed] [Google Scholar]
- Kotlinska J, Liljequist S. The NMDA/glycine receptor antagonist, L-701,324, produces discriminative stimuli similar to those of ethanol. Eur J Pharmacol. 1997;332:1–8. doi: 10.1016/s0014-2999(97)01069-8. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grinenko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Labrie V, Roder JC. The involvement of the NMDA receptor D-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci Biobehav Rev. 2010;34:351–372. doi: 10.1016/j.neubiorev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Bivalent effects of MK-801 on ethanol-induced sensitization do not parallel its effects on ethanol-induced tolerance. Behav Neurosci. 2003;117:641–649. doi: 10.1037/0735-7044.117.3.641. [DOI] [PubMed] [Google Scholar]
- Molander A, Lido HH, Lof E, Ericson M, Soderpalm B. The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol. 2007;42:11–18. doi: 10.1093/alcalc/agl085. [DOI] [PubMed] [Google Scholar]
- Moraes Ferreira VM, Morato GS. D-cycloserine blocks the effects of ethanol and HA-966 in rats tested in the elevated plus-maze. Alcohol Clin Exp Res. 1997;21:1638–1642. [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Palachick B, Chen YC, Enoch AJ, Karlsson RM, Mishina M, Holmes A. Role of major NMDA or AMPA receptor subunits in MK-801 potentiation of ethanol intoxication. Alcohol Clin Exp Res. 2008;32:1479–1492. doi: 10.1111/j.1530-0277.2008.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Hesselink M, Hartmann S, Lorenz B, Wollenburg C, Quack G. Modulation of NMDA receptors by glycine--introduction to some basic aspects and recent developments. Amino Acids. 1998;14:207–216. doi: 10.1007/BF01345264. [DOI] [PubMed] [Google Scholar]
- Peoples RW, Stewart RR. Alcohols inhibit N-methyl-D-aspartate receptors via a site exposed to the extracellular environment. Neuropharmacology. 2000;39:1681–1691. doi: 10.1016/s0028-3908(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Popp RL, Lickteig RL, Lovinger DM. Factors that enhance ethanol inhibition of N-methyl-D-aspartate receptors in cerebellar granule cells. J Pharmacol Exp Ther. 1999;289:1564–1574. [PubMed] [Google Scholar]
- Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by D-cycloserine: theoretical and clinical implications. Learn Mem. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Volpicelli JR, O’Brien CP, Mackler SA. The NMDA receptor partial agonist, 1-aminocyclopropanecarboxylic acid (ACPC), reduces ethanol consumption in the rat. Pharmacol Biochem Behav. 1999;64:585–590. doi: 10.1016/s0091-3057(99)00121-5. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Poleszak E, Sowa-Kucma M, Siwek M, Dudek D, Ryszewska-Pokrasniewicz B, Radziwon-Zaleska M, Opoka W, Czekaj J, Pilc A, Nowak G. Antidepressant activity of zinc and magnesium in view of the current hypotheses of antidepressant action. Pharmacol Rep. 2008;60:588–589. [PubMed] [Google Scholar]
- Trevisan L, Petrakis IL, Pittman B, Gueorguieva R, D’Souza DC, Perry E, Limoncelli D, Krystal JH. Absence of significant interactive effects of high-dose D-cycloserine and ethanol in healthy human subjects: preliminary insights into ethanol actions at the glycine B site of NMDA glutamate receptors. Alcohol Clin Exp Res. 2008;32:36–42. doi: 10.1111/j.1530-0277.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008a;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Kiefer F, Spanagel R. D-cycloserine facilitates extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol. 2008b;43:626–629. doi: 10.1093/alcalc/agn067. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Leonardi-Essmann F, Sommer WH, Marston HM, Spanagel R. Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol Psychiatry. 2010;68:704–711. doi: 10.1016/j.biopsych.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Vyklicky L, Jr, Benveniste M, Mayer ML. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990;428:313–31. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GB, Bolanowski MA, Baganoff MP, Deppeler CL, Lanthorn TH. D-cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Res. 1990;510:158–160. doi: 10.1016/0006-8993(90)90745-w. [DOI] [PubMed] [Google Scholar]
- Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J Neurosci. 2010;30:13586–13596. doi: 10.1523/JNEUROSCI.0849-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ, Brown L, Gonzales RA. Modulation of ethanol-induced inhibition of N-methyl-D-aspartate-stimulated neurotransmitter release by glycine. Alcohol Alcohol Suppl. 1991;1:177–80. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.