Abstract
Non-genomic effects of steroid hormones on cell physiology have been reported in the brain. However, relatively little is known about the behavioral significance of these actions. Male sexual behavior is activated by testosterone partly through its conversion to estradiol via the enzyme aromatase in the preoptic area (POA). Brain aromatase activity (AA) changes rapidly which might in turn be important for the rapid regulation of behavior. Here, acute effects of Vorozole™, an aromatase inhibitor, injected IP at different doses and times before testing (between 15 and 60 min), were assessed on male sexual behavior in quail. To limit the risk of committing both types of statistical errors (I and II), data of all experiments were entered into a meta-analysis. Vorozole™ significantly inhibited mount attempts (p<0.05, size effect [g]=0.527) and increased the latency to first copulation (p<0.05, g=0.251). The treatment had no effect on the other measures of copulatory behavior. Vorozole™ also inhibited appetitive sexual behavior measured by the social proximity response (p<0.05, g=0.534) or rhythmic cloacal sphincter movements (p<0.001, g=0.408). Behavioral inhibitions always reached a maximum at 30 min. Another aromatase inhibitor, androstatrienedione, induced a similar rapid inhibition of sphincter movements. Radioenzyme assays demonstrated that within 30 min Vorozole™ had reached the POA and completely blocked AA measured in homogenates. When added to the extracellular milieu, Vorozole™ also blocked within 5 min the AA in POA explants maintained in vitro. Together, these data demonstrate that aromatase inhibition rapidly decreases both consummatory and appetitive aspects of male sexual behavior.
Keywords: non-genomic effects, Vorozole, consummatory sexual behavior, appetitive sexual behavior, rhythmic cloacal sphincter movements, meta-analysis
Introduction
Rapid effects of steroid hormones on brain physiology and behavior have been postulated for more than three decades (e.g., (Kelly, Moss, and Dudley, 1977; Selye, 1941; Yagi, 1973). Despite extensive interest in these rapid effects, our knowledge continues to be limited about the relative importance of such effects for the regulation of behavior and physiology. There are also many uncertainties about the mechanism of action that might mediate these effects (Brann, Hendry, and Mahesh, 1995; Maggi, Ciana, Belcredito, and Vegeto, 2004; Orchinik and McEwen, 1993). Perhaps the most progress has been made for the C21 steroids corticosterone and progesterone. In the case of corticosterone, rapid effects have been identified in amphibians on various measures of sexual behavior (Moore and Orchinik, 1994) and stress (Orchinik, 1998) and there is good evidence for a highly specific membrane-binding site (Evans, Searcy, and Moore, 2000; Orchinik, Murray, and Moore, 1991). For progesterone, several lines of evidence indicate rapid, membrane effects on sexual behavior in the ventral tegmental area of Syrian hamsters (DeBold and Frye, 1994; Frye and Petralia, 2003) and this steroid or its metabolites can clearly bind to membrane receptors such as the GABA-A complex (Majewska, Harrison, Schwartz, Barker, and Paul, 1986; Zhu, Bond, and Thomas, 2003; Zhu, Rice, Pang, Pace, and Thomas, 2003). In one study, testosterone was similarly shown to exert rapid effects on striated penile muscles related to the expression of male sexual behavior (Sachs and Leipheimer, 1988).
Recently, there has been substantial interest in possible rapid effects of 17β-estradiol (Beyer and Raab, 2000; Kelly and Ronnekleiv, 2002; Lee and McEwen, 2001; Maggi et al., 2004). In males among several vertebrate species, reproductive behaviors activated by testosterone require first its conversation to an estrogenic metabolite (Naftolin, Ryan, Davies, Reddy, Flores, Petro, Kuhn, White, Takaoka, and Wolin, 1975). Although it is clear that the genomic actions of estrogen are involved at least partly in mediating these effects, less is known about possible fast actions of estrogen on these sexual behaviors. One report in rats does indicate that estrogen can act in a rapid manner on male sexual behavior (Cross and Roselli, 1999) and there are several reports of estrogen acting rapidly in the brain to modulate the synthesis or release of neurotransmitters such as dopamine (Becker, 1990; Di Paolo, Rouillard, and Bédard, 1985; Thompson and Moss, 1994). Although there are many hints, we still know little about how estrogens might act at the cellular level to exert these effects (Beyer and Raab, 2000; Kelly and Ronnekleiv, 2002; Pasqualini, Olivier, Guibert, Frain, and Leviel, 1995; Toran-Allerand, 2004; Wade, Robinson, Shapiro, and Dorsa, 2001; Zhou, Watters, and Dorsa, 1996).
In the current study we adopted a different approach to the study of the possible rapid effects of estrogen on male-typical reproductive behaviors. We took advantage of our extensive knowledge about the hormonal control of male sexual behavior in male Japanese quail (Coturnix japonica). Testosterone has powerful effects on the activation of both appetitive (approaching and searching for a female) and consummatory (copulatory behavior) aspects of male sexual behavior in quail (Balthazart and Ball, 1998). The conversion of testosterone via the enzyme aromatase to 17β-estradiol is a necessary and sufficient step for the activation of both components of male sexual behavior (Ball and Balthazart, 2002; Balthazart and Ball, 1998). Most studies of this phenomenon in quail have focused on the relatively slow actions of estrogen that involve its binding to an intracellular receptor that once activated by the ligand will migrate to the cell nucleus and induce gene transcription (Adkins, Boop, Koutnik, Morris, and Pnieswski, 1980; Balthazart, Castagna, and Ball, 1997; Foidart, Harada, and Balthazart, 1994; Taziaux, Cornil, and Balthazart, 2004).
Recent work in our laboratory has, however, demonstrated that estrogen production in the brain can be rapidly (within minutes) modulated by non-genomic mechanisms in addition to the slower (hours to days) transcriptional changes that affect the concentration of the enzyme. Conditions that enhance protein phosphorylation such as the presence of high concentrations of calcium, magnesium and ATP rapidly (within min) down-regulate AA in hypothalamic homogenates. Similarly, the pharmacological mobilization of intracellular calcium with thapsigargin or the stimulation of various glutamate receptors, which also leads to increased intracellular calcium concentrations, depress within min the AA measured in quail preoptic explants (Balthazart, Baillien, and Ball, 2001a; Balthazart, Baillien, and Ball, 2001b; Balthazart, Baillien, Charlier, and Ball, 2003). Protein kinase inhibitors interfere with the calcium-induced inhibition of AA and multiple phosphorylation consensus sites are present on the deduced amino acid sequence of quail aromatase (Balthazart et al., 2003). Fast changes in the local availability of estrogens in the brain can thus be caused by aromatase calcium-dependent phosphorylations. The resulting changes in local estrogen concentrations could then rapidly regulate neuronal physiology.
In parallel, it is clear that male sexual behavior in quail is regulated at two different time domains. There is a relatively long time course that is illustrated by comparing male quail in different seasonal states (i.e. maintained on long or short photoperiods) that can be mimicked experimentally by comparing castrated quail with those treated with exogenous testosterone. In this case one sees qualitative differences in behavior (i.e. engaging in sexual behavior with a stimulus female or not) that require days or even weeks to implement fully (Balthazart, Foidart, and Hendrick, 1990b). A second temporal domain of behavioral regulation is much quicker. When males are in a reproductive state consistent with engaging in sexual behavior, their copulatory behavior is nonetheless organized into short temporal bouts (of just a few minutes of less in duration (Hutchison, 1978). It is unlikely that this degree of temporal control can be explained by the action of testosterone and its metabolites acting via genomic receptors.
Given the postulated rapid effects of 17β-estrogen described in other species, we hypothesized that 17β-estradiol could regulate male sexual responding both with a long time duration and a short duration. In this paper, we present data that test the hypothesis that 17β-estradiol regulates male sexual behavior rapidly by administering aromatase inhibitors (Vorozole™ and ATD) and testing male sexual behaviors in quail within an hour of the administration of the compound. We mimicked the effects of a rapid inhibition of aromatase activity, as could be observed following phosphorylation of the enzyme, by systemically injecting the non-steroidal aromatase inhibitor, Vorozole™, in both intact and castrated animals treated with testosterone. Various endocrine conditions of the subjects, doses of inhibitors and latencies between injection and behavioral test were explored to identify the experimental paradigm that might best reveal such rapid effects. We present here evidence supporting the hypothesis that changes in availability in 17β-estradiol rapidly regulate the expression of both appetitive and consummatory aspects of male sexual behavior in Japanese quail.
Methods
A total of 59 experiments were performed to assess the effect of the acute blockade of the local production of estrogen by aromatase on appetitive and consummatory aspects of male sexual behavior. Appetitive sexual behavior was quantified by two procedures: the measure of the learned social proximity response originally developed by Domjan and Hall (Domjan and Hall, 1986) and the measure of the rhythmic cloacal sphincter movements (RCSM). The rate of production of this response increases substantially in response to the visual presentation of a female (Balthazart and Ball, 1998; Seiwert and Adkins-Regan, 1998). It thus can be considered as a response indicative of preparatory or appetitive responses in anticipation of copulation. The consummatory sexual behavior was assessed in two different arenas: a small arena and a large arena that was used in tests that first included a measure of the learned social proximity response. The blockade of estrogen synthesis was achieved by acute systemic injections of aromatase inhibitors: a non-steroidal inhibitor, Vorozole™, and a steroidal inhibitor, androstanedienedione (ATD). The effects of these inhibitors were assessed in 5 different groups of birds that were either gonadally intact or castrated and chronically treated with testosterone. A complete description of animal treatments is given below and is summarized in table 1. In each group, subjects were used as their own control (correlated design) so that they underwent multiple testing. Different experiments were conducted with the first three groups of birds in order to determine the optimal dose and latency between injection and test. The third and fourth groups were also involved in experiments to address the question of whether systemic injections of estradiol were able to restore the inhibitory effect produced by Vorozole™. Additional experiments also tested the effect of systemic injections of the inhibitors on aromatase activity measured in the preoptic/hypothalamic area. An in vitro experiment was also performed on hypothalamic explants in order to assess the latency of the effect of Vorozole™ when it has reached the preoptic/hypothalamic region.
Table 1. Summary of the experiments performed in a correlated design.
For each group of birds, the number of experiments and the order in which they have been performed is stated. For each experiment, the following information is mentioned: the experimental conditions tested, the apparatus where the test was performed, the dependent variable measured, the number of animals tested and the number attributed to this experiment.
| Groups | Order | Experiments | Experimental apparatus | Dependent variable | N | Exp# |
|---|---|---|---|---|---|---|
| G1 - Intacts | 1 | Vor 30min-6mg/kg | Small arena | Copulation | 12 | 1 |
| 2 | Vor 15min-6mg/kg | Small arena | Copulation | 12 | 2 | |
| 3 | Vor 30min-30mg/kg | Small arena | Copulation | 12 | 3 | |
| 4 | Vor 15min-30mg/kg | Small arena | Copulation | 11 | 4 | |
| 5 | Vor 45min-30mg/kg | Small arena | Copulation | 12 | 5 | |
| 6 | Vor 30min-30mg/kg | Aquarium | RCSM | 12 | 6 | |
| 7 | Vor 30min-30mg/kg | Small arena | Copulation | 10 | 7 | |
| 8 | Vor 30min-30mg/kg | Small arena | Locomotion | 10 | 8 | |
| 9 | Vor 30min-30mg/kg | Aquarium | RCSM | 10 | 9 | |
| 10 | Vor 30min-30mg/kg | Aquarium | RCSM | 9 | 10 | |
| 11 | Vor 30min-30mg/kg | Large arena | Locomotion | 9 | 11 | |
| Look | 9 | 12 | ||||
| Copulation | 9 | 13 | ||||
| 12 | Vor 15min-30mg/kg | Large arena | Locomotion | 9 | 14 | |
| Look | 9 | 15 | ||||
| Copulation | 9 | 16 | ||||
| 13 | Vor 45min-30mg/kg | Large arena | Locomotion | 8 | 17 | |
| Look | 8 | 18 | ||||
| Copulation | 8 | 19 | ||||
| 14 | Vor 60min-30mg/kg | Large arena | Locomotion | 6 | 20 | |
| Look | 6 | 21 | ||||
| Copulation | 6 | 22 | ||||
| 15 | Vor 30min-30mg/kg | Aquarium | RCSM | 6 | 23 | |
| G2 − CX+T40 | 1 | Vor 30min-30mg/kg | Small arena | Copulation | 11 | 24 |
| 2 | Vor 45min-30mg/kg | Small arena | Copulation | 11 | 25 | |
| 3 | Vor 15min-30mg/kg | Small arena | Copulation | 11 | 26 | |
| 4 | Vor 30min-30mg/kg | Aquarium | RCSM | 11 | 27 | |
| 5 | Vor 30min-30mg/kg | Small arena | Copulation | 11 | 28 | |
| 6 | Vor 45min-30mg/kg | Small arena | Copulation | 11 | 29 | |
| 7 | Vor 45min-30mg/kg | Aquarium | RCSM | 10 | 30 | |
| 8 | Vor 45min-30mg/kg | Aquarium | RCSM | 11 | 31 | |
| 9 | Vor 60min-30mg/kg | Aquarium | RCSM | 10 | 32 | |
| 10 | Vor 30min-30mg/kg | Aquarium | RCSM | 11 | 33 | |
| 11 | Vor 15min-30mg/kg | Aquarium | RCSM | 10 | 34 | |
| G3 − CX+T20A | 1 | Vor 15min-30mg/kg | Aquarium | RCSM | 10 | 35 |
| 2 | Vor 45min-30mg/kg | Aquarium | RCSM | 15 | 36 | |
| 3 | Vor 15min-30mg/kg | Aquarium | RCSM | 10 | 37 | |
| 4 | Vor 30min-30mg/kg | Aquarium | RCSM | 13 | 38 | |
| 5 | Vor 45min-30mg/kg | Small arena | Copulation | 13 | 39 | |
| 6 | Vor 30min-30mg/kg | Aquarium | RCSM | 12 | 40 | |
| 7 | Vor +E2 (500) 30min | Aquarium | RCSM | 12 | 41 | |
| 8 | Vor +E2 (500) 15min | Aquarium | RCSM | 12 | 42 | |
| 9 | Vor +E2 (500) 15min | Aquarium | RCSM | 12 | 43 | |
| 10 | Vor 30min-30mg/kg | Aquarium | RCSM | 11 | 44 | |
| 11 | Vor +E2 (500) 45min | Aquarium | RCSM | 11 | 45 | |
| G4 − CX+T20B | 1 | Vor 30min-30mg/kg | Aquarium | RCSM | 16 | 46 |
| 2 | Vor 30min-30mg/kg | Small arena | Copulation | 16 | 47 | |
| 3 | Vor 45min-30mg/kg | Aquarium | RCSM | 16 | 48 | |
| 4 | Vor +E2 (500)-30min | Aquarium | RCSM | 16 | 49 | |
| 5 | Vor 30min-30mg/kg | Aquarium | RCSM | 16 | 50 | |
| 6 | Vor 45min-E2 30min | Aquarium | RCSM | 13 | 51 | |
| 7 | Vor 30min-E2 15min | Aquarium | RCSM | 13 | 52 | |
| 8 | Vor 30min-30mg/kg | - | Aromatase activity | 11 | 53 | |
| G5 − CX+T20C | 1 | ATD 15min-30mg/kg | Aquarium | RCSM | 13 | 54 |
| 2 | ATD 30min-30mg/kg | Aquarium | RCSM | 13 | 55 | |
| 3 | ATD 45min-30mg/kg | Aquarium | RCSM | 13 | 56 | |
| 4 | ATD 30min-150mg/kg | Aquarium | RCSM | 13 | 57 | |
| 5 | ATD 45min-150mg/kg | Aquarium | RCSM | 13 | 58 | |
| 6 | ATD 30min-150mg/kg | - | Aromatase activity | 13 | 59 |
All experiments were analyzed by appropriate analysis of the variance (ANOVA). As would be expected, results are quite variable given that we performed a number of tests exploring the appropriate dose and timing of the administration of aromatase inhibitors to reveal rapid effects on behavior. Instead of considering experiments that did not yield statistically significant results as pilot studies, while considering those that did yield such results as the actual experimental data, we decided to include all of our studies in the statistical analysis. This raises several complex issues related to analysis. If we conduct a series of individual analyses (e.g. analyses of variance) and note that some are statistically significant and others are not, we run the danger of committing both type I and type II errors. In the case of a type I error (rejection of a true null hypothesis), we would assume that the multiple analyses we conduct are all independent and ignore the fact that as one increases the number of individual analyses one increases the probability of obtaining a statistically significant effect by chance. At the same time, one could commit a Type II error (acceptance of a false null hypothesis) by underestimating the significance of a series of tests that all have the same trend but with variation in effect size such that each individual tests only reaches significance on occasion. We therefore used the strategy of meta-analysis to analyze the significance of 44 individual experiments out of the 59 experiments we conducted. Meta-analysis is designed to identify statistical patterns revealed in multiple studies (Rosenthal, 1991; Schulze, 2004; Wolf, 1986).
Animals
A total of sixty-nine Japanese quail (Coturnix japonica) served as subjects in these experiments. Birds were sexually naive prior to experimental procedures and had never been used in any other experiment. They were obtained from a local breeder at the age of 4–6 weeks. Throughout their life at the breeding colony and in the laboratory, the birds were exposed to a photoperiod simulating long days (16 hours light and 8 hours dark per day) and had food and water available ad libitum. All experimental procedures were in agreement with the Belgian laws on “Protection and Welfare of Animals” and on the “Protection of experimental animals” and the International Guiding Principles for Biomedical Research involving Animals published by the Council for International Organizations of Medical Sciences. The protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège.
Depending on the design of the experiment considered, some birds were left gonadally intact, while others were castrated. The intact birds were housed in individual cages from the day of their arrival in the laboratory. The other birds were first housed as a group in a large cage. A few days later, they were anesthetized (Hypnodil, Janssen Pharmaceutica, Beerse, Belgium, 15 mg/kg) and both testes were removed through a unilateral incision below the last rib. After they had recovered from the surgery, birds were brought back in the large cage. Approximately two weeks later, they were implanted subcutaneously with one or two 20 mm-long Silastic™ capsule (Silclear™ Tubing, Degania Silicone Ltd., Degania Bet, 15130, Israel; 1.57 mm i.d.; 2.41 mm o.d.) filled with crystalline testosterone (Fluka, cat. No. 86500). They were then housed in individual cages.
Experimental procedures
In the behavioral experiments, all the birds were used as their own control. In the following sections, an experiment thus refers as to a set of 2 to 4 behavioral tests (or trials) depending on the experimental design in which each bird was repeatedly tested in control and experimental conditions that are further detailed below. A total of 59 experiments (E) were conducted with five different groups of birds that were either left gonadally intact (Group 1) or were castrated and implanted with one (Groups 3 and 5) or two (Groups 2 and 4) 20 mm-long capsule(s) filled with testosterone. These capsules have been proven to provide circulating concentrations of testosterone in the upper range of physiological concentrations (Balthazart et al., 1990b). Groups 1 to 4 were used in experiments testing the effect of Vorozole™ and results were analyzed with meta-analytical procedures (See statistical analysis section below for more detail). Group 5 served for the assessment of the effects of ATD on sexual behavior.
All birds first received 3 to 6 pre-test trials for copulatory behavior at around 8 weeks of age (approximately two weeks after testosterone implantation in castrated birds) in order to let them acquire the copulatory pattern and confirm that all subjects were able to copulate. Subjects within each group were then assigned to two sub-groups equated according to the mean frequency of behavior (not statistically different). Additionally, measurements of the cloacal gland area (greatest length multiplied by the greatest width in mm2) of each subject were regularly taken to assess further the effects of the testosterone capsule implantation. The cloacal gland is an androgen-dependent structure (Sachs, 1967) whose size is highly correlated to plasma levels of testosterone (Delville, Sulon, and Balthazart, 1985).
Behavioral experiments were started one week after the pre-test trials. In each trial, the acute effect of a drug injected intraperitonealy was tested after 15, 30, 45 or 60 min in one the six designs described below. All drugs were diluted in propylene glycol: saline (4:1). These drugs included: the non-steroidal aromatase inhibitor, Vorozole™ (Vor or R83842 = 6-[(4-chlorophenyl)(1H-1,2,4-triazol-1-yl)methyl]-1-methyl-1H-benzotriazole, graciously provided by Dr. R. DeCoster (Janssen Research Foundation, Beerse, Belgium), androstatrienedione (ATD, purchased from Steraloids, Inc, Wilton, N.H., USA) and 17β-estradiol (E2, purchased from Sigma-Aldrich NV/SA, Bornem, Belgium). They were injected at the doses of 6 or 30 mg/kg, 30 or 150 mg/kg and 0.5 and 2.5 mg/kg, respectively.
Depending on the drug injected, each bird was tested once (ATD, E2) or twice (Vor) a week, once after injection of the drug and once after injection of the control solution (the vehicle). Consequently, each subject received either an injection of Vorozole™ every week or an injection of ATD or E2 every two weeks. This ensured that these experimental treatments had no or very limited long-term effects mediated by an action at the genomic level. Preliminary experiments had indeed revealed that the behavioral inhibition induced by Vorozole™ 30 min after injection completely disappears when birds are retested 24 hours later (data not presented). To control for possible sequential effects, the two sub-groups of subjects in one group always received the two treatments (drug vs. vehicle) in an opposite order and data are presented as the total results observed in both sub-groups. At the end of the experiment, birds were killed by decapitation. The completeness of gonadectomy and the presence of Silastic implants were checked. All birds appeared to be completely castrated and still possessed their testosterone implants.
Behavioral tests
Appetitive sexual behavior
Two measures of the appetitive behavior were quantified: the learned social proximity response and the rhythmic cloacal sphincter movements (RCSM).
The social proximity response was learned and tested in four adjacent two-compartment test cages as previously described in full detail (Balthazart, Reid, Absil, and Foidart, 1995). Briefly, each cage consisted of a large compartment that houses the experimental male subjects (90 × 90 × 50 cm) and a smaller cage for stimulus females (20 × 60 × 24 cm) that is centered on the left lateral wall of the main cage. The two compartments are separated by a vertically sliding door that can be remotely controlled by strings and pulleys. A small “window” (vertical slit, 1 cm [wide] × 15cm [high]) is located in the middle of this door and provides the male with a limited visual access to the female. This window can be closed by an opaque swinging plywood panel attached by a hinge just above the door. The lower part of that panel is attached to a string and pulley system that allows for remote lifting of the panel. A square area of the floor (30 × 30 cm), located in the middle of the lateral left wall (in front of the door/window) represents the test area for the bird’s position. When the window is open, the male present in the main chamber can only see the female located in the lateral chamber if he stands in front of the window in the test area.
A male will progressively learn to stand continuously in front of the window providing a view of the female after he has been given an opportunity to copulate with her in the experimental set-up. In the present experiments, all subjects in group 1 were tested 9 times on successive days in order to complete acquisition this response.
Each of these behavioral tests lasted a total of 25 min. The male was first introduced in the main chamber and the stimulus female was placed in the adjacent smaller cage. The “window” between the two compartments was closed at that time. Birds were given 5 min to habituate to the new environment. The window was then opened and the position of the male was recorded for 5 min. A beeper was activated and emitted a weak sound every 5 s. At each beep, the observer recorded whether the subject was actually looking through the window or not (LOOK). Looking behavior was defined as a stereotyped positioning of the head that allows the subject to focus on the female through the window. This point sampling furnished a score for the looking behavior ranging from zero (never observed) to 60 (behavior present at every beep). Data collected during this period provided a measure of the appetitive behavior of the male. At the end of this period, the door separating the two compartments was lifted and the two birds were allowed to freely interact for 5 min. During that time, the frequency and latency of the first occurrence of male sexual behaviors were directly recorded. The following behavior patterns were systematically noted: strut, neck-grab (NG), mount attempt (MA), mount (M) and cloacal contact movements (CCM) (See (Adkins and Adler, 1972; Hutchison, 1978) for a detailed description). These data provided a measure of the consummatory behavior of the birds. The female was then removed from the experimental chamber where the male stayed for another 5 min before he was returned to his home cage.
The rhythmic cloacal sphincter movements (RCSM) are produced in response to the visual presentation of a female. These sphincter muscle movements are used by reproductively active males just before copulation to produce the stiff meringue-like foam that is transferred into the female’s cloaca during copulation and that enhances the male fertilization success (Seiwert and Adkins-Regan, 1998). This foam is produced by the cloacal gland, a large sexually dimorphic, androgen-sensitive, external protuberance of the caudal lip of the cloaca (Sachs, 1967). It has been shown previously (Seiwert, 1994; Seiwert and Adkins-Regan, 1998; Thompson, Goodson, Ruscio, and Adkins-Regan, 1998) that gonadally intact, sexually active males rapidly increase the rate of these movements when they are provided with visual access to a female. Interestingly, the RCSM rate is higher when a sexual mature male is presented with visual access to a female as compared to a male suggesting that these cloacal contractions are not simply triggered by visual cues of a general sort but are preferentially induced by visual access to a sexual partner (Seiwert, 1994; Seiwert and Adkins-Regan, 1998). Their expression depends on the endocrine status of the birds: the RCSM frequency decreases after castration but increases following a systemic treatment with exogenous testosterone (Balthazart, Absil, Gerard, Appeltants, and Ball, 1998). These movements are also inhibited by lesions of the preoptic area, a brain region that is known to control appetitive as well as consummatory aspects of sexual behavior (Balthazart et al., 1998). These movements thus provide an excellent measure of male appetitive male sexual behavior in quail. We would argue that measurement of the RCSM response rate in the presence of a female is a good measure of the male’s propensity to engage in copulation per se (i.e. a measure of his underlying sexual motivation). RCSM were quantified in an aquarium (20 × 40 cm) located on a raised platform. A mirror was placed under the aquarium at a 45° angle and provided the observer with an unobstructed view of the male’s cloacal area. At the beginning of each behavioral test, the aquarium was divided into two chambers by an opaque sliding panel and glass partition. One experimental male was placed in one of the chambers and a stimulus egg-laying female was placed in the other chamber. RCSM were directly counted for 2.5 min during which the male could not see the female. The sliding panel was then removed so that the male and female were only separated by the glass barrier; the male had then visual access to the female although he could not physically interact with her. The RCSM were quantified by direct observation for an additional 2.5 min under these conditions. A single female was used to test all males on a single day and a different female was used on each test day. On a given day, males were tested in a random order so that even if the behavior of the female changed systematically during the day, this effect would be randomized in the two experimental groups.
Because it was noticed that repeated testing often lead to a decrease in the baseline production of RCSM, we also analyzed the results of these experiments after excluding subjects that did not produce at least 30 RCSM when presented to the female in the control condition. This was done to exclude subjects in which an inhibition of RCSM frequency by aromatase inhibitors was impossible or could only have a very small magnitude due to the low baseline. This baseline value was chosen arbitrarily.
Consummatory sexual behavior
Copulatory behavior was quantified either in a large arena immediately after the measure of the social proximity response described above or in a small arena (60 × 40 × 50 cm) during independent tests. In the latter case, the experimental male was introduced into the test arena that already contained a sexually mature female with which the male could freely interact during a 5 min period. The latency and frequency of the male sexual behaviors described above (NG, MA, M, CCM) were recorded during the 5 min of interactions. Males were tested in a random order that was changed on each day with a female randomly selected from a large pool so that even if the behavior of females varied during and between the days, this effect would be randomized in the two experimental groups. In the results, only analyses of MA and CCM frequencies and CCM latencies will be reported to avoid redundancy since analysis of NG and M always leads to nearly identical conclusions.
Locomotion
Like copulatory behavior, locomotor behavior was assessed either in the large arena just before the assessment of the social proximity response or in the small arena described above. The floor of large arena was divided in nine equal squares (30 × 30 cm). During the pre-experimental control period (5 min) when the female was not visible, the observer recorded the number of times the subject entered each square. The number of entries therefore provided a score for the locomotor behavior. Alternatively, the small arena was virtually divided into three equal zones by vertical lines drawn on the front glass of the arena. During a 5 min period, the observer recorded the number of times the subjects crossed the virtual boundaries in the absence of a female in the arena.
The five experimental groups of birds
Group 1
Twelve intact birds were used in 23 separate experiments to investigate the effect of different doses of Vorozole™ on sexual behavior measured after a variety of latencies. These birds will be referred to as intact or “I” birds. They were tested for copulatory behavior in the small arena (Experiments [E] 1–5 and E7), for locomotion (E8 in small arena and E11, 14, 17 and 20 in large arena), appetitive (social proximity response: E12, 15, 18 and 21) and consummatory sexual behavior in the large arena (E13, 16, 19 and 22), and for appetitive RCSM (E6, 9, 10 and 23). These tests were carried out over a period of 4.5 months during which three subjects died from unexplained reasons apparently unrelated to the treatments. This therefore reduced the number of available subjects during the last experiments on this group.
In order to test the effect of Vorozole™ on the acquired social proximity response, birds were given 9 acquisition trials and then distributed in two matched groups based on their performance during the last trial. A mixed two-way analysis of variance with the trials as the repeated measure and the groups as independent factor revealed, as expected, no group difference during the acquisition phase (F1,7 < 0.0001, p = 0.9963) and no interaction (F8,56 = 0.266, p = 0.9742) but a trial effect (F8,56 = 8.321, p < 0.0001) indicating that both groups similarly acquired the response.
Group 2
Eleven castrated birds implanted with two 20 mm long capsules of testosterone (this group will be referred to as “CX+T40” birds) were submitted to 11 separate experiments to assess after various latencies the effect of Vorozole™ on birds in which plasma testosterone concentrations were clamped at a fixed level. These subjects were included in 5 experiments quantifying copulatory behavior in the small arena (E24–26, 28–29) and 6 experiments to measure RCSM (E27, 30–34). These experiments lasted for a total of 4.5 months.
Group 3
Fifteen castrated birds implanted with one 20 mm long capsule of testosterone (so this group will be referred to as “CX+T20A” birds) were submitted to 7 experiments to assess the effect of Vorozole™ on sexual behavior in birds exposed to lower circulating levels of testosterone than in group 2. They were used in one experiment measuring copulatory behavior in the small arena (E39) and in 6 RCSM tests (E35–38 and E40). In addition, these birds were also used to test whether 17β-estradiol (E2) was able to reverse the effect of Vorozole™ on RCSM. The effect of Vorozole™ alone or combined with a simultaneous injection of E2 (0.5 mg/kg) was tested twice 15 or 30 min after the injections (4 independent experiments, E41–43 and E45). These experiments thus tested whether E2 was able to increase the low behavior frequencies observed following injection of Vorozole™. Experiments on group 3 lasted for a total of 5.5 months.
Group 4
Another group of sixteen castrated birds were implanted like group 3 with one 20 mm long capsule of testosterone; they will be referred to as “CX+T20B” birds. They were used in 8 experiments to assess the effect of 17β-estradiol on the Vorozole™-induced inhibition of sexual behavior (E46–52). To this end, the effect of Vorozole™ alone was first tested on RCSM frequencies measured after 30 (E46 an 50) and 45 min (E48) and on copulatory behavior measured 30 min after the injection (E47).
Birds were then divided into three sub-groups based on their behavioral performance (RCSM) in control conditions during the previous tests. The effect of E2 on the Vorozole™-induced inhibition was then assessed using a Latin square design (E49). The three groups were tested in a randomized order in each of three conditions: Vorozole (30 mg/kg), 17β-estradiol (E2; 2.5 mg/kg) combined with Vorozole and Vehicle (Veh). All injections were given simultaneously 30 min before testing for RCSM production. Each subject independent of his group received two injections, namely, Vor+Veh, Vor+E2 or Veh+Veh. These experiments thus tested whether Vorozole™ decreases behavior expression as compared with controls and to what extent this effect can be reversed by a concurrent injection of E2.
After this experiment, birds were left undisturbed for three weeks. We then noticed that their cloacal gland area was beginning to decrease. All birds were therefore implanted with an additional 20 mm long testosterone capsule to ensure that their circulating level of testosterone was high enough to activate the complete sequence of copulatory behaviors in the following tests. This additional manipulation presumably resulted in a plasma concentration of testosterone that was intermediate between the level provided by one and by two capsules. Two weeks later, all birds were given an opportunity to copulate for 5 min with a receptive female in order to define again three groups based on the frequencies of their sexual behaviors. They were then submitted to 2 additional experiments in the Latin square design as described above (3 tests per experiment after Vor+Veh and Vor+E2 or Veh+Veh, injections, E51–52) to assess with different latencies the potential reversal by E2 of the Vorozole™-induced inhibition. In both cases, Vorozole™ (30 mg/kg) was now injected 15 min before E2 (2.5 mg/kg) and behavioral observations were started 15 or 30 after the injection of E2 (e.g. 30 (E52) or 45 min (E51) after the injection of Vorozole™.
Four weeks after the completion of the last behavioral experiment, birds were allowed to copulate for 5 min with a receptive female in order to define 2 sub-groups equated based on the frequencies of their behaviors (E53). One group (n=6) was injected with Vorozole™ (30 mg/kg), while the other group (n=5) was injected in parallel with the vehicle (propylene glycol: saline (4:1). All birds were sacrificed 30 min after the injection in order to assess the effect of Vorozole™ on brain aromatase activity. These tests of group 4 were carried out over a period of 6 months.
Group 5
Thirteen castrated birds implanted with one 20 mm long capsule of testosterone (“CX+T20C” birds) were submitted to 6 experiments (E54–59) to assess the effect of a steroidal aromatase inhibitor, androstatrienedione (ATD), on the expression of RCSM. Two groups equated on their copulatory behavior performance during pretests were tested with 2 doses of ATD: 30 mg/kg injected 15 (E54), 30 (E55) or 45 min (E56) before testing and 150 mg/kg injected 30 (E57) or 45 min (E58) before testing.
One week after the completion of the last trial, one group was injected with ATD (150 mg/kg) and the other one with the vehicle (propylene glycol: saline (4:1)) and birds were sacrificed 30 min after the injection in order to quantify the effect of ATD on brain aromatase activity (E59). These tests were carried out over a period of 4.5 months.
Aromatase activity in preoptic area-hypothalamic homogenates
Immediately after sacrifice, brains were dissected out of the skull, frozen on dry ice and kept at −75°C until used for assays. The preoptic area (POA)-hypothalamic block (±60–80 mg) was dissected by two coronal cuts at the level of the tractus septomesencephalicus (rostral edge of the POA) and of the oculomotor nerves (caudal edge of hypothalamus), two parasagittal cuts placed approximately 2 mm lateral to the brain midline and one horizontal cut about 2 mm above the floor of the brain. This block, that contains the majority of the aromatase-expressing cells in the quail brain, was homogenized with an all-glass homogenizer in ice-cold buffer containing 150 mM KCl, 1mM Na-EDTA, 10 mM Tris-HCl pH 7.2.
Aromatase activity was quantified by measuring the tritiated water production from [1β-3H]-androstenedione (Baillien and Balthazart, 1997; Roselli and Resko, 1991). On an ice bath, triplicate aliquots (50 μl) of homogenate containing approximately 1 mg wet weight were added to 50 μl of 100 nM [1β-3H]-androstenedione and 50 μl of buffer. To initiate the assay, 50 μl of NADPH were added so as to reach a final concentration of 1.2 mM. All these steps were conducted at 4°C in 1.5 ml Eppendorf tubes which were then quickly capped and incubated for 15 min at 37°C. The reaction was stopped by cooling the sample in an ice bath and adding 0.4 ml ice-cold 10% trichloroacetic acid containing 2% activated charcoal. After centrifugation at 1200 g for 15 min supernatants were applied to small columns made of Pasteur pipettes plugged with glass beads and filled (3 cm high) with a Dowex cation exchange resin AG 50W-X4, 100–200 mesh (Biorad, Richmond, CA). The columns were then eluted with 3 × 0.6 ml distilled water. Effluents were collected in scintillation vials and 10 ml Ecoscint A (National Diagnostics, Atlanta, GA) were finally added. Vials were counted for 3 min on a Packard Tri-Carb 1600 TR Liquid Scintillation analyzer.
Within each experiment, blanks were obtained by processing brain samples in the presence of an excess (final concentration about 40 μM) of the potent and specific aromatase inhibitor, R76713 (Racemic vorozole, Janssen Pharmaceutica, Beerse, Belgium). The blank values never exceeded 140 dpm while active control samples had radioactivities ranging between 2000 to 3000 dpm. A recovery of 93 ± 2% was usually obtained from samples of 10 000 dpm tritiated water conducted throughout the entire purification procedure (incubation, centrifugation and Dowex column). Enzyme activity was expressed in fmol h−1 mg fresh weight−1 after correction of the counts for quenching, recovery, blank values and percentage of tritium in β-position in the substrate.
Aromatase activity in hypothalamic explants maintained in vitro
Immediately after sacrifice, the POA-hypothalamic block of 3 intact 8 week-old male quail was dissected as described above with the exception that the width of the block was decreased (parasagittal cuts 1–1.5 mm lateral to midline) to optimize diffusion of oxygen and of experimental compounds into the explants. This isolated block of tissue (40–60 mg) is naturally separated into two halves by the third ventricle. Each half block was immediately transferred in a test tube containing 300 μl of physiological saline (25 mM glucose, 150 mM NaCl, 4.4 mM KCl, 3.1 mM CaCl2, 1.3 mM MgSO4, 10 mM tris-Hepes (pH 7.2) containing 25 nM [1β-3H]-androstenedione and oxygenated with pure O2. One hemi-explant (the left or the right one selected randomly) always served as control while matched other half was submitted to experimental treatment (Vorozole™). Every 5 min, the 300 μl of medium were aspirated with a syringe and replaced by 300 μl fresh saline containing 25 nM [1β-3H]-androstenedione in order to assess the time-course of changes in AA. After AA had reach a steady state in these conditions, Vorozole™ (40 nM) was added to the medium for a period of 10 min (the medium added contained the inhibitor for two 5 min periods). Incubations were then carried out without inhibitor for another 30 min. Withdrawn samples were immediately cooled in an ice bath, and 400 μl ice-cold 10% trichloroacetic acid containing 2% activated charcoal were added. They were further processed to isolate the tritiated water produced by aromatization from the remaining radioactive steroids as described above.
Data analysis
Analyses of variance
Unless otherwise mentioned, all data were originally analyzed by a one or two-way mixed design analysis of the variance (ANOVA) with the treatments (Veh/VOR and Veh/E2) as repeated factors. Analyses were followed when appropriate by Tukey post-hoc tests adapted for repeated measures. Effects were considered significant for p≤0.05. All analysis were carried out with the Macintosh version of the software Statview, version 5.01 (Abacus Concept, Inc., Berkeley, CA, USA).
Meta-analysis
Meta-analyses allow summarizing a large number of studies by using individual experiments as the units of the analysis rather than the individual data. The goal of the meta-analysis is to obtain a pure number (free of the original measurement unit), called the effect size, which is an index of the relation between treatment and outcome that can be compared across studies (for further detail see Rosenthal, 1991; Schulze, 2004; Wolf, 1986). The effect size used in this study is the standardized mean difference d as defined by Cohen (Cohen, 1977) applied to the correlated design (Dunlap, Cortina, Vaslow, and Burke, 1996). This is accomplished by standardizing the raw effect size expressed in the measurement unit of the dependent variable (e.g. difference between the two groups means) by dividing it by the common standard deviation of the measures in their respective populations, also expressed in the original measurement unit (d= (ME−MC)/SD, where ME and MC are the means of the experimental and the control groups, respectively, and SD is the common standard deviation, e.g. the square root of the variance). So an effect size of 1 represents a difference between groups that has the same magnitude as the variability in these groups, measured by the common standard deviation (SD). Then, further transformations (such as various adjustments of the estimate [Cohen adjustments, Hedges adjustments, etc], weighting of the experiments based on sample size, etc) may be applied to refine the estimation of the effect size. After necessary transformations, an overall effect size can be obtained and the general significance of the studied phenomenon statistically tested (Cooper and Hedges, 1994; Rosenthal, 1991).
Here, effect sizes were calculated as Hedge’s d standardized mean difference (g) between the different groups considered (Vehicle, Vorozole™, E2, or Vorozole™+ E2). The sign assigned to “g” was based on the expected result for a given experiment. Specifically, Vorozole™ effect on behavior was predicted to be inhibitory (decreasing the frequencies of behavior and increasing the latencies of first display of the behaviors), inhibition was thus assigned a positive sign and stimulation a negative sign. Conversely, estradiol effects were predicted to be excitatory, so stimulation (or restoration of the behavior following its inhibition by Vor) was attributed a positive sign.
In order to compare the effect sizes of several experiments and test the reliability of the conclusions, the effect size computed for the different experiments sharing the same conditions (compound, dose and latency between the injection and the test) were combined. This combination is accomplished by the computation of the mean of the effect sizes of the experiments. To get an overall estimate of the probability that the combined effect reaches statistical significance, the p values of these separate experiments were also associated. This was obtained by the calculation of the Z corresponding to each of these p levels (corresponding to the unpaired t-test performed on the data) and use these Z to compute a new Z corresponding to the p value of the combined effect size (for further details see Rosenthal, 1991). As we assumed variability to be due to both sampling error and random differences across studies, to combine study-to-study variations data were fitted to a random effects model as described by Fleiss (Fleiss, 1993), which provides more conservative estimates than a fixed effects model. Random effects are also more appropriate when heterogeneity is present in the results (Normand, 1999).
The following behaviors were analyzed in this way: the mean number of entries during tests of locomotion, the mean frequency of sexual behaviors (NG; MA, M, CCM) and the mean latencies to show the first NG (Lat NG) and CCM (Lat CCM) for the consummatory behavior, the mean number of looks (social proximity response) and the mean frequency of RCSM in the presence and in the absence (basal RCSM) of the female. The different doses administered and latencies between injections and behavioral tests were used as moderator variables in these analyses. Running a meta-analysis on data we collected our-self had the advantage of avoiding the publication bias resulting from the selection of data related to the processes of submission, reviewing, and publication of scientific results. Studies identifying statistically significant effect are systematically more often published than negative studies. Consequently, when such a publication bias exists, it has to be evaluated to qualify the results of the meta-analysis and this process is associated with a fair degree of uncertainty. This problem is not present in this work in which all studies that were carried out were known and incorporated in the analysis. The meta-analysis, although rarely used in such a context, represent a powerful tool of analysis.
Results
All results of the 59 experiments assessing the behavioral effects of Vorozole™ (See Table 1 for the details of each experiment) were originally analyzed separately by analyses of the variance. These analyses identified, as expected, a number of significant effects as well as a good number of statistically insignificant results, given that a range of doses of compounds and latencies between injections and tests were investigated and some of them had obviously to be ineffective. In this paper, we therefore selected the following strategy for analyzing and presenting the results: first we present all the data that were collected and summarize the results with the use of meta-analysis. In a second step, we then analyze in more detail a few representative specific time-response curves of experiments leading to positive results to illustrate the meta-analyses.
A. Meta-analyses of the effects of Vorozole™
Effects of Vorozole on consummatory sexual behavior
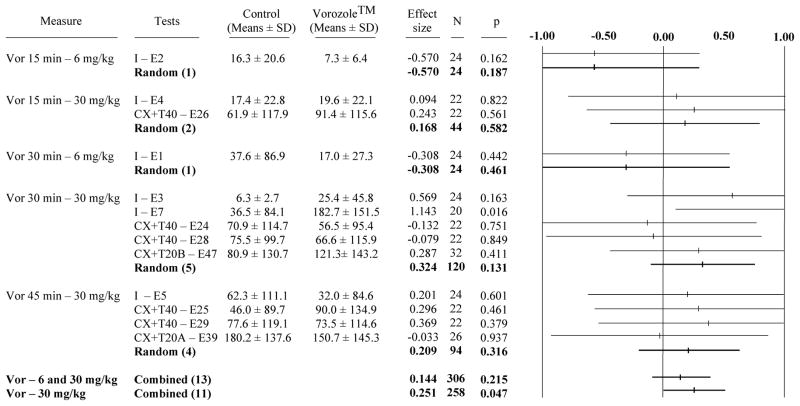
The global meta-analyses of the 13 experiments (E1–5, 7, 24–26, 28–29, 39 and 47) testing the effects of Vorozole™ on consummatory sexual behavior in the small arena identified no effect when the two doses of Vor were considered simultaneously (no effect on MA and CCM frequencies or CCM latencies). However, when the 2 studies that had used the smaller dose of Vor (6 mg/kg, E1 and E2) were excluded and the meta-analysis was focussed on the 11 studies testing the effects of the larger dose (30 mg/kg), a significant but moderate overall increase in CCM latencies was detected (effect size “g” = 0.251; p = 0.047 compared to an effect size = 0.144, p=0.215 when the two experiments with the low dose are included; see Fig. 1). This significant overall effect was associated with a significant increase in CCM latencies in only one of the 11 individual experiments (E7; p=0.016), which limits our confidence in the generality of this effect. This conclusion is further supported by the observation that no effect on MA and CCM frequencies was detected even in these analyses focussing of the large dose of aromatase inhibitor (data not shown).
Figure 1. Meta-analysis of the effect of Vorozole™ on consummatory sexual behavior evaluated by the measure of CCM latency in the small arena.
Each line represents one experiment identified by the name of the group (I: intact; CX+T40 or CX+T20: castrated plus 40 or 20 mm testosterone) followed by the number of the experiment. Average values (± SD) of results in both groups are also presented, followed by the effect size, number of observations and probability associated with the difference (calculated by t test). Since all subjects were tested in both conditions and used as their own control, this number is equal to the double of the number of subjects.
The right-hand column is a tree representation of the effect size associated with the experiment. For each experiment, a point estimate (indicated by a tick) represents the measured effect expressed as a value, namely the effect size, comparable to the effects observed in the other experiments. The horizontal bar surrounding the point estimate represents the confidence interval associated to the effect size. A point estimate of 0.00 indicates no effect. Values higher than 0.00 reflect an effect in the predicted direction (See methods for more details on the sign assignment), while values smaller than 0.00 reflect an effect in the opposite direction. The distance to 0.00 indicates the amplitude of the effect-size. If the point estimate and confidence interval fall below 0.00 the experiment would meet the criterion for statistical significance (p-value ≤ 0.05). On the contrary, if the confidence interval overlaps 0.00, the p-value would exceed 0.05 and the experiment would not be statistically significant. Some lines do not have ticks. This is due to the fact that these estimate points fall out of the scale used to present these data.
The “random” line (in bold) represents the summary of random-effects estimate for the combination of experiments performed in similar conditions of dose and timing. Data provided are from left to right: the effect size, total number of observations and associated probabilities. The two combined lines at the bottom represent the combination of all experiments (Vor - 6 and 30 mg/kg) or the combination of experiments testing only the effects of the higher dose (Vor -30 mg/kg). Values in the positive side represent an inhibitory effect on behavior (increase of the latency of CCM) while values in the negative side indicate a decrease of the CCM latency.
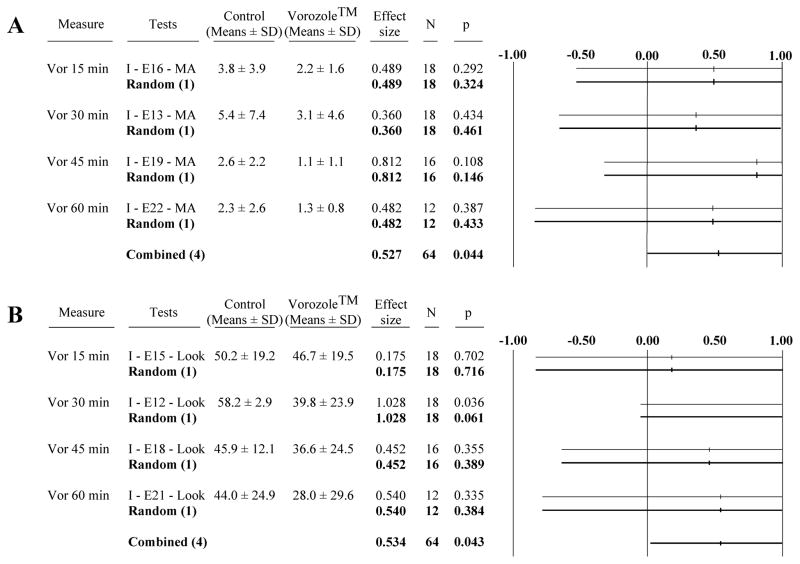
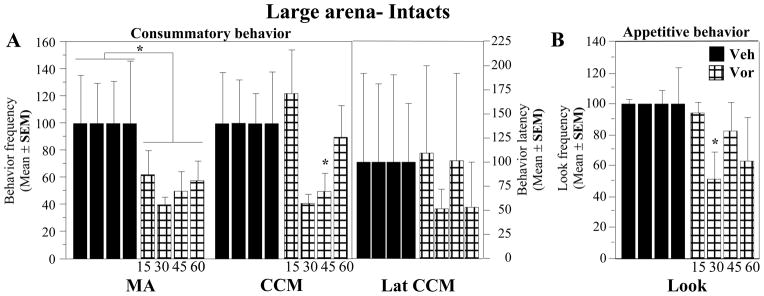
In contrast, the meta-analysis (n=4 experiments, E13, 16, 19 and 22) of copulatory behavior expressed in the large arena immediately after the quantification of the learned social proximity response indicated a pronounced significant decrease in MA frequencies in Vorozole™-injected birds associated with a relatively large effect size (g= 0.527, p=0.044; Fig. 2A). This overall effect though was not associated with significant changes in any of the individual experiments (Fig. 2A) and no effect on the CCM frequency and latency could be detected (data not shown).
Figure 2. Meta-analysis of the effect of Vorozole™ on consummatory and appetitive sexual behavior assessed in the large arena by the measure of the mount attempt frequency (A) and the social proximity response (look; B).
Data are presented as in figure 1.
Values in the positive side represent an inhibition of behavior (decrease in the frequency of MA or look) while values in the negative side indicate increases in the MA or look frequency.
Taken together, these studies therefore suggest that a single injection of Vorozole™ in male quail is able to inhibit aspects of male copulatory behavior after latencies between 15 and 60 min but overall this effect only has a very modest amplitude and is not easily replicable.
Effects of Vorozole on appetitive sexual behaviors
The meta-analysis of the results of the 4 experiments (E12, 15, 18 and 21) that quantified the learned social proximity response identified an overall significant decrease in the “Look scores” following injection of the aromatase inhibitor (g= 0.534, p=0.043; Fig. 2B). A significant effect was detected in only one of the individual experiments. This effect was observed when the latency between injection and behavioral test was 30 min. The effect of Vorozole™ was in this case associated with a very large effect size (g=1.028; E12, see Fig. 2B).
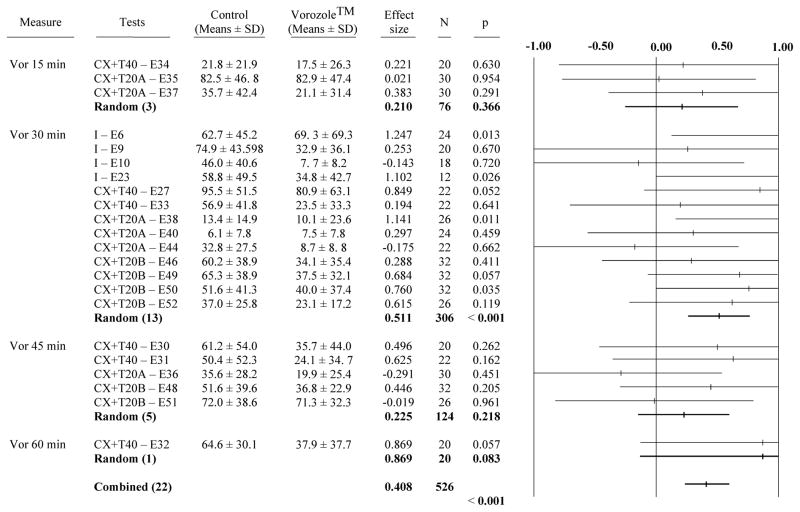
A total of 22 independent experiments assessed the action of Vorozole™ on the expression of the other measure of appetitive sexual behavior, the production of RCSM in the presence of a female (E6, 9–10, 23, 27, 30–38, 40, 44, 46, 48–52). Overall, the experiments identified a very significant effect of the inhibition on the RCSM frequencies (g= 0.408, p<0.001; Fig. 3). When analyzed separately, only 4 of these 22 experiments indicated a significant inhibition by Vorozole™ but interestingly all 4 experiments had been performed with a latency of 30 min between the injection and the test (E6, 23, 38 and 50). As a result, the partial meta-analysis considering separately the 4 groups of experiments performed with 4 different latencies between the injection and the test (15, 30, 45 or 60 min) indicated that a significant effect of the compound was only observed at the 30 min latency (g= 0.511, p<0.001; see Fig. 3).
Figure 3. Meta-analysis of the effect of Vorozole™ on appetitive sexual behavior assessed by the measure of rhythmic cloacal sphincter movements in the presence of the female.
See also figure 1 for the description of data presented. Values in the positive side represent an inhibitory effect (decrease in the frequency of RCSM) while values in the negative side indicate an increase in RCSM frequency.
Because a marked inhibition of RCSM frequencies could not obviously be induced by Vorozole™ if subjects were displaying low frequencies in control conditions, an additional analysis was performed in which subjects that showed less than 30 contractions of the cloacal gland in control condition were excluded. This analysis revealed an even larger effect size for the inhibitory effect of Vorozole™ on the RCSM produced in the presence of the female (g= 0.709; p = 0.002; data not shown).
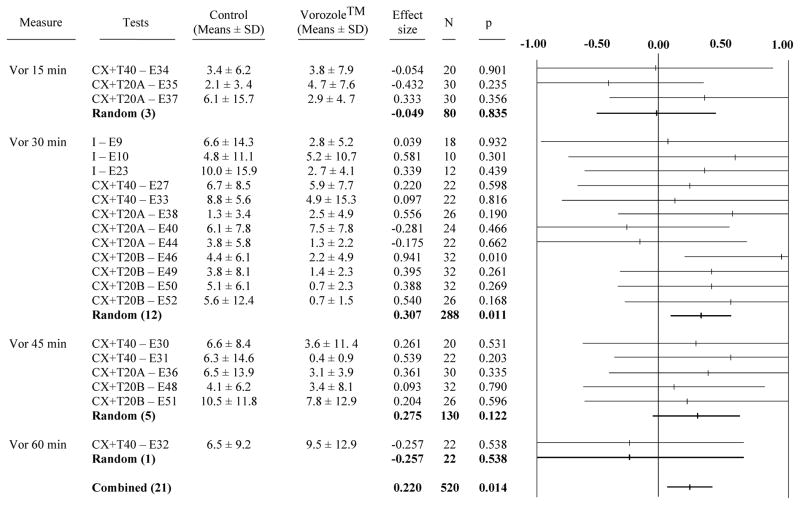
Although RCSM frequencies are typically very low during the first 2.5 min of test when the stimulus female is not visually accessible, a limited but nevertheless significant effect of Vorozole injections was detected by meta-analysis of this baseline frequency of RCSM. The amplitude of the effect was however smaller than for the RCSM expressed in response to the female (g= 0.220, p= 0.014; Fig 4). Interestingly the sub-analyses considering separately the 4 intervals between injection and tests indicated again that this effect was significant only for the 30 min latency (g=0.307; p= 0.011).
Figure 4. Meta-analysis of the effect of Vorozole™ on the measure of rhythmic cloacal sphincter movements in the absence of the female.
See also figure 1 for the description of data presented. Values in the positive side represent an inhibitory effect (decrease in the frequency of RCSM) while values in the negative side indicate an increase in the frequency of RCSM.
Together, these data clearly indicate that a single injection of Vorozole™ exerts a rapid and powerful inhibition on two measures of appetitive sexual behavior in male quail, the social proximity response and the rhythmic contractions of the cloacal gland muscles. These effects do not seem to be related to a general debilitating effect of the aromatase inhibitor. The locomotion of the subjects was indeed recorded during 5 independent experiments, 4 in the large arena (E11, 14, 17 and 20) and one in the small arena (E8). The meta-analysis of the effects of Vorozole on the amount of locomotion recorded during these 5 independent experiments indicated a negligible effect size (g= 0.079), which was far from significant (p = 0.754; data not shown).
Reversibility of Vorozole-induced inhibitions by exogenous estrogen
Assuming that Vorozole™ inhibits behavior by blocking the local estrogen production, we hypothesized that an injection of exogenous 17β-estradiol could restore behavior to its control level. This hypothesis was tested during 7 independent experiments.
In a first set of 4 experiments performed with CX+T20A birds (group 3; E41–43, 45), Vorozole™ and E2 were injected simultaneously 15 or 30 min before testing and these experiments were repeated twice. Overall, RCSM frequencies were found to be slightly increased by the E2 injections but effect size was small (g= 0.150) and far from significant (p=0.479).
In the next set of 3 experiments performed on CX+TB birds (group 4; E49 and E51–52), the injection of E2 combined with Vorozole had similarly no overall effect on the RCSM frequencies (g= −0.114, p=0.726). The overall analysis of these 7 experiments by the meta-analytical technique still did not identify a significant effect (g= 0.034, p = 0.841).
B. Analyses of variance of selected experiments illustrating inhibitory effects of Vorozole
Copulatory behavior in the large arena
In agreement with the results of the corresponding meta-analysis, the analysis by two-way ANOVA of the copulatory behavior observed in the large arena during experiments E16 (15 min), E13 (30 min), E19 (45 min) and E22 (60 min) revealed a significant inhibitory effect of the treatment (VOR 30 mg/kg) on the frequency of mount attempts (F1,5 = 10.253, p = 0.0239, Fig. 5A). No significant effect was detected on other behaviors (CCM, F1,5 = 3.356, p = 0.1265; Lat CCM, F1,5 = 0.717, p = 0.4356). No effect of time (MA, F3,15 = 0.068, p = 0.9759; CCM, F3,15 = 1.266, p = 0.3217; Lat CCM, F3,15 = 0.093, p = 0.9629) and no interaction (MA, F3,15 = 0.098, p = 0.9600; CCM, F3,15 = 1.210, p = 0.3400; Lat CCM, F3,15 = 0.356, p = 0.7854) was observed.
Figure 5. Time-response curves for the effects of Vorozole™ on appetitive and consummatory sexual behavior.
A. Effect of Vorozole™ on MA and CCM frequency and CCM latency in intacts birds tested in the large arena. B. Effect of Vorozole™ on the frequency of looks in intacts birds tested in the large arena. Data were analyzed by two -way ANOVAs with repeated factors (treatment and latency between injection and test) or by one-way ANOVA analyzing each time point separately. *= P < 0.05 by comparison with the control condition at the corresponding time.
ANOVAs considering each time point separately did not identify any significant effect (p>0.10 in all cases) except in the analysis of the CCM frequencies measured 45 min after the injection (F1.5=7.500, p = 0.0409)
The learned social proximity response
The analysis by two way ANOVA of the Look scores collected during the same experiments (E12, 15, 18 and 21) did not, however, reveal any experimental effect (treatment effect: F1,5 = 3.240, p = 0.1318; time effect: F3,15 = 1.289, p = 0.3145; interaction (F3,15 = 1.174, p = 0.3526), while the meta-analysis had identified an effect of moderate size that was just significant. It is important to note, however, that the meta-analysis was based on the mean results collected at each latency and therefore included all the data that were collected. In contrast, the ANOVA with two repeated factors excludes in all conditions the data corresponding to subjects that did not complete the entire experiment (in group 1, three birds died in their home cage before the completion of the experiment). As indicated in figure 2B, several experimental birds did not complete the entire experiments (N decreased from 18 to 12 between E12 and E18). It is therefore not unexpected that a result that was just significant in the meta-analysis became non significant in the ANOVA procedure that excluded a subset of the subjects. In agreement with the meta-analysis, the separate ANOVAs comparing look scores at each time point indicated a significant inhibition by Vorozole™ at 30 min post injection (F1.5=7.784 p=0.0384) while the effect was not significant at all other latencies (p>0.30; See Fig. 5B).
The rhythmic cloacal sphincter movements
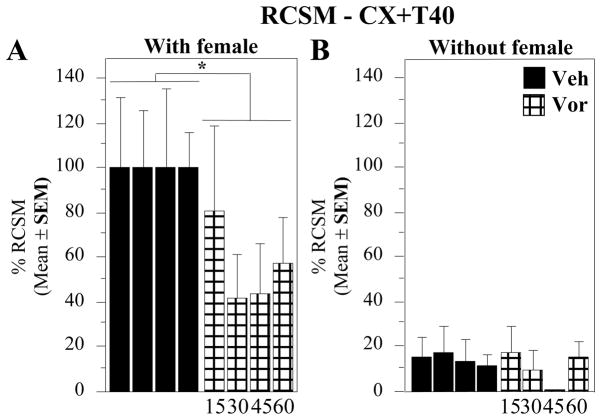
In contrast to the relatively limited effects of Vorozole™ on copulatory behavior, the aromatase inhibitor (30mg/kg) very markedly inhibited the expression of RCSM production. The two-way ANOVA performed on experiments E31 (45 min), E32 (60 min), E33 (30 min) and E34 (15 min) of CX+T40 birds revealed a significant overall inhibition of RCSM produced in the presence of the female (F1,10 = 13.422, p = 0.0052), while no significant effect was observed in the absence of the female (F1,9 = 0.710, p = 0.4211) even if a quantitative tendency to decrease was observed at 45 min after the injection (Fig. 6). No time-effect (with female: F3,27 = 0.192, p = 0.9008; without female: F3,27 = 0.727, p = 0.5448) and no interaction (with female: F3,27 = 0.460, p = 0.7127; without female: F3,27 = 0.502, p = 0.6840) were however detected in both conditions.
Figure 6. Time-response curves for the effects of Vorozole™ on the expression of rhythmic contractions of the cloacal gland muscles in the presence (left) or absence (right) of the female.
All data were normalized by expressing them as percentage of the numbers of RCSM observed in control conditions in the presence of the female at each given time point. Data were analyzed by two-way ANOVAs with repeated factors (treatment and latency between injection and test) or by one-way ANOVA analyzing each time point separately. *= P < 0.05 by comparison with the control condition at the corresponding time.
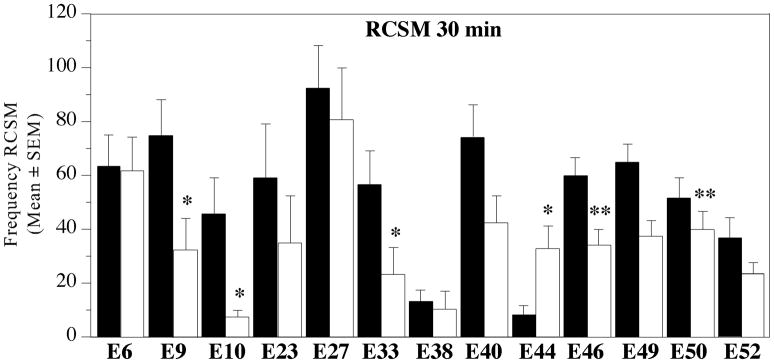
The maximal inhibition of RCSM expression by Vorozole was consistently observed after 30–45 min as indicated by the time-course analysis presented in Fig. 6 and by the meta-analysis (Fig. 3, effect significant only at 30 min). The consistency of this effect is clearly illustrated in figure 7 showing the average numbers of RCSM expressed in the presence of a female during 13 experiments comparing the injection of Vorozole™ with its vehicle 30 min after injection.
Figure 7. Effects of the injection of Vorozole™ 30 min before the test on the expression of rhythmic contractions of the cloacal gland muscles in the presence of the female during 13 independent experiments.
Data in each experiment were analyzed by a one-way ANOVA with treatment as repeated factor. *= P < 0.05 by comparison with the control condition.
In 10 out of 13 experiments a pronounced decrease in RCSM frequency was observed 30 min after the injection of Vorozole™, which was significant in 6 cases. No change (n=2) or an increase (n=1) was observed in the other experiments for reasons that are impossible to specify at this time. The overall pattern however clearly indicates a significant decrease (p<0.001 in the meta-analysis). Together, these data illustrate the inhibitory action exerted by Vorozole™ on appetitive behavior assessed by the measure of the contractions of the cloacal gland.
Reversibility of Vorozole effects by estradiol injections
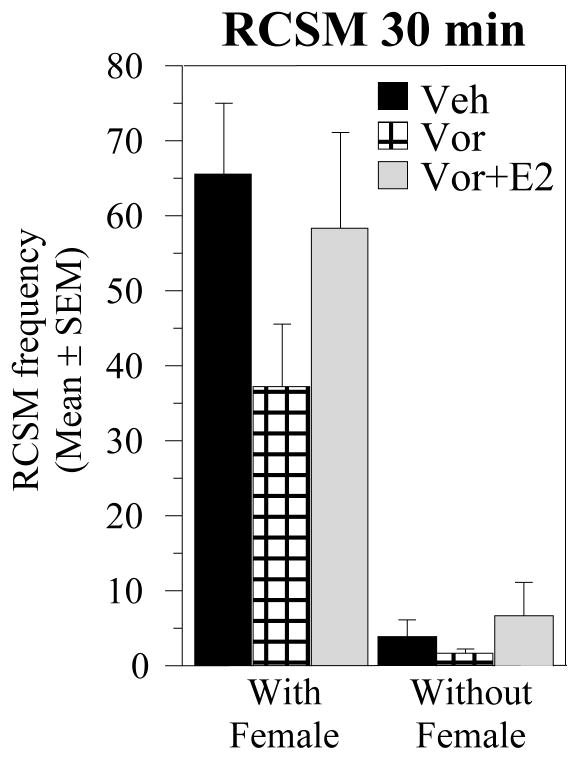
Although meta-analyses of 7 independent experiments failed to reveal an overall effect of E2 injections on the Vorozole™-induced inhibition of RCSM (see above), the inspection of the raw data indicated that weak effects might have been present at specific combinations of doses and latencies between injection and test. When E2 was injected concurrently with Vorozole™ 30 min before testing (E49), it seemed to restore partially the RCSM frequency to a level close to the control condition (see Fig 8). The difference between groups were however not significant in this experiment (F2.30 = 1.859, p = 0.1734; Fig. 8). In two other experiments, we also recorded a RCSM frequency numerically higher in the Vor+E2 than in the VOR alone group (E42 and E45). However these effects were also not significant and were not reproduced in other tests performed in the same conditions.
Figure 8. Reversibility of Vorozole™ effects by estradiol injections.
Vehicle, Vorozole™ (30 mg/kg) and estradiol (2.5 mg/kg) were all injected 30 min before testing. Data were analyzed by one-way ANOVAs with treatment as repeated factor.
ATD effects
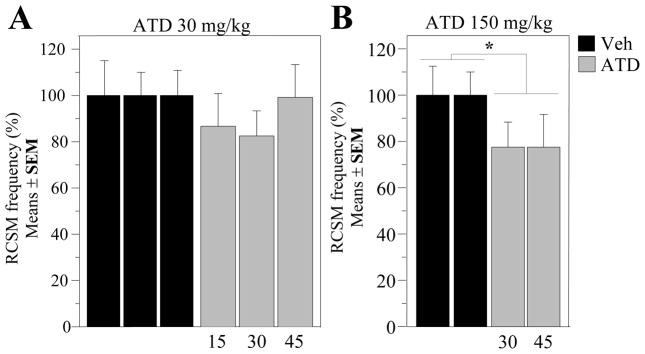
In order to test more thoroughly whether the rapid inhibitory effects of vorozole on male sexual behavior specifically resulted from the inhibition of aromatase activity, we analyzed in a fifth group of birds (castrated and implanted with a 20 mm long testosterone capsule) the effect of another aromatase inhibitor, androstatrienedione (ATD). ATD is a steroidal inhibitor which inhibits aromatase activity through a different mechanism as compared to non-steroidal inhibitors such as Vorozole™ (Simpson and Dowsett, 2002). The effects of two doses of ATD on the expression of RCSM were sequentially tested at two or three intervals between injection and tests and 10 weeks were therefore required to perform this experiment (one subgroup tested each week in each condition: ATD 30 mg/kg at latencies of 15 (E54), 30 (E55) and 45 min (E56) and ATD 150 mg/kg at latencies of 30 (E57) and 45 min (E58). During this relatively long period, some variation was observed in the baseline frequency of RCSM expressed in control conditions. All results were therefore expressed as a percentage of the RCSM frequency observed in control conditions to allow a direct comparison of all experiments (see Fig 9)
Figure 9. Time-response curves for the effects of ATD on the expression of rhythmic contractions of the cloacal gland muscles in the presence of the female.
ATD was tested at two different doses and multiple latencies between injection and test. The RCSM frequency was expressed as the percentage of the mean value of the control condition in each separate test. Data corresponding to each dose were analyzed by two-way ANOVAs with repeated factors (treatment and latency between injection and test). *= P < 0.05 by comparison with the control condition at the corresponding time.
The low dose of ATD (30mg/kg) had no significant effect on the RCSM frequencies observed in the presence and in the absence of the female (Fig 9A for data in the presence of the female). Accordingly, the two-way analyses of variance of these data with the time from injection to test (15, 30 and 45 min) and the treatment (ATD vs vehicle) as repeated factors identified no significant effects of treatment (with female: F1,12 = 1.652, p = 0.2230; without female: F1,12 = 1.467, p = 0.2492), of time (with female: F2,24 = 0.286, p = 0.7539; without female: F2,24 = 0.855, p = 0.4379) and of their interaction (with female: F2,24 = 0.981, p = 0.3893; without female: F2,24 = 2.610, p = 0.0943).
In contrast, the higher dose of ATD (150 mg/kg) rapidly inhibited the RCSM produced in the presence of the female at both time points that were tested (treatment effect: F1,12 = 7.213, p = 0.0198; Fig 9B). There was no difference between these two time points (F1,12 = 0.0005, p = 0.9825) and no interaction between the effects of the treatment and the latency before the test (F1,12 = 0.0002, p = 0.9881). No effect on RCSM displayed in the absence of the female was however observed even at this high dose of inhibitor (treatment: F1,12 = 0.6109, p = 0.273; time: F1,12 = 1.902, p = 0.1930; interaction: F1,12 = 4.564, p = 0.0539, data not shown). Together, these data demonstrate that the steroidal aromatase inhibitor, ATD, also inhibits the display of RCSM display like the non-steroidal inhibitor Vorozole™, therefore supporting the idea that aromatase inhibition itself is responsible for the rapid decrease in appetitive sexual behavior observed after injection of these compounds.
Effects of Vorozole™ and ATD on AA in preoptic area-hypothalamic homogenates
The data presented above demonstrated that the injection of Vorozole™ (30 mg/kg) results in a rapid inhibition of various aspects of male sexual behavior. Although long-term effects of this non-steroidal aromatase inhibitor on aromatase activity and on male sexual behavior have been extensively documented (e. g. (Balthazart et al., 1997; Balthazart, Evrard, and Surlemont, 1990a; Foidart et al., 1994), there are no data indicating how rapidly Vorozole™ can affect brain aromatase activity.
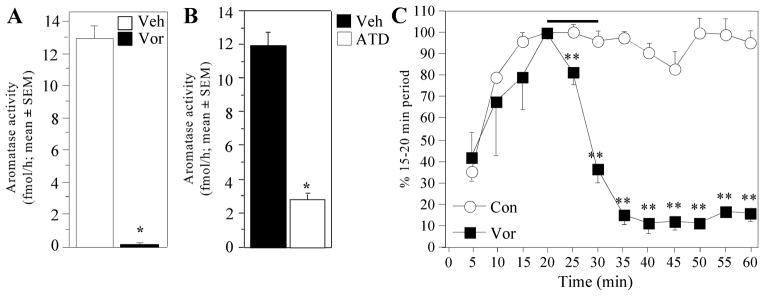
To ensure that the rapid behavioral effects described above could indeed result from the inhibition of AA in brain areas implicated in the control of male sexual behavior, we quantified here, in CX+T20B birds (Group 4) the changes in AA in hypothalamic homogenates 30 min after a single systemic injection of Vorozole™ at the dose shown to be behaviorally active (30 mg/kg). As illustrated in Fig. 10A, a dramatic decrease in AA was observed in Vorozole™-injected birds by comparison with controls (t9 = −17.918, p < 0.0001).
Figure 10. Effect of Vorozole™ and ATD on AA in the preoptic area (POA)-hypothalamus.
A. Effect of Vorozole™ injected IP in vivo 30 min before sacrifice on AA measured in vitro in POA-hypothalamus homogenates. B. Effect of ATD injected IP in vivo 30 min before sacrifice on AA measured in vitro in POA-hypothalamus homogenates. C. Effect of Vorozole™ on AA measured in paired POA-hypothalamus explants incubated in vitro and exposed for 10 min (as indicated by the horizontal bar) to 40 nM Vorozole™. In the experimental conditions, the physiological saline was removed every 5 min from the incubation milieu in order to measure the time-course of AA. The AA was measured for 30 additional min after the removal of the Vorozole™. All data are expressed in percentage of basal release, defined as the activity during the period preceding the experimental manipulation (15–20 min).
All data are means ± SEM. In (A) and (B) data were analyzed by one-way ANOVA. *= P < 0.05 by comparison with the control condition. In (C) data were analyzed by two-way ANOVA for repeated measures followed when appropriate by Tukey HSD tests comparing control and experimental conditions at each time point. Results indicated by symbols next to the corresponding data point (** = p<0.01 by comparison with the corresponding control).
This result indicated that Vorozole™ had indeed reached the POA-hypothalamus within 30 min after injection.
Behavioral experiments had also demonstrated that the injection of ATD (150mg/kg) rapidly inhibits cloacal sphincter movements. The effect of this dose injected 30 min before sacrifice on AA measured in hypothalamic homogenates was also investigated in CX+T20 birds (Group 5). As expected, a significantly lower AA was measured in aliquots from birds injected with the aromatase inhibitor than in control samples (t11 = 10.407, p < 0.0001, Fig. 10B). At the dose used in the present studies, the inhibition of enzymatic activity was however not as profound as observed with Vorozole™. This result nevertheless indicates that ATD reaches the POA-hypothalamus and inhibits AA within 30 min.
Aromatase activity in hypothalamic explants maintained in vitro
One could, however, still argue that, in vivo, the inhibitor had not penetrated the target neurons and therefore had not been able to affect estrogen production. The inhibition observed during the assays described above would have been caused by the process of tissue homogenization that disrupted cell membranes and thus made possible a direct interaction between the inhibitor and the enzyme.
To investigate this hypothesis and specifically test whether Vorozole™ is able to rapidly modulate aromatase activity in intact neurons, left and right explants of POA-hypothalamus were incubated in vitro in oxygenated glucose-saline in the presence of 25 nM [1β-3H]-androstenedione. Cumulative aromatase activity in these explants was measured every 5 min by quantifying the amount of tritiated water that had been released in the incubation medium. Because, absolute levels of AA differed from an explant to another, all measures were expressed as a percentage of the value obtained in control conditions at 20 min when AA had reached an equilibrium before any treatment had been applied (see Fig. 10C).
At that time, Vorozole™ (or its vehicle) was added for 10 min to one explant in each pair (left or right chosen randomly) at a concentration of 40 nM. Explants were then returned to the control conditions. The 40 nM concentration was chosen to be well above the previously determined IC50 for this inhibitor (3 nM, (Wouters, De Coster, Krekels, Van Dun, Beerens, Haelterman, Raeymaekers, Freyne, Van Gelder, Venet, and Janssen, 1989).
As illustrated in figure 10C, the addition of Vorozole™ dramatically decreased AA within five minutes of its application but the enzymatic activity did not recover following washout of the inhibitor during the next 30 min.
The analysis of these data (time points from 5 to 60 min) revealed non significant effect of the treatment (F1,2 = 15.281, p = 0.0596), a significant change in AA with time (F11,22 = 17.484, p < 0.0001) and most importantly a significant interaction between these two factors (F11,22 = 16.658, p < 0.0001).To identify the origins of this interaction, separate ANOVA with two repeated factors as above (group and time) were subsequently performed on the data collected before (times 5–20) and after (25–60) exposure of the explants to the aromatase inhibitor. These analyses confirmed that, as expected, interactions largely resulted from differences between treatments after but not before the treatment.
Before treatment (times 5–20), AA evolved in a similar manner in both groups of explants (progressive saturation of the explant in radioactive substrate and release of produced tritiated water). There was therefore a very strong time effect in the analysis (F3,6=13.522, p=0.0044) but no group difference (F2,4=0.675, p=0.4977) and no interaction (F2,4=1.059, p=0.4334).In contrast, after the addition of Vorozole™ in the medium (times 25–60), overall changes with time were still observed (F7,14=24.781, p<0.0001) but they were associated with significant differences between groups (F1,2=24.995, p=0.0378) and with a significant interaction of groups with time F7,14=12.687, p<0.0001).
Post hoc analyses with Tukey HSD tests comparing control and experimental values at each time point based on the residual variance of the interaction in the general ANOVA confirmed these results. Exposure to Vorozole™ rapidly (within 5 min) inhibited AA (time = 25 min). The effect reached its maximum after 15 min (time = 35 min) and no recovery was observed so that the difference between control explants and explants that had been exposed to Vorozole™ remained significant until the end of the experiment (time = 60). This experiment thus demonstrates that Vorozole™ when present in the preoptic-hypothalamic region rapidly blocks aromatase activity in intact cells within 5 min.
Discussion
While rapid effects of estrogens on cell physiology have been extensively documented in a variety of tissues including the brain, few attempts have been made to assess the functional significance of these rapid effects at the organismal level. In 1999, Cross and Roselli demonstrated that a single estradiol injection rapidly facilitates chemoinvestigation and mounting of the female in castrated male rats (Cross and Roselli, 1999) and more recently, we identified similar stimulatory effects of estrogens on male sexual behavior in quail (Cornil, Evrard, Ball, and Balthazart, 2003). We show here that the acute inhibition of the production of estrogens by an injection of the non-steroidal aromatase inhibitor, Vorozole™, rapidly affects various aspects of male sexual behavior in quail. Several lines of evidence indicate that these behavioral effects are specific to the blockade of estrogen action of appetitive behavior and performance, namely: (1) The steroidal aromatase inhibitor, ATD, produces the same effect on the expression of RCSM as the non-steroidal inhibitor, Vorozole™; (2) Systemic injections of Vorozole™ or ATD markedly decrease hypothalamic aromatase activity measured in homogenates within 30 min indicating that the aromatase inhibitor reaches the preoptic-hypothalamic region within this latency; (3) In hypothalamic explants, Vorozole™ inhibits aromatase activity within 5 min indicating that when present in the tissue the inhibitor very rapidly blocks the enzyme. (4) Finally, vorozole™ does not impair locomotion or the contraction ability of the cloacal gland (see below) suggesting that the behavioral inhibitions do not result from non-specific effects on the subjects mobility. Several aspects of these results deserve discussion.
Meta-analyses of multiple experiments
Meta-analytical techniques are extensively used in the social, behavioral and biomedical fields because they offer quantitative and objective methods for reviewing broad sets of data that are more accurate than qualitative and narrative reviews (Rosenthal, 1991; Schulze, 2004; Wolf, 1986). Meta-analytic techniques may be also used to analyze results of multiple experiments within a study (Kotiaho and Tomkins, 2002; Wolf, 1986). In this context, the advantage is that one avoids the publication bias, which constitutes the major weakness of meta-analytical procedures. Indeed, Kotiaho and Tomkins (2002) argue that meta-analyses conducted with published data rarely fail because unpublished data (that generally do not reach statistical significance) are usually unavailable. The present study clearly illustrates how useful meta-analysis could be to analyze a mix of significant and non-significant data that contain consistent trends. However, a potential problem in this context resides in the use of related studies. In theory, meta-analytical methods can only be applied to independent studies but the notion of independency as applied to meta-analyses is far from being devoid of ambiguity. According to the literature, “the assumption of independency is frequently violated because studies often report more than one effect size based on the same sample” (Martinussen and Bjornstad, 1999). This notion of multiple results coming from the same study is apparently trivial but may be interpreted in two different ways in the meta-analytic context. On the one hand, this situation is met when several dependent variables are measured in an experiment, testing the difference between two independent groups to provide different indicators of the same construct. For example, two groups of subjects (males vs females) can be compared on multiple measures such as administering shocks, hitting, and delivering noxious noise (see Martinussen and Bjornstad, 1999). Then, an effect size estimate can be calculated for each measure. If all of the obtained effect size estimates are included in a meta-analysis, the independence assumption is not met. This is the most frequent situation described in the literature but it does not correspond to our design.
On the other hand, our experiments used correlated designs with subjects serving as their own controls. The obvious advantage of this design is that it limits the between groups variance and consequently increases the chance to identify a between-treatment difference. For this reason, the correlated designs have generally greater statistical power than the equivalent between-subjects designs. However, a problem arises when one tries to introduce results from correlated designs in a meta-analysis. The correlation existing between the groups tends to skew the estimation of the effect size. Because they generally analyze published data, the meta-analysts often do not have access to the actual means and standard deviations but rather to the t-test values relative to the effect of interest. The t-test values for correlated observations (paired t-test) are larger than t values for independent observations (unpaired t-test) because the existence of the correlation between measures reduces the standard error of the difference between the means, making the difference across conditions more prominent. Dunlap et al. (Dunlap et al., 1996) argue that this correlation does not change the size of the effect when it is calculated with the mean and the standard deviation of the mean. It simply makes the effect more noticeable. Consequently, using this paired t-test to compute the effect size estimation leads to an overestimation of the effect size but Dunlap et al. proposed a corrected formula removing this overestimation. They also argued that, when it is possible, the standardized mean difference d as defined by Cohen’s (Cohen, 1977) must be estimated from the means and standard deviations.
Finally, one could argue that, if different subjects had been used for each of the experiments, the data would be more independent from each other than in the present case, in which five groups of birds were run in several experiments each. It might thus be that trends in the data, obtained from experiments on the same groups of animals, provide less generalizable information than if the same number of experiments had been performed on different groups of animals. Each technique possesses its advantages and drawbacks. A single method cannot, at the same time, avoid the publication bias (one of the major critics against meta-analytical methods), maximize the chance of identifying a between-treatment difference (by using subjects as their own controls) and preserve a perfect independency between data. To maximize independency of the data, it might have been desirable to perform each of the present experiments with different groups of birds but the prodigious number of animals needed would then probably raise ethical concerns as well as practical problems. The partial replications with different groups of subjects that are already present in this study provide, however, a reasonable support of the general character of the findings.
To summarize, in the present paper, we analyzed by meta-analyses a large number of experiments carried out on 4 different groups of subjects. Although, multiple experiments were sequentially conducted on the same subjects, we assumed in the analyses that each experiment was independent from the others since effects of treatments could vanish between experiments and additionally all birds were submitted in a counterbalanced manner to the same injections and behavioral tests. Furthermore, these data were analyzed with a method specially adapted for the computation of effect size in correlated designs suggested by the literature (Dunlap et al., 1996). All meta-analyses were also performed with the most conservative assumptions to avoid as much as possible the statistical risk of type I (rejecting a true null hypothesis). These conclusions presented here are therefore well supported by the data and their method of analysis.
These analyses identified significant experimental effects associated with very low probabilities of occurrences under the null hypothesis and in a few cases, very large effect sizes. These results therefore support the existence of biologically reliable effects of single injections of Vorozole™ despite the fact that separate analyses by t-tests or ANOVAs detected a mix of significant and non-significant results. In several cases, the same treatment applied sequentially in the same conditions to the same group of subjects produced significant as well as non-significant effects.
Despite this variability, the meta-analyses of the entire set of experiments clearly indicate that the non-steroidal aromatase inhibitor, Vorozole™, reduces both appetitive and consummatory aspects of male sexual behavior. Several aspects of the experiments may contribute to explain this variability of the action of Vorozole™. It was noticed for example that repeated testing reduces the baseline levels of RCSM displayed so that the difference between the numbers of RCSM produced following control or Vorozole™ treatment may fluctuate substantially. Accordingly, the meta-analysis of the RCSM data after exclusion of subjects that showed less than 30 contractions of the gland in control conditions identified a larger effect size than the meta-analysis of data collected on all subjects. Differences in the endocrine condition of the subjects or in their experience with the test procedures could similarly contribute to the variation in the results and additional experiments would be required to dissect the origins of this variability.
Comparison of the effects on appetitive and consummatory aspects of sexual behavior
Although Vorozole™ significantly reduced aspects of both appetitive and consummatory sexual behavior, the most reliable effect concerned the inhibition of rhythmic cloacal sphincter movements. This difference might be taken as an indication that Vorozole™ more readily affects appetitive sexual behavior than sexual performance. Consummatory sexual behavior has been described as a stereotyped sequence of reflexes leading to satiety or quiescence. In contrast, appetitive behavior is a highly variable suite of actions presumably determined by the context that leads to the approach and the establishment of an initial contact with a potential mate (Everitt, 1990; Timberlake and Silva, 1995). In natural conditions, appetitive behavior always precedes the display of consummatory behavior but this is not necessarily the case in the confined testing environment of the laboratory. In small cages, contacts between mates may occur without any searching behavior and lead to the performance of the stereotyped sequence of reflexes characterizing consummatory behavior. As a consequence, if Vorozole™ mainly or exclusively affects sexual appetitive responses, in laboratory settings, consummatory behavior could still be expressed even after injection of this inhibitor.
If this interpretation is correct, then the study of Vorozole™ effects on copulatory behavior should produce different effects depending on cage size and on the associated proximity of the partners. Accordingly, during the present experiments, Vorozole™ more effectively inhibited copulatory behavior in the large than in the small arena. This difference may thus relate simply to the probability of encounter between the partners in subjects in which approaching behavior has been inhibited by Vorozole™. Alternatively, the large arena may constitute an environment more stressful than the small arena, which would inhibit the expression of male sexual behavior. This idea is indirectly supported by the observation that males usually display fewer cloacal contact movements in the large than in small arena.
Specificity of effects on appetitive sexual behavior
The expression of RCSM was always tested here both in the absence and then in the presence of a female. Previous work indicates that RCSM produced in reaction to the view of a female are an appetitive sexual response (i.e., they are indicative of the propensity of the animal to pursue a motivated consummatory behavior i.e. copulation), while those expressed in her absence are not; they are, for example, not affected by changes in plasma testosterone levels nor by lesions of the preoptic area (Balthazart et al., 1998; Seiwert, 1994; Seiwert and Adkins-Regan, 1998). The low numbers of RCSM displayed when the female is not in view were thus considered as a control for the gland mobility in a non-sexual context.
It was therefore somewhat unexpected that Vorozole™ inhibited here the RCSM display both in the absence and the presence of the female. Although the size of the effect in the absence of the female was much smaller than in her presence, this inhibition in both conditions may thus suggest that the rapid effects of Vorozole™ did not result from an action on appetitive behavior but from an impairment of the cloacal gland mobility. However, it has recently been shown that, following repeated testing, the expression of RCSM can be conditioned to an arbitrary stimulus previously coupled to the female. These conditioned RCSM are clearly related to the animal’s propensity to engage in copulatory behavior and their expression is controlled by long-term changes in steroid concentrations (Cornil, Holloway, Taziaux, and Balthazart, 2004b; Holloway, Balthazart, and Cornil, 2005).
During testing performed in the present experiments, males were not intentionally exposed to a focal conditioning stimulus but the repeated introduction into the testing aquarium may have served as a weak conditioned stimulus. We indeed observed that, in this design, the number of RCSM displayed in the absence of the female tends to increase during successive tests suggesting that the animals learn to anticipate the view of the female (Cornil and Taziaux, Unpublished observations). It should be noted, however, that if the placement into the cage is one component of the conditioned stimulus exposure described in previous papers (Cornil et al., 2004b; Holloway et al., 2005), it is not the only one. This is clearly illustrated by the fact that the conditioned stimulus used by Holloway et al. elicited a mean level of RCSM that was much higher than the level elicited by the placement into the cage in the present design.
Consequently, the Vorozole™-induced inhibition of basal RCSM expressed before the female was visible is likely to reflect a reduction of the appetitive conditioned response to the placement into the aquarium (due to a devaluation of the reinforcing stimulus, i.e. the female) rather than an impairment of the mobility of cloacal gland. It was indeed recently shown that the production of RCSM in response to a conditioned stimulus is affected by long-term inhibitions of testosterone aromatization (Cornil et al., 2004b). The present study would therefore indicate that this response might also be rapidly affected by the aromatase inhibitor.
Cellular mechanisms: In vivo clearance of estrogens
The observation that an aromatase inhibitor rapidly inhibits a behavior known to be estrogen-dependent implies that locally produced estrogens should rapidly be cleared from the tissue as soon as aromatase activity is interrupted. Estrogenic hormones are catabolized to hormonally inactive (or less active) water-soluble metabolites that are excreted in the urine and/or feces. The metabolic elimination of estrogens relies on oxidative metabolism (largely hydroxylations by cytochromes P450 enzymes (Zhu and Conney, 1998) and conjugations by glucuronidation (Barbier and Bélanger, 2003), sulfonation (Song, 2001; Song and Melner, 2000) and/or O-methylation (Männistö and Kaakkola, 1999). While hydroxylated products retain some estrogenic activity, conjugations appear to markedly decrease hormonal activity.
The metabolism of estrogens mostly takes place into the liver, but detectable levels of activity of metabolic enzymes are also expressed in other tissues including the brain. High levels of 2- and 4-hydroxylases have, for example, been identified in the brain, notably the preoptic-hypothalamic region, in various species including quail (Balthazart, Stoop, Foidart, Granneman, and Lambert, 1994; Lambert and van Oordt, 1982; Timmers, Granneman, Lambert, and van Oordt, 1988; Zhu and Conney, 1998). The 2-and 4-hydroxyestrogens (also called catecholestrogens) are biologically active compounds but they are rapidly metabolized by the catecholamine-O-methyltransferases (COMT) into methoxyestrogens that have a weaker hormonal activity (Männistö and Kaakkola, 1999). COMT is also widely distributed in the brain (Männistö and Kaakkola, 1999) so that estrogens can be effectively transformed in less active compounds. Significant glucuronidase and sulfotransferase activities have also been identified in the brain (Albert, Barbier, Vallée, Beaudry, Bélanger, and Hum, 2000; Miki, Nakata, Suzuki, Darnel, Moriya, Kaneko, Hidaka, Shiotsu, Kusaka, and Sasano, 2002; Purinton and Wood, 2000). As suggested by Song and Melner (2000), the inactivation in target tissues by these enzymes could function as a molecular switch to control local estrogen activity.
Altogether these data demonstrate that active pathways for estrogen catabolism are thus present in the brain. It is thus conceivable that blockade of estrogen production with Vorozole™ results in a rapid drop of local estrogen concentration leading to rapid disappearance of estrogenic effects on behavior.
Reversibility of the Vorozole™-induced inhibition
If Vorozole™ and ATD rapidly block the expression of sexual behavior by inhibiting estradiol production, it is somewhat surprising that the co-injection of estradiol was not able to restore sexual behavior to the control level. Pronounced trends towards restoration were observed in some experiments but these effects were never statistically significant.
This is potentially explained by the fact that the dose of estradiol injected systemically is not high enough to restore the local concentrations of E2 provided by the aromatization of testosterone. Interestingly, previous experiments analyzing the long-term genomic effects of E2 could only activate sexual behavior in castrated male quail with extremely high doses of estradiol or estradiol benzoate that are associated with clear toxic effects (Adkins et al., 1980; Balthazart et al., 1995; Balthazart, Schumacher, and Malacarne, 1985). It is thus likely that the level of peripheral E2 required to activate sexual behavior in the brain is higher that the concentration inducing toxic effects in the periphery. An effective activation of the relevant brain mechanisms could only be obtained by a local production of estrogens through aromatization.
Site and mechanisms of rapid action of estrogens
The latencies of the rapid behavioral effects of aromatase inhibitors (30–45 min) are compatible with both genomic (slow) and non-genomic (fast) effects of estrogens, although the relatively short latencies observed suggest the existence of at least one non-genomic component. They could therefore be mediated by the classical nuclear receptors (ERα or ERβ) or by more novel membrane/cytoplasmic receptors whose nature is still subject to debate (Driggers and Segars, 2002; Maggi et al., 2004; Nadal, Diaz, and Valverde, 2001; Revankar, Cimino, Sklar, Arterburn, and Prossnitz, 2005; Segars and Driggers, 2002; Toran-Allerand, 2004). Estrogen effects (genomic as well as non genomic) occur in most parts of the brain (McEwen and Alves, 1999) and thus cannot provide any information on the potential sites implicated in the behavioral effects observed here.
Aromatase is, in contrast, less widely distributed. In quail, dense populations of aromatase-immunoreactive neurons are clustered in only four brain areas: the preoptic area, the bed nucleus of the stria terminalis, the medio-basal hypothalamus and to a lesser extent the nucleus taeniae of the amygdala (Foidart, Reid, Absil, Yoshimura, Harada, and Balthazart, 1995). Among those locations, the medial preoptic area is established as a key area in the control of both appetitive and consummatory aspects of male sexual behavior (Balthazart et al., 1998; Panzica, Viglietti-Panzica, and Balthazart, 1996) and therefore also represents a likely potential target for rapid effects of estrogens on sexual behavior. Rapid cellular effects of estrogens in this region have also been previously reported thus strongly supporting the idea that aromatase inhibition in the area could rapidly affect behavior (Kelly et al., 1977; Lagrange, Ronnekleiv, and Kelly, 1997; Zhou et al., 1996); see also introduction). Alternative sites of action are however also possible both within and outside of the brain (e.g. in aromatase cells located in the dorsal horn of the spinal cord (Evrard and Balthazart, 2002).
Independent of the site and mechanism of action, the present study indicates that the inhibition of estrogen production rapidly inhibits the expression of several aspects of male sexual behavior. This likely reflects the rapid decrease of estrogen concentration (following metabolism to less active or inactive compounds) and thus estrogen action in targets that presumably include the preoptic area. Similar changes in estrogen availability might occur spontaneously in physiological conditions. The release of testosterone from the testes could vary rapidly in response to various stimuli from the environment such as the presence of or interaction with a sexual partner (Ball and Balthazart, 2002; Harding, 1981; Wingfield and Wada, 1989). A bolus of testosterone could then reach the preoptic area and be rapidly metabolized by the local aromatase into estrogens that could contribute to the activation of male sexual behavior.
Alternatively, aromatase activity in the preoptic area could vary rapidly under the influence of various stimuli. It is generally thought that brain aromatase activity is controlled by steroids that act essentially by enhancing the transcription of the aromatase gene and thus the concentration of the enzymatic protein (Abdelgadir, Resko, Ojeda, Lephart, McPhaul, and Roselli, 1994; Balthazart and Foidart, 1993; Hutchison, Steimer, and Hutchison, 1986; Roselli, Abdelgadir, and Resko, 1997; Roselli, Ellinwood, and Resko, 1984; Roselli, Horton, and Resko, 1987; Roselli and Resko, 2001; Steimer and Hutchison, 1981). In addition, it has also recently become clear that aromatase activity can be rapidly (within min) modulated by calcium-dependent phosphorylation processes that completely block enzyme activity (Balthazart et al., 2001a; Balthazart et al., 2001b). These changes in aromatase activity can be induced by the activation of glutamate or dopamine receptors (Balthazart, Baillien, and Ball, 2002; Balthazart et al., 2003). Since glutamate and dopamine inputs and receptors are present in the preoptic area (Absil, Das, Ball, Casto, and Balthazart, 1992; Bailhache and Balthazart, 1993; Ball, Casto, and Balthazart, 1995; Balthazart and Absil, 1997; Cornil, Foidart, Minet, and Balthazart, 2000) and these receptors are expressed at the surface of aromatase cells themselves (Cornil, Seutin, Motte, and Balthazart, 2004c), it is therefore likely that afferent inputs related to a variety of environmental changes are able to rapidly affect estrogen production in the preoptic area.
This mechanism coupled to the rapid metabolism of the local estrogens can thus rapidly down-regulate brain estrogen availability independently from gonadal secretion. Although this mechanism was originally identified during in vitro work on homogenates or on brain explants maintained in culture, rapid changes in aromatase activity also occur in vivo during the expression of sexual behavior (Cornil, Dalla, Papadopoulou-Daifoti, Baillien, Ball, and Balthazart, 2004a). Together with the present results these data thus strongly suggest that rapid changes in aromatase activity and in the estrogens availability constitute critical events in the short-term control of mating behavior.
Acknowledgments
Supported by grants from the National Institutes of Health (NIH/NIMH R01 MH50388) to GFB and JB and grants from the Belgian FRFC (2.4555.01) and the French Community of Belgium (ARC99/04-241) to JB. CAC is a BAEF Postdoctoral Fellow.
References
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- Absil P, Das S, Ball GF, Casto JM, Balthazart J. Dopaminergic control of male copulatory behavior in quail: receptor autoradiography and pharmacological studies. Soc Neurosci Abstr. 1992;18:1071–0. [Google Scholar]
- Adkins EK, Adler NT. Hormonal control of behavior in the japanese quail. J Comp Physiol Psychol. 1972;81(1):27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Boop JJ, Koutnik DL, Morris JB, Pnieswski EE. Further evidence that androgen aromatization is essential for the activation of in male quail. Physiol Behav. 1980;24:441–446. doi: 10.1016/0031-9384(80)90233-4. [DOI] [PubMed] [Google Scholar]
- Albert C, Barbier O, Vallée M, Beaudry G, Bélanger A, Hum DW. Distribution of uridine diphosphate-glucuronsyltransferase (UGT) expression and activity in cynomolgus monkey tissues: evidence for differential expression of steroid-conjugating UGT enzymes in steroid target tissues. Endocrinology. 2000;141:2472–2480. doi: 10.1210/endo.141.7.7583. [DOI] [PubMed] [Google Scholar]
- Bailhache T, Balthazart J. The catecholaminergic system of the quail brain: immunocytochemical studies of dopamine β-hydroxylase and tyrosine hydroxylase. J Comp Neurol. 1993;329:230–256. doi: 10.1002/cne.903290206. [DOI] [PubMed] [Google Scholar]
- Baillien M, Balthazart J. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Steroid Biochem Mol Biol. 1997;63(1–3):99–113. doi: 10.1016/s0960-0760(97)00080-0. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 2. Academic Press; San Diego, CA: 2002. pp. 649–798. [Google Scholar]
- Ball GF, Casto JM, Balthazart J. Autoradiographic localization of D1-like dopamine receptors in the forebrain of male and female Japanese quail and their relationship with immunoreactive tyrosine hydroxylase. J Chem Neuroanat. 1995;9:121–133. doi: 10.1016/0891-0618(95)00075-i. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in japanese quail are differentialy regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18(16):6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol. 2001a;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001b;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail. Comp Biochem Physiol [B] 2002;132:37–55. doi: 10.1016/s1096-4959(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1–16. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. The japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Ann Rev Sex Res. 1998;9:96–176. [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Aromatase inhibition blocks the activation and sexual differentiation of appetitive male sexual behavior in japanese quail. Behav Neurosci. 1997;111(2):381–397. [PubMed] [Google Scholar]
- Balthazart J, Evrard L, Surlemont C. Effects of the non-steroidal inhibitor R76713 on testosterone-induced sexual behavior in the japanese quail (Coturnix coturnix japonica) Horm Behav. 1990a;24:510–531. doi: 10.1016/0018-506x(90)90039-z. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol. 1993;44(4–6):521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Hendrick C. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol Behav. 1990b;47:83–94. doi: 10.1016/0031-9384(90)90045-6. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Reid J, Absil P, Foidart A. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behav Neurosci. 1995;109(3):485–501. [PubMed] [Google Scholar]
- Balthazart J, Schumacher M, Malacarne G. Interaction of androgens and estrogens in the control of sexual behavior in male Japanese quail. Physiol Behav. 1985;35:157–166. doi: 10.1016/0031-9384(85)90330-0. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Stoop R, Foidart A, Granneman JCM, Lambert JGD. Distribution and regulation of estrogen-2-hydroxylase in the quail brain. Brain Res Bull. 1994;35:339–345. doi: 10.1016/0361-9230(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Barbier O, Bélanger A. The cynomolgus monkey (Macaca fascicularis) is the best animal model for the study of steroid glucuronidation. J Steroid Biochem Mol Biol. 2003;85:235–245. doi: 10.1016/s0960-0760(03)00235-8. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118(2):169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Beyer C, Raab H. Nongenomic effects of oestrogen: embryonic mouse midbrain neurones respond with a rapid release of calcium from intracellular stores. Eur J Neurosc. 2000;10:255–262. doi: 10.1046/j.1460-9568.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- Brann DW, Hendry LB, Mahesh VB. Emerging diversities in the mechanism of action of steroid hormones. J Steroid Biochem Mol Biol. 1995;52(2):113–133. doi: 10.1016/0960-0760(94)00160-n. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Academic Press; New York: 1977. [Google Scholar]
- Cooper H, Hedges LV. The handbook of research synthesis. 1994. [Google Scholar]
- Cornil C, Foidart A, Minet A, Balthazart J. Immunocytochemical localization of ionotropic glutamate receptors subunits in the adult quail forebrain. J Comp Neurol. 2000;428:577–608. [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Ball GF, Balthazart J. Sexual performance rapidly changes preoptic dopaminergic activity in quail. Soc Neurosci Abstr. 2004a;30:88.5. [Google Scholar]
- Cornil CA, Evrard HC, Ball GF, Balthazart J. Rapid effects of 17b-estradiol and vorozole, an aromatase inhibitor, on male sexual behavior in japanese quail. Soc Neurosci Abstr. 2003;29:726.5. [Google Scholar]
- Cornil CA, Holloway KS, Taziaux M, Balthazart J. The effects of aromatase inhibition on testosterone-dependent conditioned rhythmic cloacal sphincter movement in male Japanese quail. Physiol Behav. 2004b;83(1):99–105. doi: 10.1016/j.physbeh.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Seutin V, Motte P, Balthazart J. Electrophysiological and neurochemical characterization of neurons of the medial preoptic area in Japanese quail (Coturnix japonica) Brain Res. 2004c;1029(2):224–240. doi: 10.1016/j.brainres.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Cross E, Roselli CE. 17β-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- DeBold JF, Frye CA. Genomic and non-genomic actions of progesterone in the control of female hamster sexual behavior. Horm Behav. 1994;28:445–453. doi: 10.1006/hbeh.1994.1042. [DOI] [PubMed] [Google Scholar]
- Delville Y, Sulon J, Balthazart J. Hormonal correlates of gonadal regression and spontaneous recovery in Japanese quail exposed to short day-lengths. Arch Int Physiol Bioch. 1985;93:123–133. doi: 10.3109/13813458509079598. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Rouillard C, Bédard P. 17b-estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117:197–203. doi: 10.1016/0014-2999(85)90604-1. [DOI] [PubMed] [Google Scholar]
- Domjan M, Hall S. Sexual dimorphism in the social proximity behavior of Japanese quail (Coturnix coturnix japonica) Journal of Comparative Psychology. 1986;100:68–71. [PubMed] [Google Scholar]
- Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab. 2002;13(10):422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods. 1996;1(2):170–177. [Google Scholar]
- Evans SJ, Searcy BT, Moore FL. A subset of kappa opioid ligands bind to the membrane glucocorticoid receptor in an amphibian brain. Endocrinology. 2000;141(7):2294–2300. doi: 10.1210/endo.141.7.7587. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Evrard H, Balthazart J. Localization of oestrogen receptors in the sensory and motor areas of the spinal cord in japanese quail (Coturnix japonica) J Neuroendocrinol. 2002;14:1–15. doi: 10.1046/j.1365-2826.2002.00857.x. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. The statistical basis of meta-analysis. Statistical Methods in Medical Research. 1993;2(2):121. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- Foidart A, Harada N, Balthazart J. Effects of steroidal and non-steroidal aromatase inhibitors on sexual behavior and aromatase-immunoreactive cells and fibers in the quail brain. Brain Res. 1994;657:105–123. doi: 10.1016/0006-8993(94)90958-x. [DOI] [PubMed] [Google Scholar]
- Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical reexamination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8000:18165. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM. Mitochondrial benzodiazepine receptors in the ventral tegmental area modulates sexual behaviour of cycling of hormone-primed hamsters. J Neuroendocrinol. 2003;15:677–686. doi: 10.1046/j.1365-2826.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- Harding CF. Social modulation of circulating hormone levels in the male. Amer Zool. 1981;21:223–231. [Google Scholar]
- Holloway KS, Balthazart J, Cornil CA. Androgen mediation of conditioned rhythmic cloacal sphincter movements in Japanese quail (Coturnix japonica) J Comp Psychol. 2005;119(1):49–57. doi: 10.1037/0735-7036.119.1.49. [DOI] [PubMed] [Google Scholar]
- Hutchison JB, Steimer T, Hutchison RE. Formation of behaviorally active estrogen in the dove brain: induction of preoptic aromatase by intracranial testosterone. Neuroendocrinology. 1986;43:416–427. doi: 10.1159/000124558. [DOI] [PubMed] [Google Scholar]
- Hutchison RE. Hormonal differentiation of sexual behavior in Japanese quail. Horm Behav. 1978;11:363–387. doi: 10.1016/0018-506x(78)90038-7. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The effects of microelectrophoretically applied estrogen, cortisol and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of female rat. Exp Brain Res. 1977;30:53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, editor. Hormones, Brain and Behavior. Vol. 3. 2002. pp. 361–380. [Google Scholar]
- Kotiaho JS, Tomkins JL. Meta-analysis, can it ever fail? Oikos. 2002;96(3):551–553. [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Lambert JGD, van Oordt PGWJ. Catecholestrogens in the brain of the female trout, Salmo gairdneri. Gen Comp Endocrinol. 1982;46:41–0. doi: 10.1016/0016-6480(82)90166-6. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Ann Rev Pharmacol Toxicol. 2001;41:569–91. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: Mechanisms and nonreproductive functions. Ann Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacity of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628. [PubMed] [Google Scholar]
- Martinussen M, Bjornstad JF. Meta-analysis calculations based on independent and nonindependent cases. Educational and Psychological Measurements. 1999;59(6):928–950. [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J Clin Endocrinol Metab. 2002;87(12):5760–5768. doi: 10.1210/jc.2002-020670. [DOI] [PubMed] [Google Scholar]
- Moore FL, Orchinik M. Membrane receptors for corticosterone: a mechanism for rapid behavioral responses in an amphibian. Horm Behav. 1994;28:512–519. doi: 10.1006/hbeh.1994.1049. [DOI] [PubMed] [Google Scholar]
- Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News Physiol Sci. 2001;16:251–255. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Normand ST. Tutorial in biostatistics. Meta-analysis: formulating, evaluating, combining, and reporting. Statistics in Medicine. 1999;18:321. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Orchinik M. Glucocorticoids, stress, and behavior: shifting the timeframe. Horm Behav. 1998;34(3):320–7. doi: 10.1006/hbeh.1998.1488. [DOI] [PubMed] [Google Scholar]
- Orchinik M, McEwen BS. Novel and classical actions of neuroactive steroids. Neurotransmissions. 1993;9:1–6. [Google Scholar]
- Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on the male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem. 1995;65(4):1651–1657. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- Purinton SC, Wood CE. Ovine fetal estrogen sulfotransferase in brain regions important for hypothalamus-pituitary-adrenal axis control. Neuroendocrinology. 2000;71(4):237–242. doi: 10.1159/000054541. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signalling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44(4):351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Ellinwood WE, Resko JA. Regulation of brain aromatase activity in rats. Endocrinology. 1984;114:192–200. doi: 10.1210/endo-114-1-192. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Time-course and steroid specificity of aromatase induction in rat hypothalamus-preoptic area. Biol Reprod. 1987;37:628–633. doi: 10.1095/biolreprod37.3.628. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. In vitro assay of aromatase activity in the central nervous system. In: Greenstein B, editor. Neuroendocrine research methods. Vol. 2. Harwood Academic Publishers; Chur, Switzerland: 1991. pp. 937–951-0. [Google Scholar]
- Roselli CE, Resko JA. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J Steroid Biochem Mole Biol. 2001;79:247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Vol. 6. Sage Publication; 1991. [Google Scholar]
- Sachs BD. Photoperiodic control of the cloacal gland of the Japanese quail. Science. 1967;157:201–203. doi: 10.1126/science.157.3785.201. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Leipheimer RE. Rapid effect of testosterone on striated muscle activity in rats. Neuroendocrinology. 1988;48(5):453. doi: 10.1159/000125049. [DOI] [PubMed] [Google Scholar]
- Schulze R. Meta-analysis. A comparaison of approaches. Hogrefe & Huber Publisher; 2004. [Google Scholar]
- Segars JH, Driggers PH. Estrogen action and cytoplasmic signaling cascades. Part I: membrane-associated signaling complexes. Trends Endocrinol Metab. 2002;13(8):349–354. doi: 10.1016/s1043-2760(02)00633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwert CM. The neuromuscular system controlling foam production in japanese quail: an investigation of structure and function. 0. Cornell University; Ithaca, NY: 1994. [Google Scholar]
- Seiwert CM, Adkins-Regan E. The foam production system of the male japanese quail: characterization of structure and function. Brain Behav Evol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- Selye H. Anesthetic effect of steroid hormones. Proc Soc Exp Biol Med. 1941;46:116–121. [Google Scholar]
- Simpson ER, Dowsett M. Aromatase and its inhibitors: significance for breast cancer therapy. Recent Prog Horm Res. 2002;57:317–38. doi: 10.1210/rp.57.1.317. [DOI] [PubMed] [Google Scholar]
- Song WC. Biochemistry and reproductive endocrinology of estrogen sulfotransferase. Ann NY Acad Sci. 2001;948:43–50. doi: 10.1111/j.1749-6632.2001.tb03985.x. [DOI] [PubMed] [Google Scholar]
- Song WC, Melner MH. Steroid tranformation enzymes as critical regulators of steroid action in vivo. Endocrinology. 2000;141(5):1587–1589. doi: 10.1210/endo.141.5.7526. [DOI] [PubMed] [Google Scholar]
- Steimer T, Hutchison JB. Androgen increases formation of behaviourally effective oestrogen in dove brain. Nature. 1981;292:345–347. doi: 10.1038/292345a0. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Cornil CA, Balthazart J. Aromatase inhibition blocks the expression of sexually-motivated cloacal gland movements in male quail. Behav Processes. 2004;67(3):461–469. doi: 10.1016/j.beproc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatum nucleus taeniae in the sexual behavior of male japanese quail (Coturnix japonica): a comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Timberlake W, Silva KM. Appetitive behavior in ethology, psychology, and behavior systems. In: Thompson NS, editor. Perspective in Ethology. Plenum Press; New-York: 1995. pp. 211–253. [Google Scholar]
- Timmers RJM, Granneman JCM, Lambert JGD, van Oordt PGWJ. Estrogen-2-hydroxylase in the brain of the male african catfish, Clarias gariepinus. Gen Comp Endocrinol. 1988;72:190–203. doi: 10.1016/0016-6480(88)90202-x. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145(3):1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)a and ERb exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142(6):2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Wada M. Changes in plasma levels of testosterone during male-male interactions in the song sparrow, Melospiza melodia: Time course and specificity of response. J Comp Physiol [A] 1989;166:189–194. [Google Scholar]
- Wolf FM. Meta-analysis Quantitative methods for research synthesis. Vol. 59. Sage Publication; Iowa city: 1986. [Google Scholar]
- Wouters W, De Coster R, Krekels M, Van Dun J, Beerens D, Haelterman C, Raeymaekers A, Freyne E, Van Gelder J, Venet M, Janssen PAJ. R 76713, a new specific non-steroidal aromatase inhibitor. J Steroid Biochem. 1989;32:781–788. doi: 10.1016/0022-4731(89)90453-6. [DOI] [PubMed] [Google Scholar]
- Yagi K. Changes in firing rates of single preoptic and hypothalamic units following an intravenous administration of estrogen in the castrated female rat. Brain Res. 1973;53:343–352. doi: 10.1016/0006-8993(73)90219-9. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19(1):1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. PNAS. 2003;100(5):2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membbrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. PNAS. 2003;100(5):2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]