Abstract
The population of adults with a congenital heart defect (CHD) is increasing, due to improved survival after cardiac surgery. To accommodate the specialised care for these patients, a profound interest in the epidemiology of CHD is required. The exact size of the current population of adults with CHD is unknown, but the best available evidence suggests that currently overall prevalence of CHD in the adult population is about 3000 per million. Regional differences in CHD prevalence have been described, due to both variations in incidence and in mortality. Knowledge of demographic variations of CHD may lead to new aetiological insights and may be useful for preventive therapies. Socioeconomic status, education, urbanisation, climatological factors, ethnicity and patient-related factors, such as comorbidity, lifestyle and healthcare-seeking behaviour, may play a role in CHD incidence and mortality. The higher risk of several major cardiac outcomes in males with CHD might well explain at least partly the increased mortality rate in men. Regional differences in quality of life among CHD patients have been reported and although methodological differences may play a role, sociocultural differences warrant further attention. Socioeconomic outcomes in CHD patients, such as lower education, more unemployment and less relationships, might have a different impact on quality of life in different cultures. To gain more insight into demographic differences around the world large international multicentre studies on the epidemiology of CHD are needed.
Keywords: Congenital heart disease, Epidemiology, Demography
Introduction
Adults with congenital heart disease (CHD) form a steadily growing population. As most heart defects can be operated on in early childhood, over 90 % of children with CHD now survive into adulthood. To accommodate the specialised care for these patients, a sparked interest in the epidemiology of CHD is required. The exact size of the current population of adults with CHD is unknown. Moreover, reports on regional variations in prevalence, due to variations of incidence and mortality of CHD, are scarce. In this review, demographic variations in global CHD are highlighted. Knowledge of demographic variations is not only useful to identify the extent of the global health problem, but also to gain more insight into the underlying mechanisms of CHD.
Prevalence
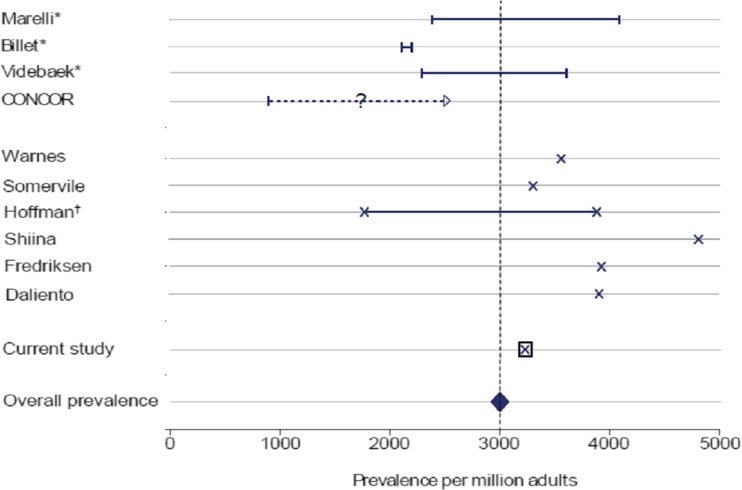
In order to anticipate the future burden of this population on care systems, an increasing number of studies have emerged in order to estimate the size of the adult CHD population. However, there is a large heterogeneity in study methodology, definitions of CHD and classifications. Consequently, interpretation can be difficult. In a recent systematic review a comprehensive overview of publications on the prevalence of CHD in adults was presented [1]. The best available evidence suggests that overall prevalence of CHD in the adult population is about 3000 per million (Fig. 1).
Fig. 1.
Studies reporting adult congenital heart disease prevalence. Caption: * Range between prevalence estimates excluding and including unspecified cases (cross-sectional studies); † Range between prevalence estimates with and without treatment (calculated) (Van der Bom et al., Am Heart J 2012)
Given a prevalence of 0.3 % within a world population of around 4.4 billion adults, a total number of 13 million adult CHD survivors worldwide can be estimated. These patients are being followed in more than 15,000 hospitals worldwide. However, a large number of them, 30–60 %, are lost to follow-up [2]. Worldwide, an urgent need is felt to identify those lost patients in order to offer them the care they need. A pro-active approach for recruitment is imperative.
Obviously, prevalence estimates are not valid for underserved areas, where CHD patients most often do not receive the required healthcare to survive. The differences in mortality between the industrialised and Third World are striking, from 3 % to 20 %, respectively. Furthermore, the mortality from CHD is likely under-reported in Third World nations because access to diagnosis is more difficult, and the great majority of studies only report data from patients in tertiary centres. Efforts are being made to improve the level of care to all adults with CHD worldwide, and recently the International Society of Adult Congenital Heart Disease (www.ISACHD.org) initiated an international Working Group with the aim to deliver care in cost effective, logistically acceptable, and socially adequate modalities in regions with specific societal, economic, and political situations.
Regional differences
Birth prevalence of CHD is generally assumed to be around 0.8 %. However, this does not take into consideration regional differences. Bernier et al. described a large regional variety in birth prevalence [3]. The authors report an incidence of CHD varying between 1.2 and 17 per 1000. The incidence in Taiwan and Iceland, for example, was reported to be more than 5 times higher than the incidence in UK, USA, France or Sweden. The study methods (including clinical, echocardiographic, and pathological) and populations (newborns versus school-age children, cohorts born in a hospital versus cases referred to a cardiologist or surgeon) of the reports and the proportions of different defects were variable enough to make it difficult to draw definite conclusions. However, more knowledge about these regional variations would increase our insight into causes and underlying mechanisms of CHD.
Regional differences in CHD prevalence may be due both to variations in incidence and in mortality. Differences in mortality may be due to variations in socioeconomic status, education, urbanisation, climatological factors, travel distance, ethnicity and patient-related factors, such as comorbidity, lifestyle and health care-seeking behaviour. Even in a small country as the Netherlands, mortality in the CHD population was shown to be significantly higher in the Northern, more rural, region than in other parts of the country [4].
A difference in mortality due to CHD is also seen between different socioeconomic groups in developed countries. For instance, an analysis of death certificates by the Centers for Disease Control in the United States has shown that mortality from CHD is generally higher in blacks than in whites, despite the incidence of CHD being slightly lower in the former [5].
Race and ethnicity
Although race and ethnicity are often difficult to discriminate from socioeconomic and other lifestyle factors, genetic factors undoubtedly may play a role in ethnic variations of CHD. In Asia relatively more right-sided and less left-sided lesions have been reported [3]. These findings confirm the results of Jacobs et al. who, in a Hong Kong population, found that white children had more left ventricular obstructive lesions, whereas Chinese children had more right ventricular outflow tract lesions [6]. Race and ethnic differences in mortality in CHD have been demonstrated by Gilboa et al.[7]. By means of death certificates filed in the United States the authors calculated annual CHD mortality by age at death, race ethnicity, and sex. From 1999 to 2006, there were 41,494 CHD-related deaths and 27,960 deaths resulting from CHD (age standardised mortality rates, 1.78 and 1.20 per 100,000, respectively). During this period, mortality resulting from CHD was consistently higher among non-Hispanic blacks compared with non-Hispanic whites.
Gender differences
Gender differences in the incidence of congenital heart defects at birth are very well known. Atrial septal defect, mitral valve prolapse, patent ductus arteriosus and common atrium show a clear female dominance, while transposition of the great arteries, aortic valve stenosis, aortic coarctation and tetralogy of Fallot occur more frequently in males [8].
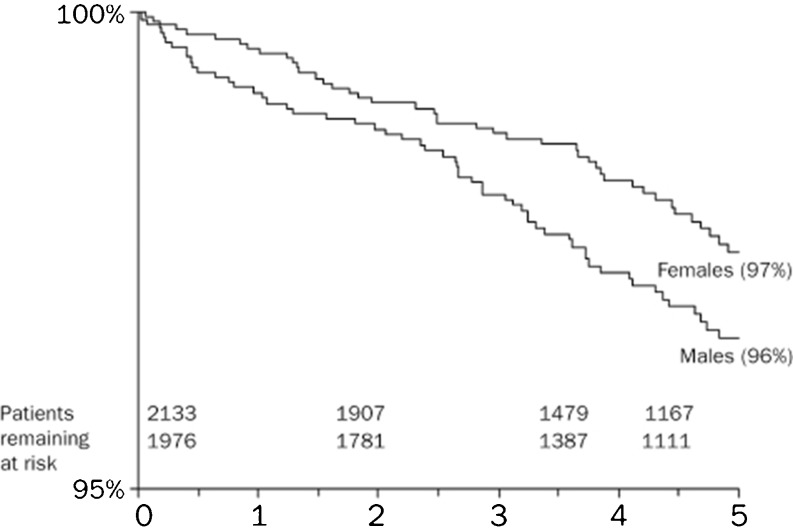
In a large European survey of over 4000 CHD patients from 24 countries it was found that males are more likely to die from CHD than females [9]. Adjusting for type of defect and age, it was found that cumulative mortality within 5 years was greater in the male (4 %) than in the female (3 %) population (Fig. 2). Significantly more men in this cohort were smokers (90.8 % versus 81.5 % women), which was shown to be a risk factor for increased mortality [10]. Other causes of increased mortality among men may be related to gender differences in late complications. In a population of 7414 adult CHD patients women had a 33 % higher risk of pulmonary hypertension, a 33 % lower risk of aortic outcomes, a 47 % lower risk of endocarditis and a 55 % lower risk of cardioverter-defibrillator (ICD) implant (all P < 0.05) [11]. After adjustment for age and adjustment for underlying cardiac defect a slightly lower risk of (mainly supraventricular) arrhythmias in women was found. No gender difference was demonstrated in ventricular arrhythmias, a major indication for ICD implant. The authors suggested that the lower ICD implant rate in women may result from gender bias, which has been reported previously in coronary heart disease [12]. Gender differences in events after ICD implant have not been described [12, 13] implying an equal benefit from ICD implant.
Fig. 2.
Crude 5-year cumulative survival curves for men and women, aggregated over all defects. (Engelfriet et al., Neth Heart J 2009)
The risk of several major cardiac outcomes in adults with CHD appears to vary by gender and might well explain at least partly the increased mortality rate in men with CHD.
Seasonal variation
A variation in seasonal mortality of CHD patients was observed in a study of 231 deaths among 8595 CHD patients, although without reaching statistical significance [4]. A trend was seen with the highest cardiac mortality in the fall (32.7 % versus 22.3 %, 23.2 %, 21.8 % in winter, spring and summertime respectively). Per season, modes of death were equally distributed.
A seasonal variation in mortality can be explained by climatic factors, behavioural changes, psychosocial factors or concomitant infections. Over 25 % of cardiovascular mortality was preceded by infection in the study by Zomer et al. [4].
Quality of life
A remarkable difference between transatlantic regions concerns the quality of life in CHD patients. Whereas most European studies suggest favourable outcomes in terms of the emotional functioning of adults with CHD, most American studies indicate poorer emotional functioning among this population [14]. Although methodological differences may play a role, sociocultural differences in the long-term management of individuals with medical conditions warrant further attention. As suggested by Kovacs et al. Dutch adults with CHD might fare better because of their healthcare system that has been heralded for almost full universal access and impressive QOL indicators and which includes provisions for long-term care and mental health treatment. Also in the general population there are sizeable differences in happiness between countries [15]. These differences are consistent across indicators and quite stable through time. There is solid empirical support for the view that these differences result from the fact that some societies provide their citizens with better living conditions than others. The bulk of the variance in happiness can be explained by nation characteristics such as economic prosperity, social security, political freedom, and social equality.
Therefore, socioeconomic outcomes in CHD patients, such as lower education, more unemployment and less relationships [16], might have a different impact on quality of life in different cultures. Cultural differences affect patients’ attitudes about medical care and their ability to understand, manage, and cope with the course of an illness, the meaning of a diagnosis, and the consequences of medical treatment. Unfortunately, the expectation of many healthcare professionals has been that patients will conform to mainstream values.
Conclusion
Undoubtedly, the greatest challenge of CHD worldwide remains to find ways to improve care globally. Even though major strides have been made, many populations still do not have access to appropriate care.
Knowledge of demographic variations of CHD may lead to new aetiological insights and may be useful for preventive therapies. However, geographic studies are associated with major problems of data quality, bias, confounding, and presentation which can seriously complicate their interpretation.
Geographical variations in CHD prevalence can be explained by variations in socioeconomic status, education, urbanisation, climatological factors, ethnicity and patient-related factors, such as comorbidity, lifestyle and healthcare-seeking behaviour. Therefore, using data from multiple sources, with adjustment for the imperfect nature of each, is an important strategy in CHD studies. Ideally, evidence-based knowledge on epidemiology of CHD should be obtained from large international multicentre studies.
Conflicts of interest
None
Financial disclosures
None
References
- 1.Van der Bom T, Bouma BJ, Meijboom FJ et al. The prevalence of adult congenital heart disease, results from a systematic review and evidence based calculation. Am Heart J. 2012;in press. [DOI] [PubMed]
- 2.Vis JC, Velde ET, Schuuring MJ, et al. Wanted! 8000 heart patients: identification of adult patients with a congenital heart defect lost to follow-up. Int J Cardiol. 2011;149(2):246–7. doi: 10.1016/j.ijcard.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Bernier PL, Stefanescu A, Samoukovic G, et al. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13(1):26–34. doi: 10.1053/j.pcsu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Zomer AC, Vaartjes I, Uiterwaal CS, et al. Circumstances of death in adult congenital heart disease. Int J Cardiol. 2012;154(2):168–72. doi: 10.1016/j.ijcard.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Boneva RS, Botto LD, Moore CA, et al. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–2381. doi: 10.1161/01.CIR.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs EG, Leung MP, Karlberg J. Distribution of symptomatic congenital heart disease in Hong Kong. Pediatr Cardiol. 2000;21:148–157. doi: 10.1007/s002469910025. [DOI] [PubMed] [Google Scholar]
- 7.Gilboa SM, Salemi JL, Nembhard WN, et al. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122(22):2254–63. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perloff JK. Congenital heart disease in adults: a new cardiovascular subspecialty. Circulation. 1991;84:1881–1890. doi: 10.1161/01.CIR.84.5.1881. [DOI] [PubMed] [Google Scholar]
- 9.Engelfriet P, Mulder BJM. Gender differences in adult congenital heart disease. Neth Heart J. 2009;17(11):414–417. doi: 10.1007/BF03086294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelfriet PM, Drenthen W, Pieper PG, et al. Smoking and its effects on mortality in adults with congenital heart disease. Int J Cardiol. 2008;127(1):93–7. doi: 10.1016/j.ijcard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Verheugt CL, Uiterwaal CS, Velde ET, et al. Gender and outcome in adult congenital heart disease. Circulation. 2008;118(1):26–32. doi: 10.1161/CIRCULATIONAHA.107.758086. [DOI] [PubMed] [Google Scholar]
- 12.Staniforth AD, Sporton SC, Robinson NM, et al. Is there a sex bias in implantable cardioverter-defibrillator referral and prescription? Heart. 2004;90:937–938. doi: 10.1136/hrt.2003.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pires LA, Sethuraman B, Guduguntla VD, et al. Outcome of women versus men with ventricular tachyarrhythmias treated with the implantable cardioverter defibrillator. J Cardiovasc Electrophysiol. 2002;13:563–568. doi: 10.1046/j.1540-8167.2002.00563.x. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs AH, Sears SF, Saidi AS. Biopsychosocial experiences of adults with congenital heart disease: review of the literature. Am Heart J. 2005;150(2):193–201. doi: 10.1016/j.ahj.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Piet Ouweneel and Ruut Veenhoven. Cross-national differences in happiness; Cultural Bias or Societal Quality? In: 'Contemporary issues in cross-cultural psychology, Bleichrodt, N & Drenth, P.J. editors. Amsterdam, Swets & Zeitlinger, The Netherlands, 1991: 168–16
- 16.Zomer AC, Vaartjes I, Uiterwaal CS, et al. Social burden and lifestyle in adults with congenital heart disease. Am J Cardiol. 2012;109(11):1657–63. doi: 10.1016/j.amjcard.2012.01.397. [DOI] [PubMed] [Google Scholar]