Abstract
Anion and fluid secretion are both defective in cystic fibrosis (CF); however, the transport mechanisms are not well understood. In this study, Cl− and HCO3− secretion was measured using genetically matched CF transmembrane conductance regulator (CFTR)-deficient and CFTR-expressing cell lines derived from the human airway epithelial cell line Calu-3. Forskolin stimulated the short-circuit current (Isc) across voltage-clamped monolayers, and also increased the equivalent short-circuit current (Ieq) calculated under open-circuit conditions. Isc was equivalent to the HCO3− net flux measured using the pH-stat technique, whereas Ieq was the sum of the Cl− and HCO3− net fluxes. Ieq and HCO3− fluxes were increased by bafilomycin and ZnCl2, suggesting that some secreted HCO3− is neutralized by parallel electrogenic H+ secretion. Ieq and fluid secretion were dependent on the presence of both Na+ and HCO3−. The carbonic anhydrase inhibitor acetazolamide abolished forskolin stimulation of Ieq and HCO3− secretion, suggesting that HCO3− transport under these conditions requires catalysed synthesis of carbonic acid. Cl− was the predominant anion in secretions under all conditions studied and thus drives most of the fluid transport. Nevertheless, 50–70% of Cl− and fluid transport was bumetanide-insensitive, suggesting basolateral Cl− loading by a sodium–potassium–chloride cotransporter 1 (NKCC1)-independent mechanism. Imposing a transepithelial HCO3− gradient across basolaterally permeabilized Calu-3 cells sustained a forskolin-stimulated current, which was sensitive to CFTR inhibitors and drastically reduced in CFTR-deficient cells. Net HCO3− secretion was increased by bilateral Cl− removal and therefore did not require apical Cl−/HCO3− exchange. The results suggest a model in which most HCO3− is recycled basolaterally by exchange with Cl−, and the resulting HCO3−-dependent Cl− transport provides an osmotic driving force for fluid secretion.
Key points
The mechanisms of anion and fluid transport by airway submucosal glands are not well understood and may differ from those in surface epithelium.
The Calu-3 cell line is often used as a model for submucosal gland serous cells and has cAMP-stimulated fluid secretion; however, it does not actively transport chloride under short-circuit conditions.
In this study we show that fluid secretion requires chloride, bicarbonate and sodium, that chloride is the predominant anion in Calu-3 secretions, and that a large fraction of the basolateral chloride loading during cAMP stimulation occurs by Cl−/HCO3− exchange.
The results suggest a novel cellular model for anion and fluid secretion by Calu-3 and submucosal gland acinar cells
Introduction
The airways are protected by a microscopic layer of airway surface liquid (ASL) which contains water, inorganic ions, lipids, mucins and proteins. The ASL humidifies inspired air and contains antimicrobial factors that defend the epithelium from inhaled pathogens. Its depth (5–30 μm in healthy airways; Widdicombe, 2002; Boucher, 2003) is reduced in cystic fibrosis (CF) due to abnormal transepithelial salt and water transport (Tarran et al. 2006), and this diminishes mucociliary clearance and leads to recurrent infections and inflammation. Despite the importance of ASL in airway host defense and pathophysiology, the relationship between anion and fluid secretion by airway epithelia remains poorly understood.
The cystic fibrosis transmembrane conductance regulator (CFTR) is the product of the CF gene and contributes to airway secretion. It is a phosphorylation-regulated, non-rectifying anion channel of ∼7 pS conductance (Hanrahan et al. 1994), which serves as the rate-limiting step during cAMP-stimulated secretion by many epithelia (e.g. Klyce & Wong, 1977) including those in the airways (Frizzell & Hanrahan, 2011). Although the CFTR channel pore is permeable to Cl− and HCO3− (Gray et al. 1990; Poulsen et al. 1994; Linsdell et al. 1997) and secretion of both anions is reduced in CF (Widdicombe et al. 1985; Smith & Welsh, 1992), the relative contributions of CFTR, SLC26A transporters and other pathways for apical HCO3− efflux remain controversial (Lee et al. 1999; Ishiguro et al. 2009; Kim & Steward, 2009; Garnett et al. 2011).
Anion secretion has been studied extensively in the human airway cell line Calu-3, which spontaneously differentiates into predominantly serous-like cells that express CFTR and antimicrobial proteins, and a smaller population of goblet-like cells, which contain mucin granules. When cultured on porous supports at the air–liquid interface (ALI), Calu-3 monolayers generate a robust basal short-circuit current (Isc) that cannot be ascribed to net Cl− or Na+ fluxes (Shen et al. 1994; Haws et al. 1994; Shan et al. 2011) and is thought to be mediated by active HCO3− secretion (Lee et al. 1998). Forskolin stimulates transepithelial 36Cl− fluxes in both directions under Isc conditions without causing detectable net Cl− secretion, and the Isc is insensitive to the NKCC1 inhibitor bumetanide (Devor et al. 1999). These findings and subsequent pH-stat measurements suggest that forskolin stimulates electrogenic HCO3− transport under Isc conditions (Devor et al. 1999; Krouse et al. 2004).
Various cellular models have been proposed to explain Calu-3 anion transport. According to one scheme, HCO3− secretion occurs by Na+-coupled entry at the basolateral membrane and exit through apical CFTR channels (Devor et al. 1999) and/or via pendrin-mediated apical anion exchange (Garnett et al. 2011). Net HCO3− secretion is stimulated by forskolin; therefore, the fluid produced during forskolin stimulation is expected to be alkaline. By contrast, secretagogues that hyperpolarize Calu-3 cells elicit mainly Cl− transport, as shown by the net 36Cl− flux measured during stimulation by the potassium channel activator 1-EBIO (Devor et al. 1999). Despite much progress, the mechanisms and relationship between anion transport and fluid secretion remain uncertain in Calu-3 cells and in gland serous cells. HCO3− appears to be the only actively secreted anion under Isc conditions, consistent with bumetanide-insensitive fluid secretion by native airway glands (Corrales et al. 1984), yet most studies indicate that the pH of native gland secretions and airway surface liquid is near neutrality or slightly acidic (Kyle et al. 1990; Coakley et al. 2003; Song et al. 2006; reviewed by Fischer & Widdicombe, 2006).
In the present study, net HCO3− transport was measured under open- and short-circuit conditions using an automated pH-stat, and 36Cl− fluxes and the volume and anion composition of fluid secreted by polarized Calu-3 monolayers were measured under comparable conditions. Forskolin-stimulated Isc was identical to the net HCO3− flux, further evidence that net HCO3− transport mediates the Isc as suggested previously (Devor et al. 1999; Ballard et al. 1999). However, although fluid secretion was strictly dependent on the presence of HCO3−, the predominant anion in the secreted fluid was Cl−. These and other results indicate that most HCO3− entering the cells by basolateral cotransport with Na+ is recycled to the basolateral side in exchange for Cl−, and suggest a revised model in which fluid secretion is mainly driven by HCO3−-dependent Cl− transport. A preliminary account of these results was presented at the 36th International Congress of Physiological Sciences, Kyoto, Japan (2009).
Methods
Cell culture
Two genetically modified Calu-3 cell lines were used: a CFTR knock-down cell line which stably expresses a 21-mer shRNA that specifically targets CFTR mRNA transcripts, and a control cell line, which expresses shRNA bearing four mutations that reduce its ability to silence cftr (Sizt and Alter cell lines, respectively; Palmer et al. 2006). Both cell lines were cultured in Eagle's minimum essential medium (EMEM; Wisent Bioproducts, St. Bruno, Qc) containing 7% fetal bovine serum (FBS). To allow comparison with previous studies, control experiments were also performed using parental Calu-3 cells (HTB-55, American Type Culture Collection, Manassas, VA, USA) cultured in EMEM containing 15% FBS.
Cells were seeded at 5 × 105 cells cm−2 on Snapwells (1.12 cm2; Costar, Corning Life Sciences, Lowell, MA, USA) for studies of Isc and HCO3− secretion, and at the same density on Transwells (4.67 cm2, Corning) for measuring fluid secretion rate and composition. Fresh medium was placed on the basolateral side 1 day after plating and the apical medium was removed to establish an air–liquid interface (ALI). Any fluid that appeared spontaneously on the apical surface was removed after 3 days. Cultures were maintained in a humidified 5% CO2 incubator at 37°C and studied after 21–25 days. Polarization of the monolayers with respect to CFTR function was confirmed by imposing a transepithelial Cl− gradient and measuring the forskolin-stimulated current after permeabilization of the basolateral or apical membrane with nystatin (100 μg ml−1 apical; 360 μg ml−1 basolateral).
Solutions and media
To measure HCO3− secretion under control pH-stat conditions, monolayers were mounted in modified Ussing chambers and the apical surface was bathed with unbuffered solution containing (mmol l−1): 120 NaCl, 5 KCl, 1.2 MgCl2 and 1.2 CaCl2. The basolateral side was bathed with 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2 and 10 glucose. Nominally Na+-free solution was prepared by replacing NaHCO3 with equimolar choline bicarbonate, and NaCl was replaced with N-methyl-d-glucamine chloride. Nominally Cl−-free solution was prepared by replacing Cl− salts with gluconate salts, and the [Ca2+] was increased from 1.2 to 4 mmol l−1 by adding calcium gluconate to compensate for gluconate binding. Nominally HCO3−-free solution was prepared by replacing 25 mmol l−1 NaHCO3 with 25 mmol l−1 Na-Hepes and gassing with 100% O2. All solutions were adjusted to pH 7.4. When studying basolateral anion exchange, pH-stat was performed on the basolateral side after permeabilizing the apical membrane with nystatin and adding 25 mmol l−1 NaHCO3 on the apical side. Apical addition of choline bicarbonate gave the same result as NaHCO3, presumably because the current induced by the apical-to-basolateral gradient of 170 → 145 Na+ present after apical addition of NaHCO3 (25 mmol l−1) was obscured by sodium pump current. In control experiments, osmotic gradients produced by adding 50 mmol l−1 mannitol on the apical side had no effect on Ieq or HCO3− flux. In pH-stat experiments, the unbuffered side was stirred with 100% O2 while the opposite side containing HCO3− was bubbled with 95% O2–5% CO2. Both sides were gassed with 100% O2 during experiments with bilateral HCO3−-free solution.
To maintain viability during long fluid secretion experiments, EMEM medium containing essential amino acids was placed on the basolateral side. The salts of amino acids (l-histidine·HCl, l-lysine·HCl) and vitamins (choline chloride, pyridoxine·HCl, thiamine·HCl) contributed 0.6 mmol l−1 Cl− to the nominally Cl−-free medium. This Cl− contamination probably did not affect the results since adding this concentration of Cl− during pH-stat measurements had no detectable effect on Ieq, which is a measure of the net Cl−+ HCO3− flux under open-circuit conditions. Monolayers bathed with basolateral 25 mmol l−1 HCO3− medium were kept in 5% CO2–95% air, which was nominally saturated with H2O during fluid secretion measurements unless otherwise noted. Control experiments performed with Transwells mounted on an orbital shaker in a 5% CO2–95% O2 atmosphere to allow better oxygenation yielded only slightly higher fluid secretion rates (data not shown). Humidified air (0.035% CO2) was used when measuring fluid secretion with nominally HCO3−-free basolateral medium. Tracer fluxes were measured using the same solutions as during pH-stat experiments (i.e. with a basolateral-to-apical HCO3− gradient) except apical pH was maintained using Hepes rather than by titrating with acid. In some experiments the basolateral membrane was permeabilized by adding nystatin from a 1000× stock solution in DMSO (final nystatin concentration 100 μg ml−1). The CFTR inhibitor GlyH-101 was added from a 1000× stock solution to give a final concentration of 100 μmol l−1 and 0.1% DMSO.
Immunoblotting
After SDS-PAGE on 8% gels, proteins were transferred to nitrocellulose membranes as described previously (Luo et al. 2009) and probed with the following monoclonal antibodies: M3A7, which recognizes an epitope between amino acids 1365 and 1395 in the second nucleotide binding domain of CFTR (1:5000, gift from J.R. Riordan and T.J. Jensen, UNC Chapel Hill, NC, USA; Kartner & Riordan, 1998), TUB-1A2, which binds to the C-terminus of α-tubulin (1:5000, Sigma) and α5, which binds the α-subunit of avian Na+/K+-ATPase (1:200, mAb gift from R.W. Mercer, Washington University, St Louis, MO, USA; Takeyasu et al. 1988). Blots were washed, incubated with secondary antibody conjugated to horseradish peroxidase (1:1000), visualized by enhanced chemiluminescence (Amersham Biosciences) and analysed using ImageJ (Rasband, 2011).
Measurement of equivalent short-circuit current (Ieq) and HCO3− secretion
Inserts were mounted in modified Ussing chambers (Physiologic Instruments, Inc., San Diego, CA, USA) at 37°C. Initial studies were carried out under voltage clamp to allow comparison of net HCO3− flux with Isc; however, in all subsequent experiments, net HCO3− secretion was measured under open-circuit conditions and compared with Ieq, which was calculated from Ohm's law using the spontaneous transepithelial potential (Vt) and resistance (Rt). Rt was determined from the small deflections in Vt produced by bipolar current pulses (1 μA, 1 s duration, 99.9 s interval, duty cycle 100.9 s) delivered by the voltage clamp (VCC200, Physiologic Instruments, Inc.). Data were digitized (Powerlab 8/30, AD Instruments, Montreal, QC, Canada) and analysed using Chart5 software.
HCO3− transport was measured using pH-stat under both open- and short-circuit conditions. A mini-pH electrode (pHG200-8, Radiometer Analytical) connected to the automated titration workstation (TitraLab 854, Radiometer) delivered 1 μl aliquots of 10 mmol l−1 HCl or 5 mmol l−1 H2SO4 to maintain the pH constant at 7.400 ± 0.002. To avoid offsets, the ‘metal’ option for cell grounding was selected in the Titralab firmware, the pHC4000 pH electrode was connected to the E1 input of the titrator, and the current electrode of the voltage clamp in the same half-chamber was connected to the GND input of the Titralab.
The amount of acid required to maintain pH constant was used to calculate the rate of HCO3− secretion. The volume of each half chamber was 4 ml. Solutions containing 25 mmol l−1 HCO3− were stirred vigorously with 95% O2–5% CO2. Nominally HCO3−-free solutions were bubbled with 100% O2.
Volume and composition of the secreted fluid
Fluid was aspirated from the apical surface and fresh media containing replacement ions, activators or inhibitors were added on the basolateral side to begin fluid secretion assays. In some experiments, agents were added to the apical side in a small volume of Krebs–Henseleit solution (mmol l−1: 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2 and 10 mannitol), which was subtracted from the volume measured at the end of the experiment when calculating secretion. Apical fluid was collected at 24 h intervals using a pipettor and the time averaged fluid secretion rate was calculated after subtracting the volume of vehicle if added on the apical side. Although cumulative volume increased linearly for at least 3 days, the instantaneous secretion rate declined over the course of each 24 h collection period, therefore total volume secreted under these conditions is sub-maximal. A low rate of evaporation was observed from siliconized Transwells in control measurements (∼0.27 μl cm−2 h−1) despite being kept in a humidified incubator. However, when cultures were coated with a film of water-saturated hexadecane (a solvent with low vapour pressure which eliminates evaporation), the volume of secreted fluid that could be retrieved from cell monolayers was not altered. High osmotic permeability apparently enables sufficient H2O diffusion through the monolayer to compensate for evaporative H2O loss from the surface of uncoated cultures so that apical fluid volume is determined simply by the quantity of solute on the surface. Since correcting for evaporation would cause overestimation of active fluid transport, no correction was applied. The Cl− concentrations of secretions and media were measured using an ADVIA 1650 biochemistry system (Bayer) after 10-fold dilution in distilled H2O. pH was measured using a micro-electrode (9826BN, Orion), which was either kept inside the 5% CO2 incubator to reduce equilibration time or immersed in the sample while bubbling with 5% CO2. The Henderson–Hasselbalch equation was used to calculate the equilibrium HCO3− concentration:
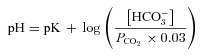 |
(1) |
where pK = 6.09 and 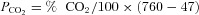 . No corrections were made for small errors in water vapour pressure (slightly less than 47 mmHg), laboratory elevation above sea level (∼31 m) or daily fluctuations in barometric pressure.
. No corrections were made for small errors in water vapour pressure (slightly less than 47 mmHg), laboratory elevation above sea level (∼31 m) or daily fluctuations in barometric pressure. 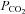 in the incubator was set using the built-in infrared controller and confirmed independently within ±0.01% using an external non-dispersive infrared (NDIR) sensor (GMM221, Vaisala, Lake Villa, IL, USA).
in the incubator was set using the built-in infrared controller and confirmed independently within ±0.01% using an external non-dispersive infrared (NDIR) sensor (GMM221, Vaisala, Lake Villa, IL, USA).
Tracer fluxes
Calu-3 monolayers were mounted in modified Ussing chambers and equilibrated for 20 min. H36Cl (generous gift of W.S. Marshall, St Francis-Xavier University, Antigonish, NS, Canada) was added to one side of the monolayer and neutralized, yielding a final Cl− concentration of 125.3 mmol l−1. Duplicate 400 μl samples were taken from the cold side after 20 min (to allow mixing), and again at 15 min intervals with replacement by 800 μl saline. Residual radioactivity was calculated (with correction for dilution) at the beginning of each subsequent flux period and samples were counted in 5 ml scintillation solution (Tri-Carb 2810 TR, PerkinElmer, Woodbridge, ON, Canada). Two 10 μl samples taken from the hot side at the beginning and end of each experiment were averaged and used to calculate mean specific activity of the tracer, which ranged between 6500 and 8000 cpm μmol−1. Counts on the cold side at the end of the sample period were several-fold above background. Unidirectional fluxes were measured in parallel experiments at the same time. Rt ranged between 200 and 300 Ω cm2 under resting conditions.
To measure 36Cl fluxes under conditions similar to those when monitoring fluid secretion, monolayers were stimulated with 10 μmol l−1 forskolin and 36Cl− was added to basolateral serum-free EMEM medium (2.5 ml) or to apical Krebs–Henseleit solution (400 μl) in Transwells rather than in Ussing chambers. Mean specific activity was determined for each experiment by averaging the number of counts on the hot side at the beginning and end of the 24 h flux period. Jsm (serosal-to-mucosal 36Cl− flux), Jms (mucosal-to-serosal 36Cl− flux) and Jnet (net 36Cl− flux) were calculated by counting 100 μl samples from the cold side, after correction for residual tracer and replacement volume.
Data analysis
Either Isc or Ieq was determined at 100 s intervals, and HCO3− net flux rate was calculated every 5 min. Basal values were those obtained immediately before adding forskolin. Steady-state fluxes during stimulation were calculated 30–60 min after forskolin. Paired or unpaired Student's t tests with P < 0.05 were used for single comparisons, whereas one-way analysis of variance followed by the Bonferonni post hoc test was used for multiple comparisons.
Results
HCO3− secretion accounts for the short-circuit current (Isc)
Electrogenic HCO3− secretion across control monolayers was measured under voltage-clamp and compared with the Isc measured simultaneously. Unstimulated HCO3− secretion and Isc were both low but increased ∼8-fold after forskolin (10 μmol l−1) was added to the basolateral side (Fig. 1). The rate of HCO3− secretion measured using pH-stat with the transepithelial potential clamped at 0 mV was equal to the Isc within measurement error (net HCO3− secretion: 2.14 ± 0.36 μeq cm−2 h−1; Isc: 2.30 ± 0.27 μeq cm−2 h−1; Fig. 1), evidence that basal and forskolin-stimulated Isc were both due to net HCO3− secretion. This confirms previous suggestions based on the large discrepancy between Isc and the net 36Cl− and 22Na+ fluxes (Lee et al. 1998; Devor et al. 1999), but differs from pH-stat results obtained during 1-EBIO stimulation, when HCO3− secretion accounted for 70% of the Isc and the discrepancy was due to acid secretion by H+/K+-ATPase (Krouse et al. 2004). The effects of 1-EBIO were not examined in the present study. The pH-stat technique required unbuffered solution on the apical side and therefore a transepithelial HCO3− gradient; however, the basolateral-to-apical gradient of 25 mmol l−1 HCO3− did not cause overestimation of HCO3− secretion since the Isc was not noticeably affected when 25 mol l−1 HCO3−/5% CO2 was added acutely on the apical side (data not shown), consistent with a previous report (Krouse et al. 2004). The stimulated Isc was similar whether symmetrical HCO3− solutions or pH-stat conditions were used, further evidence that passive diffusion due to the HCO3− gradient contributed little to Isc.
Figure 1. Relationship between short-circuit current (Isc) and HCO3− secretion.
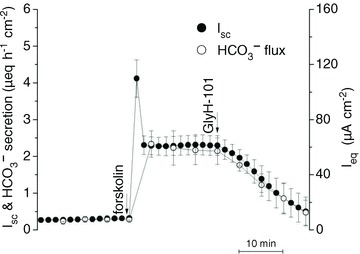
Forskolin (10 μmol l−1) and GlyH-101 (100 μmol l−1) were added to control Calu-3 cells expressing mutated shRNA. Note the close agreement between Isc and HCO3− secretion, and their sensitivity to the CFTR channel inhibitor GlyH-101 (n= 4). •, Isc, short-circuit current; º, HCO3− secretion as measured using pH-stat.
HCO3− secretion is CFTR dependent
To evaluate the role of CFTR during HCO3− and fluid transport, protein expression and anion secretion were compared in control and CFTR knock-down monolayers. The immunoblot in Fig. 2A shows CFTR expression. For comparison, Lanes 1 and 2 show baby hamster kidney (BHK) cells stably transfected with wild-type or ΔF508-CFTR, Lanes 3 and 4 show parental Calu-3 cells, and Lanes 5 and 6 show shRNA control and CFTR knock-down Calu-3 cells, respectively.
Figure 2. CFTR expression and HCO3− secretion.
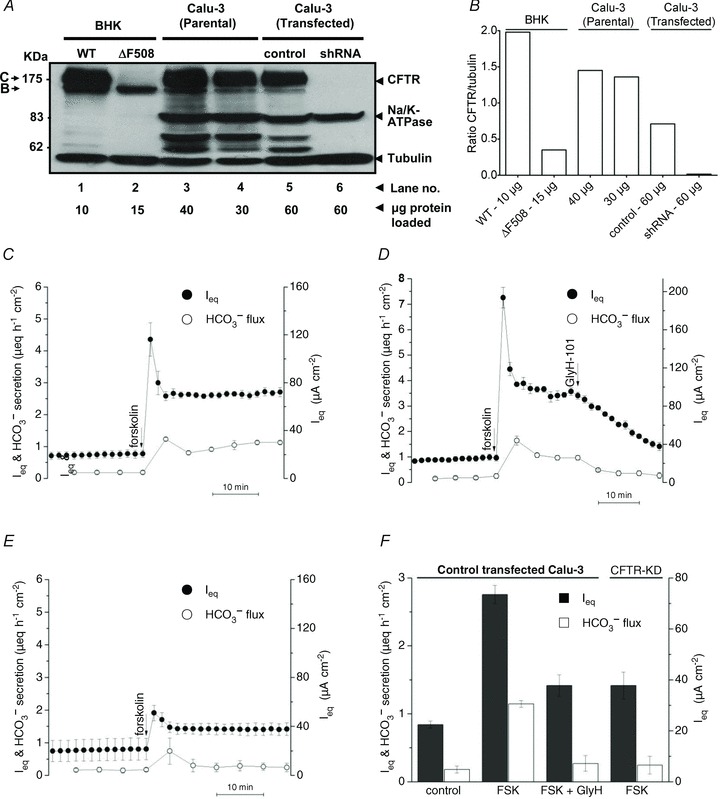
A, CFTR expression in stable cell lines. Lane 1, baby hamster kidney (BHK) cells transfected with wild-type CFTR expressed both mature and immature CFTR. Lane 2, BHK cells transfected with ΔF508 CFTR expressed only immature (band B) CFTR. Lanes 3 and 4, parental, and Lane 5 control transfected Calu-3 cells also expressed both bands B and C. Lane 6, CFTR was not detectable in shRNA knock-down cells. Comparisons of band intensities and μg protein loaded indicate the following relative expression levels: BHK ≫ Calu-3 (Parental) > Calu-3 (control transfected) ≫ Calu-3 (shRNA transfected). This blot is representative of two experiments. B, mean densities of CFTR normalized to tubulin (n= 2). C, forskolin (10 μmol l−1) stimulated Ieq and HCO3− secretion across wild-type Calu-3 cells under open-circuit conditions (n= 12). D, CFTR inhibitor GlyH-101 (100 μmol l−1) nearly abolished forskolin-stimulated Isc and HCO3− secretion (n= 6). E, the forskolin response was greatly reduced in CFTR-knock-down Calu-3 cells (n= 6). •, Ieq; •, HCO3− secretion. F, summary of forskolin-stimulated Ieq (filled bars) and HCO3− secretion (open bars) shown in C, D and E. Means ± SEM.
BHK cells expressed mature (i.e. complex glycosylated or ‘band C’ polypeptide) and immature (core-glycosylated, band B) wild-type CFTR, whereas only band B was detected in BHK cells expressing ΔF508 CFTR (Lanes 1 and 2, respectively). Mature and immature CFTR glycoforms were also found in parental cells (Lanes 3 and 4), and in cells expressing control (i.e. altered) shRNA (Lane 5). CFTR was usually not detectable in cells that expressed shRNA targeting CFTR transcripts (Lane 6). Blots were also probed with anti-tubulin antibody to control for variations in protein loading, and with anti-Na+/K+-ATPase antibody to assess non-specific changes in membrane protein expression. Tubulin and Na+/K+-ATPase α subunit levels were not affected by control or CFTR-specific shRNA. Densitometry of CFTR expression revealed >95% reduction in band C compared with the control cell line (Fig. 2B), consistent with previous estimates (Palmer et al. 2006). Band C was lower in shRNA control cells than in parental cells after normalization to tubulin, despite the presence of four mutations in the shRNA which were intended to reduce its binding to CFTR mRNA transcripts (Fig. 2B). Cells expressing control shRNA (referred to previously as ‘alter’ by (Palmer et al. 2006) were used as the control cell line in subsequent transport experiments, bearing in mind CFTR expression is lower in these control cells than in parental Calu-3 cells used for previous studies.
The effect of knocking down CFTR expression on HCO3− secretion was also investigated under open-circuit conditions using the pH-stat technique (Fig. 2C). In marked contrast to Isc, the Ieq calculated from transepithelial voltage and resistance was higher than the simultaneously measured HCO3− flux; mean Ieq was 2.76 ± 0.13 μeq cm−2 h−1 (n= 12) during forskolin stimulation whereas net HCO3− transport was 1.14 ± 0.05 μeq cm−2 h−1 (n= 12, Fig. 2F). This difference between Ieq and net HCO3− flux (∼1.55 μeq cm−2 h−1) was current carried by Cl− because, as shown below (Table 1), a net Cl− flux of 1.5 μeq cm−2 h−1 in the secretory direction was observed when unidirectional 36Cl fluxes were measured under these conditions (i.e. open-circuited and with the same 25 mmol l−1 HCO3− gradient that was present during pH-stat experiments). Also, the discrepancy between Ieq and net HCO3− flux was abolished in Cl−-free solution. Ieq and net HCO3− secretion were both inhibited by the CFTR channel blocker GlyH-101; however, this inhibition developed slowly (100 μmol l−1; ∼20 min, Fig. 1 and 2D; Muanprasat et al. 2004), perhaps due to slow diffusion through the hydrophobic mucus gel on the apical surface. Alternatively, GlyH-101 inhibition is voltage dependent therefore its affinity may decline as CFTR channels become progressively blocked and the membrane potential hyperpolarizes. The stimulation of Ieq and HCO3− secretion by forskolin was greatly reduced in CFTR knock-down cells compared with control cells (compare Fig. 2E and C), although the decrease in CFTR channel function was somewhat less than the decrease in CFTR protein expression observed on immunoblots (65%vs. 95% reduction). Figure 2F summarizes the mean Ieq (filled bars) and HCO3− flux (open bars) across control vs. CFTR-deficient cells. HCO3− was secreted by CFTR knock-down cells during forskolin stimulation at a rate that was similar to unstimulated control monolayers, and to stimulated control monolayers treated with the CFTR inhibitor GlyH-101. Taken together the results confirm that forskolin stimulates electrogenic HCO3− secretion and are consistent with CFTR channels mediating part of this flux.
Table 1.
Unidirectional 36Cl fluxes across Calu-3 cell monolayers
| Jsm | Jms | Jnet | Ieq | Rt | Vt | |
|---|---|---|---|---|---|---|
| Control | 1.10 ± 0.12 | 0.53 ± 0.11 | 0.58 ± 0.06 | 0.81 ± 0.06 | 375 ± 47 | −8.14 ± 0.68 |
| Forskolin (10 μmol l−1) | 3.13 ± 0.13*** | 1.63 ± 0.15*** | 1.51 ± 0.02*** | 2.75 ± 0.13*** | 289 ± 30 | −21.31 ± 0.97*** |
| Bumetanide (20 μmol l−1) | 1.95 ± 0.08+++ | 1.22 ± 0.06++ | 0.73 ± 0.05+++ | 2.15 ± 0.10+++ | 301 ± 32 | −17.35 ± 0.64+++ |
Experiments were performed under open-circuit, pH-stat conditions (i.e. basolateral 25 mm HCO3−, apical 0 mm HCO3−). The apical solution was stirred with 100% O2 and buffered at pH 7.4 using 10 mmol l−1 tricine. Forskolin was added to both sides. Bumetanide was added basolaterally. Jsm (serosal-to-mucosal 36Cl− flux), Jms (mucosal-to-serosal 36Cl− flux), Jnet (net 36Cl− flux), Ieq (equivalent short-circuit current) have units of μeq cm−2 h−1. Rt (transepithelial resistance) has units of Ohms cm2 electrical resistance, no time units. Values are mean ± SEM, n= 4 (36Cl− fluxes) or 8 (Ieq, Rt and Vt). ***P < 0.001, forskolin vs. control; +++P < 0.001; ++P < 0.01, bumetanide vs. forskolin.
The stimulated Ieq under open-circuit, pH-stat conditions in Fig. 2C was 2.55 μeq cm−2 h−1, higher than the HCO3− flux of 1.0 μeq cm−2 h−1 measured simultaneously. To examine if the discrepancy of ∼1.55 μeq cm−2 h−1 is carried by Cl−, unidirectional 36Cl− tracer fluxes were measured under the same conditions, i.e. open circuited and with a basolateral → apical HCO3− gradient and apical O2 bubbling. Apical pH was maintained by 25 mm Hepes during tracer experiments rather than by titration with acid. As shown in Table 1, net Cl− secretion (Jnet) was observed under basal conditions. Forskolin increased 36Cl− fluxes in both directions, but stimulation of the secretory flow was larger and resulted in a 3-fold increase in the net flux. Thus, Cl− was secreted under open circuit/pH-stat conditions (in marked contrast to Isc conditions), and the net flux rate (1.51 ± 0.02 μeq cm−2 h−1) accounted for the discrepancy of 1.55 μeq cm−2 h−1 between Ieq and net HCO3− flux measured using the pH-stat.
Proton secretion neutralizes some of the transported bicarbonate
Acid secretion was also investigated because, if present, it could cause underestimation of the HCO3− flux. Airway epithelial cells secrete H+ by multiple mechanisms (Acevedo & Steele, 1993; Fischer et al. 2002; Coakley et al. 2003; Inglis et al. 2003), two of which are electrically silent (H+/K+-ATPase and Na+/H+ exchange) and thus capable of increasing the discrepancy between Ieq and HCO3− secretion. The other two mechanisms (electrogenic vacuolar H+-ATPase and H+ channels) would be invisible in these experiments because they would cause equivalent reductions in current and net HCO3− flux.
To assess the role of the non-gastric isoform of H+/K+-ATPase, the effect of apical ouabain on Ieq and HCO3− secretion was investigated (Fig. 3A).
Figure 3. Effect of proton transport inhibitors on Ieq and net HCO3− secretion across Calu-3 ‘Alter’ shRNA control cells.
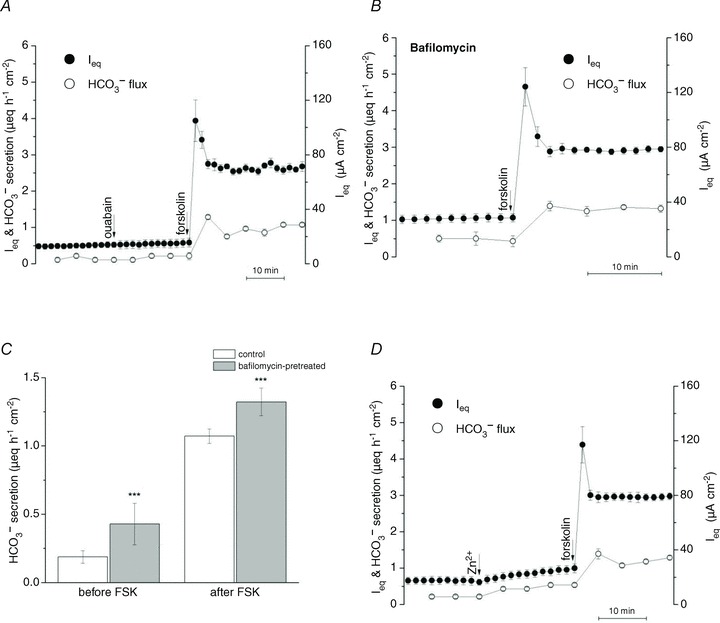
A, ouabain (100 μmol l−1) had no effect when added to the apical side to inhibit putative H+/K+-ATPase (n= 3), B and C, pretreatment with the V-ATPase inhibitor bafilomycin (50 nmol l−1), increased basal- and forskolin-stimulated HCO3− secretion (n= 3). D, apical addition of ZnCl2 (nominally 1 mmol l−1) to block putative H+ channels increased Ieq and HCO3− secretion (n= 3). •, Ieq; º, HCO3− secretion. ***P < 0.001.
Ouabain (100 μmol l−1) did not alter basal or forskolin-stimulated Ieq or HCO3− secretion, suggesting little role of ouabain-sensitive H+/K+-ATPase activity with an apical solution containing 5 mmol l−1 K+ and bubbled with 100% O2. Apical amiloride (1 mmol l−1) also had no effect (data not shown), evidence against significant apical proton secretion via Na+/H+ exchange under these conditions. By contrast, pretreating cells with the vacuolar H+-ATPase inhibitor bafilomycin for 30 min increased Ieq and HCO3− secretion by ∼0.25 μeq cm−2 h−1, and similar increases were obtained under basal and forskolin-stimulated conditions (Fig. 3B and C). This implies that forskolin did not cause insertion of additional proton pumps into the apical membrane (Paunescu et al. 2010). Finally, acute exposure to ZnCl2 (1 mmol l−1), which inhibits acid secretion by blocking apical H+ (HVCN1) channels in airway epithelial cells (Iovannisci et al. 2010), increased Isc and HCO3− secretion by ∼0.25 μeq cm−2 h−1 (Fig. 3D), implicating proton channels in H+ efflux. Taken together these inhibitor studies indicate that HCO3− secretion is 30% higher across Calu-3 monolayers than the measured net flux, but is partially neutralized by parallel H+ secretion. These electrogenic H+ transport mechanisms would not cause any discrepancy between Ieq and HCO3− net flux and are thus compatible with the close agreement between the unidentified Ieq and 36Cl− net flux and the net HCO3− flux and Isc under voltage clamp.
Fluid secretion is CFTR dependent
Dehydration is a hallmark of CF airway secretions, and suggests a role of CFTR in fluid secretion. To test if fluid transport by Calu-3 cells is CFTR dependent, apical fluid was removed at time 0 h and secretions were collected from control and CFTR knock-down cultures at 24 h intervals under control conditions and during forskolin stimulation (Fig. 4A). Little fluid was produced by control monolayers that were left untreated or exposed only to vehicle (0.1% basolateral DMSO). However, fluid secretion increased to >40 μl day−1 during stimulation with forskolin (10 μmol l−1), and a similar volume was produced for several days, leading to the cumulative secretion of ∼130 μl.
Figure 4. CFTR-dependent fluid secretion by control and CFTR knock-down Calu-3 cells.
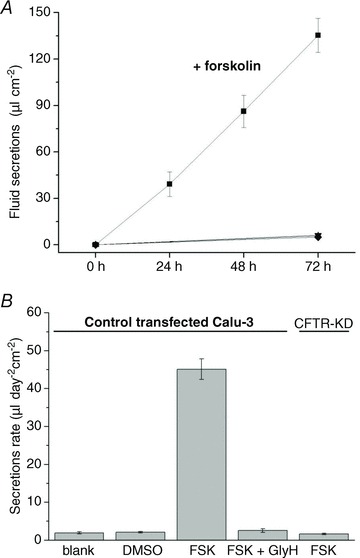
A, fluid secretion under different conditions: ▪, control Calu-3 cells stimulated by 10 μmol l−1 forskolin; ▴, untreated; ▾, 0.1% DMSO or •, forskolin + GlyH-101 (100 μmol l−1)-treated control Calu-3 cells; and ♦, CFTR knock-down Calu-3 cells treated with forskolin (n= 6 each condition). B, summary of results in A. Means ± SEM.
Figure 4B shows the time-averaged fluid secretion rate measured daily for 3 days under each condition. GlyH-101 (100 μmol l−1) nearly abolished forskolin-stimulated fluid secretion when added to the apical side in 400 μl PBS, suggesting CFTR channel activity is required for fluid transport.
CFTR mediates apical HCO3− conductance
The next series of experiments used control and CFTR knock-down Calu-3 cells to examine whether CFTR can directly mediate HCO3− flux. Monolayers were voltage-clamped at 0 mV, the basolateral membrane was permeabilized using nystatin (360 μg ml−1), and an apical → basolateral gradient of 25 mm HCO3− was imposed. Adding forskolin to control monolayers under these conditions caused a rapid (i.e. negative) Isc, which was blocked by the inhibitor CFTRinh-172 (10 μmol l−1; Ma et al. 2002), suggesting ion diffusion through CFTR channels (Gray et al. 1990; Poulsen et al. 1994; Linsdell et al. 1997; Illek et al. 1997) (Fig. 5A).
Figure 5. CFTR-dependent HCO3− conductance at the apical membrane of permeabilized control and CFTR knock-down (CFTR-KD) Calu-3 monolayers.
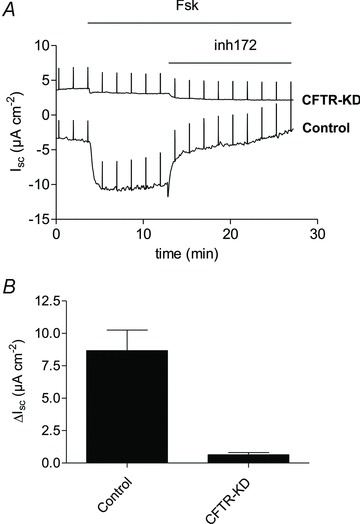
The basolateral membrane was permeabilized using nystatin and an apical-to-basolateral HCO3− gradient 25 → 0 mmol l−1 was imposed across the monolayer. A, forskolin (Fsk, 10 μmol l−1) stimulated a large inward current of ∼8 μeq cm−2 h−1. Forskolin-stimulated inward current in control cells was abolished by the CFTR inhibitor CFTRinh-172 (inh172). Forskolin and CFTRinh-172 had little effect on CFTR-KD monolayers. B, summary of short-circuit currents measured across control (n= 2) and CFTR-KD (n= 3) cells in the presence of a bicarbonate gradient. Forskolin-stimulated secretion (ΔIsc) was almost abolished in CFTR-KD cells. Means ± SEM, P < 0.01.
Forskolin did not stimulate Isc across CFTR knock-down cells under these conditions (Fig. 5A). Figure 5B shows the mean Isc measured across basolaterally permeabilized monolayers. Although these results do not exclude other apical exit mechanisms, they do show that forskolin-stimulated Isc is CFTRInh-172-sensitive and reduced by 88% in CFTR knock-down cells, consistent with the decrease in CFTR protein expression. The simplest interpretation is that CFTR channels can conduct HCO3− and mediate electrogenic HCO3− secretion at the apical membrane under these conditions.
Electrogenic anion transport (Ieq) requires Na+ and CO2/HCO3−
To examine the ionic requirements of HCO3− transport under open-circuit conditions, Ieq and the appearance of HCO3− on the apical side were measured after bilateral removal of CO2/HCO3−, Na+ or Cl− (Fig. 6A–D). The basal and forskolin-stimulated Ieq were both abolished in nominally CO2/HCO3−-free solution (Fig. 6B).
Figure 6. Effects of ion substitutions on anion and fluid transport.
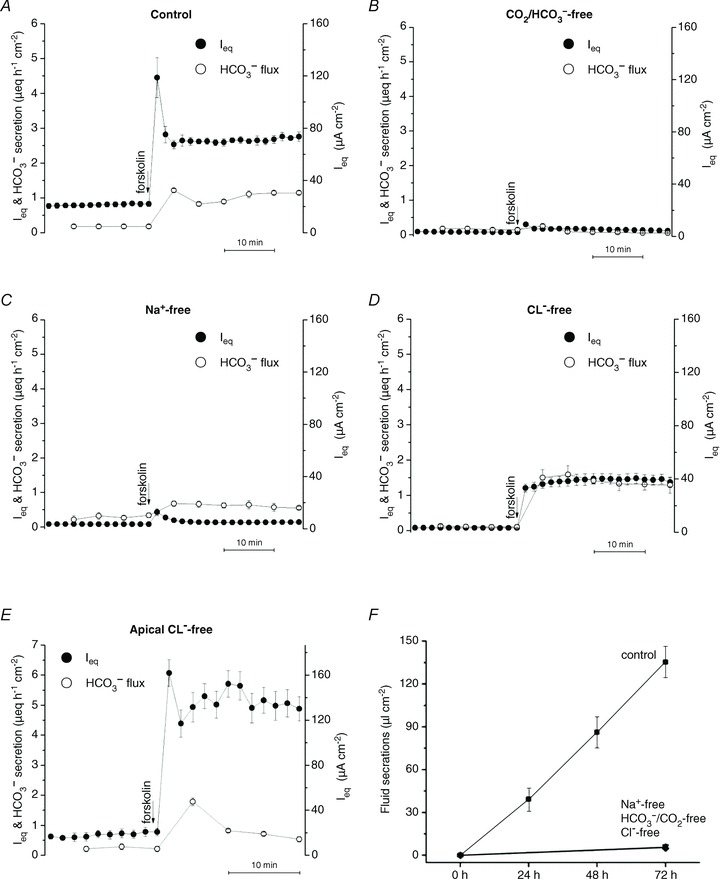
A, the response of Calu-3 ‘Alter’ shRNA control cells to 10 μmol l−1 forskolin in control solutions (n= 3). B–D, effect of 10 μmol l−1 forskolin on Calu-3 cells bathed bilaterally with solutions lacking HCO3−/CO2, Na+ or Cl−, respectively (n= 6 for each). Forskolin-stimulated HCO3− secretion was abolished in the nominal absence of HCO3− or Na+, but was increased ∼25% in Cl−-free conditions. E, monolayers were bathed with apical Cl−-free solution, then stimulated with 10 μmol l−1 forskolin. Panels A–E: •, Ieq; º, HCO3− secretion. F, forskolin-stimulated fluid transport was abolished in the nominal absence of Na+, HCO3−/CO2 or Cl−. ▪, normal medium; ▴, Na+-free medium; ▾, Cl−-free medium; ♦, HCO3−/CO2-free medium (n= 6 each condition; means ± SEM).
This dependence of Ieq on CO2/HCO3− is consistent with Na+–nHCO3− (NBC)-mediated cotransport at the basolateral membrane (Devor et al. 1999), but also with H2CO3 synthesis and subsequent Na+/H+ exchange (Cuthbert et al. 2003). Forskolin had little effect on Ieq under nominally Na+-free conditions but did induce electrically silent HCO3− secretion of >0.5 μeq cm−2 h−1 in the absence of sodium (Fig. 6C). Importantly, transepithelial HCO3− transport during forskolin stimulation was 25% higher under bilateral Cl−-free conditions than when Cl− was present, despite a 50% reduction in Ieq (from 2.76 ± 0.13 to 1.35 ± 0.32 μeq cm−2 h−1; compare mean values indicated by open circles in Fig. 6A and E). This argues strongly against Cl−/HCO3− exchange as the mechanism of apical HCO3− exit under these conditions. Ieq was identical to net HCO3− secretion under symmetrical Cl−-free conditions (1.32 ± 0.31 vs. 1.32 ± 0.19 μeq cm−2 h−1, respectively), providing further evidence that HCO3−-independent Ieq measured in control solutions is carried by Cl−. The large transient Ieq normally observed immediately after forskolin addition (e.g. Fig. 6A) was eliminated under bilateral Cl−-free conditions and therefore may reflect Cl− and K+ redistribution when CFTR channels are activated. When Cl− was removed only from the apical side to generate a favourable Cl− gradient, forskolin caused more robust stimulation of Ieq and a larger discrepancy between Ieq and HCO3− flux consistent with an increased Cl− flux (Fig. 6E).
Cl− is the predominant anion in Calu-3 secretions
If net HCO3− secretion accounts for Isc and is mediated by the sodium–bicarbonate cotransporter NBCe1 and CFTR, why does fluid secretion depend on Cl−? To investigate this, the pH of apical fluid on control (alter) and CFTR knock-down monolayers was measured. Surprisingly, the pH of the fluid was in the range 7.2–7.6 when equilibrated with 5% CO2 (Table 2).
Table 2.
Composition of fluid secreted by Calu-3 monolayers
| Control transfected | CFTR knock-down | Initial medium | |
|---|---|---|---|
| Measured [Cl−] (mmol l−1) | 120 ± 5 | 123 ± 6 | 135 ± 16 |
| Measured pH | 7.55 ± 0.04 | 7.28 ± 0.02 | 7.43 ± 0.03 |
| Calculated [HCO3−] (mmol l−1) | 31.35 ± 2.1 | 16.08 ± 0.52*** | 24.4 ± 2.2 |
| Osmotic pressure (mosmol l−1) | 309 ± 16 | 301 ± 6 | 310 ± 16 |
Fluid was collected daily during forskolin (10 μmol l−1) stimulation and pooled for analysis. Values are mean ± SEM, n= 3–12 each condition.
P < 0.001 comparing CFTR knock-down vs. control transfected.
The Cl− concentration in fluid secreted by control shRNA transfected, CFTR knock-down and parental Calu-3 cell lines was ∼ 120 mmol l−1. Thus, Cl− was the predominant anion and was 2- to 4-fold higher than the [HCO3−] calculated using the Henderson–Hasselbalch equation. [HCO3−] was similar in secretions collected after several hours or several days of forskolin stimulation (data not shown), and the osmotic pressure of secretions at the end of collection periods was equal to that of the basolateral medium within measurement error (Table 2). Somewhat higher pH (7.7) and HCO3− levels (50 mmol l−1) were obtained using parental Calu-3 cells (Table 2), presumably due to their higher CFTR expression; nevertheless, even they produced Cl−-rich secretions, suggesting most fluid is osmotically driven by transepithelial Cl− rather than by HCO3− secretion despite the close correspondence between Isc and HCO3− secretion (Fig. 1), and the absence of detectable 36Cl− net flux under Isc conditions (Devor et al. 1999).
The net Cl− flux needed to account for fluid transport at the observed rate of fluid secretion and [Cl−] of the secretions shown in Table 3 was estimated to be small; nevertheless, we attempted to measure it in Ussing chambers under open-circuit conditions.
Table 3.
Unidirectional 36Cl fluxes across Calu-3 cells under open-circuit conditions
| Jsm | Jms | Jnet | Ieq | RT | VT | |
|---|---|---|---|---|---|---|
| Control | 0.73 ± 0.22 | 0.60 ± 0.19 | 0.13 ± 0.23 | 1.05 ± 0.16 | 344 ± 29 | −9.77 ± 0.35 |
| Forskolin (10 μmol l−1) | 1.62 ± 0.21*** | 1.57 ± 0.25*** | 0.05 ± 0.14 | 2.85 ± 0.19*** | 276 ± 20*** | −21.09 ± 0.70*** |
Experiments were performed in Ussing chambers with symmetrical Cl− and HCO3− concentrations. Forskolin was added to both sides. Jsm, Jms, Jnet, Ieq: μeq cm−2 h−1; Rt: ohms cm2 h−1 (see Table 1). Values are mean ± SEM, n= 6.
P < 0.001, forskolin vs. control.
It was not possible to detect a significant difference between the large unidirectional 36Cl− fluxes (Table 3). Since forskolin caused a modest elevation of apical [HCO3−] (see Table 2, control transfected), unidirectional 36Cl− fluxes were also measured when apical [HCO3−] was increased to 30 and 50 mmol l−1 (Table 4). Net Cl− flux was observed with asymmetrical [HCO3−], although it only reached statistical significance in the experiments with 30 mmol l−1 HCO3−. A more striking effect of exposing forskolin-stimulated monolayers to apical 50 mmol l−1[HCO3−] was a ∼2-fold increase in both unidirectional Cl− fluxes (P < 0.01).
Table 4.
Effect of apical HCO3− on unidirectional 36Cl− fluxes across Calu-3 cells under open-circuit conditions
| Jsm | Jms | Jnet | Ieq | RT | VT | |
|---|---|---|---|---|---|---|
| Control 25 HCO3− | 0.85 ± 0.10 | 0.77 ± 0.16 | 0.08 ± 0.10 | 0.78 ± 0.11 | 364 ± 23 | −7.61 ± 0.61 |
| Forskolin 25 HCO3− | 1.75 ± 0.18*** | 1.60 ± 0.18*** | 0.15 ± 0.17 | 2.70 ± 0.14*** | 266 ± 14*** | −19.26 ± 0.63*** |
| Forskolin 30 HCO3− | 1.53 ± 0.12++ | 1.27 ± 0.10+++ | 0.25 ± 0.11++ | 2.71 ± 0.12 | 262 ± 16 | −19.03 ± 0.74 |
| Forskolin 50 HCO3− | 2.77 ± 0.15††† | 2.50 ± 0.10††† | 0.27 ± 0.13 | 2.75 ± 0.14 | 254 ± 20 | −18.73 ± 0.56 |
Monolayers were bathed with symmetrical 25 m HCO3−, then with 30 or 50 mmol l−1 HCO3− on the apical side; 10 μmol l−1 forskolin was added to both sides. Jsm, Jms, Jnet, Ieq: μeq cm−2 h−1; Rt: ohms cm2 h−1. Values are mean ± SEM. ***P < 0.001, forskolin (25 HCO3−) vs. control. ++, +++, P < 0.01, P < 0.001, forskolin (30 HCO3−) vs. forskolin (25 HCO3−). †††P < 0.001, forskolin (50 HCO3−) vs. forskolin (30 HCO3−), control and forskolin (25 HCO3−) (n= 8 each) and forskolin (30 HCO3−) and forskolin (50 HCO3−) (n= 4 each).
HCO3−, Na+ and Cl− are all required for forskolin-stimulated fluid secretion
The low [HCO3−] and high [Cl−] of Calu-3 secretions prompted us to study the ionic requirements for fluid secretion and Ieq. Fluid transport was reduced from 45.1 ± 7.9 μl cm−2 day−1 to 0.8 ± 0.4 μl cm−2 day−1 when monolayers were bathed with nominally HCO3−-free/low 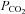 medium (Fig. 6F), and a similar decrease to 1.0 ± 0.8 μl cm−2 day−1 was observed in nominally Na+-free medium. These effects of removing HCO3− and Na+ were anticipated since Ieq also depends on these ions (e.g. Fig. 6B and C). More surprising was the effect of replacing Cl− with gluconate, which nearly abolished fluid secretion (from 45.1 ± 7.9 μl cm−2 day−1 to 2.0 ± 0.7 μl cm−2 day−1; Fig. 6F). This contrasts with the effect on Ieq, 60% of which persisted in nominally Cl−-free solution (Fig. 6D). Thus, forskolin-stimulated HCO3− secretion depends on the simultaneous presence of Na+ and HCO3− but not Cl−, whereas fluid secretion requires all three ions (Fig. 6F).
medium (Fig. 6F), and a similar decrease to 1.0 ± 0.8 μl cm−2 day−1 was observed in nominally Na+-free medium. These effects of removing HCO3− and Na+ were anticipated since Ieq also depends on these ions (e.g. Fig. 6B and C). More surprising was the effect of replacing Cl− with gluconate, which nearly abolished fluid secretion (from 45.1 ± 7.9 μl cm−2 day−1 to 2.0 ± 0.7 μl cm−2 day−1; Fig. 6F). This contrasts with the effect on Ieq, 60% of which persisted in nominally Cl−-free solution (Fig. 6D). Thus, forskolin-stimulated HCO3− secretion depends on the simultaneous presence of Na+ and HCO3− but not Cl−, whereas fluid secretion requires all three ions (Fig. 6F).
Ion substitution effects and the high [Cl−] of secretions suggested that most fluid is osmotically driven by transepithelial Cl− rather than HCO3− transport. Inhibitor studies in the next section examine the role of NKCC1 cotransport and basolateral anion exchange in basolateral Cl− loading.
Some basolateral Cl− entry is independent of NKCC
Previous studies of NKCC1's contribution to anion secretion by Calu-3 cells have yielded varying results, perhaps due to different experimental conditions. In the present work, the effect of bumetanide on Isc was measured and compared with its effects on Ieq, net HCO3− flux, tracer fluxes, and fluid transport under open-circuit conditions. Basolateral bumetanide (20–50 μmol l−1) did not affect Isc during stimulation by forskolin (data not shown), in good agreement with a previous study (Devor et al. 1999). However, under open-circuit conditions bumetanide (20 μmol l−1) inhibited ∼20% of the basal (Fig. 7A) and forskolin-stimulated (Fig. 7B) Ieq, which monitors both Cl− and HCO3− transport. Bumetanide inhibition of the Ieq component carried by Cl− was ∼35% in control-transfected Calu-3 cells (Fig. 7A and B), similar to the results with parental cells (Huang et al. 2011) and partial inhibition of Isc noted previously during stimulation by the hyperpolarizing secretagogues 1-EBIO and thapsigargin (Lee et al. 1998; Krouse et al. 2004).
Figure 7. Effects of bumetanide on forskolin-stimulated Ieq and fluid secretion.
A, basolateral pretreatment with the NKCC1 inhibitor bumetanide (20 μmol l−1) partially inhibited basal Ieq (∼20%) but did not prevent forskolin-stimulated Ieq or HCO3− secretion (n= 6). B, basolateral bumetanide (20 μmol l−1) inhibited ∼20% of the Ieq (•) without affecting HCO3− secretion (º) (n= 6). C, forskolin-stimulated fluid secretion was inhibited ∼15% by bumetanide (▴) when compared with forskolin alone (▪) (n= 6 each condition; means ± SEM).
Net 36Cl flux under these conditions confirmed the bumetanide-sensitive component of Ieq was mediated by Cl− transport (see Table 1) and confirmed that a fraction of the transepithelial Cl− transport is mediated by NKCC1 when the basolateral membrane is hyperpolarized, either by current flowing through paracellular and transcellular shunt pathways under open-circuit conditions (loop current), or by activation of Ca2+-activated potassium channels under short-circuit conditions. Bumetanide (20 μmol l−1) reduced fluid secretion from 33.5 ± 6.98 to 26.6 ± 1.39 μl cm−2 day−1; however, the other 70–80% of the fluid secretion was insensitive to this inhibitor despite the high [Cl−] of the secreted fluid (Fig. 7C). Inhibition of fluid transport was not further increased by raising bumetanide concentration to 50 μmol l−1 (data not shown). Partial inhibition of fluid transport by bumetanide paralleled its effect on Cl−-dependent Ieq in Ussing chambers, reinforcing the conclusion that substantial Cl− and fluid transport is independent of NKCC1.
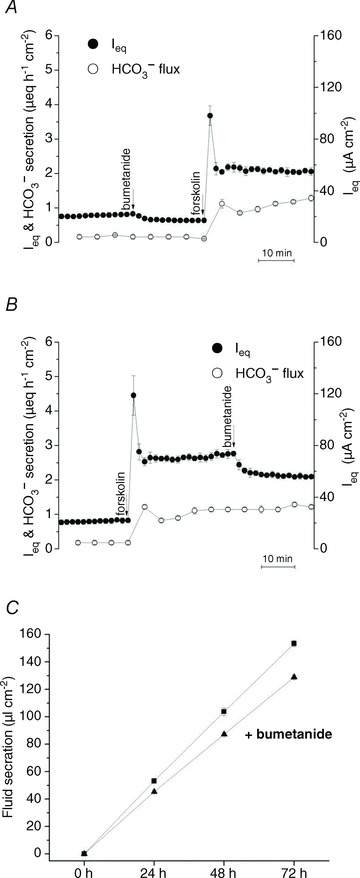
Evidence for basolateral Cl− loading by anion exchange
To examine if HCO3− efflux through basolateral anion exchangers could serve as an alternative Cl− entry mechanism during anion and fluid secretion, experiments were designed to monitor the ‘trans’ effect of anions on the flow of HCO3− through the basolateral membrane (Fig. 8). The apical membrane was permeabilized using nystatin (100 μg ml−1) while monolayers were bathed with symmetrical HCO3−-free solution. After the current reached a plateau, 25 mmol l−1 NaHCO3 was added on the apical side to generate an apical → basolateral HCO3− gradient and pH-stat was used to monitor the appearance of HCO3− on the basolateral side.
Figure 8. Basolateral anion exchange mediates forskolin-stimulated anion and fluid secretion by Calu-3 cells.
A and B, monolayers were incubated in HCO3−/CO2-free or Cl− and HCO3−/CO2-free solutions, respectively, and gassed with 100% O2. The apical membrane was permeabilized using nystatin (100 μg ml−1), and HCO3− was added to the apical side, which was switched to bubbling with 95% O2–5% CO2. HCO3− flux to the basolateral side was increased in the presence of basolateral Cl− (A, n= 4), but not when Cl− was replaced with gluconate (B, n= 4). C, monolayers were incubated in HCO3−/CO2-free solutions, in which Cl− was replaced with NO3− (n= 4). The apical membrane was permeabilized and HCO3− re-introduced as described in A and B. Basolateral HCO3− flux was observed under these conditions, indicating HCO3−/NO3− exchange. •, Ieq; º, HCO3− flux. D, fluid secretion was observed during the first 24 h after Cl− was replaced with NO3− but was nearly abolished after gluconate replacement. ▪, normal medium; ▴, low-Cl− gluconate medium; ▾, low-Cl− nitrate medium (n= 4 each condition; means ± SEM).
As shown in Fig. 8A, apical nystatin induced a large current that was apparently due to electrogenic Na+ absorption since it was inhibited by basolateral ouabain in control experiments (data not shown) as has been reported for other epithelia (Lewis et al. 1978).
After Na+ current had stabilized, imposing a transepithelial HCO3− gradient with Cl− solution bathing the basolateral (trans) side caused Ieq to decrease and HCO3− to appear in the basolateral compartment (Fig. 8A). When the same experiment was performed with gluconate solution on the basolateral (trans) side, no HCO3− flux to the basolateral side was detected by pH-stat (Fig. 8B). However, robust HCO3− flux was produced with basolateral (trans) NO3− solution (Fig. 8C). No osmotically induced changes in Ieq or HCO3− flux were observed in control experiments when 50 mmol l−1 mannitol was added instead of 25 mmol l−1 NaHCO3 (Supplementary Fig. S1). These results indicate HCO3− flow through the basolateral membrane depends on the nature of the trans-anion and are consistent with the activity of basolateral anion exchangers that carry HCO3−, Cl− or NO3− but not gluconate ions.
Since NO3− substituted for Cl− during basolateral anion exchange, we wondered if it could also support fluid transport. Forskolin stimulated robust fluid secretion when monolayers were bathed with nominally Cl−-free NO3− medium on the basolateral side (Fig. 8D). The rate of fluid transport from basolateral NO3− solution was >60% of that measured with Cl− solution during the first 24 h stimulation, then gradually declined, presumably because Cl− is needed for other cellular processes to maintain viability. Nevertheless, NO3− did sustain fluid secretion for many hours, evidence that its basolasteral entry is supported by HCO3− efflux in permeabilized monolayers. HCO3− recycling through basolateral anion exchangers could load Calu-3 cells with either Cl− or NO3−, and both these anions are permeant through apical CFTR channels (Linsdell et al. 1997).
Electrogenic HCO3− secretion requires carbonic anhydrase
To study the role of HCO3− synthesis in anion and fluid secretion, the effects of acetazolamide (100 μmol l−1) on Ieq and HCO3− transport were assayed in Ussing chambers under pH-stat conditions, and on fluid transport in parallel secretion assays. Acetazolamide abolished forskolin-stimulated HCO3− secretion and Ieq (Fig. 9A), suggesting a large fraction of the secreted HCO3− arises from the intracellular hydration of CO2, as suggested previously (Krouse et al. 2004).
Figure 9. Effect of acetazolamide on anion and fluid secretion by Calu-3 cells.
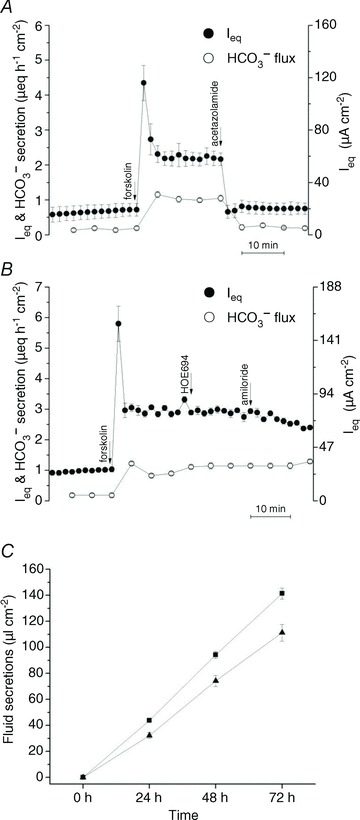
A, the carbonic anhydrase inhibitor acetazolamide (100 μmol l−1) abolished forskolin-stimulated secretion (n= 6). B, sequential addition of the NHE inhibitors HOE694 (10 μmol l−1) and amiloride (1 mmol l−1) to the basolateral side had no effect on Ieq or HCO3− secretion (n= 3). C, acetazolamide caused a small reduction in forskolin-stimulated fluid secretion by Calu-3 cells. •, Ieq; º, HCO3− secretion. ▪, without acetazolamide; ▴, with acetazolamide (n= 6). Means ± SEM.
Formation of the weak acid H2CO3 and efflux of HCO3− might be expected to stimulate Na+/H+ exchangers (NHEs) and other pHi regulatory mechanisms. However, the NHE inhibitor amiloride (1 mmol l−1; IC50= 24 μmol l−1) and the NHE1 isoform-specific inhibitor HOE-694 (20 μmol l−1; IC50= 0.16 μmol l−1; Counillon et al. 1993) did not affect HCO3− transport or Ieq (Fig. 9B). This suggests pHi regulation is dependent on the nature of the secretagogue and is mediated by basolateral HCO3− influx via NBCe1 and apical H+ extrusion during forskolin stimulation. Acetazolamide abolished forskolin-stimulated Ieq (Fig. 9A) but only inhibited fluid secretion by 27% (Fig. 9C).
Origin of the basal Ieq and HCO3− secretion
Calu-3 monolayers have basal currents of 0.5–1.0 μeq cm−2 h−1; however, the nature of this constitutive transport remains uncertain. Since Calu-3 cells express purinergic (Communi et al. 1999) and adenosine receptors (Cobb et al. 2002) and respond to prostaglandins (Palmer et al. 2006b), we examined whether these autocrine signals might be responsible for the basal current. Ieq and HCO3− transport were unaffected by adding apyrase (10 units ml−1) to metabolize ATP released from the cells, 8-SPT (100 μmol l−1) to prevent activation of A2B adenosine receptors, or indomethicin (100 μmol l−1) to inhibit prostaglandin synthesis (Fig. 10A–C).
Figure 10. Effects of autocrine signalling inhibitors on Ieq and HCO3− secretion.
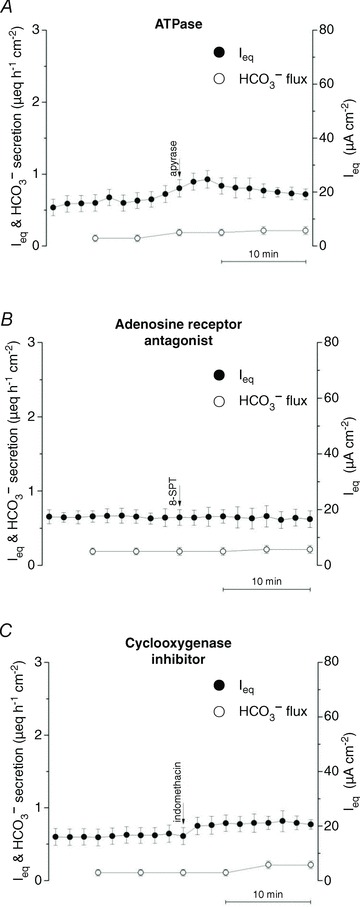
Adding A, the ATPase apyrase (10 units ml−1) (n= 3) or B, the adenosine receptor antagonist 8-SPT (100 μmol l−1) (n= 3) apically to block purinergic signalling had no effect on basal Ieq or HCO3− secretion. C, the cyclo-oxygenase inhibitor indomethacin (100 μmol l−1) did not inhibit Ieq or HCO3− secretion when added bilaterally (n= 3). •, Ieq; º, HCO3− secretion (n= 3 for each condition; means ± SEM).
We then examined the role of CFTR and whether there is tonic adenylyl cyclase activity. GlyH-101 (100 μmol l−1), which blocks the CFTR channel with an IC50≍ 5 μmol l−1 at −60 mV (Muanprasat et al. 2004), strongly inhibited both basal Ieq and HCO3− transport (Fig. 11).
Figure 11. Inhibitor effects suggest basal Ieq and HCO3− secretion depend on CFTR and on Ca2+-dependent, membrane-bound adenylyl cyclase activity, but not on HCO3− -stimulated soluble adenylyl cyclase.
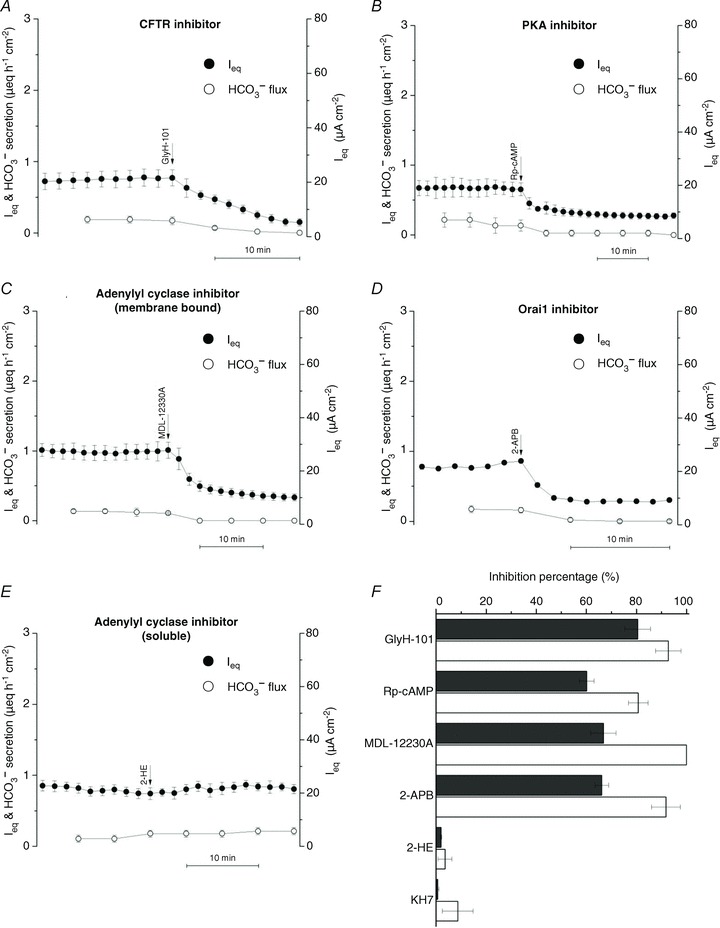
A, basal Ieq was abolished by apical addition of the CFTR open channel blocker GlyH-101 (100 μmol l−1, n= 4). B, basal Ieq and net HCO3− secretion were inhibited by bilateral addition of 200 μmol l−1 Rp-cAMPS, a competitive inhibitor of cAMP-dependent PKA (n= 4). C, basal Ieq and HCO3− secretion were reduced by basolateral MDL-12330A (200 μmol l−1), an inhibitor of membrane-bound adenylyl cyclase (n= 4). D, inhibition of basal Ieq and HCO3− secretion by apical 2-APB (100 μmol l−1), an inhibitor of store-operated Ca2+ channels (n= 3). E, an inhibitor of HCO3−-stimulated soluble isoform of adenylyl cyclase, 2-HE (20 μmol l−1), had no effect on Ieq or HCO3− secretion (n= 3 each) when added bilaterally. F, summary of inhibitor effects on basal anion transport. ▪, Ieq; □, HCO3− secretion.
These results suggest CFTR mediates basal secretion. As a further test for the involvement of CFTR, the dependence of basal current on PKA was investigated. Adding 100 μmol l−1 Rp-cAMPS (Rp-adenosine-3′,5′-cyclic mono-phosphorothioate triethylamine salt; IC50 for inhibition of PKA = 4.9 μmol l−1), a membrane-permeant competitive inhibitor of PKA, reduced basal Ieq and HCO3− secretion by 63% and 88%, respectively (Fig. 11B), evidence that much of the basal secretion is mediated by PKA in resting cells and can be rapidly down-regulated by a phosphatase when PKA is inhibited.
The sensitivity of basal current to PKA inhibitors implies constitutive elevation of cAMP, which may be localized near the apical membrane. There are nine conventional membrane-bound adenylyl cyclases, and one soluble isoform is stimulated by HCO3− and proposed to function as a HCO3− sensor in various cells (Chen et al. 2000), including Calu-3 (Wang et al. 2005). Inhibitors were used to examine the role of these adenylyl cyclases. An antagonist of conventional membrane-bound adenylyl cyclases, MDL-12330A (cis-N-(2-phenylcyclopentyl) azacyclotridec-1-en-2-amine; Guellaen et al. 1977), reduced Ieq and HCO3− secretion by 68% and 100%, respectively (200 μmol l−1; IC50 <10 μmol l−1; Fig. 11C). Since Ca2+-activated adenylyl cyclase might stimulate CFTR, 2-APB (100 μmol l−1), a non-specific inhibitor of store-operated Ca2+ entry, was also tested (Fig. 11D). Remarkably, 2-APB was a potent inhibitor of basal anion secretion, reducing Ieq and HCO3− secretion 65–90%. This suggests the membrane-bound adenylyl cyclase is indeed activated by elevated Ca2+ near the membrane. By contrast, bilateral addition of 2-hydroxyestradiol (2-HE; 20–50 μmol l−1), an inhibitor of soluble adenylyl cyclase (Fig. 11E), had no effect on basal Ieq or HCO3− transport. The same negative result was obtained using a chemically unrelated inhibitor KH7 (50 μmol l−1, IC50= 3–10 μmol l−1 in vivo; data not shown). The effects of these inhibitors on Ieq and HCO3− transport are summarized in Fig. 11F. Taken together they suggest the basal current in Calu-3 cells is mediated by CFTR channels which are partially activated by PKA. This tonic activity may be due to local production of cAMP by a membrane-bound adenylyl cyclase in response to store-operated Ca2+ entry.
Discussion
Electrogenic HCO3− and Cl− secretion by airway epithelia
Electrogenic HCO3− secretion by airway epithelium was initially hypothesized because some of the Isc across dog trachea could not be explained by Cl− and Na+ net fluxes measured using radiotracers (Al-Bazzaz & Al-Awqati, 1979). The unidentified component of the Isc was increased by the non-specific phosphodiesterase inhibitor aminophylline and therefore stimulated by cAMP (Al-Bazzaz & Jayaram, 1981). HCO3− secretion was later confirmed in human airway cell cultures and shown to be defective in cystic fibrosis (Smith et al. 1994). Mechanistic studies of HCO3− transport have been complicated by its dependence on other ions, the lack of a useful radiotracer, and the unconserved nature of HCO3− itself, which may be synthesized and/or degraded during secretion.
Bumetanide (100 μm) had little effect on fluid secretion by submucosal glands in cat trachea (Corrales et al. 1984), which suggests the glands use mechanisms other than NKCC1 unlike airway surface epithelial cells, which are mainly bumetanide-sensitive. Cl− secretion by freshly isolated equine trachea declined ∼70% when CO2 and HCO3− were removed under Isc conditions, suggesting basolateral HCO3−/Cl− exchange (Tessier et al. 1990). Further evidence for basolateral anion exchange (and apical bicarbonate conductance) was obtained in human nasal primary cells by measuring intracellular pH during extracellular Cl− replacement (Paradiso et al. 2003). In summary, HCO3− secretion is stimulated by cAMP and defective in CF airway epithelia, and much of the electrogenic anion secretion is insensitive to bumetanide, although the precise role of HCO3− in Cl− and fluid secretion by airway epithelia is not well understood.
The normal pH of ASL and gland secretions is in the range 6.2–7.2 (Fischer & Widdicombe, 2006). Weakly buffered solutions placed on cultures of surface epithelium become acidic, and pH falls more rapidly on CF than non-CF monolayers, consistent with a defect in parallel HCO3− secretion (Coakley et al. 2003). The buffer capacity of gland fluid collected in the presence vs. absence of HCO3− (14 vs. 3.7 mmol l−1 at pH 7, respectively) suggests that these secretions normally contain about 10 mmol l−1 HCO3− (Song et al. 2006). Secretions from pig glands are 0.2–0.4 units more acidic when secreted in the presence of CFTR inhibitors, mimicking the low pH seen with CF glands (Song et al. 2006). A reduction in the HCO3− concentration and pH of ASL in CF may inhibit mucociliary clearance by altering the release or microrheology of mucus (Quinton, 2010), reducing ciliary beat frequency (Schmid et al. 2010) or suppressing innate immunity (Fischer, 2011).
The human airway cell line Calu-3 was used in the present study because it forms polarized monolayers, displays cAMP-stimulated bicarbonate and fluid secretion, expresses the serous cell markers, and responds to gland secretagogues such as vasoactive intestinal peptide and epinephrine (reviewed by Shan et al. 2011). The present results confirm that anion and fluid secretion by Calu-3 monolayers are less sensitive to bumetanide than surface epithelial cell primary cultures and resemble cat tracheal glands in this regard (Corrales et al. 1984). Although a systematic comparison of the secretion by Calu-3 and human submucosal gland cells remains to be carried out, this result implies that Calu-3 is a useful model for cAMP-stimulated secretion by submucosal gland serous cells although it lacks apical Ca2+-activated Cl− conductance (Moon et al. 1997).
Results consistent with previous studies
Some results in this study can be explained by the current model for Calu-3 cells, which is based on tracer flux and inhibitor studies under Isc conditions (Devor et al. 1999), pH-stat experiments (Krouse et al. 2004) and fluid secretion measured using a virtual gland technique (Irokawa et al. 2004). According to that scheme, Isc is mediated by electrogenic HCO3− transport and results from Na+-coupled HCO3− basolateral entry and apical exit through CFTR channels (Kreindler et al. 2006). This section relates the present results obtained under mostly open-circuit, pH-stat conditions with those earlier studies of Isc.
Isc is mediated by electrogenic HCO3− transport
The rate of HCO3− secretion measured in the present study by automated pH-stat was equal to the simultaneously measured Isc, and both were stimulated by forskolin and abolished by the CFTR inhibitor Inh-172. Close agreement between Isc and HCO3− flux is consistent with previous studies of unstimulated (Lee et al. 1998) and forskolin-stimulated Calu-3 monolayers (Devor et al. 1999). The 36Cl− net fluxes measured under open-circuit conditions in Table 1 suggest that membrane hyperpolarization leads to a discrepancy between Isc and HCO3− net flux, as suggested previously during stimulation with 1-EBIO (Devor et al. 1999). Although forskolin also induced some Cl− secretion in the present study, this is expected since current flowing through shunt pathways would hyperpolarize the basolateral membrane under open-circuit conditions in concert with apical depolarization (Tamada et al. 2001). Thus, the present results extend earlier findings to open-circuit conditions that would exist during fluid transport.
The present results with H+ transport inhibitors suggest HCO3− secretion is actually ∼30% higher than the net flux measured by pH-stat. Proton secretion by H+/K+-ATPase has been demonstrated previously during 1-EBIO stimulation (Krouse et al. 2004) but was not observed in the present work. The different results obtained using forskolin vs. 1-EBIO may be due to their opposite effects on the membrane potential. Forskolin depolarizes the apical membrane (Tamada et al. 2001) and should favour electrogenic H+ efflux pathways whereas 1-EBIO would cause membrane hyperpolarization and favour electroneutral acid secretion. Electrogenic H+ secretion would be inconspicuous in the present study because it would cause identical reductions in current and HCO3− net flux. H+/K+-ATPase activity during 1-EBIO stimulation would consume HCO3− without affecting the current and therefore would increase the unidentified component of the Isc, as was demonstrated previously (Krouse et al. 2004).
Ieq depends on Na+ and CO2/HCO3−
Basal and forskolin-stimulated Ieq were abolished in Na+ or HCO3−-free solution, consistent with the ionic dependence of Isc reported previously (Devor et al. 1999). However, we noticed that ∼50% of the HCO3− net flux measured under pH-stat conditions persisted despite the nominal absence of Na+, suggesting an additional source of HCO3− besides NBCe1 (Krouse et al. 2004). This flux may reflect carbonic anhydrase-catalysed synthesis and apical efflux since forskolin-stimulated HCO3− secretion was abolished by acetazolamide (Fig. 9A), although we cannot exclude basolateral HCO3− loading through anion exchangers, which may operate in reversed mode due to the favourable transepithelial HCO3− gradient under pH-stat conditions.
Forskolin-stimulated HCO3− secretion requires carbonic anhydrase
Acetazolamide abolished forskolin-stimulated Isc and HCO3− secretion, as observed previously during stimulation by other secretagogues (Krouse et al. 2004). The simplest explanation for this inhibition is that secreted HCO3− must be synthesized in the epithelial cells by carbonic anhydrase to sustain electrogenic secretion, even when NBCe1 is available to mediate basolateral HCO3− loading. Acetazolamide inhibition was surprisingly rapid, perhaps because carbonic anhydrase activity and the supply of intracellular bicarbonate are limiting when intracellular pCO2 and [HCO3−] are low due to HCO3−/CO2-free solution on the apical side. It is interesting to consider the role of carbonic anhydrase activity in intracellular pH regulation. Forskolin-stimulated HCO3− efflux effectively converts the weak acid H2CO3 to a strong acid H+, and cells must neutralize this acid load to sustain a high rate of HCO3− secretion during steady-state forskolin stimulation. Basolateral HCO3− entry via NBCe1 probably mediates most of this pHi regulation, as occurs during recovery from ammonium-induced acid loads (Inglis et al. 2002). The use of basolateral HCO3− entry to neutralize intracellular H+ may generate CO2 that can be reused for carbonic anhydrase-catalysed HCO3− synthesis, and this may be important when intracellular CO2 and HCO3− are low and rate limiting. Future studies should examine whether the sensitivity to acetazolamide is a universal feature of HCO3− secretion or a consequence of using pH-stat conditions.
Further evidence that CFTR mediates apical membrane HCO3− and Cl− conductance
When the basolateral membrane was permeabilized using nystatin and an apical-to-basolateral HCO3− gradient was imposed, subsequent addition of forskolin produced a (reversed) Isc across control monolayers that was sensitive to the inhibitor CFTRinh-172. Although GlyH-101 has also been shown to inhibit the Cl− channel/exchanger Slc26a9 (Bertrand et al. 2009), the anion exchangers Slc26a3, -a6 and -a11 (Stewart et al. 2011), and mitochondrial function (Kelly et al. 2010), similar results were obtained using CFTR knock-down cells. Together these results demonstrate the HCO3− conductance of CFTR under these conditions, consistent with previous studies of Calu-3 monolayers (Illek et al. 1997; Tamada et al. 2001; Krouse et al. 2004) and with the single channel 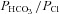 permeability ratio, which ranges between 0.13 and 0.26 (e.g. 0.13, Gray et al. 1990; 0.26, Poulsen et al. 1994; 0.16, Hanrahan et al. 1994; and 0.25, Linsdell et al. 1997). Various factors could influence permeability of the CFTR pore to Cl− and HCO3−. The pore is partially blocked by intracellular anions and permeability is enhanced when extracellular Cl− is replaced with [HCO3−] (Li et al. 2011).
permeability ratio, which ranges between 0.13 and 0.26 (e.g. 0.13, Gray et al. 1990; 0.26, Poulsen et al. 1994; 0.16, Hanrahan et al. 1994; and 0.25, Linsdell et al. 1997). Various factors could influence permeability of the CFTR pore to Cl− and HCO3−. The pore is partially blocked by intracellular anions and permeability is enhanced when extracellular Cl− is replaced with [HCO3−] (Li et al. 2011). 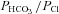 may also be modulated through the WNK1-OSR1/SPAK kinase pathway during stimulated secretion (Park et al. 2010).
may also be modulated through the WNK1-OSR1/SPAK kinase pathway during stimulated secretion (Park et al. 2010).
Forskolin-stimulated Isc was less affected by shRNA than CFTR protein expression, consistent with studies using a different CFTR-deficient Calu-3 cell line (MacVinish et al. 2007). Indeed, basal Ieq and HCO3− flux were similar in control and CFTR-deficient monolayers even though pharmacological studies indicate basal anion secretion is mediated by CFTR. This result would be explained if some of the band C protein that had been knocked down was not on the cell surface, or if the decline in conductance caused by silencing CFTR was partly offset by an increase in driving force.
Modifications to existing models
This section considers how existing models for Calu-3 transport might be revised to accommodate the results from this study, which were obtained under conditions that have not been used previously to study Calu-3 (i.e. open circuit, pH-stat).
1. Fluid and Cl− secretion are HCO3− dependent, but most fluid is driven by the net flux of Cl− (or NO3−)
The [Cl−] of secretions was ∼120 mmol l−1, or about 4-fold higher than the [HCO3−]. This implies that most fluid secretion is driven by transepithelial Cl− transport rather than HCO3− flux during forskolin stimulation despite the fact that active Cl− transport is not detected (Devor et al. 1999), and the Isc can be fully explained by net HCO3− flux (Fig. 1). We note, however, that a very low rate of Cl− transport would be sufficient to sustain fluid secretion at the observed rate and it is difficult to detect a small net flux when it is the difference between two large unidirectional fluxes measured across different monolayers (see section 3 below).
A related finding was that much of the Cl− transport that drives fluid secretion is independent of NKCC1. This conclusion was based on anion selectivity and pharmacological data. Robust fluid secretion was observed for 24 h when the basolateral side was bathed with nominally Cl−-free solution containing NO3−, which does not bind to the highly selective anion site on NKCC transporters and therefore is not transported (Kinne et al. 1986). Further evidence for NKCC1-independent Cl− entry is the relative insensitivity of fluid secretion to bumetanide. These results suggest other basolateral Cl− entry pathways such as anion exchangers are important for fluid secretion. NO3− is carried by many anion exchangers including AE2 (Humphreys et al. 1994).
Although HCO3− is secreted by Calu-3 cells under open-circuit conditions, its concentration in the secreted fluid is low compared with Cl−. If anion secretion drives fluid transport, the higher concentration of Cl− suggests that it provides more of the osmotic driving force for fluid secretion. Some of the HCO3− entering via NBCe1 returns to the basolateral side, apparently by exchanging for Cl− (or NO3−). The anion exchangers that mediate this basolateral Cl− loading were not identified at the molecular level in the present study; however, AE2 is the most likely candidate since it is expressed at the basolateral membrane of Calu-3 cells (Loffing et al. 2000). Recent studies of an AE2-deficient Calu-3 cell line indicate that AE2 mediates essentially all Cl−/HCO3− exchange at the basolateral membrane and plays an important role in fluid secretion; nevertheless, there is another bumetanide-insensitive mechanism for Cl− loading at the basolateral membrane in AE2 cells that remains to be identified (Huang et al. submitted).
Basolateral anion exchange was demonstrated in the present study by permeabilizing the apical membrane, imposing an apical-to-basolateral HCO3− gradient, and examining the effect of anions in the basolateral solution on the HCO3− flux. Nystatin is Na+ permeable, thus apical nystatin permitted apical Na+ entry and produced a large transepithelial current, which was mediated by the basolateral sodium pump since it was ouabain sensitive. This response had variable kinetics due to the use of different batches of nystatin (compare Fig. 8A and B with Fig. 8C). We speculate that this results from variations in the aggregation state of nystatin and rate of incorporation into the apical membrane. The time course of permeabilization did not affect our conclusions regarding anions on the ‘trans’ side, however, because Na+ currents were allowed to stabilize before studying the effects of basolateral (trans) anions on HCO3− flux.
2. Calu-3 secretions are only weakly alkaline
HCO3−-driven fluid transport was expected to generate strongly alkaline secretions having pH > 8; however, the maximal pH achieved during forskolin stimulation in the present experiments was ∼7.6. The calculated [HCO3−] in secretions was slightly higher than in the basolateral medium (33 vs. 25 mmol l−1), and higher apical [HCO3−] was achieved by parental Calu-3 monolayers, which have higher CFTR expression (∼50 mm[HCO3−]; D. Kim, unpublished observations, Fig. 2A); nevertheless, secretions were always less alkaline than expected for fluid driven by HCO3− transport. The values obtained with parental cells were generally consistent with previous studies in a virtual gland preparation (74 mm HCO3− (Irokawa et al. 2004), and cyclophilin B shRNA control Calu-3 cells cultured on transwells (60 mm; Garnett et al. 2011). Silencing CFTR reduced the [HCO3−] of secretions by ∼50% in the present study, whereas CFTR knock-down had no effect on the pH and HCO3− concentration in previous studies (Garnett et al. 2011), perhaps because those knock-down cells had higher residual CFTR expression (28%vs. <5%). The [HCO3−] is reduced in CF gland secretions (Song et al. 2006), albeit from a lower starting value (i.e. 13.8 mmol l−1 vs. 3.5 mmol l−1[HCO3−] in non-CF vs. CF glands, respectively).
In the present study, silencing CFTR expression caused reductions in the volume and total Cl− content by 10-fold and also caused a 2-fold decrease in the [HCO3−] of the secretions. Similar changes in CF airways may contribute to disease symptoms by altering mucus release or rheology, innate immunity or ciliary beating (Shan et al. 2011).
3. A low rate of net Cl− secretion is sufficient to drive fluid secretion
HCO3− secretion accounted for ∼40% of the Ieq under pH-stat conditions. The remainder (1.5 μeq cm−2 h−1) was carried by Cl−, as revealed by measuring 36Cl− fluxes under the same conditions. Nevertheless, Cl− net flux was negligible during previous short-circuit current experiments (Devor et al. 1999) and under open-circuit conditions in the present study (Table 1), making it difficult to understand how Calu-3 monolayers could produce Cl−-rich fluid. However, a net Cl− flux of 0.21 μeq cm−2 h−1 would be sufficient to account for the Cl− content of secretions, and such low rates could not be determined reliably in Ussing chambers. In preliminary tracer experiments performed in Transwells over 24 h, a net Cl− flux of 0.23 μeq cm−2 h−1 was observed; therefore this low rate of Cl− transport could drive fluid secretion (J. Shan, personal observation).
Elevating mucosal HCO3− to 50 mmol l−1 with 25 mmol l−1[HCO3−] on the serosal side increased the forward and back fluxes of 36Cl− (Table 4). Although the mechanism of this increase was not studied in detail, it may be secondary to an increase in intracellular pH. The basolateral anion exchanger AE2 is stimulated 5- to 10-fold by raising pHi from 6.8 to 7.3 (Alper, 2009). Increasing its turnover by raising pHi should increase 36Cl− flux in both directions if the basolateral anion exchange is rate limiting.
Cl−, HCO3− and fluid secretion by Calu-3
The present results suggest a revised model of fluid and electrolyte secretion by Calu-3 cells (Fig. 12). Most fluid secretion is driven by Cl− secretion and 50–70% of the basolateral Cl− loading occurs by anion exchange, which is increased during forskolin-stimulated secretion. This scheme differs from a model in which NKCC mediates basolateral Cl− entry (Devor et al. 1999). We hypothesize that basolateral Cl− loading via basolateral anion exchange increases during forskolin stimulation, in contrast to another recent model for Calu-3 in which forskolin stimulation was proposed to inhibit basolateral anion exchange (Garnett et al. 2011).
Figure 12. Scheme for anion transport by Calu-3 cells under the conditions used in this study.
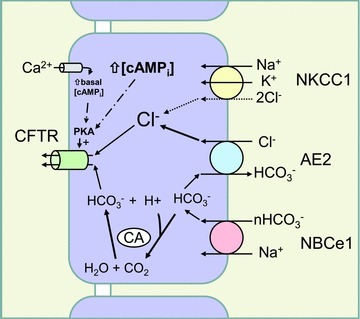
In resting cells, an apical Ca2+ microdomain produced by store-operated Ca2+ entry causes partial activation of CFTR through stimulation of membrane-bound adenylyl cyclase and local elevation of [cAMP]. Secretagogues that further elevate [cAMPi] stimulate apical Cl− and HCO3− efflux, creating an acid load that may further increase CFTR open probability (Chen et al. 2009). Regulation of pHi by HCO3− that enters via NBCe1 provides CO2 for bicarbonate synthesis by carbonic anhydrase, which sustains HCO3− secretion. Most bicarbonate entering via NBCe1 is recycled basolaterally by anion exchange during Cl− loading. Not shown in this scheme are other anion exchangers and Na+/H+ exchangers that are active under other conditions; i.e. Na+/H+ exchange is likely to mediate pHi regulation during stimulation by secretagogues that hyperpolarize the basolateral membrane.
Forskolin stimulates a large transient current which is abolished in Cl−-free solution and therefore probably mediated by apical Cl− and basolateral K+ efflux. While the decline in intracellular Cl− activity would favour basolateral anion exchange, cell shrinkage produced by the loss of these solutes may trigger a volume regulatory increase and stimulation of Cl− entry through NKCC1 (Jiang et al. 1997). In this scheme for Calu-3 cells, most apical HCO3− efflux is sustained by intracellular HCO3− synthesis, which is catalysed by carbonic anhydrase. The acid load produced by forskolin-stimulated HCO3− efflux may be neutralized by HCO3− entry through NBCe1, whereas H+ extrusion via electroneutral NHE1 (Cuthbert et al. 2003) and H+/K+-ATPase (Krouse et al. 2004) mediate pHi regulation during the response to hyperpolarizing secretagogues.
Calu-3 monolayers had significant basal Ieq and HCO3− secretion. Basal activity was sensitive to inhibitors of CFTR (GlyH-101), PKA catalytic subunit (Rp-cAMPS) and membrane-bound adenylyl cyclase (MDL-12330A). Adenylyl cyclase may be constitutively activated by Ca2+ entry through the store-operated Ca2+ entry channel Orai1. A similar mechanism would explain the high basal activity of CFTR in other tissues such as the sweat duct (Quinton, 1983) and small airways (Wang et al. 2006). The present results do not allow discrimination of conductances that are strictly dependent on CFTR activity (Bertrand et al. 2009).
Relevance to other epithelia
Early work identified cAMP-stimulated anion conductance (Klyce & Wong, 1977; Shorofsky et al. 1983; Welsh et al. 1983) and demonstrated basolateral Cl− loading by furosemide- and bumetanide-sensitive cotransporters in native tissues (Frizzell et al. 1979) and cell lines (Dharmsathaphorn et al. 1985), including airway epithelial cell cultures (Widdicombe et al. 1985). Calu-3 is used as a model for submucosal gland serous cells, whereas only surface epithelial cells have been studied extensively in primary culture. Perhaps the best evidence that the Calu-3 model shown in Fig. 12 may apply to glands in native tissue comes from early studies of cat trachea, where bumetanide (100 μm) caused negligible inhibition of gland secretion (see Fig. 7 in Corrales et al. 1984). More recent studies of droplets secreted under oil also indicate a substantial bumetanide-insensitive component in gland fluid secretion (Joo et al. 2002). Approximately half of the vasoactive intestinal peptide (VIP)-stimulated fluid secretion by pig and human glands was insensitive to bumetanide, and replacing CO2/bicarbonate with Hepes (which should inhibit basolateral Cl− loading by anion exchangers) reduced secretion rate by 67%; thus, HCO3− removal caused greater inhibition than bumetanide addition. In summary, the present Calu-3 results are consistent with some previous studies and suggest fluid secretion by glands is less dependent on NKCC compared with other epithelia such as airway surface cells.
Acetazolamide abolished forskolin-stimulated HCO3− secretion and strongly inhibited Ieq in the present study, evidence for the involvement of carbonic anhydrase during HCO3− secretion (Cuthbert et al. 2003; Krouse et al. 2004; Smith & Welsh, 1992). Catalysing HCO3− synthesis may supply HCO3− for secretion while HCO3− entry via NBCe1 maintains pHi while promoting basolateral HCO3− recycling and Cl− entry by exchangers.
This paper presents a model for secretion by Calu-3 cells in which basolateral anion exchange mediates a major portion of Cl− loading during secretion. A similar mechanism may explain the surprisingly weak sensitivity of gland secretion to bumetanide that has been reported in native airway tissues (e.g. Corrales et al. 1984). Basolateral anion exchangers such as AE2 are expressed in Calu-3 (Loffing et al. 2000) and intestinal epithelia (Alper, 2009), and intestinal fluid secretion is defective in Ae2−/− knock-out mice (Gawenis et al. 2010). Studies of an AE2 knock-down cell line in the following companion paper provide direct evidence for the role of this anion exchanger in fluid secretion by Calu-3 cells (Huang et al. 2012).
Acknowledgments
We thank Drs R. Beaulieu (Hôpital Hôtel Dieu) and D. Bowie (McGill University) for access to equipment, J. Riordan (UNC Chapel Hill, NC) and R. Mercer (Washington University, St Louis, MO, USA) for antibodies, W. Marshall (St Francis-Xavier University, Antigonish, NS, Canada) for H36Cl, R. Bridges (Rosalind Franklin University, The Chicago Medical School) and Cystic Fibrosis Foundation Therapeutics Inc. for CFTR inhibitors, and S. Alper (Harvard University) for comments on the manuscript. This study was supported by Cystic Fibrosis Canada, a Seed Grant to J.W.H. from the Canadian Institutes of Health Research program entitled ‘Novel Alternatives to Antibiotics’, and NIH contract grant AI50494 to S.M.O. J.W.H. is affiliated with the Groupe d'étude des protéines membranaires (GEPROM) and the Groupe de recherche axé sur la structure des protéines (GRASP).
Glossary
- ALI
air–liquid interface
- ASL
airway surface liquid
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- Jnet
net 36Cl− flux
- Jms
mucosal-to-serosal 36Cl− flux
- Jsm
serosal-to-mucosal 36Cl− flux
Author contributions
J.S. and J.W.H.: conception and design of the experiments; J.S., J.L., J.H. and R.R.: collection, analysis and interpretation of data, M.L.P., S.C.F. and S.M.O.: essential advice and reagents, J.S., S.M.O. and J.W.H.: drafting the article or revising it critically for important intellectual content. The work was conducted in the Cystic Fibrosis Translational Research Centre, Department of Physiology, McGill University. All authors approved the final version for publication.
Supplementary material
Supplementary Fig. S1
References
- Acevedo M, Steele LW. Na+–H+ exchanger in isolated epithelial tracheal cells from sheep. Involvement in tracheal proton secretion. Exp Physiol. 1993;78:383–394. doi: 10.1113/expphysiol.1993.sp003692. [DOI] [PubMed] [Google Scholar]
- Al-Bazzaz F, Jayaram T. Ion transport by canine tracheal mucosa: effect of elevation of cellular calcium. Exp Lung Res. 1981;2:121–130. doi: 10.3109/01902148109052308. [DOI] [PubMed] [Google Scholar]
- Al-Bazzaz FJ, Al-Awqati Q. Interaction between sodium and chloride transport in canine tracheal mucosa. J Appl Physiol. 1979;46:111–119. doi: 10.1152/jappl.1979.46.1.111. [DOI] [PubMed] [Google Scholar]
- Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ST, Trout L, Bebök Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol Lung Cell Mol Physiol. 1999;277:L694–L699. doi: 10.1152/ajplung.1999.277.4.L694. [DOI] [PubMed] [Google Scholar]
- Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol. 2009;133:421–438. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflügers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- Chen J-H, Cai Z, Sheppard DN. Direct sensing of intracellular pH by the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel. J Biol Chem. 2009;284:35495–35506. doi: 10.1074/jbc.M109.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, O’Neal WK, Boucher RC. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A. 2003;100:16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kavacs T, Sorscher EJ, Clancy JP. A2 adenosine receptors regulate CFTR through PKA and PLA2. Am J Physiol Lung Cell Mol Physiol. 2002;282:L12–L25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- Communi D, Paindavoine P, Place GA, Parmentier M, Boeynaems J-M. Expression of P2Y receptors in cell lines derived from the human lung. Br J Pharmacol. 1999;127:562–568. doi: 10.1038/sj.bjp.0702560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales RJ, Nadel JA, Widdicombe JH. Source of the fluid component of secretions from tracheal submucosal glands in cats. J Appl Physiol. 1984;56:1076–1082. doi: 10.1152/jappl.1984.56.4.1076. [DOI] [PubMed] [Google Scholar]
- Counillon L, Scholz W, Lang HJ, Pouysségur J. Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Mol Pharmacol. 1993;44:1041–1045. [PubMed] [Google Scholar]
- Cuthbert AW, Supuran CT, MacVinish LJ. Bicarbonate-dependent chloride secretion in Calu-3 epithelia in response to 7,8-benzoquinoline. J Physiol. 2003;551:79–92. doi: 10.1113/jphysiol.2003.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K, Mandel KG, Masui H, McRoberts JA. Vasoactive intestinal polypeptide-induced chloride secretion by a colonic epithelial cell line. J Clin Invest. 1985;75:462–471. doi: 10.1172/JCI111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. Function of proton channels in lung epithelia. Wiley Interdiscip Rev Membr Transp Signal. 2011;1:247–258. doi: 10.1002/wmts.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Widdicombe JH, Illek B. Acid secretion and proton conductance in human airway epithelium. Am J Physiol Cell Physiol. 2002;282:C736–C743. doi: 10.1152/ajpcell.00369.2001. [DOI] [PubMed] [Google Scholar]
- Frizzell RA, Field M, Schultz SG. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol Renal Fluid Electrol Physiol. 1979;236:F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Frizzell RA, Hanrahan JW. Physiology of chloride and fluid secretion. In: Riordan JR, Boucher RC, Quinton PM, editors. Cystic Fibrosis: Molecular Basis, Physiological Changes, and Therapeutic Strategies. Cold Spring: Cold Spring Harbor Press; 2011. [Google Scholar]
- Garnett JP, Hickman E, Burrows R, Hegyi P, Tiszlavicz L, Cuthbert AW, Fong P, Gray MA. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J Biol Chem. 2011;286:41069–41082. doi: 10.1074/jbc.M111.266734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawenis LR, Bradford EM, Alper SL, Prasad V, Schull GE. AE2 Cl−/HCO3−exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon. Am J Physiol Gastrointest Liver Physiol. 2010;298:G493–G503. doi: 10.1152/ajpgi.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Pollard CE, Harris A, Coleman L, Greenwell JR, Argent BE. Anion selectivity and block of the small conductance chloride channel on pancreatic duct cells. Am J Physiol Cell Physiol. 1990;259:C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- Guellaen G, Mahu JL, Mavier P, Berthelot P, Hanoune J. RMI 12330 A, an inhibitor of adenylate cyclase in rat liver. Biochim Biophys Acta. 1977;484:465–475. doi: 10.1016/0005-2744(77)90102-4. [DOI] [PubMed] [Google Scholar]
- Hanrahan JW, Tabcharani JA, Chang X-B, Riordan JR. A secretory Cl channel from epithelial cells studied in heterologous expression systems. In: Gerenscer G, editor. Electrogenic Cl− Transporters in Biological Membranes. Springer Verlag, Berlin; 1994. pp. 193–220. [Google Scholar]
- Haws C, Finkbeiner WE, Widdicombe JH, Wine JJ. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl− conductance. Am J Physiol Lung Cell Mol Physiol. 1994;266:L502–L512. doi: 10.1152/ajplung.1994.266.5.L502. [DOI] [PubMed] [Google Scholar]
- Huang J, Shan J, Alper SL, Hanrahan JW. Secretion by the human airway epithelial cell line Calu-3 depends on basolateral chloride loading by AE2. Proc Physiol Soc. 2011;23:160. doi: 10.1113/jphysiol.2012.236919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Shan J, Kim D, Liao J, Evagelidis A, Alper SL, Hanrahan JW. Basolateral chloride loading by AE2: role in fluid secretion by the human airway epithelial cell line Calu-3. J Physiol. 2012;590:xxxx–xxxx. doi: 10.1113/jphysiol.2012.236919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am J Physiol Cell Physiol. 1994;267:C1295–C1307. doi: 10.1152/ajpcell.1994.267.5.C1295. [DOI] [PubMed] [Google Scholar]
- Illek B, Yankaskas JR, Machen TE. cAMP and genistein stimulate HCO3− conductance through CFTR in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 1997;272:L752–L761. doi: 10.1152/ajplung.1997.272.4.L752. [DOI] [PubMed] [Google Scholar]
- Inglis SK, Finlay L, Ramminger SJ, Richard K, Ward MR, Wilson SM, Olver RE. Regulation of intracellular pH in Calu-3 human airway cells. J Physiol. 2002;538:527–539. doi: 10.1113/jphysiol.2001.012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis SK, Wilson SM, Olver RE. Secretion of acid and base equivalents by intact distal airways. Am J Physiol Lung Cell Mol Physiol. 2003;284:L855–L862. doi: 10.1152/ajplung.00348.2002. [DOI] [PubMed] [Google Scholar]
- Iovannisci D, Illek B, Fischer H. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J Gen Physiol. 2010;136:35–46. doi: 10.1085/jgp.200910379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irokawa T, Krouse ME, Joo NS, Wu JV, Wine JJ. A ‘virtual gland’ method for quantifying epithelial fluid secretion. Am J Physiol Lung Cell Mol Physiol. 2004;287:L784–L793. doi: 10.1152/ajplung.00124.2004. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol. 2009;133:315–326. doi: 10.1085/jgp.200810122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Chernova MN, Alper SL. Secondary regulatory volume increase conferred on Xenopus oocytes by expression of AE2 anion exchanger. Am J Physiol Cell Physiol. 1997;272:C191–C202. doi: 10.1152/ajpcell.1997.272.1.C191. [DOI] [PubMed] [Google Scholar]
- Joo NS, Saenz Y, Krouse ME, Wine JJ. Mucus secretion from single submucosal glands of pig. Stimulation by carbachol and vasoactive intestinal peptide. J Biol Chem. 2002;277:28167–28175. doi: 10.1074/jbc.M202712200. [DOI] [PubMed] [Google Scholar]
- Kartner N, Riordan JR. Characterization of polyclonal and monoclonal antibodies to cystic fibrosis transmembrane conductance regulator. Methods Enzymol. 1998;292:629–652. doi: 10.1016/s0076-6879(98)92049-3. [DOI] [PubMed] [Google Scholar]
- Kelly M, Trudel S, Brouillard F, Bouillaud F, Colas J, Nguyen-Khoa T, Ollero M, Edelman A, Fritsch J. Cystic fibrosis transmembrane regulator inhibitors CFTR(inh)-172 and GlyH-101 target mitochondrial functions, independently of chloride channel inhibition. J Pharmacol Exp Ther. 2010;333:60–69. doi: 10.1124/jpet.109.162032. [DOI] [PubMed] [Google Scholar]
- Kim D, Steward MC. The role of CFTR in bicarbonate secretion by pancreatic duct and airway epithelia. J Med Invest. 2009;56:336–342. doi: 10.2152/jmi.56.336. [DOI] [PubMed] [Google Scholar]
- Kinne R, Kinne-Saffran E, Schölermann B, Schütz H. The anion specificity of the sodium-potassium-chloride cotransporter in rabbit kidney outer medulla: studies on medullary plasma membranes. Pflügers Arch. 1986;407:S168–S173. doi: 10.1007/BF00584947. [DOI] [PubMed] [Google Scholar]
- Klyce SD, Wong RKS. Site and mode of adrenaline action on chloride transport across the rabbit corneal epithelium. J Physiol. 1977;266:777–799. doi: 10.1113/jphysiol.1977.sp011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreindler JL, Peters KW, Frizzell RA, Bridges RJ. Identification and membrane localization of electrogenic sodium bicarbonate cotransporters in Calu-3 cells. Biochim Biophys Acta. 2006;1762:704–710. doi: 10.1016/j.bbadis.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Krouse ME, Talbott JF, Lee MM, Joo NS, Wine JJ. Acid and base secretion in the Calu-3 model of human serous cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1274–L1283. doi: 10.1152/ajplung.00036.2004. [DOI] [PubMed] [Google Scholar]
- Kyle H, Ward JPT, Widdicombe JG. Control of pH of airway surface liquid of the ferret trachea in vitro. J Appl Physiol. 1990;68:135–140. doi: 10.1152/jappl.1990.68.1.135. [DOI] [PubMed] [Google Scholar]
- Lee MC, Penland CM, Widdicombe JH, Wine JJ. Evidence that Calu-3 human airway cells secrete bicarbonate. Am J Physiol Lung Cell Mol Physiol. 1998;274:L450–L453. doi: 10.1152/ajplung.1998.274.3.L450. [DOI] [PubMed] [Google Scholar]
- Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl−/HCO3−exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Wills NK, Eaton DC. Basolateral membrane potential of a tight epithelium: ionic diffusion and electrogenic pumps. J Membr Biol. 1978;41:117–148. doi: 10.1007/BF01972629. [DOI] [PubMed] [Google Scholar]
- Li MS, Holstead RG, Wang W, Linsdell P. Regulation of CFTR chloride channel macroscopic conductance by extracellular bicarbonate. Am J Physiol Cell Physiol. 2011;300:C65–C74. doi: 10.1152/ajpcell.00290.2010. [DOI] [PubMed] [Google Scholar]
- Linsdell P, Tabcharani JA, Rommens JM, Hou Y-X, Chang X-B, Tsui L-C, Riordan JR, Hanrahan JW. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol. 1997;110:355–364. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffing J, Moyer BD, Reynolds D, Shmukler BE, Alper SL, Stanton BA. Functional and molecular characterization of an anion exchanger in airway serous epithelial cells. Am J Physiol Cell Physiol. 2000;279:C1016–C1023. doi: 10.1152/ajpcell.2000.279.4.C1016. [DOI] [PubMed] [Google Scholar]
- Luo Y, McDonald K, Hanrahan JW. Trafficking of immature DeltaF508-CFTR to the plasma membrane and its detection by biotinylation. Biochem J. 2009;419:211–219. doi: 10.1042/BJ20081869. [DOI] [PubMed] [Google Scholar]
- Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJV, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVinish LJ, Cope G, Ropenga A, Cuthbert AW. Chloride transporting capability of Calu-3 epithelia following persistent knockdown of the cystic fibrosis transmembrane conductance regulator, CFTR. Br J Pharmacol. 2007;150:1055–1065. doi: 10.1038/sj.bjp.0707175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S, Singh M, Krouse ME, Wine JJ. Calcium-stimulated Cl− secretion in Calu-3 human airway cells requires CFTR. Am J Physiol Lung Cell Mol Physiol. 1997;273:L1208–L1219. doi: 10.1152/ajplung.1997.273.6.L1208. [DOI] [PubMed] [Google Scholar]
- Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJV, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ML, Lee SY, Carlson D, Fahrenkrug S, O’Grady SM. Stable knockdown of CFTR establishes a role for the channel in P2Y receptor-stimulated anion secretion. J Cell Physiol. 2006;206:759–770. doi: 10.1002/jcp.20519. [DOI] [PubMed] [Google Scholar]
- Paradiso AM, Coakley RD, Boucher RC. Polarized distribution of HCO3− transport in human normal and cystic fibrosis nasal epithelia. J Physiol. 2003;548:203–218. doi: 10.1113/jphysiol.2002.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139:620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Fluid Electrol Physiol. 2010;298:F643–F654. doi: 10.1152/ajprenal.00584.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton PM. Chloride impermeability in cystic fibrosis. Nature. 1983;301:421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Role of epithelial HCO3− transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2010;299:C1222–C1233. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. 2011. ImageJ. National Institutes of Health, Bethesda, Maryland USA http://imagej.nih.gov/ij/, 1997–2011.
- Schmid A, Sutto Z, Schmid N, Novak L, Ivonnet P, Horvath G, Conner G, Fregien N, Salathe M. Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. J Biol Chem. 2010;285:29998–30007. doi: 10.1074/jbc.M110.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Huang J, Liao J, Robert R, Hanrahan JW. Anion secretion by a model epithelium: More lessons from Calu-3. Acta Physiol. 2011;202:523–531. doi: 10.1111/j.1748-1716.2011.02253.x. [DOI] [PubMed] [Google Scholar]
- Shen B-Q, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am J Physiol Lung Cell Mol Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- Shorofsky SR, Field M, Fozzard HA. Electrophysiology of Cl− secretion in canine trachea. J Membr Biol. 1983;72:105–115. doi: 10.1007/BF01870318. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Karp PH, Welsh MJ. Defective fluid transport by cystic fibrosis airway epithelia. J Clin Invest. 1994;93:1307–1311. doi: 10.1172/JCI117087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2006;290:C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL. SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol. 2011;301:C289–C303. doi: 10.1152/ajpcell.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyasu K, Tamkun MM, Renaud KJ, Fambrough DM. Ouabain-sensitive (Na++ K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the α-subunit of an avian sodium pump. J Biol Chem. 1988;263:4347–4354. [PubMed] [Google Scholar]
- Tamada T, Hug MJ, Frizzell RA, Bridges RJ. Microelectrode and impedance analysis of anion secretion in Calu-3 cells. JOP. 2001;2:219–228. [PubMed] [Google Scholar]
- Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127:591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier GJ, Traynor TR, Kannan MS, O’Grady SM. Mechanisms of sodium and chloride transport across equine tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 1990;259:L459–L467. doi: 10.1152/ajplung.1990.259.6.L459. [DOI] [PubMed] [Google Scholar]
- Wang X, Lytle C, Quinton PM. Predominant constitutive CFTR conductance in small airways. Resp Res. 2006;6:7. doi: 10.1186/1465-9921-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lam CS, Wu F, Wang W, Duan Y, Huang P. Regulation of CFTR channels by HCO3sensitive soluble adenylyl cyclase in human airway epithelial cells. Am J Physiol Cell Physiol. 2005;289:C1145–C1151. doi: 10.1152/ajpcell.00627.2004. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Smith PL, Frizzell RA. Chloride secretion by canine tracheal epithelium: III. Membrane resistances and electromotive forces. J Membr Biol. 1983;71:209–218. doi: 10.1007/BF01875462. [DOI] [PubMed] [Google Scholar]
- Widdicombe JH. Regulation of the depth and composition of airway surface liquid. J Anat. 2002;201:313–318. doi: 10.1046/j.1469-7580.2002.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JH, Welsh MJ, Finkbeiner WE. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc Natl Acad Sci U S A. 1985;82:6167–6171. doi: 10.1073/pnas.82.18.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


