Summary
Prostate cancer risk can be modified by environmental factors, however the molecular mechanisms affecting susceptibility to this disease are not well understood. As a result of a series of recently published studies, the steroidal lipid, cholesterol, has emerged as a clinically relevant therapeutic target in prostate cancer. This review summarizes the findings from human studies as well as animal and cell biology models which suggest that high circulating cholesterol increases risk of aggressive prostate cancer, while cholesterol lowering strategies may confer protective benefit. Relevant molecular processes that have been experimentally tested and might explain these associations are described. We suggest that these promising results now could be applied prospectively to attempt to lower risk of prostate cancer in select populations.
Keywords: Prostate Cancer, Cholesterol, Statin, Castration-resistance
Introduction
Prostate cancer (PCa) is the most common non-skin cancer and the second leading cause of cancer-related death in men. While most PCas are treatable at an early stage, advanced PCa is fatal. Although much is now known about the disease, its high frequency and mortality in developed nations reinforces the need for strategies to reduce incidence and prevent progression to advanced cancer.
Like other malignancies, PCa has a genetic component, though it is not well defined. Men are at higher risk if there is a family history of the disease, or if they are of African decent. It is also apparent that environmental factors play a large role in PCa risk, likely explaining some of the increased risk seen in developed countries and in the observation that risk of disease increases among migrants from poorer or non-Western countries (reviewed in [1,2]). Regional diet is a potentially important source of variation in PCa risk [1,2]. Western diets are complex, with a variety of components that may exacerbate disease risk, including estrogenic compounds, hormones, carcinogens, red meat, and an excess of fat and cholesterol.
Cholesterol, a steroidal lipid that makes up about one-third of the lipid content of the plasma membrane, is an essential membrane component of animal cells [3,4]. By virtue of its unique steroid chemistry, cholesterol has critical effects[c1] on membrane fluidity and structure as well as being essential in steroidogenesis [3]. Yet high levels of cholesterol can cause cytotoxicity, in large part due to the propensity of cholesterol to become oxidized. Consequently the cholesterol content of cells is very tightly regulated, despite wide variations in serum cholesterol level. Multiple mechanisms, including regulated cholesterol uptake, synthesis, conversion to esters, bile acids and steroid hormones, as well as efflux operate to maintain the correct intracellular cholesterol concentration [4]. Nevertheless, all cells are potentially subject to pathologic loss of homeostatic control over cholesterol metabolism, potentially leading to multiple effects on tumor cell growth, apoptosis and sensitivity or resistance to external agents.
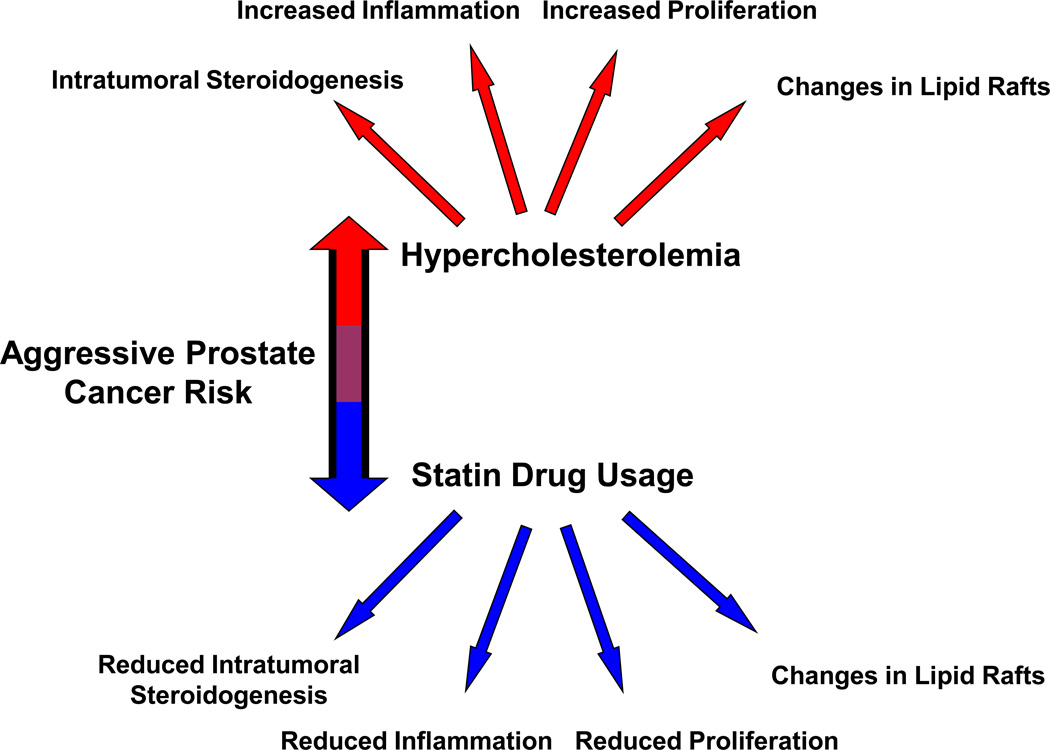
Evidence for a role for cholesterol in PCa has been increasing over the past decade, with a number of new studies supporting an important role for this lipid in PCa progression. Here we summarize this new information, place it in context with older reports and explore potential mechanisms that may provide unifying explanations for these observations (Fig. 1).
Figure 1. Graphical abstract of the contents of this review.
Red represents increased PCa risk; blue represents decreased PCa risk.
EVIDENCE
Epidemiology
Four types of epidemiological studies shed light on the role of cholesterol in PCa incidence and progression: Large population studies of cholesterol and disease, observational studies of cholesterol and PCa, observational studies of cholesterol-lowering drugs and PCa, and randomized trials of cholesterol-lowering drugs that report on cancer.
A minority of large population studies published between 1980–2000 of overall disease incidence, mortality and cholesterol level include PCa in the analysis, and we tabulated the total number of PCa cases combined to be only 1,652 [5]. We have reviewed these reports previously and conclude that, collectively, these studies point to a modest association between low cholesterol and increased PCa risk [5]. This result is likely explained by an effect of pre-existing cancer that is now known to result in reduced circulating cholesterol levels [5] (see section on mechanisms that follows for more on this point). A more recent study of overall disease incidence, mortality and cholesterol level did include sufficient patients with PCa to permit a more complete analysis. Kitahara et al. [6] report on a large prospective study in Korea which collected data on 756,604 men including 2,490 PCa cases. They found that the 329 men in the highest quintile of total cholesterol (≥ 240 mg/dL) had an increased risk of PCa [HR 1.24 95% CI (1.07–1.44), p-trend = .001] when compared to the 366 men in the lowest quintile (<160 mg/dL).
Most of the reports cited above include limited numbers of PCas, were generally of relatively short duration, and for the most part never include late stage disease (except regarding mortality). In contrast, a number of studies that specifically address the potential association between serum cholesterol level and PCa have large enough cohort sizes, many more advanced cases, and stratify disease by grade, thus permitting a more comprehensive analysis. Thompson and colleagues [7] found no cholesterol-PCa association (n=100). The Asia Pacific Cohort Studies Collaboration 2007 [8] reported on mortality and found a statistically insignificant larger number of deaths in the population with the highest cholesterol using tertile analysis. Platz et al. [9] in a case-control analysis of men in the Health Professionals Follow-up Study showed that patients (n=698) with low cholesterol had a lower risk of high-grade PCa [OR 0.61 95% CI (0.39–0.98)]. In a separate study, Platz et al. [10] examined 1,251 incident PCas and found that men with low cholesterol (<200 mg/dL) had a lower risk of high-grade disease. Mondul et al. [11] examined 438 incident PCas and found that men with cholesterol <240 mg/dL were at lower risk of developing high grade PCa then men with cholesterol >240 mg/dL. Hemelrijck et al. [12] examined 200,660 men of which 5,112 developed PCa and found no association between cholesterol and PCa. However, in a later report, Hemelrijck and colleagues [13] found that after excluding the first 3 years of follow-up, high density lipoprotein (HDL) was inversely associated with PCa risk [HR 0.79 95% CI (0.68–0.92), p trend= 0.003], when comparing the highest (>63.8 mg/dL) with the lowest (<43.7 mg/dL) cohort in quartile analysis. In addition, it was observed that increased total cholesterol (TC)/HDL lipid ratios of >5.45 were related to increased PCa risk [HR 1.26 95% CI (1.07–1.49), p trend= 0.005] when compared to ratios of <3.44. Furthermore, LDL (low density lipoprotein)/HDL ratios of >3.70 were associated with increased risk in comparison to ratios <2.11 [HR 1.21 95% CI (1.03–1.41), p trend= 0.026]. In a study that included 578 PCa deaths, Batty et al. [14] report a greater number of cancer deaths in the highest cholesterol tertile. Farwell et al. [15] demonstrated a significant relationship between total serum cholesterol and PCa risk with a 45% increased risk of total PCa [HR 1.45 95% CI (1.07–1.97)] and a 204% increased risk of high-grade PCa [HR 3.04 95% CI (1.65–5.60)] for patients in the highest quartile of TC (>237mg/dL) when compared to the lowest (<176mg/dL). Shafique and colleagues [16] found that of the 650 men that developed PCa, cholesterol level was positively associated with incidence of high-grade PCa (Gleason score ≥8[c2]). In the adjusted analysis, the association was greatest [HR 2.28 95% CI (1.27–4.10)] when comparing the 2nd highest cholesterol quintile (235.9–258.7 mg/dL) to the first (< 195.3 mg/dL). Mondul et al. [17] using the Alpha-Tocopherol Beta-Carotene Cancer Prevention study cohort, examined a population of smokers of which 2,041 developed PCa. They observed, after excluding the first 10 years of follow-up, that men with higher TC (≥240 vs. <200 mg/dl) were at increased risk of overall [HR 1.22 95% CI (1.03–1.44), p-trend = 0.01] and advanced PCa [HR 1.85 95% CI (1.13–3.03), p-trend = 0.05]. In addition, when comparing men in the highest quintile of TC/HDL ratios to the lowest, there was an increased risk of overall [HR, 1.20 95% CI (1.02–1.41)] and advanced PCa [HR 1.44 95% CI (1.02–2.05)]. In a prospective population-based study, Kok et al. [18] examined 2,118 men who reported never using cholesterol-lowering drugs. Of the 43 PCa cases, the adjusted analysis showed that higher total and LDL cholesterol were significantly associated with an increased risk of overall and advanced PCa.
In aggregate, these reports suggest that men with hypercholesterolemia are at increased risk for PCa or late stage, aggressive disease.
Another stream of epidemiologic data linking cholesterol to PCa risk comes from studies of cholesterol-lowering drugs (principally 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, collectively referred to as "statins") and PCa risk [19–25].
Statins are used clinically to reduce LDL levels and improve cardiovascular health. They inhibit the rate-limiting step in cholesterol synthesis in the liver (the primary target organ), thereby resulting in lower circulating cholesterol. Because statins inhibit an early step in cholesterol synthesis, they also reduce upstream non-cholesterol synthetic intermediates. Much of the activity of statins in vitro can be attributed to a reduction in these non-cholesterol products. In vivo statins are almost exclusively found in the liver and most of their pleiotropic effects can be ascribed to cholesterol lowering activities [5,26]. We have argued from the known pharmacology and pharmacokinetics of statins that it is unlikely that they accumulate in the prostate in sufficient concentrations for long enough periods of time to have any sustained local effect[5,26]. Most likely any effect of statins on the prostate is a result of their potent cholesterol-lowering activities; consequently, studies examining statins and PCa risk largely address the effect of cholesterol lowering on disease incidence or severity.
Platz et al. [24] analyzed potential statin drug effects specifically on PCa in a study powered to examine differences in cancer incidence as well as progression in 2,579 PCa cases, including 316 cases of advanced disease. The adjusted relative risk of castration-resistant PCa among statin users was 0.51 95% CI (0.30–0.86) and of metastatic or fatal disease was 0.39 95% CI (0.19–0.77) for statin users vs. nonusers. These authors also showed that advanced disease risk was lower with longer statin use. In contrast to advanced disease, the authors reported no association between statin use and overall PCa risk. Several more studies from independent groups largely confirmed the conclusions of Platz et al. [19,22–24] that statins reduce the risk of aggressive PCa.
Although there have been some conflicting accounts, with two studies reporting no association [27,28], one study showing a positive association between statin use and PCa risk [29] and one study showing statin users had a lower 5-year biochemical recurrence-free survival rate [30], a series of reports in the last several years buttress support for a protective effect of these drugs.
A retrospective study by Mondul et al. [31] studying 2,399 men who underwent prostatectomy, showed patients were less likely to have non-organ confined disease if taking a statin [OR 0.66 95% CI (0.50–0.85)]. The 16% of men who used a statin were also less likely to have high-grade PCa among men with a preoperative PSA ≥10ng/ml [OR 0.35 95% CI (0.13–0.93), p= 0.02]. In addition, patients who used statins for ≥1 year had a lower risk of PCa recurrence with the hazard ratio (HR) of 0.77 95% CI (0.41–1.42) compared to nonusers.
A study by Tan et al. [32] examining 4,204 men who underwent prostate biopsy showed that the men taking statins (24.3% of the total) were less likely to be positive on a digital rectal examination (5.3% vs. 8.9%, OR 0.7, p <0.01), and less likely to have a Gleason score ≥7 (61.4% vs. 72.4%, OR 0.78, p=0.02) or high volume PCa (27.2% vs. 31.4%, p<0.01). Adjusted analysis showed that statin use at any time decreased the incidence of PCa [RR 0.92 95% CI (0.85–0.98)] and high-grade PCa [RR 0.76, 95% CI (0.67–0.85)]. Patients with >5 years of statin use also had a decreased incidence of high-grade PCa [RR 0.75 95% CI (0.53–0.94)] vs. patient who never used a statin.
Using a population-based cohort of 2,447 men who were followed from 1990–2007, Breau et al. [25] found that the 634 men using statins had a decreased risk of PCa diagnosis [HR 0.36 95% CI (0.25–0.53)] and high-grade PCa [HR 0.25 95% CI (0.11–0.58)]. Patients using statins were also at decreased risk of undergoing prostate biopsy [HR 0.31 95% CI (0.24–0.40)]. In addition, patients who used statins for the longest period had the lowest risk of these outcomes (all tests for trend p<0.05).
In a retrospective cohort study of 55,875 men in the veterans population who were followed from 1997–2007, Farwell and colleagues [15] reported that statin users were 31% less likely [HR 0.69 95% CI (0.52–0.90)] to be diagnosed with PCa when compared to men taking antihypertensive medication. Additionally, patients using statins were 14% less likely [HR 0.86 95% CI (0.62 to 1.20)] to be diagnosed with low-grade PCa and 60% less likely [HR 0.40 95% CI (0.24 to 0.65)] to be diagnosed with high-grade PCa. This report is of particular interest because the patient population was well controlled for access and attention to medical care. One of the confounding problems in interpreting the observational studies is whether there is an extraneous difference between the cohorts taking statins vs. those not taking statins. Consequently, one explanation for some of the study results is that statin users have greater access to health care or are more health conscious. In these cases, cancer might be diagnosed earlier, resulting in a greater incidence of early stage disease. Because the two groups compared in Farwell et al. [15], statin takers vs. takers of antihypertensive drugs, are seemingly equally invested in health care, this confounder is diminished.
A retrospective cohort study by Kollmeier et al. [33] identified 1,681 patients who between 1995 and 2007 were treated for PCa with radiotherapy of which 382 patients were taking a statin. The 5- and 8-year PSA relapse-free survival (PRFS) rates for statin patients were 89% and 80%, compared with 83% and 74% for those not taking statins (p = 0.002). In a multivariate analysis, statin use was associated with improved PRFS [(HR 0.69 95% CI (0.50–0.97), p = 0.03]. Additionally, when examining patients diagnosed in a high risk group, statin users showed improved PRFS [HR 0.52 95% CI (0.30–0.91), p = 0.02] when compared to those not taking a statin. Statin use was not associated with improved distant metastasis-free survival.
A study by Alizadeh et al. [34] included 381 patients treated with either radiotherapy or brachytherapy for low-risk (n = 152), intermediate-risk (n = 142), or high-risk (n = 87) localized PCa. 45.1% were taking statins, 37.0% were taking anticoagulants (AC) and 27.6% used both. Exclusive users of statins compared with users of neither drug class had a lower adjusted odds ratio [OR 0.29 95% CI (0.09–0.88), p = 0.03] of having a PSA level >20 ng/mL. Concomitant AC and statin use was also associated with a lower likelihood of a PSA level >20 ng/mL, and a greater likelihood of a PSA <10 ng/ml. Similarly, exclusive statin use was associated with a greater probability of having a PSA level <10 ng/mL [OR 2.9 95% CI (1.3–6.8), p = 0.012]. A significant effect was also found between the concomitant use of statins and ACs with high-risk localized PCa [OR 0.43 95% CI, (0.21–0.87), p = 0.02], although no association was found with lowrisk localized PCa (p =0.3).
A case-control study by Marcella et al. [35] examined cases between 1997 and 2000 in which PCa was the underlying cause of death. With 1:1 matching for age, race and observation time, they identified 379 cases and controls. The adjusted analysis showed a decrease in PCa deaths among statin users compared to their age-matched controls [OR 0.45 95% CI (0.29–0.71), p=0.0006)]. Further analysis showed that highpotency statins (cerivastatin, atorvastatin, & simvastatin) were associated with a significant risk reduction [OR 0.27 95% (CI 0.15–0.48), p <0.0001], while low-potency statins (pravastatin, lovastatin, & fluvastatin) were associated with a non-significant risk reduction [(OR 0.69 95% CI (0.33–1.45)].
In summary, recent observational studies of statin effects on PCa risk, which contain large numbers of subjects, are largely supportive of the hypothesis that statins reduce risk of advanced PCa.
The recent literature indicates that long-term statin therapy is chemopreventive against aggressive PCa, while large randomized trials of statin drugs that report on cancer (including PCa) do not support this claim. We have reviewed this literature previously and have cited several concerns about the all-cancer studies: their relatively short duration, relatively few PCa cases, no recording of PCa grade or stage, large crossover of patients from control to statin groups due to usual care requirements, and the over-representation of pravastatin, the least potent statin, to explain why these studies are inconclusive concerning statin use and PCa [5,26,36]. Nothing published in the past 2 years has altered this conclusion.
Pre-clinical studies
Schaffner and colleagues provided the initial evidence that reducing cholesterol levels systemically altered prostate cell growth and/or survival. These investigators showed that prostate regression was selectively induced in dogs and rodents by oral administration of hypocholesteremic agents, such as the polyene macrolide candicidin (reviewed in [1]). However, progress on the role of cholesterol in PCa incidence and progression in animal models stalled for many years until reports by Zhuang et al. [37] and Solomon et al. [38] renewed interest. Using a xenograft model, they found that hypercholesterolemia accelerated the growth of prostatic tumors, while hypocholesterolemia had the opposite effect and retarded tumor growth. In these studies, hypercholesterolemia was associated with increased tumor cell proliferation, activated Akt (a critical kinase in PCa progression), and increased intratumoral androgen levels[37–39]. Others have used spontaneous PCa models to reinforce the conclusions of Zhuang et al. and Solomon et al. Using the TRAMP mouse model, an autochthonous model of PCa [40], hypercholesteremia was shown to result in increased tumor volume and progression as well as increased tumor incidence and metastases to the lung. Histologically, these tumors showed increased proliferation, angiogenesis, expression of cyclin D1 and expression of scavenger receptor class B type 1(SR-B1). Taken together, these reports suggest that circulating cholesterol affects multiple pathways and physiologic mechanisms, findings that may underlie the observations in humans described above.
Cholesterol-sensitive mechanisms in prostate cancer progression
There are a number of possible ways that excess cholesterol could affect responses by malignant cells, including effects on cell proliferation, inflammation, membrane organization and steroidogenesis. Here we will briefly outline the case for some of the potential mechanisms.
Cell proliferation
It has been known for decades that cholesterol is critical for the proliferation of animal cells, and its synthesis is tightly synchronized to cell cycle progression.
Studies examining a specific effect of cholesterol on the cell cycle have shown that reducing cholesterol levels through synthesis blockades induce cells to undergo growth arrest. Whether low cholesterol leads to growth arrest because of the paucity in materials for membrane synthesis, and/or because of a more specific regulatory role is not clear. However, certain observations suggest that cholesterol plays an essential role in cell cycle progression in animal cells. For example, in several different organisms, absence of their main membrane sterol causes growth arrest despite the availability of sufficient amounts of a related, membrane-compatible sterol (e.g., replacing cholesterol with ergosterol). In these cases, a small amount of native sterol, an amount insufficient for membrane synthesis, is required to restore cell cycle progression ([41] and references within).
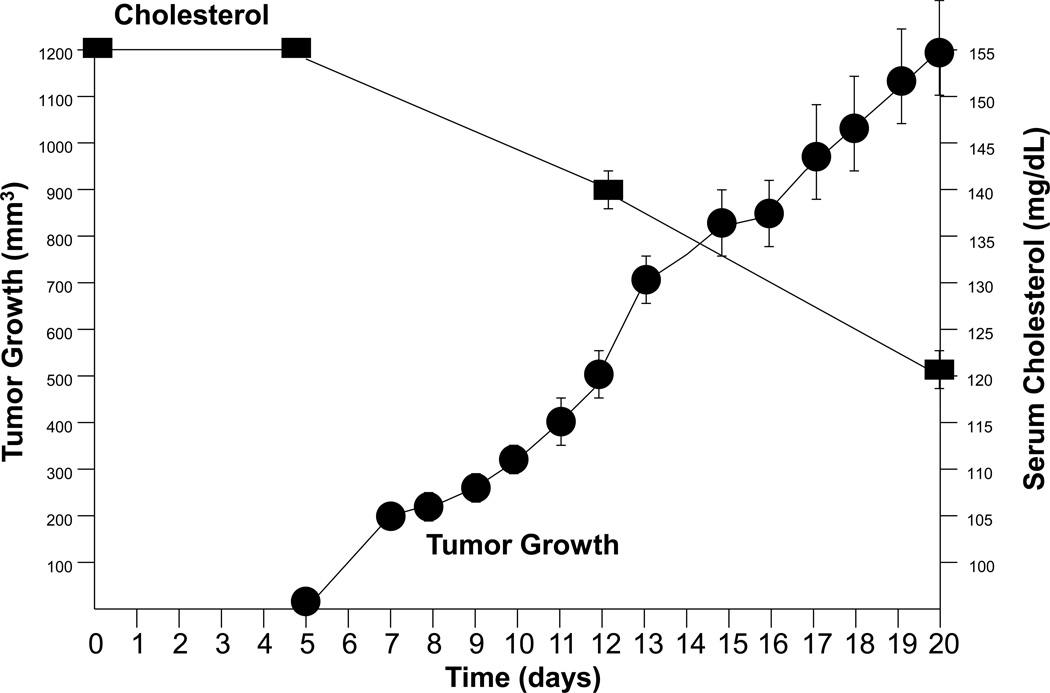
Regardless of any role for cholesterol in cell cycle control, the rapid growth and high metabolic rate of cancer cells requires a large amount of cholesterol. In fact so much cholesterol may be required that extant cancer is known to lower serum cholesterol. Examples of this phenomenon include: Sherwin et al., who reported that men developing cancer had a 22.7-mg/dL decrease in their cholesterol levels vs. matched survivors. Similarly, Keys et al. found that men who died of cancer within two years of the study’s initiation had cholesterol levels 9.5% lower than the mean of all men at entry. Men dying from cancer one year after their last cholesterol measurement had concentrations that were 24–35 mg/dL less than controls (i.e. those not dying); those succumbing 2–5 years after cholesterol measure had levels 4–5 mg/dL less than controls; and those dying of cancer 6–10 years after cholesterol measure had concentrations 2 mg/dL less than controls (reviewed in [5]). The phenomenon of tumor growth resulting in reduced circulating cholesterol is also recapitulated in pre-clinical models of PCa (Fig. 2).
Figure 2. Relationship of prostatic tumor growth to serum cholesterol level in a pre-clinical model.
Normocholesterolemic immunodeficient mice had their serum cholesterol determined and 5 days later were implanted with subcutaneous LNCaP tumors (4 per mouse). Tumor growth and serum cholesterol were monitored until the experiment was complete (day 20). Tumor growth is plotted as tumor volume (mm3) vs. time (days) and serum cholesterol level is plotted as cholesterol concentration (mg/dL) vs. time (days) ± SEM. Note that as tumor volume increases, serum cholesterol level decreases.
These observations reveal the tendency of cancer to reduce circulating cholesterol levels, possibly a result of the requirement by malignant cells for increased rates of metabolism, a phenomenon related to the Warburg effect, in which an abundance of macromolecules is needed by the tumor cells to support rapid growth. Prior to the advent of the statin era it was thought that these findings pointed to hypocholesterolemia as a potential risk factor for cancer, but this notion has been disproven and it is now known that existing cancer reduces circulating cholesterol levels, with low cholesterol an effect of cancer, not its cause [5]. Because of the essential role of cholesterol in tumor cell proliferation, cholesterol lowering may induce substantial apoptosis in PCa cells progressing through the cell cycle [41].
Inflammation
The pathological consequences of hypercholesterolemia in humans are prominently seen in the formation of atherosclerotic lesions, with high LDL levels resulting in the accumulation of LDL particles in the arterial intima, where they are enzymatically modified to become inflammatory agonists. Accumulation of these deposits over time results in the inflammatory and pathological changes that are the hallmarks of atherosclerosis. Clinical consequences of atherosclerosis can be reversed 20–40% by prolonged treatment with statins, with risk reduction proportional to the extent of LDL lowering. These and other data identify cholesterol, and its synthetic products (e.g. cholesteryl esters), as major mediators of inflammation within the cardiovascular system.
Many human cancers (~20%) are thought to develop as a consequence of chronic inflammatory or infectious conditions. Increasing evidence now links pathologic or premalignant changes in the prostate, as well as PCa, with inflammation (reviewed in [42]).
Human CD4+ and CD8+ T cells recognize antigenic sequences present in PSA, suggesting that prostatic secretory products, may result in autoimmunity. IL-6 and IL-8, inflammatory mediators, are also mitogens for prostate cells, hinting at a potential role for inflammatory components in disrupting prostate tissue homeostasis by altering the balance between cell growth and death [42].
In the human prostate, focal areas of epithelial atrophy are often accompanied by inflammatory infiltrates, a condition recently named “proliferative inflammatory atrophy” (PIA). Frequent transitions between areas of PIA, or proliferative atrophy without inflammatory infiltrate, and high-grade prostatic intraepithelial neoplasia (PIN) have been described [42].
Inflammatory processes preceding neoplastic changes are also observed in preclinical models. For example, neonatal estrogen imprinting of the prostate causes lobespecific inflammation, hyperplasia, and PIN-like lesions in adult animals[42]. Investigations into PCa susceptibility loci have identified a number of genes involved in immunity, including RNASEL, MSR1, and TLR4. Other loci involved in inflammation have also been associated with increased PCa risk [42]. In total, although the data are incomplete, these observations suggest that the prostate is susceptible to several types of inflammatory disruptions leading to pathology, with cholesterol potentially contributing to sustained inflammation. This also points to the intriguing possibility that the action of statins on PCa risk could potentially be accounted for by the drugs’ anti-inflammatory activity. It is also important to point out that cholesterol promotes PCa progression in immunodeficient pre-clinical models, suggesting that inflammation alone is unlikely to explain the PCa-cholesterol association [37–39].
Membrane organization
Studies of the biophysical properties of the plasma membrane, of the behavior of glycosylphosphatidylinositol (GPI)-anchored proteins in membranes, of membrane organelles termed caveolae, and of intracellular transport processes provide evidence for the existence of a distinct type of cholesterol rich-membrane microdomain generally referred to as the lipid raft [1–3]. These domains contain high concentrations of cholesterol and fatty acids with long saturated acyl chains (e.g. sphingolipids), relative to other plasma membrane domains. The acyl chain composition of the lipids in the membrane is a major determinant of lipid segregation into rafts, with cholesterol providing structural order to the fluid lipid bilayer. At high concentrations, the tight packing of these components causes rafts to exist in a liquid-ordered phase, in contrast to the liquid-disordered state of the wider membrane [1–3].
In their simplest form, lipid rafts are small heterogeneous membrane domains consisting of cholesterol, sphingolipids, and glycolipids. They variably contain cohorts of (GPI)-anchored proteins, src family kinases, G proteins and other components. Many studies, indicate that lipid rafts, as discrete domains within the ‘lipid sea’, serve as privileged sites for certain types of cell signaling, signal pathway cross-talk and signal amplification [1,2].
Our current understanding of lipid rafts is incomplete and certain ideas about rafts have proven controversial. One serious concern stems from results of single molecule tracking studies that have suggested that large stable raft domains are unlikely to exist. Consistent with these findings, we argue that rafts are small and heterogeneous, but are subject to alteration in size and composition as a consequence of specific perturbations (e.g. excess cholesterol) [1,2]. This paradigm is in agreement with even the most skeptical reports on the lipid raft hypothesis, and is in fact important for understanding how excess cholesterol may affect cells in general, and prostate epithelial cells specifically.
Signals critical to PCa cell survival and progression are transmitted through rafts. Studies [43–45] have demonstrated that particular proteins, critical for PCa growth and survival, are regulated by lipid rafts and that alteration in membrane cholesterol affects in measurable ways the signals generated by these molecules. Specifically it has been demonstrated that a subpopulation of epidermal growth factor receptor (EGFR) within lipid rafts of PCa cells is far more active, and much more highly phosphorylated than the cohort of receptor in non-raft membranes, and that signaling by the EGFR to downstream effectors is disrupted when cholesterol is targeted [44,45]. It has also been shown that a subpopulation of Akt found within rafts exhibits profoundly different substrate specificity than non-raft Akt. This raft-resident Akt is inhibited by cholesterol level reduction [43]. Signaling by LXRs (liver X receptors) leads to PCa cell apoptosis by downregulating the level of phosphorylated Akt present in rafts, a process precipitated by LXR-stimulated cholesterol efflux and reversed by the addition of exogenous cholesterol. The aggregate data suggest that cholesterol regulates lipid raft dynamics, which in turn affects vital signaling pathways, with increased cholesterol acting to protect cells from apoptosis through effects on lipid rafts [46].
Steroidogenesis
Prostate tumor cells respond to androgen through the action of the androgen receptor (AR), a nuclear receptor that controls PCa cell proliferation at all stages of disease, including late-stage, castration-resistant disease. In the last few years multiple lines of evidence have converged on the hypothesis that PCa cells carry out intratumoral steroidogenesis (androgen synthesis) [47–49] sufficient to activate the AR, explaining, in part, the development of castration-resistance. Studies [47] have demonstrated that the enzymes necessary for de novo androgen synthesis are expressed in PCa tumor xenografts, and that androgen-starved PCa cells have the capacity to synthesize dihydrotestoserone (DHT) from acetic acid, indicating that the entire mevalonate-steroidogenic pathway is functionally intact [47]. Other reports have demonstrated that all enzymes necessary for testosterone and DHT synthesis are present in many human primary and metastatic PCas[49], implying that de novo steroidogenesis is not merely an experimental phenomenon, but instead a possible insidious mechanism of disease progression in the hormone-repressed state.
Cholesterol is an essential precursor in androgen synthesis; consequently, it is possible that cholesterol promotes PCa growth through effects on steroidogenesis. To test this possibility we used the in vivo LNCaP PCa xenograft model and diet-induced hypo- and hypercholesterolemia to demonstrate that circulating cholesterol levels are significantly associated with tumor size (R=0.3957, p=0.0049) and intratumoral levels of testosterone (R=0.41, p=0.0023) [39]. We also demonstrated that the xenograft tumors expressed the full spectrum of steroidogenic enzymes necessary for androgen biosynthesis from cholesterol, with the intratumoral cholesterol concentration directly correlated with expression of CYP17A, an essential enzyme required for de novo synthesis of androgens from cholesterol (R=0.4073, p=0.025) [39]. This last result suggests that cholesterol acts not only as an essential precursor, but also as a pathway agonist, stimulating the upregulation of steroidogenic gene expression. These results are in accord with those of others who demonstrated that proteins responsible for cholesterol regulation are altered during disease progression to increase the pool of available cholesterol, coincident with an increase in androgens to physiologically relevant levels [50].
Conclusion
Epidemiological observations and pre-clinical models suggest that hypercholesterolemia plays an important role in the progression of PCa, with many human and mechanistic studies now supporting roles for cholesterol in PCa progression and for cholesterol-lowering statin drugs in retarding PCa growth. Cholesterol functions as a mediator of cell proliferation, membrane dynamics, inflammation and steroidogenesis, thus providing multiple avenues for this lipid to contribute to PCa progression.
It will be fascinating to watch as this new knowledge is exploited prospectively to reduce the number of advanced PCa cases in whole populations through strategies that are specifically focused on cholesterol reduction. One such approach would be to employ enhanced cholesterol lowering for chemoprevention in at-risk populations by using the most potent statins and/or combination therapy with statin and non-statin drugs. It is also possible that in the next decade we will witness the use of cholesterol-lowering regimens as adjuvant therapy to treat existing PCa to slow progression and/or render the cancer more susceptible to conventional therapies. We suggest that sufficient mechanistic and human data are now available to support applying these strategies clinically in the case of patients eligible for or desiring disease management by active surveillance.
Highlights.
Hypercholesterolemia increases the risk of aggressive prostate cancer.
Cholesterol-lowering statin drugs reduce the risk of aggressive prostate cancer.
Hypercholesterolemia may elicit multiple mechanisms that effect prostate cancer.
Hypercholesterolemia may be a risk factor for developing castration-resistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91:54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 2.Hager MH, Solomon KR, Freeman MR. The role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab Care. 2006;9:379–385. doi: 10.1097/01.mco.0000232896.66791.62. [DOI] [PubMed] [Google Scholar]
- 3.Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 4.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 5.Solomon KR, Freeman MR. The complex interplay between cholesterol and prostate malignancy. Urol Clin North Am. 2011;38:243–259. doi: 10.1016/j.ucl.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson MM, Garland C, Barrett-Connor E, Khaw KT, Friedlander NJ, Wingard DL. Heart disease risk factors, diabetes, and prostatic cancer in an adult community. Am J Epidemiol. 1989;129:511–517. doi: 10.1093/oxfordjournals.aje.a115162. [DOI] [PubMed] [Google Scholar]
- 8.Huxley R. The impact of modifiable risk factors on mortality from prostate cancer in populations of the Asia-Pacific region. Asian Pac J Cancer Prev. 2007;8:199–205. [PubMed] [Google Scholar]
- 9. Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008;123:1693–1698. doi: 10.1002/ijc.23715. First definitive epidemiological report tying cholesterol to the risk of advanced prostate cancer.
- 10. Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, Neuhouser ML, Klein EA, Thompson IM, Jr, Kristal AR. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2807–2813. doi: 10.1158/1055-9965.EPI-09-0472. Confirms the results of Platz et al. 2008.
- 11.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21:61–68. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Hemelrijck M, Garmo H, Holmberg L, Walldius G, Jungner I, Hammar N, Lambe M. Prostate cancer risk in the Swedish AMORIS study. the interplay among triglycerides, total cholesterol, and glucose. Cancer. 2011;117:2086–2095. doi: 10.1002/cncr.25758. [DOI] [PubMed] [Google Scholar]
- 13.Van Hemelrijck M, Walldius G, Jungner I, Hammar N, Garmo H, Binda E, Hayday A, Lambe M, Holmberg L. Low levels of apolipoprotein A-I and HDL are associated with risk of prostate cancer in the Swedish AMORIS study. Cancer Causes Control. 2011;22:1011–1019. doi: 10.1007/s10552-011-9774-z. [DOI] [PubMed] [Google Scholar]
- 14.Batty GD, Kivimaki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London. forty years of follow-up in the Whitehall study. Cancer Causes Control. 2011;22:311–318. doi: 10.1007/s10552-010-9691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farwell WR, D'Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011;103:885–892. doi: 10.1093/jnci/djr108. Excellent, well controlled study showing a relationship between statin drug usage and prostate cancer risk.
- 16.Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years' follow up. BMC Cancer. 2012;12:25. doi: 10.1186/1471-2407-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control. 2011;22:1545–1552. doi: 10.1007/s10552-011-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kok DE, van Roermund JG, Aben KK, den Heijer M, Swinkels DW, Kampman E, Kiemeney LA. Blood lipid levels and prostate cancer risk; a cohort study. Prostate Cancer Prostatic Dis. 2011;14:340–345. doi: 10.1038/pcan.2011.30. [DOI] [PubMed] [Google Scholar]
- 19.Flick ED, Habel LA, Chan KA, Van Den Eeden SK, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry CP, Jr, et al. Statin Use and Risk of Prostate Cancer in the California Men's Health Study Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2218–2225. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 20.Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, Dalton SO, Sorensen HT, Olsen JH. Cancer risk among statin users. a population-based cohort study. Int J Cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 21.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2213–2217. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 23.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-Lowering Drugs and Prostate Cancer Risk. A Population-based Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226–2232. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 24. Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. First study demonstrating an effect of statin drugs on the risk of advanced prostate cancer.
- 25.Breau RH, Karnes RJ, Jacobson DJ, McGree ME, Jacobsen SJ, Nehra A, Lieber MM, St Sauver JL. The association between statin use and the diagnosis of prostate cancer in a population based cohort. J Urol. 2010;184:494–499. doi: 10.1016/j.juro.2010.03.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon KR, Freeman MR. Do the cholesterol-lowering properties of statins affect cancer risk? Trends Endocrinol Metab. 2008;19:113–121. doi: 10.1016/j.tem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Fowke JH, Motley SS, Barocas DA, Cookson MS, Concepcion R, Byerly S, Smith JA., Jr The associations between statin use and prostate cancer screening, prostate size, high-grade prostatic intraepithelial neoplasia (PIN), and prostate cancer. Cancer Causes Control. 2011;22:417–426. doi: 10.1007/s10552-010-9713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs EJ, Newton CC, Thun MJ, Gapstur SM. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011;71:1763–1771. doi: 10.1158/0008-5472.CAN-10-2953. [DOI] [PubMed] [Google Scholar]
- 29.Chang CC, Ho SC, Chiu HF, Yang CY. Statins increase the risk of prostate cancer. a population-based case-control study. Prostate. 2011;71:1818–1824. doi: 10.1002/pros.21401. [DOI] [PubMed] [Google Scholar]
- 30.Ritch CR, Hruby G, Badani KK, Benson MC, McKiernan JM. Effect of statin use on biochemical outcome following radical prostatectomy. BJU Int. 2011;108:E211–E216. doi: 10.1111/j.1464-410X.2011.10159.x. [DOI] [PubMed] [Google Scholar]
- 31.Mondul AM, Han M, Humphreys EB, Meinhold CL, Walsh PC, Platz EA. Association of statin use with pathological tumor characteristics and prostate cancer recurrence after surgery. J Urol. 2011;185:1268–1273. doi: 10.1016/j.juro.2010.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan N, Klein EA, Li J, Moussa AS, Jones JS. Statin use and risk of prostate cancer in a population of men who underwent biopsy. J Urol. 2011;186:86–90. doi: 10.1016/j.juro.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Kollmeier MA, Katz MS, Mak K, Yamada Y, Feder DJ, Zhang Z, Jia X, Shi W, Zelefsky MJ. Improved biochemical outcomes with statin use in patients with high-risk localized prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:713–718. doi: 10.1016/j.ijrobp.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Alizadeh M, Sylvestre MP, Zilli T, Van Nguyen T, Guay JP, Bahary JP, Taussky D. Effect of Statins and Anticoagulants on Prostate Cancer Aggressiveness. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 35.Marcella SW, David A, Ohman-Strickland PA, Carson J, Rhoads GG. Statin use and fatal prostate cancer. A matched case-control study. Cancer. 2011 doi: 10.1002/cncr.26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman MR, Solomon KR, Moyad M. Statins and the risk of cancer. Jama. 2006;295:2720–2721. doi: 10.1001/jama.295.23.2720-b. author reply 2721–2722. [DOI] [PubMed] [Google Scholar]
- 37. Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. First demonstration that hypercholesterolemia accelerates the growth of prostatic tumors in a pre-clinical model.
- 38. Solomon KR, Pelton K, Boucher K, Joo J, Tully C, Zurakowski D, Schaffner CP, Kim J, Freeman MR. Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol. 2009;174:1017–1026. doi: 10.2353/ajpath.2009.080551. Confirms and extends the observations of Zhuang et al. using isocaloric diets and the hypocholesterolemic agent ezetimibe.
- 39. Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of Circulating Cholesterol Levels on Growth and Intratumoral Androgen Concentration of Prostate Tumors. PloS One. 2012;7:e30062. doi: 10.1371/journal.pone.0030062. Demonstrates that circulating cholesterol, intratumoral androgen level and tumor growth were associated in a pre-clinical model of prostate cancer.
- 40. Llaverias G, Danilo C, Wang Y, Witkiewicz AK, Daumer K, Lisanti MP, Frank PG. A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am J Pathol. 2010;177:3180–3191. doi: 10.2353/ajpath.2010.100568. Reiterates the points of Zhuang et al. and Solomon et al. using a spontaneous tumor model.
- 41.Dong P, Flores J, Pelton K, Solomon KR. Prohibitin is a cholesterol-sensitive regulator of cell cycle transit. J Cell Biochem. 2010;111:1367–1374. doi: 10.1002/jcb.22865. [DOI] [PubMed] [Google Scholar]
- 42.Freeman MR, Solomon KR. Cholesterol and benign prostate disease. Differentiation. 2011;82:244–252. doi: 10.1016/j.diff.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adam RM, Mukhopadhyay NK, Kim J, Di Vizio D, Cinar B, Boucher K, Solomon KR, Freeman MR. Cholesterol sensitivity of endogenous and myristoylated Akt. Cancer Res. 2007;67:6238–6246. doi: 10.1158/0008-5472.CAN-07-0288. Demonstrates for the first time that, Akt a kinase critical in prostate cancer, is regulated by lipid rafts.
- 44.Freeman MR, Cinar B, Kim J, Mukhopadhyay NK, Di Vizio D, Adam RM, Solomon KR. Transit of hormonal and EGF receptor-dependent signals through cholesterol-rich membranes. Steroids. 2007;72:210–217. doi: 10.1016/j.steroids.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]
- 46.Pommier AJ, Alves G, Viennois E, Bernard S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 47. Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. First report, using a pre-clinical model showing that tumor androgens increase during CRPC progression in correlation to PSA up-regulation and that all enzymes necessary for androgen synthesis are expressed in prostate cancer tumors and may be upregulated during CRPC progression.
- 48. Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–120. doi: 10.1016/j.mce.2008.08.013. Demonstrates for the first time that prostate cancer cells in advanced stages of the disease could synthesize androgens from cholesterol and hence are not dependent upon testicular and/or adrenal androgens.
- 49.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer. a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD, Nelson CC, Guns ES, Wasan KM. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castrationresistant prostate cancer in a mouse xenograft model. Prostate. 2010;70:390–400. doi: 10.1002/pros.21072. Using a pre-clinical prostate cancer model these authors demonstrate that proteins responsible for cholesterol regulation are altered during disease progression to increase intratumoral cholesterol levels with a coincident increase in androgens.