Abstract
Stimulation of the plasma membranes of granulocytes results in an oxidative metabolic response. This response can be measured by measuring the reduction of oxidizable substrates, such as Nitro Blue Tetrazolium, as well as by measuring the energy released as light (chemiluminescence). While investigating the oxidative response of human granulocytes, we observed a marked variation in the chemiluminescence response when leukocytes were suspended in a balanced salt solution without gelatin or any other protein. We performed systematic study to investigate the role of protein in suspensions of human polymorphonuclear leukocytes. Final results were identical with human serum, albumin, fetal calf serum, and gelatin; gelatin was used as the protein source in most experiments. Polymorphonuclear leukocytes suspended in Hanks balanced salt solution without gelatin decreased in numbers during incubation at room temperature (approximately 50 percent after 60 min). Cell structures were observed on the walls of the tubes containing leukocyte suspensions without gelatin. Numbers of polymorphonuclear leukocytes were stable in suspensions containing gelatin. A chemiluminescence response which peaked at approximately 10 min and was sustained for at least 30 min was observed in suspensions of polymorphonuclear leukocytes without gelatin. This surface attachment-stimulated chemiluminescence occurred in the absence of either soluble or particulate stimuli. Chemiluminescence was inhibited by either superoxide dismutase or sodium azide and did not occur with suspensions of granulocytes from patients with chronic granulomatous disease. We postulate that both superoxide- and myeloperoxidase-dependent oxidative metabolic reactions are induced during the adherence of polymorphonuclear leukocytes to surfaces. Gelatin or other proteins in leukocyte suspending media are necessary when assays are performed to evaluate the metabolic responses of these cells to particulate or soluble stimuli.
Full text
PDF
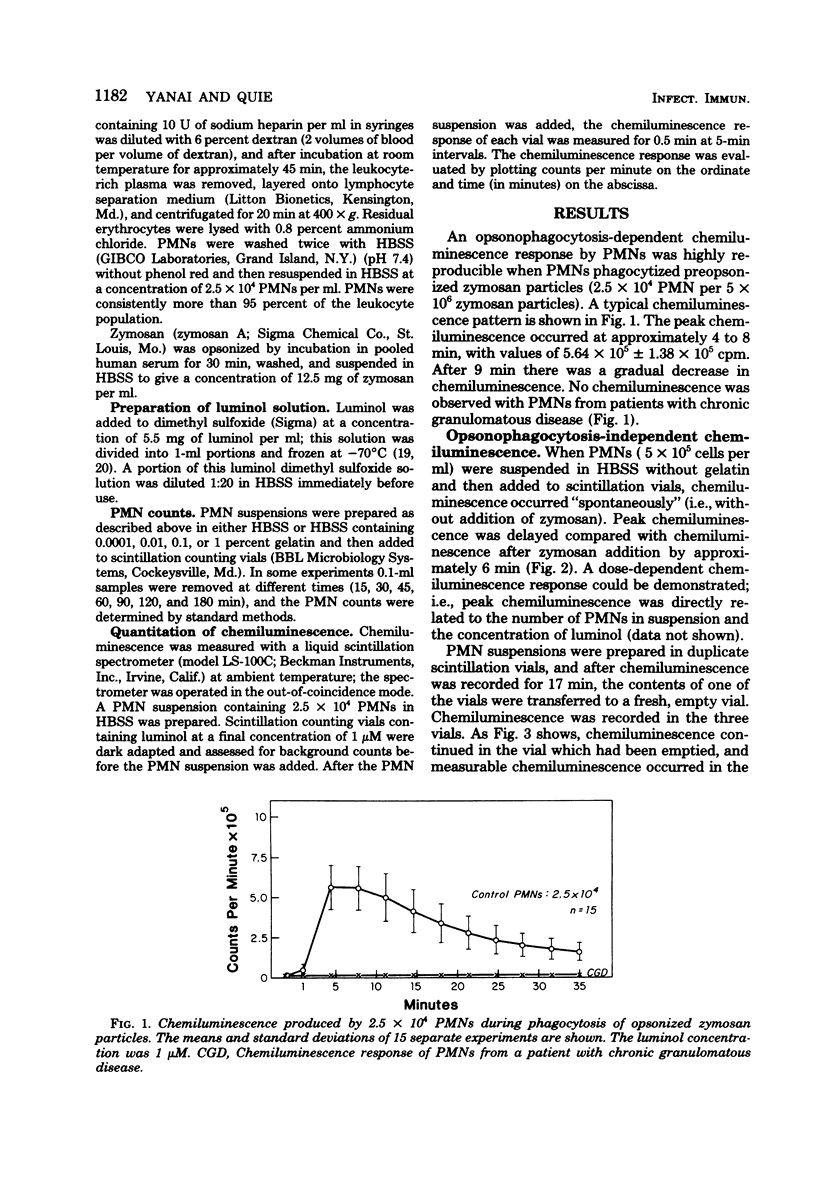
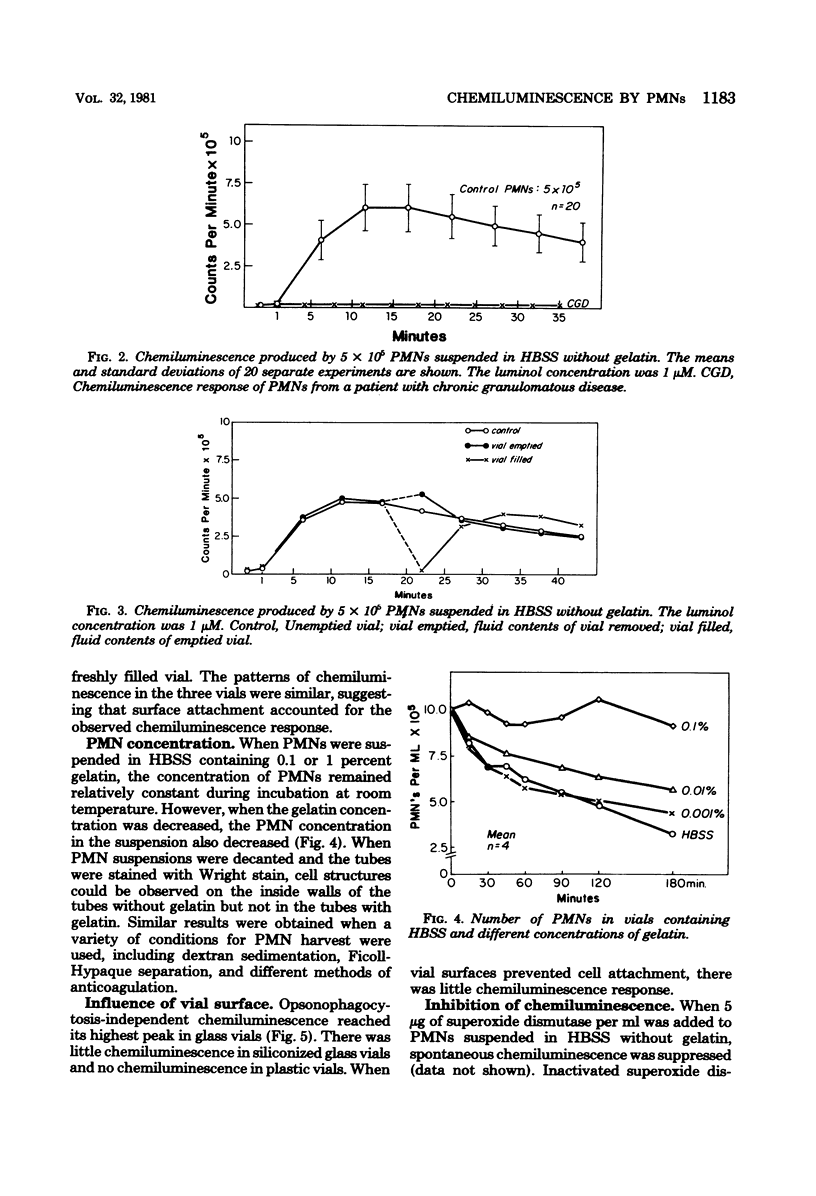
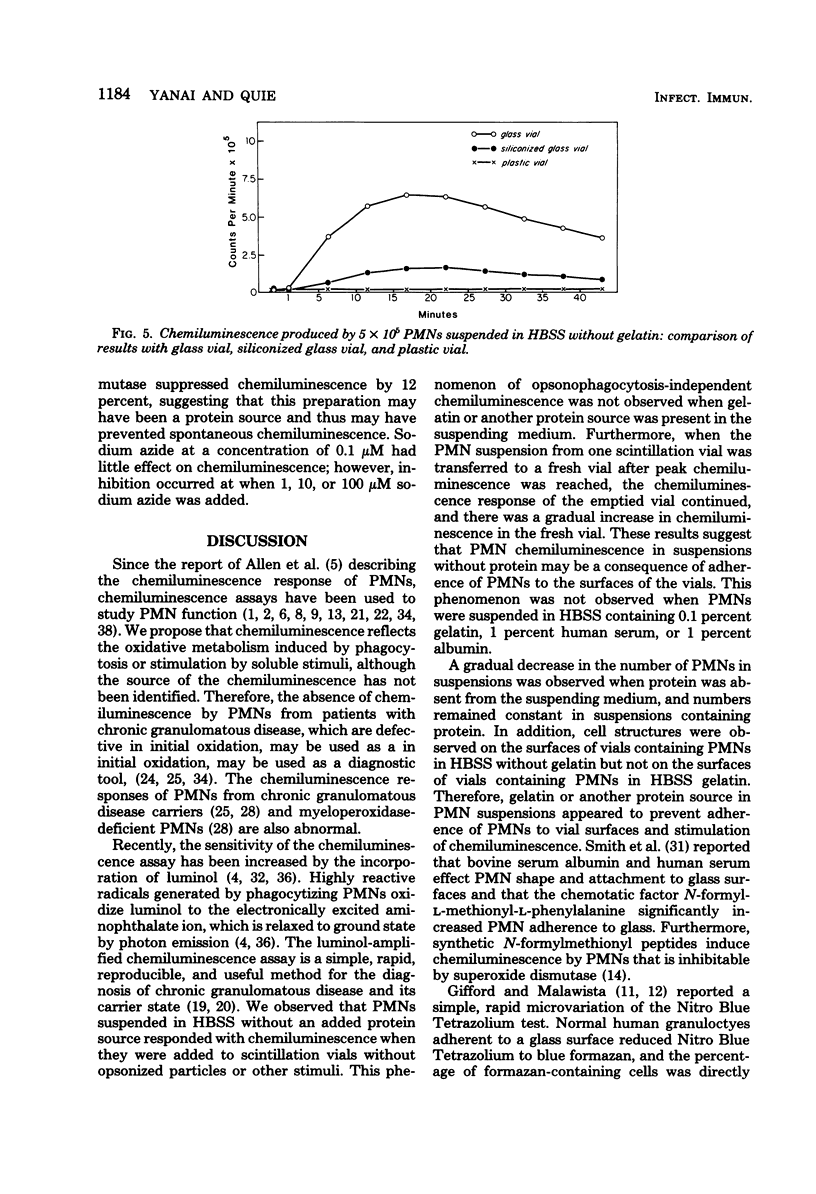


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. C. Halide dependence of the myeloperoxidase-mediated antimicrobial system of the polymorphonuclear leukocyte in the phenomenon of electronic excitation. Biochem Biophys Res Commun. 1975 Apr 7;63(3):675–683. doi: 10.1016/s0006-291x(75)80437-2. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Allen R. C. The role of PH in the chemiluminescent response of the myeloperoxidase-halide-HOOH antimicrobial system. Biochem Biophys Res Commun. 1975 Apr 7;63(3):684–691. doi: 10.1016/s0006-291x(75)80438-4. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Yevich S. J., Orth R. W., Steele R. H. The superoxide anion and singlet molecular oxygen: their role in the microbicidal activity of the polymorphonuclear leukocyte. Biochem Biophys Res Commun. 1974 Oct 8;60(3):909–917. doi: 10.1016/0006-291x(74)90401-x. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Allred C. D., Solberg C. O., Hill H. R. Chemiluminescence by polymorphonuclear leukocytes from patients with active bacterial infection. J Infect Dis. 1980 Jan;141(1):14–26. doi: 10.1093/infdis/141.1.14. [DOI] [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. H., Malawista S. E. A simple rapid micromethod for detecting chronic granulomatous disease of childhood. J Lab Clin Med. 1970 Mar;75(3):511–519. [PubMed] [Google Scholar]
- Gifford R. H., Malawista S. E. The nitroblue tetrazolium reaction in human granulocytes adherent to a surface. Yale J Biol Med. 1972 Apr;45(2):119–132. [PMC free article] [PubMed] [Google Scholar]
- Grebner J. V., Mills E. L., Gray G. H., Quie P. G. Comparison of phagocytic and chemiluminescence response of human polymorphonuclear neutrophils. J Lab Clin Med. 1977 Jan;89(1):153–159. [PubMed] [Google Scholar]
- Hatch G. E., Gardner D. E., Menzel D. B. Chemiluminescence of phagocytic cells caused by N-formylmethionyl peptides. J Exp Med. 1978 Jan 1;147(1):182–195. doi: 10.1084/jem.147.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L. The metabolism of leukocytes. Semin Hematol. 1968 Apr;5(2):156–165. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- Mills E. L., Rholl K. S., Quie P. G. Luminol-amplified chemiluminescence: a sensitive method for detecting the carrier state in chronic granulomatous disease. J Clin Microbiol. 1980 Jul;12(1):52–56. doi: 10.1128/jcm.12.1.52-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E. L., Rholl K. S., Quie P. G. X-linked inheritance in females with chronic granulomatous disease. J Clin Invest. 1980 Aug;66(2):332–340. doi: 10.1172/JCI109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E. L., Thompson T., Björkstén B., Filipovich D., Quie P. G. The chemiluminescence response and bactericidal activity of polymorphonuclear neutrophils from newborns and their mothers. Pediatrics. 1979 Mar;63(3):429–434. [PubMed] [Google Scholar]
- Nelson R. D., Mills E. L., Simmons R. L., Quie P. G. Chemiluminescence response of phagocytizing human monocytes. Infect Immun. 1976 Jul;14(1):129–134. doi: 10.1128/iai.14.1.129-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G. Disorders of phagocyte function: biochemical aspects. Prog Clin Biol Res. 1977;13:157–169. [PubMed] [Google Scholar]
- Quie P. G., Mills E. L., Holmes B. Molecular events during phagocytosis by human neutrophils. Prog Hematol. 1977;10:193–210. [PubMed] [Google Scholar]
- Quie P. G. Pathology of bactericidal power of neutrophils. Semin Hematol. 1975 Apr;12(2):143–160. [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Mendelson D. S., Metz E. N. The effect of sodium azide on the chemiluminescence of granulocytes--evidence for the generation of multiple oxygen radicals. J Lab Clin Med. 1977 Jun;89(6):1333–1340. [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Winston D. J., Van Dyke K. In vitro evaluation of opsonic and cellular granulocyte function by luminol-dependent chemiluminescence: utility in patients with severe neutropenia and cellular deficiency states. Infect Immun. 1978 Oct;22(1):41–51. doi: 10.1128/iai.22.1.41-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A. C., PILLEMER L. The properdin system and immunity. V. The bactericidal activity of the properdin system. J Exp Med. 1956 May 1;103(5):553–575. doi: 10.1084/jem.103.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L. S., Keele B. B., Jr, Johnston R. B., Jr Inhibition of phagocytosis-associated chemiluminescence by superoxide dismutase. Infect Immun. 1974 Jun;9(6):1051–1056. doi: 10.1128/iai.9.6.1051-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]



