Abstract
Objective
To prospectively evaluate the clinical course of patients with severe aortic stenosis (AS) and identify factors associated with treatment selection and patient outcome.
Methods
Patients diagnosed with severe AS in the Rotterdam area were included between June 2006 and May 2009. Patient characteristics, echocardiogram, brain natriuretic peptide (NT-proBNP), and treatment strategy were assessed at baseline, and after 6, 12, and 24 months. Endpoints were aortic valve replacement (AVR) / transcatheter aortic valve implantation (TAVI) and death.
Results
The study population comprised 191 patients, 132 were symptomatic and 59 asymptomatic at study entry. Two-year cumulative survival of symptomatic patients was 89.8 % (95 % CI 79.8–95.0 %) after AVR/TAVI and 72.6 % (95 % CI 59.7–82.0 %) with conservative treatment. Two-year cumulative survival of asymptomatic patients was 91.5 % (95 % CI 80.8–96.4 %). Two-year cumulative incidence of AVR/TAVI was 55.9 % (95 % CI 47.5–63.5 %) in symptomatic patients. Sixty-eight percent of asymptomatic patients developed symptoms, median time to symptoms was 13 months; AVR/TAVI cumulative incidence was 38.3 % (95 % CI 23.1–53.3 %). Elderly symptomatic patients with multiple comorbidities were more likely to receive conservative treatment.
Conclusions
In contemporary Dutch practice many symptomatic patients do not receive invasive treatment of severe AS. Two-thirds of asymptomatic patients develop symptoms within 2 years, illustrating the progressive nature of severe AS. Treatment optimisation may be achieved through careful individualised assessment in a multidisciplinary setting.
Keywords: Aortic valve stenosis, Clinical course, Aortic valve replacement
Introduction
The prevalence of calcified aortic stenosis (AS) increases with the ageing of the population, and represents a growing health burden [1, 2]. According to the current ESC and ACC/AHA guidelines, aortic valve replacement (AVR) is indicated in patients with severe symptomatic AS [3, 4]. Even elderly patients with multiple comorbidities are usually eligible for AVR, and if surgery is not an option, transcatheter aortic valve implantation (TAVI) is often feasible [5, 6]. Nevertheless, at least one third of patients with symptomatic AS do not undergo AVR although they have a clear indication [7–10]. Advanced age, poor left ventricular function, and comorbidities are common reasons for non-referral for AVR [8, 9, 11–13].
The aim of this study was to prospectively evaluate the clinical course of patients with severe AS in contemporary Dutch practice and identify factors associated with treatment selection and patient outcome. This information may facilitate treatment optimisation.
Methods
Patient population
The Aortic VAlve RIJNmond (AVARIJN) Study is a multicentre prospective cohort study of patients diagnosed with severe AS in seven Cardiology clinics in the wider Rijnmond area between June 2006 and May 2009. Patients 18 years and older were included if they met one of the following echocardiographic criteria: aortic valve area (AVA) ≤1 cm2, peak transaortic jet velocity (Vmax) ≥4 m/s, or aortic valve / left ventricular outflow tract velocity time integral ratio ≥4. The study protocol was approved by the medical ethics committee of Erasmus University Medical Center (MEC 2006-066); all patients provided written informed consent.
Patient characteristics, i.e. medical history, cardiovascular risk factors, symptomatic status defined as presence of dyspnoea, angina, and/or syncope at study entry [3, 4], echocardiographic data including Vmax, peak and mean aortic gradient, AVA, left ventricular ejection fraction, and low-flow/low-gradient AS (mean aortic gradient <30 mmHg and an AVA <1.0 cm2), brain natriuretic peptide (NT-proBNP), and treatment strategy (conservative or either AVR or TAVI) were assessed at baseline, and after 6, 12, and 24 months. Expected operative risk was calculated using the logistic EuroSCORE and the Society of Thoracic Surgeons’ risk model (www.euroscore.org; www.sts.org). Asymptomatic patients were invited for exercise testing at baseline; a positive exercise test outcome was defined according to the ACC/AHA guidelines [14]. Patients with a positive test stayed in the asymptomatic group.
Treatment strategies were retrieved from the patients’ medical charts. Study endpoints were AVR or TAVI and all-cause death, which were documented using the hospital information systems or information obtained through the treating physicians.
Statistical analysis
Continuous data are presented as mean (SD) or median (interquartile range) and for comparison between groups the unpaired t-test or Mann-Whitney U test was used. Categorical data are presented as counts and proportions, and comparison was done with the Chi-square test.
Kaplan-Meier analysis was used to assess patient survival and cumulative incidence of AVR/TAVI. Patient follow-up started at enrolment and ended at time of death (event), completion of study, or when the patient was lost to follow-up (censoring).
Logistic regression was used to evaluate the association between baseline characteristics and conservative treatment strategy. Cox proportional hazards analysis was used to analyse time-related events. Missing values were imputed by the mean. Univariable predictors with a p-value ≤0.05 were entered into the multivariable model using the enter method. In case of correlation between potential predictors, the potential predictor that was considered clinically most relevant was selected for the multivariable model. Age, male gender, smoking, hypertension, diabetes, dyslipidaemia, chronic obstructive pulmonary disease, carotid disease, stroke, peripheral arterial disease, previous myocardial infarction, coronary artery disease, renal failure, symptomatic status, body mass index, body surface area, systolic and diastolic blood pressure, NT-proBNP, Vmax, AVAi (indexed by body surface area), left ventricular ejection fraction, left ventricular hypertrophy (on electrocardiography), ischaemia (on electrocardiography), and aortic and mitral regurgitation ≥ grade II were considered as co-variables in the models (definitions in the Appendix). All statistical tests were two-sided and a p-value ≤0.05 was considered significant. Statistical analyses were performed using SPSS for Windows, version 15 (SPSS Inc, Chicago, Illinois) and GraphPad Prism 5 for Windows (GraphPad Software, San Diego, California).
Results
The study population consisted of 191 patients with severe AS, of whom 132 were symptomatic and 59 were asymptomatic at study entry (Table 1).
Table 1.
Patient characteristics at baseline differentiated by symptomatic status
| All | Symptomatic | Asymptomatic | P-value | |
|---|---|---|---|---|
| N = 191 | N = 132 | N = 59 | ||
| Age (yrs) | 72.6 (63.7–78.6) | 74.0 (64.4–79.2) | 69.9 (61.6–76.4) | 0.034 |
| Male gender (%) | 62 | 56 | 76 | 0.008 |
| Previous valve surgery (%) | 1 | 2 | 0 | 0.343 |
| Previous CABG (%) | 6 | 8 | 3 | 0.272 |
| Smoking (%) | 61 | 56 | 71 | 0.049 |
| Hypertension (%) | 52 | 54 | 49 | 0.554 |
| Diabetes (%) | 20 | 19 | 22 | 0.622 |
| Dyslipidaemia (%) | 49 | 49 | 47 | 0.820 |
| COPD (%) | 17 | 20 | 10 | 0.083 |
| PAD (%) | 13 | 15 | 7 | 0.108 |
| History of MI (%) | 13 | 15 | 8 | 0.207 |
| Stroke (%) | 19 | 18 | 20 | 0.725 |
| Vmax (m/s) | 4.3 ± 0.8 | 4.3 ± 0.8 | 4.2 ± 0.7 | 0.693 |
| AVA (cm2) | 0.74 (0.59–0.91) | 0.72 (0.54–0.85) | 0.80 (0.63–0.96) | 0.026 |
| LVEF (%) | 61 ± 7 | 61 ± 7 | 62 ± 6 | 0.129 |
| Low flow/low gradient AS (%) | 13 | 15 | 8 | 0.207 |
| AR grade ≥ II (%) | 17 | 18 | 14 | 0.494 |
| MR grade ≥ II (%) | 11 | 15 | 4 | 0.025 |
| LVH (%) | 27 | 28 | 24 | 0.445 |
| NT-proBNP (pmol/l) | 50 (22–153) | 89 (29–180) | 31 (13–74) | <0.001 |
| Logistic EuroSCORE (%) | 5.4 (3.1–8.2) | 6.2 (3.9–9.6) | 4.0 (2.1–6.9) | <0.001 |
| STS score (%) | 4.5 (2.8–7.6) | 5.1 (3.3–8.0) | 3.8 (2.0–6.0) | 0.002 |
CABG coronary artery bypass graft, COPD chronic obstructive pulmonary disease, PAD peripheral arterial disease, MI myocardial infarction, Vmax peak transaortic jet velocity, AVA aortic valve area, LVEF left ventricular ejection fraction, AS aortic stenosis, AR aortic regurgitation, MR mitral regurgitation, LVH left ventricular hypertrophy, NT-proBNP N-terminal pro-brain natriuretic peptide, STS Society of Thoracic Surgeons. Normal distributed variables: mean ± standard deviation; skewed distributed variables: median (interquartile range 25 and 75 %).
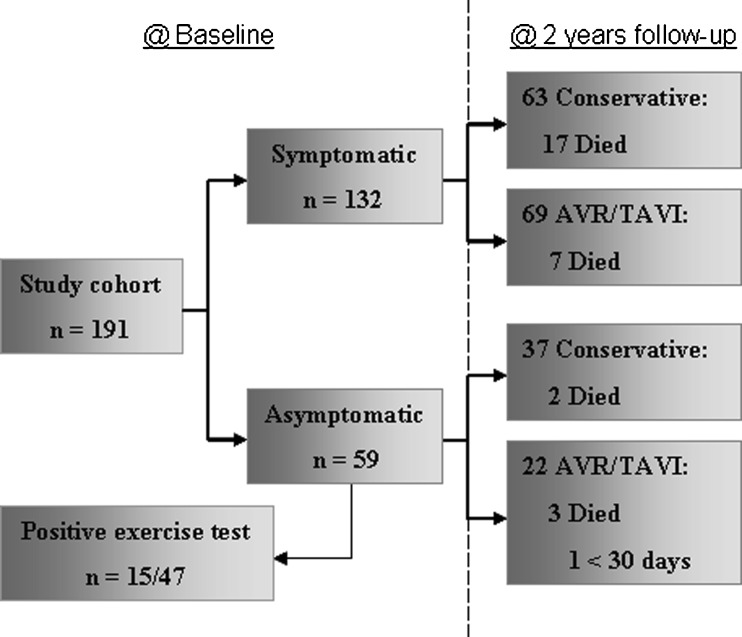
Forty-seven of the 59 patients who were asymptomatic underwent an exercise test at baseline. Of these 47 patients, 15 (32 %) tested positive (ST depression ≥2 mm (N = 10), no increase blood pressure (N = 2), collapse (N = 1), angina (N = 1), and dyspnoea (N = 2)), 25 (53 %) patients tested negative, and in 7 (15 %) patients the test was inconclusive. Twelve patients were unable to perform the exercise test due to impaired mobility, logistic reasons, or refusal.
Figure 1 displays the flow chart of patients during the study. Completeness of follow-up was 99 %; 2 patients had emigrated.
Fig. 1.
Flowchart of patient distribution during the study
Clinical course of symptomatic patients
Of the 132 symptomatic patients at baseline, 24 patients (18 %) died during follow-up of whom 7 patients after AVR/TAVI due to: pneumonia (N = 3), sudden unexpected unexplained death (N = 1), subdural haematoma (N = 1), mediastinitis (N = 1), and unknown reason (N = 1). Causes of death in the non-operated patients were congestive heart failure (N = 11), sudden unexpected unexplained death (N = 3), ruptured abdominal aortic aneurysm (N = 1), pneumonia (N = 1), and intestinal bleeding (N = 1).
Sixty-four patients (48 %) underwent AVR, 5 (4 %) TAVI, and 63 (48 %) were treated conservatively (Fig. 1). Reasons for TAVI were informed patient preference in 1 patient (age 53 years) and inoperability due to comorbidities in the other 4 patients (age >70 years).
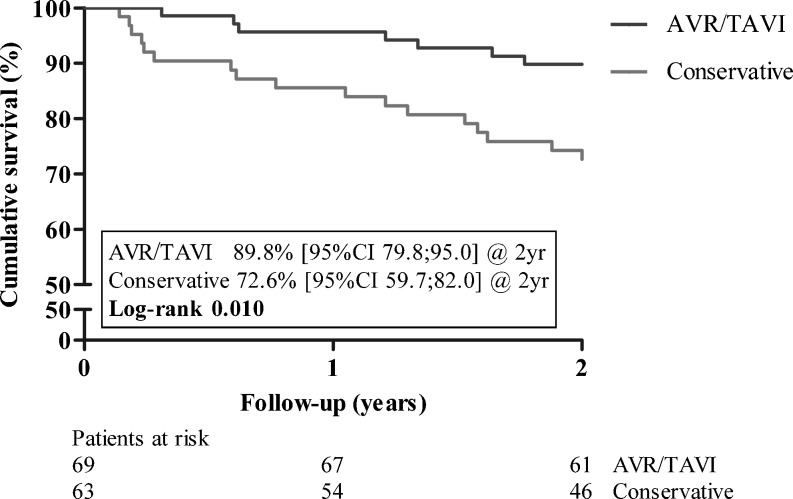
Overall cumulative survival at 2 years was 81.7 % (73.9–87.3 %). For patients receiving AVR/TAVI, 2-year cumulative survival was 89.8 % (95 % CI 79.8–95.0 %) and for patients who were treated conservatively 72.6 % (95 % CI 59.7–82.0 %) (Fig. 2). Older patient age (HR 1.05; 95 % CI 1.001–1.101; p = 0.046), previous myocardial infarction (HR 2.75; 95 % CI 1.14–6.60; p = 0.024), and a higher baseline NT-proBNP (HR 1.002; 95 % CI 1.001–1.003; p < 0.001) were independently associated with increased mortality rates. Although in the univariable model AVR/TAVI was associated with decreased mortality rates (HR 0.30; 95 % CI 0.13–0.67; p = 0.004), in the multivariable model it was no longer a significant factor (HR 0.69; 95 % CI 0.27–1.75; p = 0.430).
Fig. 2.
Patient survival for symptomatic patients differentiated by treatment strategy
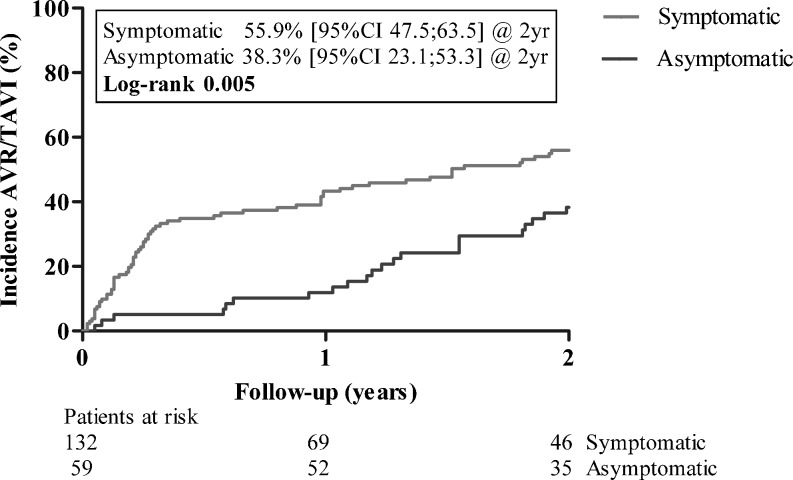
Cumulative incidence of AVR/TAVI at 2 years was 55.9 % (95 % CI 47.5–63.5 %) (Fig. 3). Factors associated with a conservative treatment strategy are displayed in Table 2. Logistic EuroSCORE in symptomatic patients was 5.1 % for those who underwent AVR/TAVI and 7.2 % for symptomatic patients who were treated conservatively (p < 0.001). Low-flow/low-gradient AS was more common in symptomatic patients who were conservatively treated compared with those who underwent AVR/TAVI (22 % versus 9 %; p = 0.013).
Fig. 3.
Cumulative incidence of AVR/TAVI differentiated by symptom status
Table 2.
Logistic regression analysis for conservative treatment in symptomatic patients at baseline
| Odds ratio | ||||
|---|---|---|---|---|
| Univariable | p-value | Multivariable* | P-value | |
| Age (yrs) | 1.09 (1.05–1.14) | <0.001 | 1.10 (1.04–1.15) | 0.001 |
| PAD (%) | 8.77 (2.42–31.25) | 0.001 | 10.99 (2.32–52.63) | 0.003 |
| Vmax (m/s) | 0.37 (0.22–0.63) | <0.001 | 0.46 (0.25–0.85) | 0.013 |
| Previous MI (%) | 5.95 (1.87–18.87) | 0.003 | 5.26 (1.30–21.28) | 0.020 |
| Hypertension (%) | 3.21 (1.56–6.58) | 0.002 | 2.72 (1.11–6.67) | 0.029 |
| MR (%) | 2.93 (1.04–8.26) | 0.042 | 0.64 (0.17–2.34) | 0.495 |
| Low flow/low gradient AS (%)** | 3.23 (1.15–9.01) | 0.025 | ||
| EuroSCORE (%)** | 1.11 (1.04–1.19) | 0.002 | ||
PAD peripheral arterial disease, Vmax peak transaortic jet velocity, MI myocardial infarction, MR mitral regurgitation, AS aortic valve stenosis, () 95 % confidence interval. Univariable p-values ≤0.05 were included in multivariable model. * Enter method. **Low flow/low gradient AS and EuroSCORE were highly correlated with ≥1 other co-variables and not entered in multivariable model
Clinical course of asymptomatic patients
Of the 59 asymptomatic patients at baseline, 5 patients died during follow-up. Three patients died after AVR due to congestive heart failure (N = 2: 1 < 30 days postoperative) and malignancy (N = 1). One patient died of a pulmonary embolism and 1 patient died of unknown cause.
Forty patients (68 %) became symptomatic, median time to symptom development was 13 months (range 1–24 months); 19 underwent AVR. In addition, 3 asymptomatic patients underwent AVR for rapidly progressing very severe AS (n = 2) and 1 for subvalvular AS with a gradient of 61 mmHg.
Overall cumulative survival at 2 years was 91.5 % (80.8–96.4 %). Of the 19 patients who became symptomatic and underwent AVR/TAVI, 2-year cumulative survival was 89.5 % (95 % CI 64.1–97.3 %). For the 21 patients who became symptomatic during follow-up but were treated conservatively, survival was 90.5 % (95 % CI 67.0–97.5 %), for the 16 patients who remained asymptomatic and were treated conservatively 100 %, and for the 3 patients who remained asymptomatic but nevertheless underwent AVR, survival was 66.7 % (95 % CI 5.4–94.5 %).
Symptom development rate was faster in patients with a higher Vmax at baseline (HR 2.06; 95 % CI 1.29–3.27; p = 0.002), those with CAD (HR 4.73; 95 % CI 1.20–18.73; p = 0.027), and prior myocardial infarction (HR 3.47; 95 % CI 1.14–10.54; p = 0.028).
Cumulative incidence of AVR/TAVI at 2 years was 38.3 % (95 % CI 23.1–53.3 %) (Fig. 3).
Discussion
This study reflects current clinical practice for adult patients with severe AS in several ways. First, a significant proportion of asymptomatic patients have a positive exercise test, underlining the importance of exercise testing in asymptomatic severe AS patients. Secondly, a considerable proportion of symptomatic patients do not undergo AVR/TAVI. In particular, elderly symptomatic patients with multiple comorbidities and a relatively low peak transaortic gradient are not likely to undergo AVR, and have a poor survival. Finally, the majority of asymptomatic patients become symptomatic over a 2-year period of time. This illustrates the progressive nature of severe AS and the need for careful and frequent ‘watchful waiting’ if a conservative strategy in the asymptomatic patients is pursued.
Challenges at diagnosis
A significant proportion of asymptomatic patients have a positive exercise test [13, 15]. The gradual decrease in physical functioning in the elderly can be attributed to advanced age, multiple comorbidities or to the worsening of AS, which might sometimes be difficult to differentiate. If it is not clear whether a patient with severe AS is symptomatic, exercise testing and/or measuring BNP can play an important role [16]. Unfortunately, the European Heart Survey shows that exercise testing is underutilised and the true number of symptomatic patients may be much higher than is currently observed [17].
Symptomatic patients
This study shows that symptomatic patients are usually older, more often female, and have more severe AS, more often concomitant mitral regurgitation, a higher NT-proBNP, and higher surgical risk scores compared with asymptomatic patients. Almost half of the symptomatic patients at study entry, as well as half of the asymptomatic patients who develop symptoms, are treated conservatively. Confirming previous reports, in particular older patients with a lower Vmax and multiple comorbidities are more likely to be treated conservatively [8, 12, 13]. Low-flow/low-gradient AS may possibly explain the association between lower Vmax and conservative treatment [18]. Although a higher EuroSCORE is associated with conservative treatment, the average EuroSCORE of conservatively treated patients in our study was only 7.2 %. However, EuroSCORE and other operative risk stratification models do not consider patient factors related to ageing, such as frailty, which become increasingly important in determining short- and long-term outcome with advancing age.[19, 20]. In this respect, there is a need for risk stratification models that better fit this elderly population.
We previously showed that important reasons for conservative treatment of symptomatic AS patients include misclassification of AS severity and symptoms, overestimation of operative risk, and patient preferences [13]. Given the survival benefit of TAVI for inoperable patients [10], patients with severe symptomatic AS should be referred for multidisciplinary heart team discussion to assess individual feasibility of invasive treatment approaches [21].
Although survival appears better in symptomatic patients who undergo AVR/TAVI versus those treated conservatively, this survival benefit disappears when corrected for patient age, NT-proBNP, and previous myocardial infarction. This suggests that patient survival is mainly driven by patient characteristics and to a lesser extent by treatment strategy. Our finding that NT-proBNP is associated with increased mortality confirms a previous report [22]. Although treatment strategy may not affect survival, it does influence quality of life [23]. In elderly patients with severe AS, quality of life should play a key role in optimising treatment strategies. With the steadily increasing application of TAVI it is expected that more elderly symptomatic AS patients will receive invasive treatment, and hopefully an improved quality of life.
Asymptomatic patients
Asymptomatic severe AS has a progressive course, evidenced by the fact that no less than two-thirds of asymptomatic patients in our study became symptomatic within 2 years. This is higher compared with a previous report in which only one third became symptomatic and may be explained by the higher prevalence of classical risk factors, more left ventricular hypertrophy, and smaller aortic valve areas in our study patients [24]. AS severity was predictive of symptom development in our study, and underlines the importance of frequent monitoring of asymptomatic patients with more severe AS. Of all asymptomatic patients who became symptomatic, less than half undergo invasive treatment, while there are also a few patients who remain asymptomatic, but actually receive AVR. This illustrates the ongoing debate on the timing of AVR in asymptomatic patients with very severe AS.
Limitations
Some elderly patients refused participation which has undoubtedly resulted in a selection bias toward younger patients with milder symptoms and less comorbidity. The 15 patients who tested positive during exercise testing remained assigned to the asymptomatic group during data analysis. Exercise test results were sent to the treating cardiologists and may have influenced treatment strategy.
Conclusions
In contemporary practice in the Rotterdam Rijnmond area nearly half of the patients with symptomatic severe AS, in particular elderly patients with multiple comorbidities, do not undergo invasive treatment. In addition, our observation that more than two-thirds of asymptomatic patients develop symptoms during a two-year period underlines the progressive nature of severe aortic stenosis and the need for stringent and frequent watchful waiting.
A systematic evidence-based multidisciplinary team approach is recommended to optimise treatment selection for symptomatic patients with severe AS. There is an urgent need to optimise patient treatment strategy by taking into account clinical factors related to AS and comorbidities, costs and benefits of treatment strategies, patient preferences, quality of life, and anticipated life expectancy.
Acknowledgements
The authors would like to thank the patients and colleagues of the participating hospitals for their enthusiastic collaboration: Albert Schweitzer Ziekenhuis, Erasmus University Medical Center, Havenziekenhuis, Maasstad Ziekenhuis, Sint Franciscus Gasthuis, Vlietland Ziekenhuis, and IJsselland Ziekenhuis.
Conflict of interest
None declared.
Appendix: Definitions
- Body surface area
calculated with DuBois and DuBois formula.
- Carotid disease
stenosis >50 %, or previous or planned surgery.
- Chronic obstructive pulmonary disease
diagnosis previously made by physician, or receiving bronchodilators.
- Congestive heart failure
hospital stay with clinical sign(s) of congestive heart failure.
- Coronary artery disease
>50 % stenosis in at least one coronary artery proved by coronary angiography, or previously coronary artery bypass grafting.
- Diabetes
diagnosis previously made by physician, or receiving blood glucose-lowering medication.
- Dyslipidaemia
diagnosis previously made by physician, or receiving lipid-lowering medication.
- Hypertension
diagnosis previously made by physician, or known blood pressure of ≥140 mmHg systolic or ≥90 mmHg diastolic on at least two measurements, or receiving blood pressure-lowering medication.
- Ischaemia
ST depression ≥1 mm at J + 60 ms in at least two electrocardiographic leads.
- Left ventricular hypertrophy
S in V1 plus R in V5/V6 > 35 mm, R in V6 > R in V5, R in I and/or aVL > 12 mm on electrocardiography at J + 60 ms.
- Myocardial infarction
diagnosis previously made by physician.
- Peripheral arterial disease
claudication, or previous or planned surgery of the lower limbs.
- Renal failure
diagnosis previously made by physician or creatinine ≥200 μmol/l.
- Smoking
smoking cigarettes or cigars for ≥5 years in the past.
- Stroke
diagnosis ‘transient ischaemic attack’ or ‘cerebrovascular accident’ previously made by physician, or neurological disease severely affecting ambulation or day-to-day functioning.
Footnotes
Helena J. Heuvelman and Martijn W. A. van Geldorp have equally contributed to this work.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Faggiano P, Antonini-Canterin F, Baldessin F, et al. Epidemiology and cardiovascular risk factors of aortic stenosis. Cardiovasc Ultrasound. 2006;4:27. doi: 10.1186/1476-7120-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48:e1–148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–68. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation. 2006;114:591–6. doi: 10.1161/CIRCULATIONAHA.106.632927. [DOI] [PubMed] [Google Scholar]
- 6.Litmathe J, Feindt P, Kurt M, et al. Aortic valve replacement in octogenarians: outcome and predictors of complications. Hellenic J Cardiol. 2011;52:211–5. [PubMed] [Google Scholar]
- 7.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 8.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–20. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 9.Charlson E, Legedza AT, Hamel MB. Decision-making and outcomes in severe symptomatic aortic stenosis. J Heart Valve Dis. 2006;15:312–21. [PubMed] [Google Scholar]
- 10.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 11.Bouma BJ, Meulen JH, Brink RB, et al. Variability in treatment advice for elderly patients with aortic stenosis: a nationwide survey in The Netherlands. Heart. 2001;85:196–201. doi: 10.1136/heart.85.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach DS, Cimino N, Deeb GM. Unoperated patients with severe aortic stenosis. J Am Coll Cardiol. 2007;50:2018–9. doi: 10.1016/j.jacc.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Geldorp MW, Gameren M, Kappetein AP, et al. Therapeutic decisions for patients with symptomatic severe aortic stenosis: room for improvement? Eur J Cardiothorac Surg. 2009;35:953–7. doi: 10.1016/j.ejcts.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106:1883–92. doi: 10.1161/01.CIR.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 15.Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J. 2005;26:1309–13. doi: 10.1093/eurheartj/ehi250. [DOI] [PubMed] [Google Scholar]
- 16.Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47:2141–51. doi: 10.1016/j.jacc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43. doi: 10.1016/S0195-668X(03)00201-X. [DOI] [PubMed] [Google Scholar]
- 18.Hachicha Z, Dumesnil JG, Bogaty P, et al. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–64. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Buth KJ, Martin BJ, et al. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–8. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 20.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 21.Head SJ, Bogers AJ, Serruys PW, et al. A crucial factor in shared decision making: the team approach. Lancet. 2011;377:1836. doi: 10.1016/S0140-6736(11)60775-7. [DOI] [PubMed] [Google Scholar]
- 22.Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J. 2004;25:2048–53. doi: 10.1016/j.ehj.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–72. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 24.Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290–5. doi: 10.1161/CIRCULATIONAHA.104.495903. [DOI] [PubMed] [Google Scholar]