Abstract
Objectives
The clinical translation of stem cell-based Regenerative Endodontics demands further development of suitable injectable scaffolds. Puramatrix™ is a defined, self-assembling peptide hydrogel which instantaneously polymerizes under normal physiological conditions. Here, we assessed the compatibility of Puramatrix™ with dental pulp stem cell (DPSC) growth and differentiation.
Methods
DPSC cells were grown in 0.05 to 0.25% Puramatrix™. Cell viability was measured colorimetrically using the WST-1 assay. Cell morphology was observed in 3-D modeling using confocal microscopy. In addition, we used the human tooth slice model with Puramatrix™ to verify DPSC differentiation into odontoblast-like cells, as measured by expression of DSPP and DMP-1.
Results
DPSC survived and proliferated in Puramatrix™ for at least three weeks in culture. Confocal microscopy revealed that cells seeded in Puramatrix™ presented morphological features of healthy cells, and some cells exhibited cytoplasmic elongations. Notably, after 21 days in tooth slices containing Puramatrix™, DPSC cells expressed DMP-1 and DSPP, putative markers of odontoblastic differentiation.
Significance
Collectively, these data suggest that self-assembling peptide hydrogels might be useful injectable scaffolds for stem cell-based Regenerative Endodontics.
Keywords: Tissue engineering, Hydrogel, Dental pulp, Regenerative Endodontics, Odontoblast, Stem cells
Introduction
The focus on conservative strategies for treatment of diseased dental pulps has led Endodontics to advance in the fields of stem cell biology, genetics, and tissue engineering. The new field of Regenerative Endodontics has emerged around the premise that it might be possible to generate a new dental pulp to treat necrotic teeth [1-4]. The discovery of dental pulp stem cells (DPSC) capable of differentiating into many cell types [5,6] has provided a significant boost to this field. Dental pulp stem cells contain specific sub-populations of specific progenitor cells making them ideally suitable to the engineering of dental tissues [7]. Of specific interest for Regenerative Endodontics, these cells can differentiate into both functional, dentin-making odontoblasts [8,9] and vascular endothelial cells [10,11]. These features are critically important, since adequate vascularization is vital for the regeneration of the dentin-pulp complex.
Despite the excitement around the clinical application of dental pulp stem cells, there are still several challenges that have to be overcome before regenerative approaches can be used routinely in Endodontics. An area of considerable interest now is the interaction between dental pulp stem cells and 3 dimensional (3-D) scaffolds. Scaffolds provide cell adhesion and enables cell proliferation, mimicking the microenvironment observed in natural tissues and organs [12]. In the context of dental pulp tissue engineering, scaffolds should have a relatively fast setting time, and provide adequate root canal modeling and adaptation [2]. Notably, at the time that this manuscript was prepared there were no commercially available scaffolds developed for dental pulp tissue engineering [13]. Puramatrix™ is a self-assembling peptide hydrogel that has been tested in differentiated primary cells and stem cells [14,15]. It supports the regeneration of functional bone in murine calvaria [16]. The hydrogel comprises a 16-mer peptide in aqueous solution, which instantly polymerizes forming a biodegradable scaffold when introduced to physiological salt conditions. This capacity renders it ideal for clinical situations requiring both biocompatibility and rapid matrix formation. Here, we hypothesized that DPSC cultured in in Puramatrix™ will survive, proliferate and differentiate into odontoblasts.
Materials and Methods
Dental pulp stem cells (DPSC) were kindly provided by Dr. Songtao Shi (University of Southern California) and cultured as previously described [6]. Briefly, cells from 4th to 8th passage were grown in α-MEM medium (Invitrogen, Grand Island, NY, USA), supplemented by 20% FBS and incubated at 37°C in 5% CO2.
Cell growth in Puramatrix™
For all Puramatrix™ (BD, Franklin Lakes, NJ, USA) preparations, cells were washed in 10% (isotonic) sucrose solution to remove salts. Compatibility of Puramatrix™ with DPSC growth was assessed by proliferation assay. Cells were suspended in 0.2% Puramatrix™ and added to the wells of a 96-well plate then gelation was induced by careful addition of an equal volume of complete culture medium to give final densities as indicated in the text. The 0.2% concentration was chosen based on injection assays performed with syringes to determine suitability for endodontic use. After gelation, assessed by microscopic examination, complete culture medium was layered onto the gel (100 μl) and the cells were finally incubated at 37°C, 5% CO2 for indicated times. Cell growth curves were performed for up to 72 hours, analyzing different cell densities from 100,000 to 800,000 cells/ml of gel. In studies investigating the effects of gel concentration on cell growth, the cells (200,000 cells/ml) were suspended in varying dilutions (0.05 to 0.25%) of Puramatrix in sucrose, and left to grow for 72 hours. After suspension samples were treated as described above.
At indicated times relative cell density was assessed by addition of WST-1 (2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) reagent to a 1:10 final concentration. WST-1 (Roche, Indianapolis, IN, USA) is a soluble tetrazolium salt converted to a deep red colored product by mitochondrial activity. Samples were incubated for 3 hours at 37°C in 5% CO2 to allow reagent color development and absorbance was measured using microplate reader at 420 nm (Tecan, Austria). Here, and throughout this manuscript, experiments were performed in triplicate wells per concentration and time point. Three independent experiments were performed to verify reproducibility of the data. Data were analyzed by one-way ANOVA followed by recommended post-hoc tests using the SigmaStat 2.0 software (SPSS, Chicago, IL, USA). Significance was determined at a p<0.05.
3-D modeling
Cells were suspended in 0.2% Puramatrix™ and cell bearing beads were formed and carefully dropping the cell suspension, using a manual pipette, into complete medium where the cell suspension instantaneously polymerized. Hydrogel beads were immobilized within a coverslip base 30 mm culture dish by setting in collagen gel (Invitrogen). Samples were fixed using paraformaldehyde (10%) then probed for actin using Alexafluor 488 phalloidin (Invitrogen) as indicated by the manufacturer. To prevent drying and to stain nuclei, Prolong Gold plus DAPI (Invitrogen) was diluted 1:3 in PBS then layered over the gel beads.
Cell differentiation using Puramatrix™
Previous studies of solid polymer scaffolds have shown that differentiation of DPSC may be stimulated by the presence of a tooth slice [17]. Thus in the present study, cells were seeded in the tooth slice model at 1×105 cells/ml, in Puramatrix™ at 0.2%. After 21 days of culture, changing the media every other day, the matrix was removed from the tooth slices for RNA extraction by Trizol (Sigma/Aldrich), as indicated by manufacturer. cDNA from the samples were used in a reverse transcriptase polymerase chain reaction (SuperScript II Platinum®, Invitrogen). The human-specific primers were designed according to published cDNA sequences of GenBank, as follows: DSPP sense 5′gaccccttcattgacctcaact’3, antisense 5′tgccatttgctgtgatgttt’3; DMP-1 sense 5′caggagcacaggaaaaggag’3, antisense 5′ctggtggtatcttgggcact’3; GAPDH sense 5′gaccccttcattgacctcaact’3, antisense 5′caccaccttcttgatgtcatc’3. The following PCR protocol was used: denaturation, 94°C for 45 seconds; annealing, 57°C for 45 seconds; and extension, 72°C for 60 seconds, for 35 cycles, then 72° C for 5 minutes and held at 4°C. The PCR products were separated by 1.5% agarose gel electrophoresis, stained with ethidium bromide and digital images were taken under ultraviolet illumination.
Results
Puramatrix™ supports the proliferation and survival of DPSC
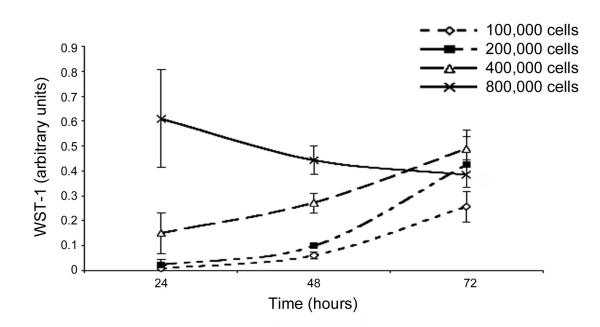
We observed that DPSC proliferate in Puramatrix™ when seeded at a density of 1-4×105 cells/ml. In contrast, at the excessively high cell density (8×105 cells/ml), DPSC cells were not able to proliferate and instead fell to around the same as the maximum 72-hour data for the other densities of cells (Fig. 1). DPSC survived in all concentrations of Puramatrix (Figure 2). Indeed, no difference was observed in DPSC proliferation in the different concentrations of Puramatrix that were tested here (p=0.1243). Therefore, 0.2% gel was used for most applications as its increased rigidity provided a more stable gel than the lower concentrations.
Figure 1.
Proliferation of DPSC in Puramatrix™ over time and cell density. DPSC cells were plated in 0.2% Puramatrix™ at a cell density of 1-8×105 cells/ml and allowed to grow for up to 72 hours. WST-1 assay was used to determine relative cell densities at indicated time points.
Figure 2.
Proliferation of DPSC according to the concentration of Puramatrix™. DPSC cells were plated in 0.05-0.25% Puramatrix™. WST-1 was used to determine relative cell densities at indicated time points.
3-D modeling of DPSC seeded in Puramatrix™
Under confocal laser microscopy, it was possible to observe that cells tended to form clusters after 24 hours, with some cells extending processes to other clusters (Fig. 3). It is remarkable that, after 72 hours, the cell shape was even more characteristic (spindle shaped) for dental pulp stem cells, and it can be observed that the cell extensions started to be more specific, suggesting the formation of cytoplasmic elongations among the different clusters (Fig. 3). After 72 hours in 3-D culture, the DPSC clusters manifested elongated structures, some tubular and some spindle shaped (Fig. 3). These structures were comprised of both single and multiple cells.
Figure 3.
Confocal microscopy images of DPSC cells cultured in Puramatrix™. In the top row, DPSC were observed after 24 hours in culture (red: actin; blue: nuclear staining). In the bottom row, DPSC were evaluated after 72 hours in culture (green: actin; blue: nuclear staining).
DPSC seeded in tooth slice/Puramatrix™ expressed putative odontoblastic markers
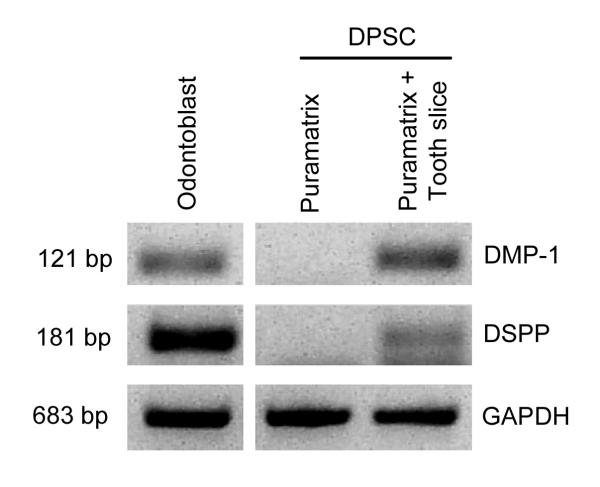
RT-PCR demonstrated that DPSC seeded in Puramatrix™ and cultured in tooth slices expressed the putative odontoblastic markers DSPP and DMP-1 (Fig. 4). Notably, DPSC seeded in Puramatrix™ without the tooth slice did not express markers of odontoblastic differentiation (Fig. 4). These findings suggested that dentin-derived factors are required for odontoblastic differentiation of DPSC, confirming previous reports [22].
Figure 4.
Expression of putative markers of odontoblastic differentiation by DPSC cells. DPSC were cultured in Puramatrix™ only, or Puramatrix™ casted within a human tooth slice. RT-PCR was used to evaluated the expression of DMP-1 and DSPP after 21 days in culture. Primary human odontoblasts scrapped from extracted teeth were used as positive controls for DMP-1 and DSPP.
Discussion
Three-dimensional scaffolds are required for cellular organization and interaction in engineered tissue [12]. However, the use of any such scaffold in the small and closed environments of the root canal provides a singular challenge. Some scaffolds described in the literature are very successful environments for cell growth in vitro but are unsuitable for the demands of clinical practice [4]. Uniquely, Puramatrix™ is a liquid that may be potentially poured into a pulp chamber and which self-polymerizes under physiological conditions to form a solid gel capable of supporting cell growth. This application is highly attractive from an endodontic standpoint as a liquid may be expected to conform more easily to the variable shape of a pulp chamber than would a solid or even moldable scaffold. For example, production of poly-l-lactic acid or poly-lactic-co-glycolic acid scaffolds, widely used for research purposes, requires use of solvents and/or pressure and molding apparatus for specific shape conformations [18,19]. The Puramatrix™ hydrogel scaffold has been used successfully in clinical applications and with favorable cell growth [14,16]. These studies led us to test the compatibility of this scaffold with dental pulp stem cells with a view to clinical pulp reconstruction. It is clear from data presented here, that dental pulp stem cells are able to survive and proliferate in a 3-D Puramatrix™ scaffold under the conditions tested. This sustained cell viability in the hydrogel unveils an injectable scaffold that could conceptually be used in regenerative endodontics and indicates the value of developing new similar scaffolds allowing stem cell therapy inside the root canal.
Data from the cell viability study presented here suggests that DPSC cells reduce or stop their growth at a density of 800,000 cells/ml. This may be explained by the fact that the growth media was not replaced during the cell viability testing, leading to a saturation/depletion of this media, avoiding cell growth. This condition intentionally mimicked the initial lack of consistent nutrient supply in an avascular scaffold, as would be the case in a freshly constructed preparation in the clinic. It may also result from the tendency of the DPSC to form spheroid-like clusters within the gel. When grown in supportive matrices, both primary endothelial cells and tumor cells have the capacity to form spheroids that have been shown to develop organized outer monolayers of cells with apoptotic or necrotic centers [20]. Thus, the burst of DPSC growth at lower cell densities in the Puramatrix™ indicates log phase proliferation of cells in localized colonies that may have spheroid-like properties. Whereas the highest cell plating density resulted in a decrease in WST-1 absorbance over three days which may have resulted from either a more rapid depletion of nutrients of from more rapid clustering due to increased cell-cell proximity followed by the necrosis/apoptosis described above. Clearly too, contact inhibition alone may contribute to the reduction of proliferation at higher densities and may account for a reduction in proliferation over time but could not alone account for the fall in absorbance seen at higher cell densities.
Regarding the gel concentration, it is clear that it is not a significant factor mediating cell growth at the gel densities tested, since no statistical differences were found. It should be noted that the Puramatrix™ concentrations used currently both included and, at the low percentage end, exceeded the manufacturer’s suggested parameters. This is notable as varying Puramatrix™ density has been shown to markedly alter growth characteristics of other primary cell types [21], however, in this case with DPSC, proliferation was not significantly affected.
An important finding of this work is that Puramatrix is an injectable scaffold that allowed for odontoblastic differentiation of dental pulp stem cells. Previous work from our laboratory showed odontoblastic differentiation within tooth slices using poly-l-lactic acid scaffolds [11,18]. In this study it is demonstrated that using Puramatrix™ in the same tooth slice model enabled induction of the expression of odontoblastic differentiation markers. In this study, we have used the dentin sialo-phosphoprotein (DSPP) and the dentin matrix protein 1 (DMP-1) as markers of odontoblastic differentiation, as we shown [11]. The expression of DSPP, responsible for encoding dentin phosphoprotein (DPP) and dentin sialoprotein (DSP), is observed during odontoblastic differentiation. Indeed, altered expression of DSPP is involved in deficient dentin synthesis, characteristic of “dentinogenesis imperfecta” [22]. Furthermore, other studies have used DSPP as a marker for odontoblastic differentiation [9,23]. In addition, the DMP-1 gene, has been reported to be present during stages of the development of the tooth, in order that it also becomes important to state odontoblastic differentiation [24,25]. Notably, we have shown that expression of these markers by dental pulp stem cells seeded in tooth slice/scaffolds and transplanted into immunodeficient mice correlates with deposition of new dentin, as determined by tetracycline staining [11]. We hypothesize that soluble factors responsible for odontoblastic differentiation diffuse from the dentin through the hydrogel [23]. Where intact tooth is filled with a hydrogel scaffold such as Puramatrix™, we may expect that the surviving dentin may itself provide some growth stimulation and morphogenic differentiation signaling to the stem cells suspended within the gel.
In summary, the development of new scaffolds suitable for Regenerative Endodontics is an important new field of dental materials research. The next step in the use of self-assembling peptide hydrogels for dental pulp tissue engineering would be to understand how this class of scaffolds behaves in clinical conditions. More specifically, it will be important to learn if the physical and mechanical properties of these scaffolds are adequate for clinical use, and to understand if they enable full odontoblastic differentiation as measured by deposition of new tubular dentin. The current study represents an initial step in the characterization of this class of scaffolds for dental pulp tissue engineering.
Conclusion
Under the experimental conditions described, dental pulp stem cells survived and proliferated in a self-assembling peptide hydrogel. This class of materials represents a promising new alternative of injectable scaffolds for dental pulp tissue engineering.
Acknowledgements
This work was funded by grant R01-DE021410 from the NIH/NIDCR (JEN) and by CAPES, Brazilian government (BNC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakashima M, Reddi H. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotech. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 2.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007;13:151–157. doi: 10.1111/j.1601-0825.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosa V, Bona AD, Cavalcanti BN, Nör JE. Tissue engineering: From research to dental clinics. Dent Mater. 2012;28:341–348. doi: 10.1016/j.dental.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell. 2007;99:465–474. doi: 10.1042/BC20070013. [DOI] [PubMed] [Google Scholar]
- 8.Huang GT, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–1073. doi: 10.1016/j.joen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 10.d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G. Human postnatal dental pulp cells codifferentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 11.Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, Santos CF, Nör JE. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89:791–796. doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 12.Bohl KS, Shon J, Rutherford B, Mooney DJ. Role of synthetic extracellular matrix in development of engineered dental pulp. J Biomater Sci Polym Ed. 1998;9:749–764. doi: 10.1163/156856298x00127. [DOI] [PubMed] [Google Scholar]
- 13.Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A. 2012;18:176–184. doi: 10.1089/ten.tea.2011.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narmoneva DA, Oni O, Sieminski AL, Zhang S, Gertler JP, Kamm RD, Lee RT. Selfassembling short oligopeptides and the promotion of angiogenesis. Biomaterials. 2005;26:4837–4846. doi: 10.1016/j.biomaterials.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Thonhoff JR, Lou DI, Jordan PM, Zhao X, Wu P. Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Res. 2008;1187:42–51. doi: 10.1016/j.brainres.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misawa H, Kobayashi N, Soto-Gutierrez A, Chen Y, Yoshida A, Rivas-Carrillo JD, Navarro-Alvarez N, Tanaka K, Miki A, Takei J, Ueda T, Tanaka M, Endo H, Tanaka N, Ozaki T. Puramatrix™ facilitates bone regeneration in bone defects of calvaria in mice. Cell Transplant. 2006;15:903–910. doi: 10.3727/000000006783981369. [DOI] [PubMed] [Google Scholar]
- 17.Gonçalves SB, Dong Z, Bramante CM, Holland GR, Smith AJ, Nör JE. Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod. 2007;33:811–814. doi: 10.1016/j.joen.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Kaigler D, Krebsbach PH, Wang Z, West ER, Horger K, Mooney DJ. Transplanted endothelial cells enhance orthotopic bone regeneration. J Dent Res. 2006;85:633–637. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 20.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieminski AL, Was AS, Kim G, Gong H, Kamm RD. The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation. Cell Biochem Biophys. 2007;49:73–83. doi: 10.1007/s12013-007-0046-1. [DOI] [PubMed] [Google Scholar]
- 22.Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, Fu G, Qian M, Yang J, Shi Y, Hu L, Han B, Wang Z, Huang W, Liu J, Chen Z, Zhao G, Kong X. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nature Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 23.Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res. 2010;89:603–608. doi: 10.1177/0022034510364487. [DOI] [PubMed] [Google Scholar]
- 24.D’Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12:2040–2049. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- 25.Bégue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT. Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci. 1998;106:963–970. doi: 10.1046/j.0909-8836.1998.eos106510.x. [DOI] [PubMed] [Google Scholar]