Abstract
We report the first implantation of a percutaneous left ventricular partitioning device in the Netherlands. This device is developed for patients with chronic heart failure due to a left ventricular apical aneurysm caused by an anterior myocardial infarction.
Introduction
Coronary artery disease is the most important cause of chronic heart failure (CHF) and symptoms of CHF are common in patients who have suffered from an anterior myocardial infarction [1–3]. After an anterior infarction left ventricular (LV) remodelling may occur, leading to left ventricular aneurysm (LVA) formation. Both systolic and diastolic functions deteriorate due to stasis of blood in the aneurysmatic cavity. Medical treatment is often limited and revascularisation is only useful in the acute setting or in the presence of documented ischaemia in viable tissue. Surgical ventricular reconstruction reduces ventricular volume, but does not show an improvement in symptoms or exercise tolerance [4].
A percutaneous ventricular partitioning device (Parachute, CardioKinetix, Inc.) has been developed for patients with CHF due to LVA. This umbrella-like device isolates the dysfunctional region of the left ventricle and may improve LV haemodynamics and functional capacity. The Parachute consists of a nitinol frame of 16 struts with anchors at each strut tip and an ePTFE membrane (Fig. 1). The device is implanted by the transfemoral route in the cathlab. The foot of the device is positioned in the apex and the Parachute is expanded after balloon inflation. The anchors at the tips of the struts will move into the myocardial wall to maintain the Parachute position.
Fig. 1.
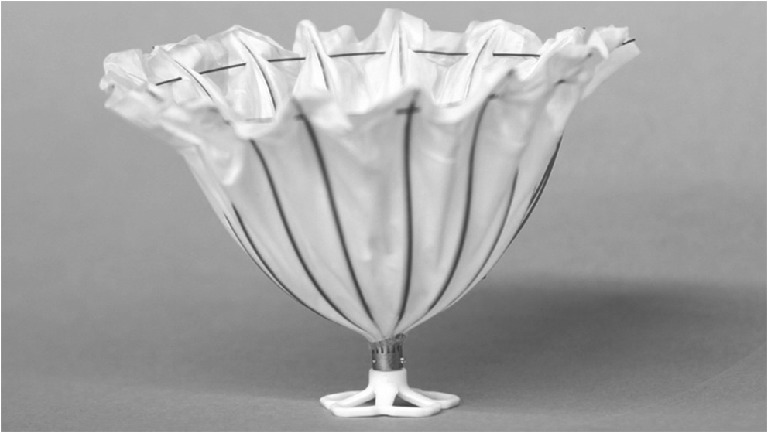
Parachute implant. The Parachute consists of a nitinol frame of 16 struts with anchors at each strut tip and an ePTFE membrane. Several sizes of the Parachute are available. The foot of the device is positioned in the apex and the Parachute is expanded after balloon inflation. The anchors at the tips of the struts will move into the myocardial wall to maintain the Parachute position
In April 2012 the first Parachute implantation in the Netherlands was performed at our institution, according to the Medical Ethics Committee approved PARACHUTE study (Percutaneous Ventricular Restoration in Chronic Heart Failure due to Ischemic Heart Disease).
Case
A 69-year-old female patient suffered an anterior infarction in 2010, followed by primary percutaneous coronary intervention of the left anterior descending coronary artery (LAD) complicated by cardiac insufficiency. She is also affected by hypertension, diabetes mellitus, obesity and renal insufficiency. She developed progressive symptoms of dyspnoea and fatigue and her functional status was New York Heart Association class III despite optimal medical therapy. She had no complaints of angina and coronary ischaemia was excluded by scintigraphy. Transthoracic echocardiogram (TTE) showed a dilated, hypertrophic left ventricle with a poor ejection fraction (35 %) and a large LVA without thrombi. Furthermore, a dilated left atrium and moderate mitral regurgitation were found. A contrast-enhanced retrospective ECG-gated cardiac CT scan showed a cardiac anatomy suitable for implantation of a 85 mm Parachute device with a short foot (Fig. 2). After informed consent, the procedure was performed in the cathlab under local anaesthesia by the transfemoral route. Prophylactic antibiotics were administered according to our protocol. A 16 Fr guide catheter was used to introduce the crimped Parachute implant with the delivery catheter into the left ventricle through the aortic valve. The foot of the implant was placed in the LVA, after which the delivery catheter balloon (20 ml) was inflated to expand the frame and the Parachute implant was successfully deployed (Fig. 3). Fluoroscopy and LV angiogram revealed that all the anchors were attached to the LV wall. LV end-diastolic pressure decreased immediately after the implantation from 19 to 14 mmHg. The femoral artery was closed by a Prostar device. Total procedure time was 57 min, 40 ml of contrast were used and the total amount of fluoroscopy was 88.3 Gy/cm2. Oral anticoagulation was restarted the evening after the procedure in combination with acetylsalicylic acid. A TTE performed the day after implantation showed a Parachute implant in the LV apex with only slight leakage. Mitral regurgitation was still grade II. No arrhythmias or other complications occurred and the patient was discharged the day after. TTE at 30 days showed an unchanged position of Parachute device. Functional tests have not yet been performed.
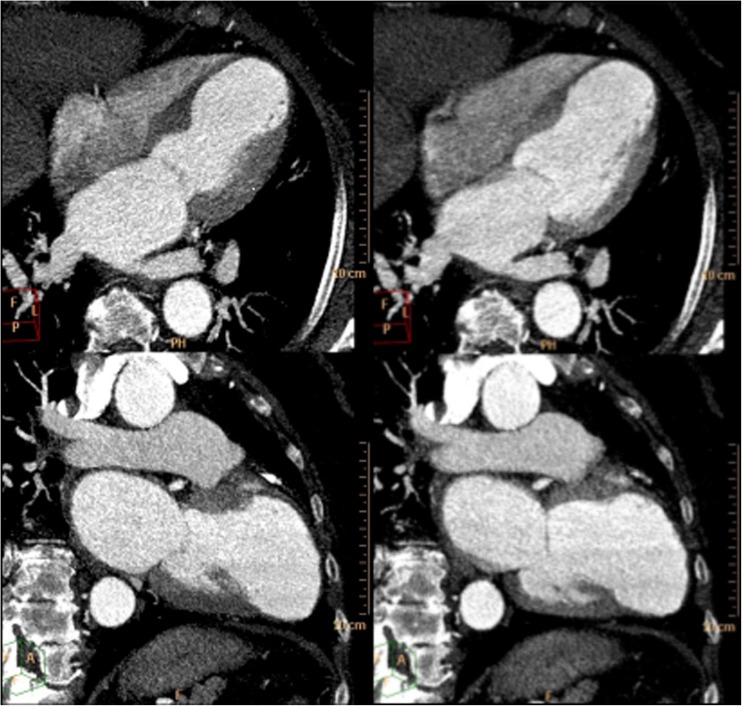
Fig. 2.
CT scan. Images reconstructed from a contrast-enhanced retrospective ECG-gated cardiac CT scan (64 CT scanner, Philips, Brilliance). End-systolic (left) and end-diastolic (right) 4- and 2-chamber views (slice thickness of 3 mm) show left ventricle dilatation and an akinetic aneurysmatic apex after myocardial infarction
Fig. 3.
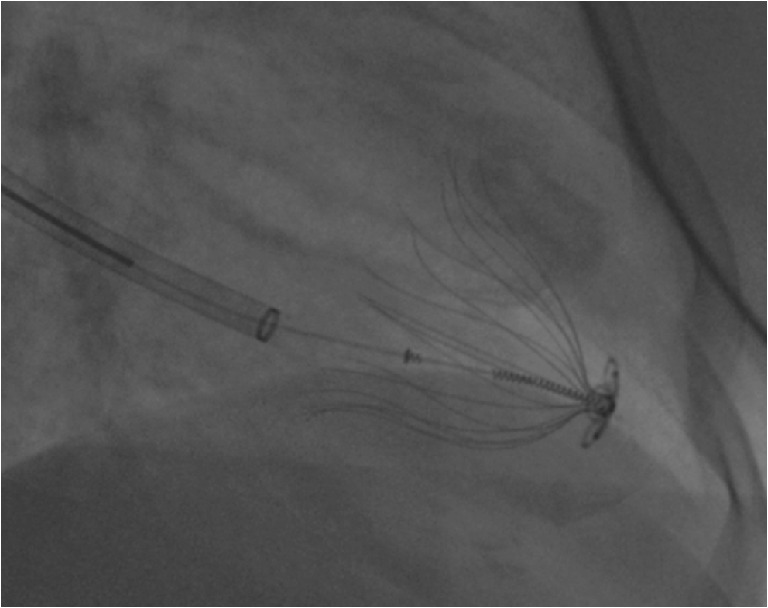
Parachute implantation. Fluoroscopic image of the Parachute device in the left ventricle. The distal foot of the device is placed in the centre of the aneurysm; the 16 struts of the Parachute implant are fully deployed and the tips are anchored in the myocardium to prevent migration of the implant
Discussion
The percutaneous ventricular partitioning device was developed in 1999 and animal studies showed haemodynamic benefits and safety; in 2006 the first implantation in a human was reported [5, 6]. A non-randomised multicentre study, previously reported after enrolment of 18 patients and recently extended to 39 patients, showed a significant improvement in LV end-systolic and end-diastolic volumes, NYHA class and quality-of-life scores at 1-year follow-up. Functional improvement was seen after 6 months. Despite one incomplete device opening with subsequent percutaneous device removal, no device or catheter malfunctions were reported. Very few complications occurred (1 inadequately attached device migrated into the LV cavity and was removed surgically, 1 patient developed a non-device-related sepsis and the device did not open completely in one patient during the procedure as mentioned above) [7, 8].
Our case demonstrates that implantation of the percutaneous ventricular partitioning device is safe and feasible under local anaesthesia by the transfemoral route. No complications occurred and fluoroscopic time and total amount of contrast were very limited. Currently, data concerning the effectiveness in large series are limited; therefore our centre is participating in, and is the principle investigator site for, the PARACHUTE trial.
Conclusion
The percutaneous ventricular partitioning device may be a promising technique for chronic heart failure patients with a left ventricular aneurysm caused by an anterior myocardial infarction. Effectiveness of the device has to be proven in large clinical trials.
Acknowledgments
Disclosures
None
References
- 1.Choudhury L, Gheorghiade M, Bonow RO. Coronary artery disease in patients with heart failure and preserved systolic function. Am J Cardiol. 2002;89:719–722. doi: 10.1016/S0002-9149(01)02345-1. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.CIR.97.3.282. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor CM, Hathaway WR, Bates ER, et al. Clinical characteristics and long-term outcome of patients in whom congestive heart failure develops after thrombolytic therapy for acute myocardial infarction: development of a predictive model. Am Heart J. 1997;133:663–673. doi: 10.1016/S0002-8703(97)70168-6. [DOI] [PubMed] [Google Scholar]
- 4.Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharkey H, Nikolic S, Khairkhahan A, et al. Left ventricular apex occluder. Description of a ventricular partitioning device. EuroIntervention. 2006;2:125–127. [PubMed] [Google Scholar]
- 6.Skowasch M, Robertson GC, Wunderlich N, et al. Percutaneous ventricular restoration in a chronic heart failure patient. EuroIntervention. 2006;2:128–131. [PubMed] [Google Scholar]
- 7.Mazzaferri EL, Jr, Gradinac S, Sagic D, et al. Percutaneous left ventricular partitioning in patients with chronic heart failure and a prior anterior myocardial infarction: results of the percutaneous ventricular restoration in chronic heart failure patients trial. Am Heart J. 2012;163:812–820. doi: 10.1016/j.ahj.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Sagic D, Otasevic P, Sievert H, et al. Percutaneous implantation of the left ventricular partitioning device for chronic heart failure: a pilot study with 1-year follow-up. Eur J Heart Fail. 2010;12:600–606. doi: 10.1093/eurjhf/hfq051. [DOI] [PubMed] [Google Scholar]