Abstract
Objective
Hypovitaminosis D may be associated with diabetes, hypertension and coronary heart disease (CHD). However because studies examining associations of all three chronic conditions with circulating 25(OH)D and 1,25(OH)2D are limited. We examined these associations in the US. Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial (n=2,465).
Research Design and Methods
Caucasian PLCO participants selected as controls in previous nested case-control studies of 25(OH)D and 1,25(OH)2D were included in this analysis. Diabetes, CHD and hypertension prevalence, risk factors for these conditions, and intake of vitamin D and calcium were collected from a base-line questionnaire.
Results
Serum levels of 25(OH)D were low (<50 nmol/L) in 29% and very low (<37 nmol/L) in 11% of subjects. The prevalence of diabetes, hypertension and CHD were 7%, 30% and 10%, respectively. After adjustment for confounding by gender, geographical location, educational level, smoking history, body mass index(BMI), physical activity, total dietary energy and vitamin D and Ca intake, only diabetes was significantly associated with lower 25(OH)D and 1,25 (OH)2D levels. Caucasians who had 25(OH)D ≥80 nmol/L were half as likely to have diabetes [odds ratio (OR) =0.5 (95% CI=0.3-0.9)] compared to those who had 25(OH)D < 37 nmol/L. Those in the highest quartile of 1,25(OH)2D (≥103 pmol/L) were less than half as likely to have diabetes [OR= 0.3 (95% CI=0.1-0.7)] than those in the lowest quartile (< 72 pmol/L).
Conclusions
The independent association of 25(OH)D and 1,25(OH)2D with diabetes prevalence in a large population is a new finding and thus these findings warrant confirmation in larger, prospective studies.
Keywords: Diabetes; Vitamin D status; 25(OH)D; 1,25(OH)2D
Introduction
Vitamin D is a steroid pro-hormone synthesized in the skin following UV exposure or through supplemental or dietary intake. In addition to maintaining bone and muscle health, vitamin D has recently been postulated to protect against immune dysfunction, cancer [1], cardiovascular conditions [2-3], hypertension [3-4], metabolic syndrome and diabetes [5].
The concentration of 25(OH)D, the major circulating form of vitamin D in the blood, rises and falls with the supply of vitamin D, on the other hand, the concentration of circulating 1,25(OH)2D (the biologically active form) is thought to be kept under tight homeostatic control, and is thus largely independent of vitamin D supply, except in severe deficiency [1].
As early as the 1980s, animal studies identified expression of the vitamin D receptor in rat pancreatic cells, and demonstrated that vitamin D deficiency inhibits the production of insulin [6-7]. To our knowledge, no large epidemiological analysis study has investigated simultaneously diabetes, hypertension and CHD risk together with vitamin D blood levels. Thus, the aim of the present study was to investigate the association between diabetes, hypertension and CHD and two serum bio-markers associated with vitamin D function, 25(OH)D and 1,25(OH)2D.
Materials and Methods
For this study, we analyzed data from subjects selected as controls in five previous case-control studies of circulating vitamin D biomarkers (25(OH)D and 1,25(OH)2D) nested within the Prostate Lung Colon and Ovarian (PLCO)Trial which was a large randomized controlled multicenter trial in the United States of 155,000 men and women at sites in Birmingham, AL; Denver, CO; Detroit, MI; Honolulu, HI; Marshfield, WI; Minneapolis, MN; Pittsburgh, PA; Salt Lake City, UT; St Louis, MO; and Washington, DC, that was designed to evaluate selected methods for the early detection of these four cancers as well as non-Hodgkins lymphoma (NHL), breast and pancreatic cancer: enrolment began November 1, 1993, and ended June 30, 2001[8]. Details of these studies of colorectal adenoma, non-Hodgkins lymphoma (NHL) and prostate, breast, and pancreatic cancer are described elsewhere [9]. Briefly, we included 399 controls used for a study of colorectal adenoma (matched to cases by gender and race), 286 controls used for a study of non-Hodgkin lymphoma, 713 controls used for a study of prostate cancer (matched to cases by age, time since screening, and year of follow-up), 932 controls used for a study of breast cancer (matched to cases by age and year of blood draw), and 350 used for a study of pancreatic cancer (matched to cases by age, gender, race and date of blood draw). Of these controls, 59 were included in more than one study; thus, in total 2621 control subjects from PLCO were included in this present data analysis. As there were only 157 non-Caucasians in these data we only present results for Caucasian subjects [25(OH)D, N=2465; 1,25(OH)2D, N=1369]. At the initial screening, all participants were asked to complete a questionnaire on medical history including diabetes, hypertension and CHD as well as demography, anthropometry, lifestyle factors (including smoking history, menopausal hormone therapy use (MHT) and vigorous physical activity (PA) during the last year), and usual dietary intake over the 12 months before enrolment (137-item food frequency questionnaire and 14 questions about intake of vitamin and mineral supplements [10]. Because of the self reported nature of the questionnaire the medical diagnosis questions were repeated on the same population five years later with 87% repeatability for diabetes, 89% for hypertension and only 55% for CHD. Daily nutrient intake from foods was calculated by multiplying the reported frequency of consumption of each food item by the nutrient composition of the imputed gender-specific portion size using the nutrient database from the U.S. Department of Agriculture [11]. Calcium and vitamin D intake were measured both from food and supplemental sources. Serum samples were collected during the baseline visit, and stored at −70 °C. Levels of the serum 25(OH)D and 1,25(OH)2D for subjects were determined at base-line using a radio-iodinated tracer assay in the laboratories of Hollis and Horst [12-13]. Replicate blinded quality control samples from two to four different individuals were included in all 25(OH)D and 1,25(OH)2D batches. The overall coefficients of variation for 25(OH)D were 16.3% for the colorectal adenoma, 11.4% for NHL, 5.9% for prostate, 8.2% for the breast study, and 4.7% for the pancreas study and for 1,25(OH)2D: 16.3% for adenoma and 12.8% for breast studies, respectively.
Dummy variables coded to represent each of the centers in the study and each of control series (from the 5 case-control studies) were included as covariates in all models as well as age, smoking, educational status, body mass index (BMI), total dietary energy and physical activity as they are known risk factors for diabetes, hypertension and CHD in previous studies. All these variables were included as a priori confounders/covariates in all multivariable regression models. Unconditional logistic regression analyses were conducted with diabetes, hypertension and CHD as health outcomes with both 25(OH)D nmol/L (categorized as >80, 50-80, 37-50 and < 37) as the independent variable for vitamin D status or 1,25(OH)2D pmol/L (in quartiles: ≥ 103, ≥86-103, ≥ 72-86 and < 72). Vitamin D and calcium intakes (diet plus supplements) were also calculated as potential independent variables and also included in all models.
Linear trends of ordered categorical variables (e.g., categories of 25(OH)D, 1,25(OH)2D, BMI and vitamin D and calcium intake) were assessed using continuous values for each variable and applying a likelihood ratio test [14]. All statistical analyses were performed using the SPSS 15 statistical package. All p-values are two-sided.
Results
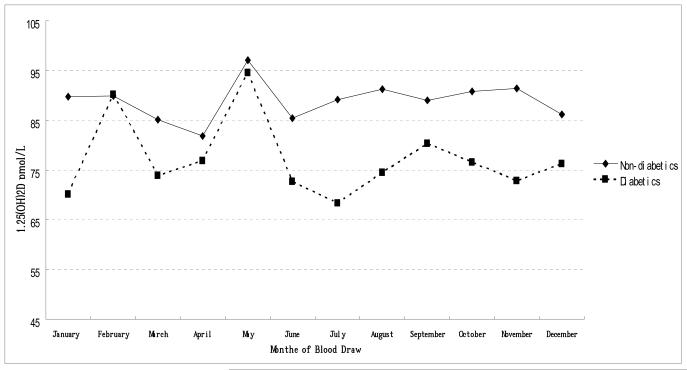
The characteristics of study participants are reported in Table 1. Serum 25(OH)D levels, varied seasonally, with the highest levels during the summer and autumn months and the lowest levels during winter and spring (unlike 1,25(OH)2D levels). Unadjusted 25(OH)D and 1,25(OH)2D values were significantly lower for diabetics (Figure 1A,B), those with hypertension and those with CHD (data not shown). As well as sunshine exposure other independent predictors of 25(OH)D levels in these data have been previously reported to be high dietary and supplement intake of vitamin D and calcium, and menopausal hormone therapy (MHT) use in women, as well as having a high BMI and low physical activity levels [9]. As 1,25(OH)2D levels are under tight metabolic control 25(OH)D and 1,25(OH)2D levels were only minimally correlated (r2=0.158 p<0.001) and also do not vary by season, and in contrast to 25(OH)D, there were no other lifestyle predictors except MHT use. Diabetes was significantly associated with lower levels of 25(OH)D and 1,25 (OH)2D in blood: after adjustment for confounding including gender, geographical location, educational level, smoking history, body mass index(BMI), physical activity, total dietary energy, and vitamin D and Ca intake and blood vitamin D (25(OH)D or 1,25(OH)2D, as appropriate), Caucasians who had 25(OH)D ≥80 nmol/L 25(OH)D were half as likely to have diabetes: [odds ratio (OR) =0.5 (95% CI=0.3-0.9)] compared to those who had 25(OH)D < 37 nmol/L (trend test: P=0.05). Those in the highest quartile of 1,25(OH)2D (≥103 pmol/L) were a third as likely to have diabetes: [OR= 0.3 (95% CI=0.1-0.7)] than those in the lowest quartile (< 72 pmol/L; trend test: P=0.02). In contrast, there were no significant associations between 25(OH)D or 1,25 (OH)2D with CHD or hypertension risk once adjustment was made for confounding (BMI was the major confounder) (Table 2). There were no gender differences with any of these chronic conditions and 25(OH)D or 1,25(OH)2D (Pinteraction > 0.05).
Table 1.
Distribution of demographic, lifestyle habits and dietary intake by low serum 25(OH)D, 1,25(OH)2D, diabetes, hypertension and coronary heart disease (CHD) status in Caucasian healthy middle-aged men and women living across the USA
| Total (n=2465) |
25(OH)D <37 nmol/L (n=269) |
1,25(OH)2D <72 pmol/L (n=352) |
Diabetes (n=170) |
Hypertension (n=724) |
CHD (n=248) |
|
|---|---|---|---|---|---|---|
| Categorical Variables † | Percentage values | |||||
| Demographics | ||||||
| Gender: Female | 47 | 46 | 71 | 33** | 55 | 23** |
| Education: above High School | 93 | 92 | 94 | 88** | 91* | 94 |
| Chronic Conditions and Lifestyle Habits | ||||||
| Diabetes | 7 | 11* | 9* | 12** | 16** | |
| Coronary Heart Disease | 10 | 12 | 10 | 24** | 19** | |
| Hypertension | 30 | 36* | 33 | 50** | 54** | |
| Lifestyle Habits | ||||||
| Menopausal Hormone Therapy (MHT) Use (Current)*1 | 54 | 45* | 41** | 43 | 52 | 45 |
| MHT Use (Ever)*2 | 71 | 63 | 66 | 57* | 69 | 59* |
| Smoking (pack years) >40 | 25 | 25 | 22 | 37** | 29* | 31* |
| Vigorous Exercise (current) ( ≥ 3 hours/week) | 40 | 28** | 36 | 35 | 36** | 43 |
| Vitamin Supplement Intake | ||||||
| Multi-vitamins (recent) | 65 | 48** | 70 | 53** | 62 | 63 |
| Continuous Variables ‡ | Mean (standard deviation) | |||||
| Age | 63(5) | 63(5) | 63(4) | 65(5)** | 64(5)** | 66(5)** |
| Anthropometry | ||||||
| Body Mass Index (BMI) (current) | 27(5) | 29(5)** | 28(6) | 30(6)** | 29(5)** | 28(5)** |
| Nutrients from Diet and supplements | ||||||
| Energy from diet (kcal/day) | 2074(857) | 2049(850) | 1939(762) | 2109(1060) | 2083(913) | 2161(849) |
| Total fat from diet (g/day) | 69(35) | 67(32) | 64(31) | 74(40)* | 69(36) | 70(35) |
| Calcium from diet and supplements (mg/day) | 1261(631) | 1022(530)** | 1353(681) | 1237(823) | 1197(609)** | 1182(550)* |
| Vitamin E from diet and supplements (mg/day) | 182(254) | 123(222)** | 210(272) | 159(240) | 183(262) | 209(271) |
| Vitamin D from diet and supplements (μg/day) | 12(9) | 7(7)** | 12(8) | 11(9) | 11(8)* | 11(8) |
Chi-Square p-value testing for difference between the category versus the converse.
n=616 women
n=813 women
p-value for significance of Pearson correlation coefficient
p < 0.01
p < 0.001
Figure 1a.
Distribution of 25(OH)D nmol/L levels by month of blood draw for diabetic and non-diabetic healthy middle-aged Caucasian US men and women
Figure 1b.
Distribution of 1,25(OH)2D nmol/L levels by month of blood draw for diabetic and non-diabetic healthy middle-aged Caucasian US men and women
Table 2.
Association between dietary vitamin D and calcium intake and serum 25(OH)D nmol/L and 1,25(OH)2D pmol/L and diabetes, hypertension and coronary heart disease prevalence in Caucasian men and women living across the USA
| Serum/Diet | n=2465 | Diabetes (n=170) | Hypertension (n=724) | CHD (n=248) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||
| 25 (OH)D‡ nmol/L | OR† | 95% CI | OR* | 95% CI | OR† | 95% CI | OR* | 95% CI | OR† | 95% CI | OR* | 95% CI | |||||||
| <37 | 269 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| ≥37-50 | 442 | 0.7 | 0.4 | 1.2 | 0.7 | 0.4 | 1.2 | 0.8 | 0.6 | 1.1 | 0.9 | 0.6 | 1.2 | 0.7 | 0.4 | 1.2 | 0.7 | 0.4 | 1.1 |
| ≥50-80 | 1239 | 0.7 | 0.4 | 1.0 | 0.7 | 0.4 | 1.1 | 0.7 | 0.6 | 1.0 | 0.9 | 0.7 | 1.2 | 1.0 | 0.6 | 1.4 | 1.1 | 0.7 | 1.7 |
| ≥80 | 514 | 0.3 | 0.2 | 0.6 | 0.5 | 0.3 | 0.9 | 0.6 | 0.5 | 0.9 | 1.0 | 0.7 | 1.4 | 0.5 | 0.3 | 0.9 | 0.7 | 0.4 | 1.3 |
| p trend | <0.001 | 0.02 | 0.004 | ns | ns | ns | |||||||||||||
| Calcium from diet & supplements mg/day (quartiles) | |||||||||||||||||||
| <791 | 595 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| ≥791-<1137 | 618 | 0.8 | 0.5 | 1.2 | 0.9 | 0.6 | 1.5 | 0.8 | 0.7 | 1.1 | 0.8 | 0.6 | 1.1 | 0.8 | 0.6 | 1.2 | 0.7 | 0.5 | 1.1 |
| ≥1137-<1597 | 621 | 0.7 | 0.4 | 1.0 | 0.8 | 0.4 | 1.3 | 0.8 | 0.7 | 1.1 | 0.8 | 0.6 | 1.1 | 0.9 | 0.6 | 1.3 | 0.9 | 0.5 | 1.3 |
| ≥1597 | 631 | 0.7 | 0.5 | 1.1 | 0.8 | 0.4 | 1.5 | 0.7 | 0.5 | 0.9 | 0.7 | 0.5 | 1.0 | 0.7 | 0.5 | 1.0 | 0.7 | 0.4 | 1.1 |
| p trend | ns | ns | 0.004 | 0.05 | 0.06 | ns | |||||||||||||
| Vitamin D from diet & supplements mcg/day(quartiles) | |||||||||||||||||||
| <4.5 | 599 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| ≥4.5-<11 | 624 | 1.2 | 0.8 | 1.9 | 1.2 | 0.7 | 1.8 | 0.9 | 0.7 | 1.1 | 1.0 | 0.7 | 1.3 | 1.3 | 0.9 | 1.8 | 1.2 | 0.8 | 1.8 |
| ≥11-<16 | 629 | 0.9 | 0.6 | 1.5 | 0.9 | 0.5 | 1.5 | 0.9 | 0.7 | 1.1 | 1.1 | 0.8 | 1.4 | 1.1 | 0.8 | 1.6 | 1.5 | 1.0 | 2.3 |
| ≥16 | 613 | 0.8 | 0.5 | 1.2 | 0.8 | 0.4 | 1.4 | 0.8 | 0.6 | 1.1 | 1.1 | 0.8 | 1.5 | 0.9 | 0.6 | 1.4 | 1.2 | 0.7 | 2.0 |
| p trend | ns | ns | ns | ns | ns | ns | |||||||||||||
| n=1369 | Diabetes (n=80) | Hypertension (n=405) | CHD (n=105) | ||||||||||||||||
| 1,25(OH)2D pmol/L | OR† | 95% CI | OR** | 95% CI | OR† | 95% CI | OR** | 95% CI | OR† | 95% CI | OR** | 95% CI | |||||||
| <72 | 352 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| ≥72-86 | 347 | 0.7 | 0.4 | 1.2 | 0.8 | 0.4 | 1.5 | 1.0 | 0.7 | 1.4 | 1.1 | 0.8 | 1.5 | 0.6 | 0.3 | 1.0 | 0.5 | 0.3 | 1.0 |
| ≥86-103 | 339 | 0.6 | 0.3 | 1.1 | 0.8 | 0.4 | 1.5 | 0.9 | 0.6 | 1.2 | 1.0 | 0.7 | 1.4 | 0.7 | 0.4 | 1.2 | 0.9 | 0.5 | 1.5 |
| ≥103 | 331 | 0.2 | 0.1 | 0.5 | 0.3 | 0.1 | 0.7 | 0.7 | 0.5 | 1.0 | 0.8 | 0.6 | 1.2 | 0.7 | 0.4 | 1.2 | 0.9 | 0.5 | 1.7 |
| p trend | <0.001 | 0.02 | 0.03 | ns | ns | ns | |||||||||||||
Unadjusted for confounders and covariates
missing data 25(OH)D n=1
Adjusted for 1,25(OH)2D, study center, different case-control study vitamin D analysis, date of blood draw, pack years of smoking, educational level, BMI, physical activity, gender, total dietary energy, dietary vitamin D and Ca plus supplements where appropriate
Adjusted for 25(OH)D level, study center, different case-control study vitamin D analysis, date of blood draw , pack years of smoking, educational level, BMI, physical activity, gender, total dietary energy. dietary vitamin D and Ca plus supplements where appropriate
Discussion
Our findings for 25(OH)D and diabetes support the results of earlier clinical and animal studies [5-7] and are consistent with other large epidemiological cross-sectional studies that have investigated the relationship between serum 25(OH)D levels and diabetes [5, 15]. Two recent prospective investigations also support this hypothesis: in the first study, which was conducted among 524 women and men in the United Kingdom, baseline 25(OH)D levels were inversely associated with glycemia and insulin resistance after a 10-year follow-up [16]. In the second study which used data from two nested case-control studies in Finland, baseline 25(OH)D levels were significantly inversely associated with type 2 diabetes risk after a 22-year follow-up, but only in men [17]. None of these studies investigated associations with 1,25(OH)2D nor investigated CHD or hypertension risk.
Several small clinical intervention studies also support that vitamin D, or its active metabolite 1,25(OH)2D, improves insulin sensitivity, even in subjects with glucose metabolism parameters classified within normal ranges [5]. The mechanisms proposed to explain this effect include potential relationships with improvements in lean mass, regulation of insulin release, altered insulin receptor expression, and specific effects on insulin action. These actions may be mediated by systemic or local production of 1,25(OH)2D or by suppression of parathyroid hormone, which may function to negatively affect insulin sensitivity [5-7, 15-19]. However, since our study is cross-sectional, we cannot rule out that being diabetic could modify the regulation of renal synthesis of 1,25(OH)2D, which would be reflected in the concentration of this hormone in blood.
Vitamin D status is of interest with respect to diabetes because of its potential as a target for intervention. Maintaining adequate vitamin D status has proven to be challenging, as sunlight is the only substantial natural source of vitamin D for humans; given the low intensity of UV light in the winter and many people’s indoor lifestyles, vitamin D supplied through sun exposure is often inadequate to avoid deficiency.
In contrast to our findings, others, but not all, have reported associations between low blood levels of vitamin D and hypertension [4]; in summary eighteen out of twenty six cross sectional studies (n= 22 - 12,644) and of the two prospective studies only one was positive [2, 5]. As increased BMI is related to both hypertension and low vitamin D levels it is important to consider it as a confounder in these studies. Only, half of the positive observational studies adjusted for BMI, it should be noted that in our data, a significant association with 25OHD or 1,25(OH)2D concentrations disappeared once BMI was added into the model. Similarly the results from small randomized control trials have been variable with less than half positive [5]. Three of the large observational studies in the US, UK and Germany reported significant associations between 25(OH)D and hypertension risk after adjustment for diabetes prevalence [2, 15, 18]. One postulated mechanism for vitamin D-mediated reduction of hypertension involves reno-protective effects i.e., increased activation of the rennin-angiotensin-aldosterone system (RAAS), which is the main regulator of electrolyte and volume homeostasis which contributes to development of arterial hypertension [3-4].
In contrast to our findings, some previous epidemiological studies have found a negative association between serum vitamin D and heart disease [3], with ten out of twelve observational studies (n=238-4839) of vascular disease (including two peripheral artery disease (PAD), five cardiovascular disease (CVD), five myocardial infarction (MI)) and six out of seven observational studies of heart failure (n=25 - 3299) showing a negative association between lower 25(OH)D levels and disease. Of these studies, only seven out of twelve adjusted for hypertension and diabetes but none of these reported all three conditions separately in the same study [2-3]. It is thought that vitamin D plays a role in maintaining cardiovascular homeostasis both through a direct action of 1,25(OH)2D on cardiomyocytes and indirect actions on circulating parathyroid hormone and calcium levels. The lack of association with CHD seen in these data could have been because of the low repeatability of the self reported nature of this outcome variable and its associated variability compared to that of hypertension or diabetes.
Strengths of the present investigation are its large sample size, relatively low deficiency levels and the fact that the vitamin D analyses were all performed by the same assay method. Moreover, our analysis is unique in reporting, in a large cohort study the association between diabetes and blood levels of 1,25(OH)2D levels: to our knowledge only associations of 1,25(OH)2D with hypertension [4], CHD [3], BMI [20] been reported in large epidemiological groups. Important limitations of our study include its cross-sectional design and the reliance upon self-report of diabetes, hypertension and CHD status, however the measures of hypertension and diabetes had high reliability on follow-up. We only had one measure of adiposity (BMI), not total body fat, which is often thought to be more accurate however a recent validation study of BMI and DEXA has reported very favorable correlations [21]. We only used education as a marker for social class however this has been well accepted as the best marker for social class on an epidemiological level [22-24]. In particular, we cannot rule out the possibility that changes in sun exposure and/or diet as a consequence of having diabetes may have led to our findings however, the associations reported here warrant investigation in large prospective studies.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Cancer Institute (NCI), the National Institutes of Health (NIH). In addition, this research was supported by U.S. Public Health Service contracts from NCI, NIH, the Department of Health and Human Services. All authors reviewed the final manuscript before submission, and none has a conflict of interest with regard to this work
Abbreviations
- OR
odds ratio
- CI
confidence interval
- BMI
body mass index
- PA
physical activity
- CHD
coronary heart disease
- 25(OH)D
25-hydroxyvitamin D
- 1,25(OH)2D
1,25-dihydroxyvitamin D
Reference
- 1.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Kendrick J, Targher G, Smits G, et al. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–60. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Nemerovski CW, Dorsch MP, Simpson RU, et al. Vitamin D and cardiovascular disease. Pharmacotherapy. 2009;29:691–708. doi: 10.1592/phco.29.6.691. [DOI] [PubMed] [Google Scholar]
- 4.Pilz S, Tomaschitz A, Ritz E, et al. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. 2009;6:621–30. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 5.Pittas AG, Lau J, Hu FB, et al. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christakos S, Friedlander EJ, Frandsen BR, et al. Studies on the mode of action of calciferol. XIII. Development of a radioimmunoassay for vitamin D-dependent chick intestinal calcium-binding protein and tissue distribution. Endocrinology. 1979;104:1495–503. doi: 10.1210/endo-104-5-1495. [DOI] [PubMed] [Google Scholar]
- 7.Norman AW, Frankel JB, Heldt AM, et al. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–5. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 8.Hayes RB, Reding D, Kopp W, et al. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21:349S–355S. doi: 10.1016/s0197-2456(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 9.Brock K, Huang WY, Fraser DR, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol. 2010;121:462–6. doi: 10.1016/j.jsbmb.2010.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subar AF, Midthune D, Kulldorff M, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;152:279–86. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 11.Tippett KS, Cypel YS. Continuing survey of food intakes by individuals, nationwide food surveys. US Department of Agriculture, Agricultural Research Service; Washington, DC: 1997. Design and operation: the continuing survey of food intakes by individuals and diet and health knowledge survey, 1994-96. [Google Scholar]
- 12.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42:1549–56. doi: 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–86. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 14.Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Scientific Publications. 1980;32:5–338. [PubMed] [Google Scholar]
- 15.Hyppönen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care. 2006;29:2244–6. doi: 10.2337/dc06-0946. [DOI] [PubMed] [Google Scholar]
- 16.Forouhi NG, Luan J, Cooper A, et al. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes. 2008;57:2619–25. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–71. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 18.Hintzpeter B, Mensink GB, Thierfelder W, et al. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–89. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 19.Teegarden D, Donkin SS. Vitamin D: emerging new roles in insulin sensitivity. Nutr Res Rev. 2009;22:82–92. doi: 10.1017/S0954422409389301. [DOI] [PubMed] [Google Scholar]
- 20.Konradsen SAH, Lindberg F, Hexeberg S, et al. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr. 2008;47:87–91. doi: 10.1007/s00394-008-0700-4. [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–8. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kant AK, Graubard BI. Ethnicity Is an Independent Correlate of Biomarkers of Micronutrient Intake and Status in American Adults. J Nutr. 2007;137:2456–63. doi: 10.1093/jn/137.11.2456. [DOI] [PubMed] [Google Scholar]
- 23.Winkleby MA, Jatulis DE, Frank E, et al. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberatos P, Link BG, Kelsey JL. The measurement of social class in epidemiology. Epidemiol Rev. 1988;10:87–121. doi: 10.1093/oxfordjournals.epirev.a036030. [DOI] [PubMed] [Google Scholar]