Abstract
The regulatory activities of mouse CD4+Foxp3+ T cells on various immune cells, including NK cells, have been well documented. Under some conditions, conventional CD4+Foxp3− T cells in the periphery are able to acquire inhibitory function on other T cells, but their roles in controlling innate immune cells are poorly defined. As a potential cellular therapy for cancer, ex vivo activated CD4+Foxp3− effector T cells are often infused back in vivo to suppress tumor growth and metastasis. Whether such activated T cells could affect NK-cell control of tumorigenesis is unclear. In the present study, we found that mitogen-activated CD4+Foxp3− T cells exhibited potent suppressor function on NK-cell proliferation and cytotoxicity in vitro, and notably facilitated B16 melanoma metastasis in vivo. Suppression of NK cells by activated CD4+Foxp3− T cells is cell-cell contact dependent and is mediated by Qa-1:NKG2A interaction, as administration of antibodies blocking either Qa-1 or NKG2A could completely reverse this suppression, and significantly inhibited otherwise facilitated melanoma metastasis. Moreover, activated CD4+Foxp3− cells from Qa-1 knockout mice completely lost the suppressor activity on NK cells, and failed to facilitate melanoma metastasis when transferred in vivo. Taken together, our findings indicate that innate anti-tumor response is counter regulated by the activation of adaptive immunity, a phenomenon we term as “activation-induced inhibition”. Thus, the regulatory role of activated CD4+Foxp3− T cells in NK-cell activity must be taken into consideration in the future design of cancer therapies.
Keywords: NK cells, T cells, suppression, melanoma, metastasis, Qa-1
Introduction
Regulation is central to immune responses and immune tolerance. Naturally-occurring CD4+Foxp3+ cells are considered to be one of the main types of T regulatory cells (Tregs), which are involved in regulating a wide range of activities including the responses to autoantigens, alloantigens, tumor antigens, pathogen-derived antigens, and even the maintenance of normal pregnancy1. CD25 has been used as a traditional, albeit imperfect, marker for CD4+Foxp3+ Tregs. Nonetheless, peripheral mature CD4+CD25− T cells can also be induced to acquire inhibitory function in vivo2,3,4,5,6,7. For example, both CD4+CD25+ and CD4+CD25− T cells from tolerant mice could mediate suppression, although CD4+CD25+ T cells were more efficient than CD4+CD25− T cells8. CD4+CD25− T cells activated by anti-CD3 monoclonal antibody were able to inhibit the activation and proliferation of bystander CD4+ T cells in a cell contact-dependent manner, reversible by exogenous IL-2 and anti-Glucocorticoid Induced Tumor Necrosis Factor Receptor (GITR)9. In some of these studies when Foxp3 expression was examined, CD4+CD25− T cells are still Foxp3+5,7, whereas in others, they are clearly Foxp3−6. These data suggest that T cells with regulatory activities are likely to be extremely diverse, but it is not clear how such diverse regulatory T cells assume common responsibility in regulating productive immune responses, which often involve cells in both adaptive and innate immune systems.
NK cells are one of the most important innate immune cells for protection against virus infection and tumor growth10, and are best characterized by their ability of cytotoxicity towards target cells without pre-activation and immunoregulatory cytokine production11,12. NK-cell activation induced by a set of defined surface receptors has been well elucidated12,13. However, signals delivered to NK cells from other cells, especially from adaptive immune cells, have not been adequately studied. The anatomical proximity of NK cells and T cells increases the probability of direct interactions between them14. Several recent studies show that naturally occurring CD4+CD25+ T cells could inhibit NK-cell function through TGF-β1, most likely in its membrane-bound form15,16,17. However, there is no evidence regarding the influence of activated CD4+CD25− T cells on NK-cell function. In the present study, we polyclonally activated Treg-depleted CD4+CD25− T cells with Concanavalin A (Con A), and examined the effects of pre-activated CD4+CD25− T cells on NK-cell-mediated cytotoxicity both in vitro and in vivo. Our data indicate that activated CD4+CD25− T cells dampen NK-cell cytotoxicity and facilitate melanoma metastasis via Qa-1-dependent pathway.
Results
Activated CD4+ T cells exhibited potent inhibitory effect on NK-cell cytotoxicity and proliferation
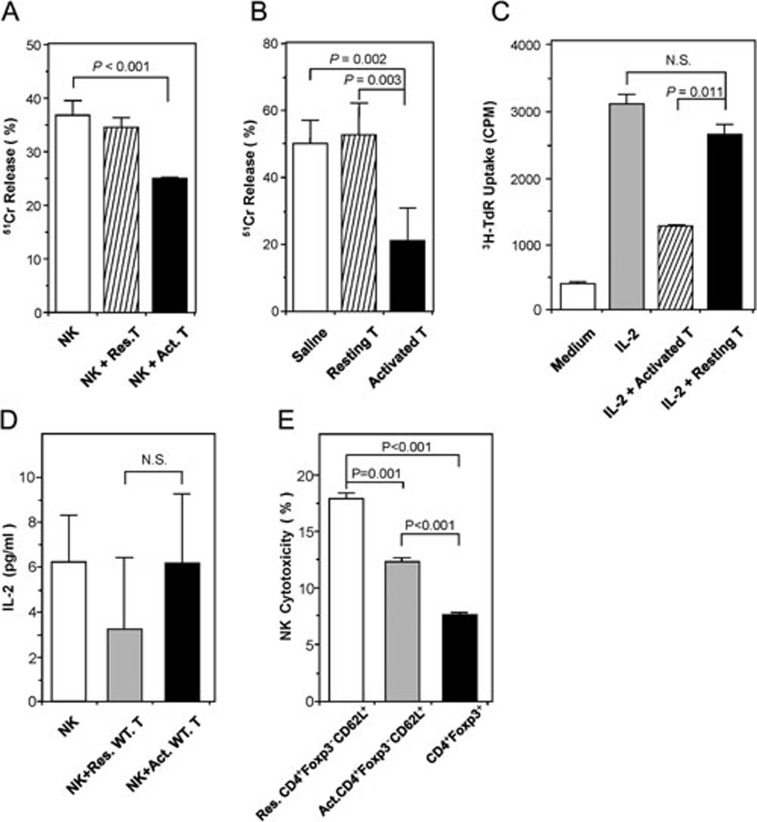
To determine the impact of activated CD4+ effector T cells on NK-cell cytotoxicity, we MACS-purified CD4+CD25− T cells, stimulated with Con A, and then co-cultured them with freshly isolated syngeneic NK cells in an in vitro 51Cr-release cytotoxicity assay. We found that activated T cells, but not resting T cells, significantly inhibited NK-cell cytotoxicity. NK-cell lysis of YAC-1 target cells was inhibited by as much as 32% in the presence of activated CD4+ effector T cells (Figure 1A).
Figure 1.
The effect of activated CD4+ T cells on NK-cell cytotoxicity and proliferation. (A) Activated CD4+ T cells inhibited NK-cell function in vitro. NK cells were co-cultured with target YAC-1 cells alone, or in the presence of either resting CD4+CD25− T cells or CD4+CD25− T cells activated overnight with Concanavalin A (10 μg/ml). Data are shown as mean percentages of 51Cr release (± SD) after 48 h in vitro culture, and are representative of three independent experiments. (B) Activated CD4+ T cells inhibited NK-cell function in vivo. Nude mice (n = 3/group) were injected twice with saline or 2 × 106 of either resting or Con A-activated syngeneic CD4+CD25− T cells on days 0 and 1. On day 4, cytolytic activities of splenocytes on YAC-1 cells were determined ex vivo by 51Cr release assay. Data are shown as mean percentages of 51Cr release (±SD), and are representative of three independent experiments. (C) Activated CD4+ T cells inhibited NK-cell proliferation in vitro. Freshly isolated NK cells were stimulated with 100 U/ml IL-2, with or without the fixed (0.5% glutaraldehyde) Con A-activated CD4+ T cells. NK-cell proliferation was analyzed after 96 h by 3H-thymidine incorporation. The data are representative of three independent experiments. (D) IL-2 concentrations assayed in NK-T-cell co-culture. NK cells were co-cultured with either Con A-activated or resting T cells without exogenous IL-2 for 24 h. IL-2 concentrations in the supernatants were determined by mouse IL-2 ELISA kit. Data are representative of three independent experiments. (E) Con A-activated CD4+Foxp3−CD62L+ T cells inhibited NK cytotoxicity in vitro. CD4+Foxp3−CD62L+ T cells were sorted from mononuclear cells by FACS and then activated by Con A (10 μg/ml). Meanwhile, CD4+Foxp3+ T cells were sorted by FACS and served as control. Each type of FACS-sorted cells was co-cultured with NK cells. NK cytotoxicity was determined by FACS as described in Materials and Methods. The data are representative of two independent experiments.
To test whether activated CD4+ T cells could also inhibit NK-cell cytotoxicity in vivo, we adoptively transferred syngeneic resting or Con A-activated CD4+ T cells into BALB/c nude mice, which are athymic and lack of mature T cells. After 3 days, NK cells in the spleen were harvested to measure their cytotoxicity towards YAC-1 cells. Strikingly, cytotoxicity of NK cells from BALB/c nude mice receiving activated, but not resting, CD4+ T cells was reduced by 58% as compared to that from mice receiving saline control (Figure 1B).
To assess whether activated CD4+ T cells could exert an inhibitory effect on NK-cell proliferation, we stimulated NK cells with exogenous IL-2 (100 U/ml) alone or together with resting or Con A-activated CD4+ effector T cells. These T cells were fixed with 0.5% glutaraldehyde post-activation to prevent thymidine incorporation by themselves. Compared to the medium, IL-2 potently stimulated NK-cell proliferation. However, in the presence of activated T cells, IL-2-stimulated NK-cell proliferation was significantly inhibited. In contrast, resting T cells fixed with 0.5% glutaraldehyde did not apparently affect IL-2-stimulated NK-proliferation (Figure 1C).
Because activated T cells expressing IL-2 receptors could compete with NK cells for IL-2, we measured IL-2 concentrations in the supernatants of NK-T-cell co-cultures in the absence of exogenous IL-2. The data showed that there were no significant differences between the groups added with resting or Con A-activated T cells (Figure 1D).
As CD4+CD25− T cells are a mixture of naïve, effector, memory and small number of contaminating Foxp3+ regulatory T cells, we used Foxp3GFP knockin mice to FACS-sort CD4+Foxp3−CD62L+ naïve T cells and CD4+Foxp3+ Treg cells based on GFP expression, and tested their effects on NK cytotoxicity. Compared to resting CD4+Foxp3−CD62L+ naïve T cells, Con A-activated naïve T cells were found to inhibit NK cytotoxicity by 31% (Figure 1E, bar 2 vs. bar 1, P = 0.001). Even at 10-fold less cell number (NK:Treg = 1:1) than activated naïve T cells (NK:T = 1:10), CD4+Foxp3+ Treg cells potently inhibited NK cytotoxicity by 56% (Figure 1E, bar 3 vs. bar 1, P < 0.001). This suggests that activated naïve T cells may inhibit NK cytotoxicity in a mechanism different from that by Foxp3+ Treg cells.
Adoptive transfer of activated CD4+ T cells facilitated melanoma metastasis in SCID mice through suppressing NK-cell function
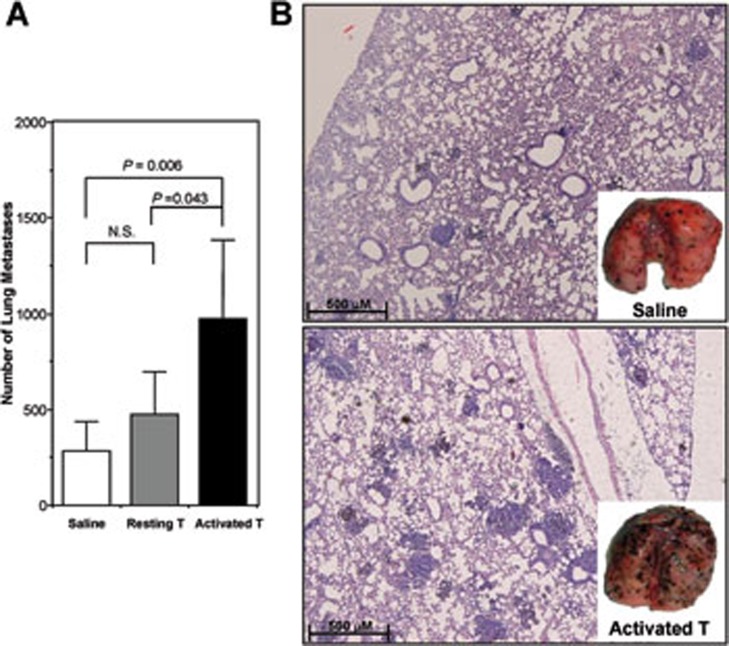
Because of the crucial role of NK cells in preventing tumorigenesis, we tested whether activated CD4+ T cells could affect NK-cell control of tumor growth and metastasis. We set up a B16 melanoma model in SCID mice, and adoptively transferred either resting or Con A-activated CD4+ T cells at the time of tumor cell inoculation. Compared to saline-treated control group, the number of metastatic melanoma nodules in the lung was significantly higher in the mice receiving activated, but not resting, CD4+ T cells (Figure 2A). Histological examination confirmed the gross observation by eye (Figure 2B). Compared to saline, transferred resting CD4+ T cells did not exhibit beneficial effect in this melanoma lung metastasis model, although they did provide protection in a liver metastasis model18.
Figure 2.
Activated CD4+ T cells facilitated B16 melanoma metastasis in SCID mice. Sex- and age-matched SCID mice were injected with 2.5 × 105 B16 tumor cells via tail vein on day 0. On days 1 and 3, 106 of resting or Con A-activated CD4+ T cells were adoptively transferred (i.v.). The control group was injected with an equal volume of saline. Tumor metastasis was assessed on day 10 in the lung. (A) Metastatic nodules on the lung surface were quantified with the aid of a dissecting microscope. Data are recorded as mean ± SD (n = 5), and Student's t test is used to compare saline and cell transfer groups. The results are representative of two independent experiments including five mice in each group. (B) Histological examination (HE staining, ×100) of melanoma formation in lung tissues confirmed that there was a significantly increased number of visible melanoma nodules on the lung surface (insets) in mice transferred with activated CD4+ T cells (bottom), as compared with those treated with saline (upper).
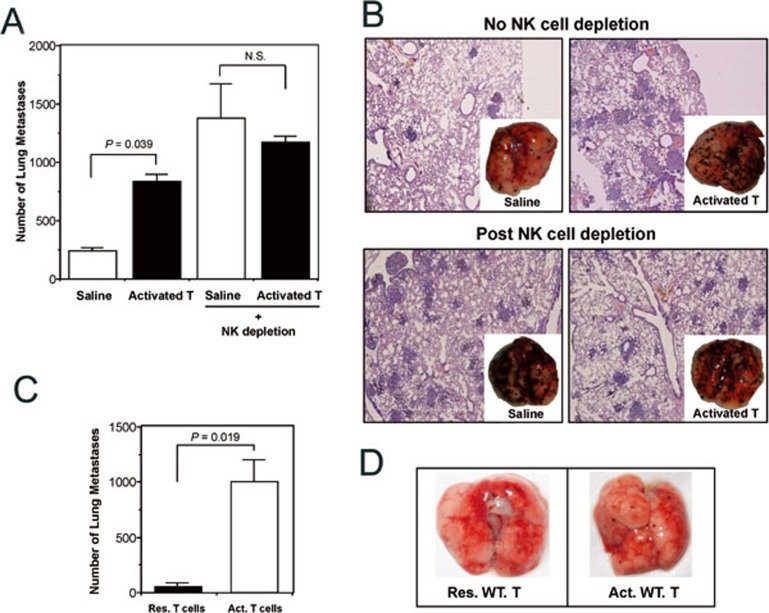
To determine whether NK cells were involved in the facilitation of melanoma metastasis by activated CD4+ T cells, we depleted NK cells with antibody PK136. The results showed that NK-cell depletion markedly enhanced tumor metastasis into the lung (Figure 3A and 3B). Under these conditions, adoptively transferred activated CD4+ T cells did not significantly influence the growth and metastasis of tumor cells (Figure 3A and 3B), suggesting that suppression of NK-cell function was involved in activated CD4+ T cell-facilitated melanoma metastasis. To further dissect the effect of transferred T cells from endogenous T and B cells on NK cells, RAG-1−/− mice were used as recipients of adoptive T-cell transfer. The results showed that although RAG-1−/− mice are more resistant to melanoma metastasis due to heightened NK-cell activities, transfusion of Con A-activated T cells compromised such innate immunity, and dramatically facilitated melanoma metastasis in the RAG-1−/− mice (Figure 3C and 3D).
Figure 3.
Activated CD4+ T cells facilitated melanoma metastasis through suppressing NK-cell function. Inoculation of B16 melanoma cells (on day 0) and transfer of Con A-activated CD4+ T cells into SCID or RAG-1−/− mice was carried out as described in Materials and Methods. NK cell depletion antibody (PK136) was injected (i.p.) on days -1, 0 and 1. (A) The visible melanoma metastatic nodules on the lung surface were counted on day 10. After NK cell depletion, there was no longer significant difference in tumor metastasis between animals treated with saline or activated T cells. Data are recorded as mean ± SD (n = 5), and student's t test is used to compare saline and cell transfer groups. (B) Histological examination (HE staining, ×100) of melanoma formation in lung tissues of mice with (bottom) or without (upper) NK cell depletion. Photo insets are representative of five animals of each group. (C) Sex- and age-matched RAG-1−/− mice were injected with 2 × 105 B16 tumor cells via tail vein on day 0. On days 1 and 3, 106 of resting or Con A-activated CD4+Foxp3− T cells were adoptively transferred. Tumor metastasis was assessed on day 10 on the lung surface. Data are recorded as mean ± SD (n = 6), and Student's t test is used to compare the groups receiving resting or Con A-activated CD4+Foxp3− T cells. (D) Representative photos of dissected lungs in C.
Activated CD4+ T cells are Foxp3−, and their suppression on NK-cell cytotoxicity was TGF-β and adenosine independent
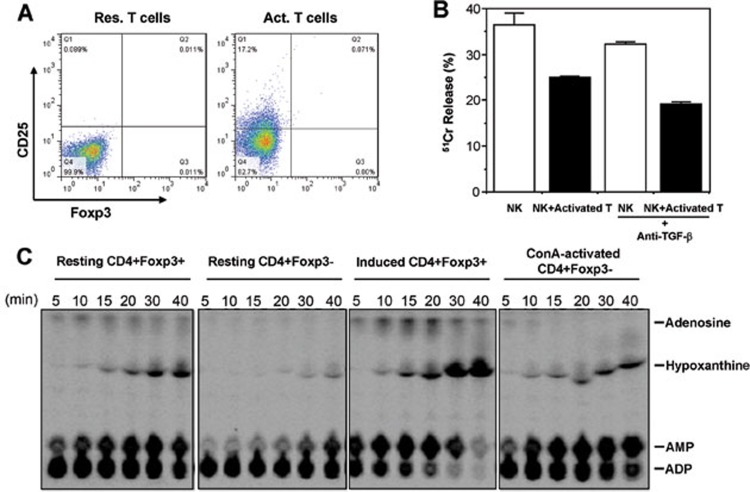
In several different experiments, after stimulation with Con A in the presence of APC, about 20%-50% of FACS-sorted CD4+Foxp3(GFP)− T cells became CD25-positive. However, these activated CD4+ T cells did not express Foxp3 (Figure 4A). Thus, activated naïve CD4+ T cells can suppress NK-cell function in a manner independent of Foxp3 expression.
Figure 4.
Suppression of NK cytotoxicity by activated CD4+ T cells was Foxp3, TGF-β and adenosine independent. (A) Flow cytometry analysis of GFP(Foxp3) in resting (left panel) and Con A-activated (right panel) CD4+CD25− T cells from Foxp3GFP knockin mice. (B) Inhibition of NK-cell cytotoxicity by Con A-activated CD4+Foxp3− T cells was TGF-β independent. NK cytotoxicity in the presence of Con A-activated CD4+Foxp3− T cells was compared with or without anti-TGF-β1 added into the co-culture system. Data are shown as mean percentages of 51Cr release (±SD) and are representative of three independent experiments. (C) Hydrolysis of extracellular 14C-radiolabeled ADP to adenosine was catalyzed by natural and TGF-β-induced CD4+Foxp3+ Tregs (Panels 1 and 3). Resting or Con A-activated CD4+Foxp3− T cells did not generate immunosuppressive adenosine (Panels 2 and 4).
To test whether like CD4+Foxp3+ Tregs, activated CD4+Foxp3− T cells could suppress NK cytotoxicity via TGF-β, we added antibodies against TGF-β1 into the co-culture. Still, neutralizing TGF-β1 failed to reverse the inhibitory effect of activated T cells on NK-cell cytotoxicity (Figure 4B). Another recently revealed mechanism of Treg action is that by co-expressing ecto-enzymes CD39 (ectonucleoside triphosphate diphosphohydrolase-1; EC 3.6.1.5) and CD73 (ecto-5′-nucleotidase; EC 3.1.3.5), Tregs can hydrolyze ATP/ADP into AMP and eventually to adenosine, which is immunosuppressive19. We thus FACS-sorted CD4+Foxp3(GFP)− cells from Foxp3GFP knockin mice, stimulated them with Con A in the presence of syngeneic APC, and after activation re-sorted the activated CD4+Foxp3(GFP)− cells and tested whether they did produce adenosine. The result showed that both natural Treg (nTreg) and in vitro TGF-β-induced Treg (iTreg) produced copious amounts of adenosine, whereas Con A-activated CD4+Foxp3− T cells failed to maintain a meaningful pool of adenosine, even though their CD39 enzymatic activity was increased to catalyze ADP→AMP (Figure 4C). In fact, upon activation, elevated CD39 activity can be observed also in CD4+Foxp3(GFP)− cells activated by plate-bound anti-CD3/CD28 (data not shown), but these cells do not produce adenosine as they do not express CD7319. Therefore, adenosine-mediated immunoregulation, which contributes to Treg suppressive activity, is not responsible for NK suppression by activated CD4+Foxp3− T cells.
Activated CD4+Foxp3− T cells suppress NK-cell cytotoxicity via Qa-1
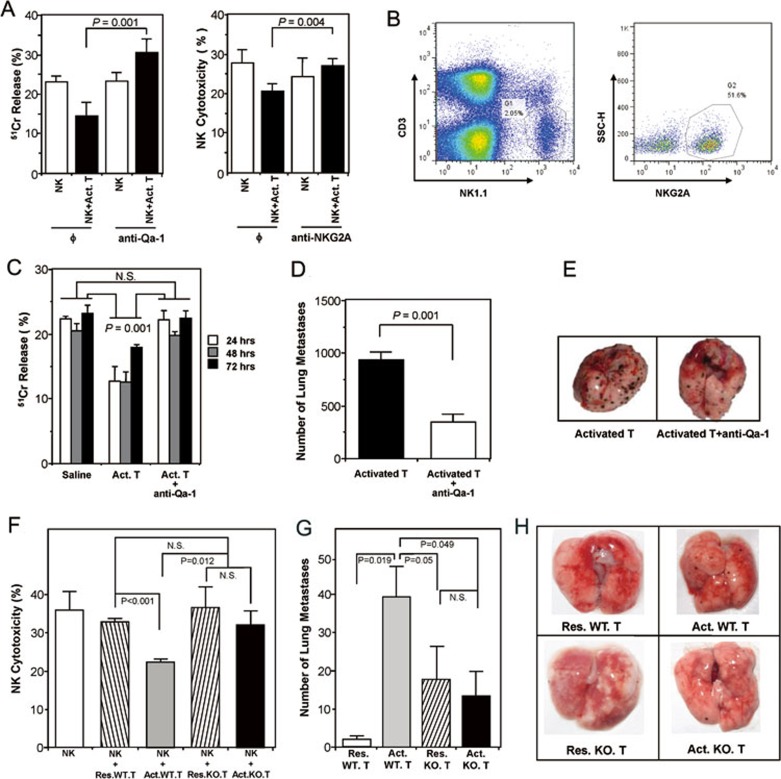
In mice, the nonpolymorphic major histocompatibility complex class Ib molecule Qa-1 is the homolog of human leukocyte antigen E (HLA-E), which forms a heterodimer with β2-microglobulin that presents peptides derived in TAP-dependent fashion from the major histocompatibility complex class Ia leader sequences (Qdm) and other self and foreign antigens20,21,22. The interaction between Qa-1 and nonclonally distributed NK cell inhibitory receptor CD94-NKG2A could dampen the cytotoxicity of NK cells and a subpopulation of CD8+ CTL23. Moreover, Qa-1 has been reported to be induced on Con A-activated splenic T cells24. We therefore tested in our system whether activated CD4+Foxp3− T cells suppress NK-cell cytotoxicity via Qa-1. Indeed, anti-Qa-1-blocking antibody could reverse the inhibitory effect of activated CD4+ T cells on NK-cell lytic activity against target cells in vitro (Figure 5A, left panel). As NKG2A is the ligand of Qa-1, we thus analyzed the expression of NKG2A on NK cells. Around 51.6% of CD3−NK1.1+ NK cells were NKG2A-positive (Figure 5B). Similar to blocking Qa-1, blocking NKG2A was also able to reverse the inhibitory effect of activated CD4+ T cells on NK cytotoxicity (Figure 5A, right panel).
Figure 5.
Suppression of NK-cell cytotoxicity by Con A-activated CD4+Foxp3− T cells was Qa-1 dependent. (A) Anti-Qa-1 or anti-NKG2A antibody abrogated the suppression of activated CD4+ T cells on NK-cell cytotoxicity in vitro. Data shown are representative of three independent experiments. (B) Percentage of NKG2A-positive cells in the NK population. (C) Qa-1 antibody could reverse the inhibition of NK function by activated CD4+ T cells in vivo. SCID mice (n = 3/group) were injected with saline or Con A-activated CD4+Foxp3− T cells (Act. T) or Act. T plus anti-mouse Qa-1 antibody. At the indicated times, cytolytic activities of splenocytes were determined ex vivo in a 51Cr release assay. (D) Metastatic nodules on the lung surface of animals treated with activated CD4+ T cells in the presence or absence of anti-Qa-1. Data were recorded as mean ± SD, and the Student's t test was used to compare between groups. (E) Photos of dissected lungs from the two groups (n = 5/group) in D. The visible melanoma nodules on the lung surface were significantly reduced in animals treated with anti-Qa-1 as compared with those non-treated. (F) Con A-activated T cells from Qa-1 knockout mice lost their inhibitory function on NK cytotoxicity in vitro. Data were mean ± SD, and representative of two independent experiments. (G) Con A-activated CD4+Foxp3− T cells from Qa-1 knockout mice failed to facilitate B16 melanoma metastasis in RAG-1−/− mice. Sex- and age-matched RAG-1−/− mice were injected with B16 melanoma cells via tail vein one day before transferring resting or activated CD4+Foxp3− T cells from wild-type or Qa-1 knockout mice. Ten days later, tumor metastasis was assessed by counting melanoma nodules on the lung surface. Error bars indicate mean ± SD (n = 6) and statistical significance was tested by Student's t test. (H) Photos of dissected lungs from the 4 groups (n = 6/group) in G. There was a significantly decreased number of visible melanoma nodules on the lung surface in Qa-1 knockout mice as compared with those wild-type animals.
We then administered anti-Qa-1 together with Con A-activated CD4+Foxp3− T cells into SCID mice, and tested NK-cell cytotoxicity in vivo after 24, 48 and 72 h. The result showed that NK-cell function suppressed by adoptively transferred activated CD4+ T cells could be reinstated after infusion of anti-Qa-1 antibody (Figure 5C). We further performed in vivo experiments in the tumor model, where SCID mice inoculated with B16 melanoma cells were transferred with Con A-activated CD4+Foxp3− T cells, together with or without anti-Qa-1 antibody. Strikingly, melanoma metastasis enhanced by activated T cells was almost completely blocked by anti-Qa-1, as shown by the significantly reduced number of metastatic nodules in the lungs of mice that received the antibody than those did not (Figure 5D-5E). In addition to Qa-1 blocking antibody, we used Qa-1 knockout mice to demonstrate the involvement of Qa-1 in NK-cell inhibition. In vitro, activated CD4+Foxp3− T cells from Qa-1 knockout mice were unable to inhibit NK cytotoxicity (Figure 5F). Furthermore, adoptive transfer of Con A-activated Qa-1 knockout T cells into RAG-1−/− mice bearing melanoma did not favor melanoma metastasis as their wild-type counterpart did (Figure 5G-5H). These data strongly support the notion that activated T cells could facilitate tumor development via Qa-1-dependent suppression of NK cytotoxicity.
Discussion
Innate and adaptive immunity are two categories of immune responses with an interdependent and interactive relationship that maintains the homeostasis of the body. Accumulating clinical and experimental data have demonstrated that the adaptive immune lymphocytes not only provide reinforcement to the ongoing innate response, but also under certain circumstances act as negative regulators in the innate response25,26,27.
Naturally-occurring CD4+Foxp3+ T cells are considered to be one of the main regulatory cell types, which inhibit the proliferation and effector functions of a large panel of immune cells17. Besides the thymus-derived Foxp3+ natural Tregs, conditionally-induced T regulatory cells type 1 (Tr1) and Th3 cells are generally regarded as distinct populations following peripheral immune activation28. In vitro, Tr1 cells derive from repetitive stimulation in the presence of IL-10. They are anergic, Foxp3−, but can suppress immune responses via cell-cell contact and/or the production of high levels of IL-10 and TGF-β29. Th3 cells cloned from orally tolerized mice produce high amounts of TGF-β, low amounts of IL-4 and IL-10, but no IFN-γ or IL-2 upon TCR ligation, and suppressed EAE in a TGF-β-dependent fashion30. Because of the intimate role of TGF-β in Foxp3 induction, the boundary between Foxp3+ Tregs and Th3 cells becomes less clear. Transient overexpression of TGF-β upon TCR ligation differentiates antigen-specific Th0 cells into regulatory Foxp3+CD25+/− Th3 cells in vitro. Repetitive antigen stimulation results in sustained expression of Foxp3 in Th3 cells that acquire regulatory function independent of surface expression of CD2531,32.
In addition to these well-documented regulatory cell types, we observed that pre-activated CD4+CD25− T cells, being Foxp3− as examined by intracellular Foxp3 staining and by using Foxp3GFP mice, counter-intuitively suppressed NK cytotoxicity, suggesting that fully stimulated effector T cells may act, under certain conditions, as a type of regulatory cells to restrain NK activity. The mechanism underlying such regulation involves Qa-1. Although Qa-1 mRNA can be detected in many cell types, surface expression of functional Qa-1-β2-microglobulin heterodimer is only detectable on activated T, B lymphocytes and dendritic cells22,33. In line with this, Con A-activated T cells were reported to express Qa-1 molecule24. Dissection of the NK-cell population revealed that the NKG2A+ but not NKG2A− subset of NK-cells-mediated lysis of Qa-1-deficient CD4 cells. Antibody blockage of Qa-1:NKG2A interaction resulted in potent NK-dependent elimination of activated-autoreactive T cells and amelioration of autoimmune EAE34. These findings emphasize that the interaction between Qa-1 on activated T cells and NKG2A on NK cells protects activated T cells from NK lysis and is essential for clonal expansion and development of immunologic memory.
However, Qa-1:NKG2A interaction may function as a double-edged sword, i.e., activated T cells through expression of Qa-1 can disarm NK cells via the NKG2A inhibitory receptor. Our data in a simplified adoptive transfer model showed that blockade of Qa-1:NKG2A interaction liberated NK cells from the inhibitory action of activated CD4+ T cells, and restored otherwise suppressed NK control on tumor metastasis. Obviously, the role of Qa-1:NKG2A interaction in tumor immunity in immunocompetent hosts is very complicated, as we were surprised that, compared to wild-type animals, metastasis of B16 melanoma cells in Qa-1 knockout mice was dramatically enhanced (data not shown). Recently, Lu et al.34 found that Qa-1-deficient CD4 cells failed to undergo antigen-induced or homeostatic expansion due to increased susceptibility to NK cell lysis. Re-expression of Qa-1-Qdm on autoreactive CD4 cells rescued expansion of self-reactive T cell clones. Whether Qa-1 deficiency negatively affects APC-T cell priming and activation, needs to be further studied.
Infusion of ex vivo activated tumor-specific T cells back into the tumor-bearing host has been attempted in the treatment of cancer patients35,36,37, but its therapeutic capacity is marginal. For example, in a Phase I study, even when 6 billion of tumor-reactive CTLs were transferred back to melanoma patients every 2-3 weeks, there was only a modest clinical benefit with disease stabilization in roughly half of the cases. There were no formal partial clinical responses (defined as >50% reduction in cross-sectional area of all measurable metastases) or complete responses (defined as regression of all measurable metastatic disease)38. One of the reasons responsible for the poor clinical response is believed to be the higher prevalence of CD4+Foxp3+ Tregs at the tumor microenvironment, which potently suppresses NK and other immune cells39. Our study raised another intriguing possibility that fully stimulated, Qa-1-expressing, CD4+Foxp3− effector T cells (or CD8+ CTLs) when adoptively transferred in vivo might also suppress the tumoricidal activities of NK cells. Such suppression is conditional, i.e., dependent on the net outcome of negative and positive signals provided by T cells, such as T-cell-derived stimulatory cytokines for NK cells (IL-214 and IFN-γ). Our finding that Con A-activated CD4+Foxp3− T cells fixed by formalin to block secretion possessed even higher inhibitory capacity than those unfixed ones (data not shown) supports this view.
For cancer biotherapy, most strategies aimed to exploit the highly specific nature of adaptive immune responses do not work well as predicted. We caution that this may be because the feedback control by adaptive cells on innate cells is not adequately considered. If attention is solely drawn to enhancing T cells while neglecting the potential “activation-induced inhibition”, one may inadvertently blunt innate immunity against tumor. On the other hand, our finding on the involvement of Qa-1, a specific molecule underpinning this “activation-induced inhibition”, may provide opportunities for further manipulation. For instance, it can be envisioned that anti-Qa-1 may function as an immune-enhancer if co-administered with ex vivo expanded anti-tumor T cells. Such manipulation, nevertheless, needs to be carefully controlled by dose and timing, lest any possible negative effects on APC-T cell priming, as well as unleashed overzealous innate responses that could sometimes lead to pathological consequences25.
In summary, we demonstrated for the first time that activated CD4+Foxp3− T cells could attenuate tumor surveillance by inhibition of NK cytotoxicity in membrane Qa-1-dependent manner. This activated CD4+Foxp3− “suppressor” T cell population is distinct from previously known natural and induced regulatory T cells based on their different regulatory mechanisms. We therefore refer this phenomenon as “activation-induced inhibition”, which provides a new link between T and NK cells. These findings challenge the current dogma that adoptive transfer of activated autologous T cells favors the host for fighting against tumors.
Materials and Methods
Ethics statement
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal studies were approved by IACUC at both the Third Military Medical University (protocol #: SCXK20070003) and Beth Israel Deaconess Medical Center, Boston, USA (protocol #: 053-2008).
Animals
Wild-type, nude and SCID mice in BALB/c background were purchased from the Experimental Animal Department of the Third Military Medical University, Chongqing, China. Qa-1 knockout mice in C57BL/6 background were reported previously34, and were kind gifts from Dr Harvey Cantor (Dana-Faber Cancer Institute, USA). Foxp3GFP knockin mice (C57BL/6) were generated as previously reported40. RAG-1−/− mice (C57BL/6) were purchased from Model Animal Research Center of Nanjing University, China. All animals were maintained under specific pathogen-free conditions and used at 6-8 weeks of age.
Cell preparation
All the cells used in the experiments were obtained from mouse spleens. Live, single cell suspensions were prepared by Ficoll density gradient centrifugation after lysing erythrocytes. The negative enrichment strategy was used for isolation of CD4+CD25− T cells following depletion of CD4+CD25+ T cells with mouse Treg isolation kit (Miltenyi, Germany). CD4+Foxp3+(GFP+), CD4+Foxp3−(GFP−) cells and CD4+Foxp3−CD62L+ (GFP−CD62L+) cells were sorted by FACS from Foxp3GFP knockin mice. NK cells were isolated from BALB/c splenocytes by magnetic cell separation using the NK cell isolation kit (Miltenyi). The purity of each cell type was determined by FACS with PE- or FITC-conjugated antibodies against CD3, CD4, CD25 and CD49b, and was typically >95%.
Detection of intracellular Foxp3 expression
Foxp3 protein was detected by using a mouse Regulatory T cell Staining Kit (eBioscience, USA), according to the manufacturer's instructions. Briefly, BALB/c splenocytes were surface-stained with FITC-conjugated anti-CD4 and APC-conjugated anti-CD25, followed by intracellular staining with PE-conjugated anti-Foxp3. PE-labeled Rat IgG2a served as an isotype control. Cells were then washed and analyzed by FACS. In some experiments when Foxp3GFP knockin mice were used, Foxp3 expression was tracked by GFP expression in flow cytometry.
Adenosine generation assay
CD4+Foxp3+(GFP+) and CD4+Foxp3−(GFP−) cells were FACS-sorted from Foxp3GFP knockin mice40, and used as resting natural Tregs (nTreg) and effector T cells (Teff), respectively. For cell activation, Teff cells were stimulated with plate-bound anti-CD3 (10 μg/ml) and soluble anti-CD28 (1 μg/ml) in the presence of TGF-β (5 ng/ml) to generate induced Tregs (iTreg)41. Teff cells were also stimulated with anti-CD3/CD28 alone or with 10.0 μg/ml Concanavalin A (Sigma, USA) in the presence of syngeneic T-cell-depleted splenocytes for 3 days. At the end of culture, viable GFP+ (iTreg) or GFP− (activated Teff) cells were again FACS-sorted. All the cell populations (2 × 105) were added with 14C-ADP at 2.0 mCi/ml (GE Healthcare, USA). At the indicated times, aliquots were removed and analyzed for the presence of 14C-ADP hydrolysis products by thin layer chromatography (TLC), as reported previously19.
NK-cell cytotoxicity assay
A standard 51Cr-release method was used to assay NK-cell cytotoxicity42. Briefly, YAC-1 cells (China Cell Storage Center) were labeled with 51Cr in RPMI-1640 growth medium. NK cells and YAC-1 cells were added in triplicate into a 96-well plate at an Effector:Target (E:T) ratio of 100:1 for 4 h at 37 °C. In some experiments, freshly isolated NK cells and target cells were co-cultured with activated CD4+ T cells at a ratio (NK:T) of 1:10 alone or in the presence of 10 μg/ml blocking antibodies against CD25 (BD Biosciences), TGF-β1 (R&D Systems), NKG2A or Qa-1 (both from Santa Cruz). In comparison, freshly isolated NK cells were also co-cultured with natural Treg cells (CD4+GFP+) at the ratio of 1:1. IL-2 concentrations in the supernatants from the co-culture were determined with an ELISA Kit (R&D Systems). NK-cell cytotoxicity was determined 24 h post co-culture. For that, radioactivities of the supernatants were measured by a γ-counter (Shanghai Medical Instrument Company, China). The spontaneous release and maximal release controls were also set up42. Specific cytotoxicity was calculated as follows:
Percent 51Cr Release = (cpm experimental − cpm spontaneous release)/(cpm maximum release − cpm spontaneous release) × 100
The inhibition by different subsets of T cells on NK-cell cytotoxicity was calculated as follows:
Percent Inhibition = (Percent 51Cr Release of experimental group − Percent 51Cr Release of control group)/Percent 51Cr Release of control group × 100
NK-cell cytotoxicity on YAC-1 target cells was also measured by FACS. Briefly, target cells were stained with CFSE (5.0 μM) for 10 min. NK cells were co-cultured with CFSE-labeled YAC-1 cells at a ratio of 100:1 for 3 h at 37 °C, 5% CO2. Target cell killing was determined by FACS on the basis of PI uptake. Percent cytotoxicity = (Number of CFSE+PI+ cells)/(Number of total CFSE+ cells) × 10042.
NK-cell proliferation assay
Activated CD4+CD25− T cells and resting CD4+CD25− T cells were fixed with 0.5% glutaraldehyde, carefully washed with PBS, and co-cultured with NK cells in 96-well U-bottom plates in the presence of IL-2 (100 U/ml) for 96 h at 37 °C in a 5% CO2 incubator. Cells were pulsed with 3H-TdR (0.2 μCi per well) 8 h before harvest. Radioactivity of each sample in triplicate (means ± SD) was determined by a liquid scintillation counter (Beckman, USA).
Adoptive transfer of activated T cells
Isolated BALB/c CD4+CD25− T cells were incubated with Con A (10 μg/ml) in the presence of syngeneic T-cell-depleted splenocytes for 48 h. Then, purified 1.5 × 106 Con A-activated CD4+ T cells or naïve CD4+CD25− T cells were injected on days 0 and 2 via the tail vein into BALB/c nude mice. Same volume of saline was injected into control mice. 24 h later, NK cells were harvested from the spleen, and the cytotoxicity of NK cells against YAC-1 cells was tested as described above.
B16 melanoma metastasis in SCID and RAG-1−/− mice
As described previously15, 2 × 105 B16 tumor cells were injected into either SCID or RAG-1−/− mice and wild-type C57BL/6 mice via the tail vein on day 0. On days 1 and 3, 106 Con A-activated or resting T cells were infused into mice. On day 10, mice were sacrificed and lung metastasis of B16 tumor cells was determined by quantifying the melanoma nodules. In some experiments, depletion of NK cells in SCID mice43 was achieved by injecting (i.p.) 100 μg of anti-NK1.1 (PK136) monoclonal antibody on days −1, 0 and 1 of tumor cell inoculation.
In vivo administration of antibodies
For each mouse, a total of 200 μg anti-Qa-1 (Santa Cruz, clone 6A8.6F10, mouse IgG1) or anti-NKG2A (Santa Cruz, clone 16A11, mouse IgG2b) was injected (i.v.) on day 0 of transferring T cells or tumor cells. The cytotoxicity of purified splenic NK cells against YAC-1 cells was tested after 24, 48 and 72 h. Lung metastasis of B16 tumor cells was determined by counting the melanoma nodules on day 10.
Statistics
Results were expressed as means ± SD. Data were analyzed with Student's t test and Difference Square Analysis where appropriate. P < 0.05 was considered significant.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81027004, 30571922) to JW and GL, Hi-Tech Research and Development Program of China (863 program; 2012AA020504) to JW, State Key Laboratory Funding (SKLZZ200808, SKLZZ200820) to JW and GL, and a grant from JDRF (1-2007-551) to WG. The authors acknowledge the kind assistance of Dr Harvey Cantor (Dana-Faber Cancer Institute, USA) for the Qa-1 knockout mice, Thomas FitzGibbon for manuscript editing and Dr Liyan for graph preparation.
References
- Waldmann H, Cobbold S. Regulatory T cells: context matters. Immunity. 2009;30:613–615. doi: 10.1016/j.immuni.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J Immunol. 2000;165:3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- Feunou P, Poulin L, Habran C, Le Moine A, Goldman M, Braun MY. CD4+CD25+ and CD4+CD25− T cells act respectively as inducer and effector T suppressor cells in superantigen-induced tolerance. J Immunol. 2003;171:3475–3484. doi: 10.4049/jimmunol.171.7.3475. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Moriizumi E. CD4+CD25− T cells in aged mice are hyporesponsive and exhibit suppressive activity. J Immunol. 2003;170:1675–1682. doi: 10.4049/jimmunol.170.4.1675. [DOI] [PubMed] [Google Scholar]
- Degauque N, Lair D, Braudeau C, et al. Development of CD25− regulatory T cells following heart transplantation: evidence for transfer of long-term survival. Eur J Immunol. 2007;37:147–156. doi: 10.1002/eji.200635879. [DOI] [PubMed] [Google Scholar]
- Han Y, Guo Q, Zhang M, Chen Z, Cao X. CD69+ CD4+ CD25− T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol. 2009;182:111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- Schartner JM, Singh AM, Dahlberg PE, Nettenstrom L, Seroogy CM. Recurrent superantigen exposure in vivo leads to highly suppressive CD4+CD25+ and CD4+CD25− T cells with anergic and suppressive genetic signatures. Clin Exp Immunol. 2009;155:348–356. doi: 10.1111/j.1365-2249.2008.03827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H. Both CD4(+)CD25(+) and CD4(+)CD25(−) regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002;168:5558–5565. doi: 10.4049/jimmunol.168.11.5558. [DOI] [PubMed] [Google Scholar]
- Lu Y, Xu W, Zhou Y, Lv P, Gao XM. Regulatory activity of activated murine peripheral CD4+CD25− T cells: a possible mechanism of feedback regulation on adaptive immunity. Scand J Immunol. 2006;64:500–506. doi: 10.1111/j.1365-3083.2006.01842.x. [DOI] [PubMed] [Google Scholar]
- Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- Carayannopoulos LN, Yokoyama WM. Recognition of infected cells by natural killer cells. Curr Opin Immunol. 2004;16:26–33. doi: 10.1016/j.coi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Arnon TI, Markel G, Bar-Ilan A, et al. Harnessing soluble NK cell killer receptors for the generation of novel cancer immune therapy. PLoS One. 2008;3:e2150. doi: 10.1371/journal.pone.0002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- Barao I, Hanash AM, Hallett W, et al. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralainirina N, Poli A, Michel T, et al. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;81:144–153. doi: 10.1189/jlb.0606409. [DOI] [PubMed] [Google Scholar]
- Sun X, Wu Y, Gao W, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich CJ, Waltrip R, Hermel E, et al. T cell recognition of QA-1b antigens on cells lacking a functional Tap-2 transporter. J Immunol. 1992;149:3773–3777. [PubMed] [Google Scholar]
- Lo WF, Ong H, Metcalf ES, Soloski MJ. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- Sullivan BA, Kraj P, Weber DA, Ignatowicz L, Jensen PE. Positive selection of a Qa-1-restricted T cell receptor with specificity for insulin. Immunity. 2002;17:95–105. doi: 10.1016/s1074-7613(02)00343-6. [DOI] [PubMed] [Google Scholar]
- Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- Kim KD, Zhao J, Auh S, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Medzhitov R. Not so fast: adaptive suppression of innate immunity. Nat Med. 2007;13:1142–1144. doi: 10.1038/nm1007-1142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Yang X, Auh SL, Kim KD, Tang H, Fu YX. Do adaptive immune cells suppress or activate innate immunity. Trends Immunol. 2009;30:8–12. doi: 10.1016/j.it.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MA, Baxter AG. The genetics of immunoregulatory T cells. J Autoimmun. 2008;31:237–244. doi: 10.1016/j.jaut.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178:179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. II. TGF-beta-transgenic Th3 cells rescue IL-2-deficient mice from autoimmunity. J Immunol. 2007;178:172–178. doi: 10.4049/jimmunol.178.1.172. [DOI] [PubMed] [Google Scholar]
- Soloski MJ, DeCloux A, Aldrich CJ, Forman J. Structural and functional characteristics of the class IB molecule, Qa-1. Immunol Rev. 1995;147:67–89. doi: 10.1111/j.1600-065x.1995.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AE, Brenner MK, Dotti G. Adoptive T-cell immunotherapy of chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2008;21:375–389. doi: 10.1016/j.beha.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HW, Liao CH, Ying C, Chang CJ, Lin CM. Ex vivo expansion of dendritic-cell-activated antigen-specific CD4+ T cells with anti-CD3/CD28, interleukin-7, and interleukin-15: potential for adoptive T cell immunotherapy. Clin Immunol. 2006;119:21–31. doi: 10.1016/j.clim.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Ohshita A, Kawabuchi Y, et al. Adoptive immunotherapy of cancer using activated autologous lymphocytes--current status and new strategies. Hum Cell. 2003;16:183–189. doi: 10.1111/j.1749-0774.2003.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Ishikawa R, Biron CA. Transforming growth factor-beta expression and natural killer cell responses during virus infection of normal, nude, and SCID mice. J Immunol. 1993;151:4874–4890. [PubMed] [Google Scholar]
- Kulesza J, Hoser G, Wasilewska D, Kawiak J. NK cell depletion and recovery in SCID mice treated with anti-NK1.1 antibody. Folia Histochem Cytobiol. 2006;44:93–96. [PubMed] [Google Scholar]