Abstract
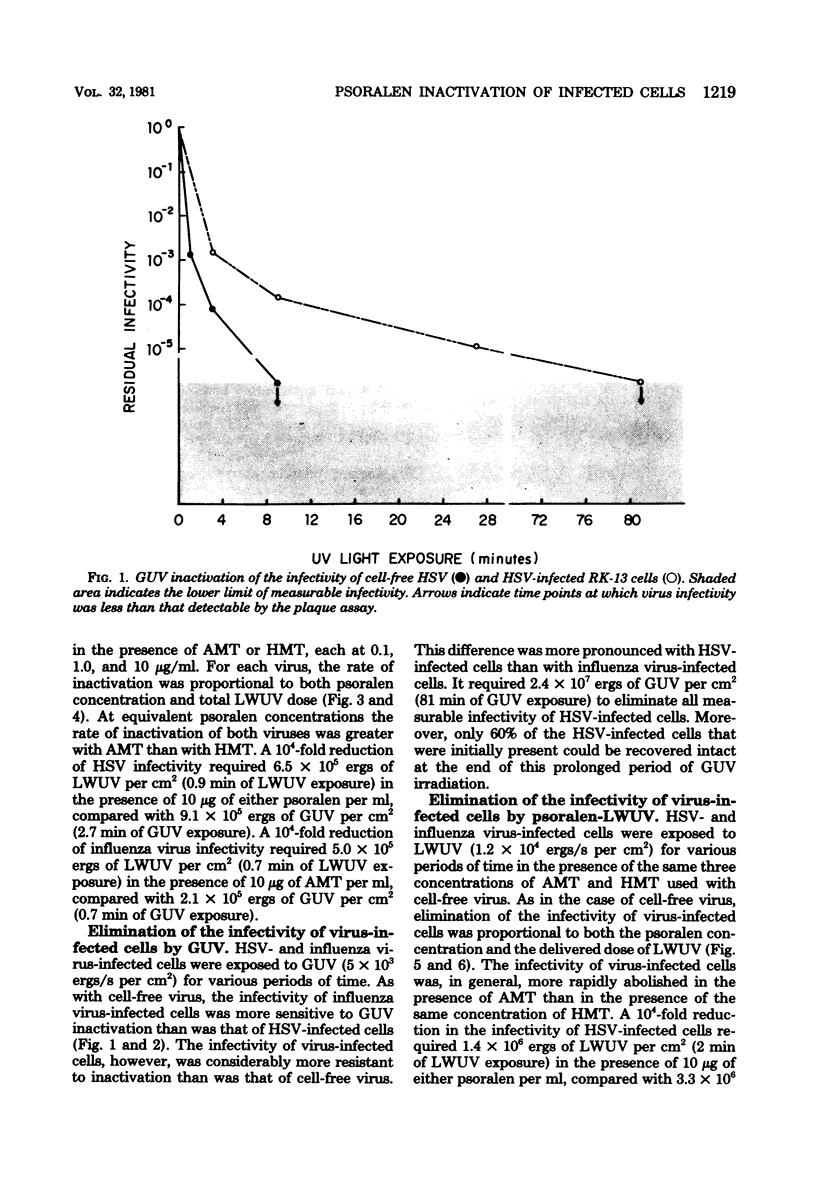
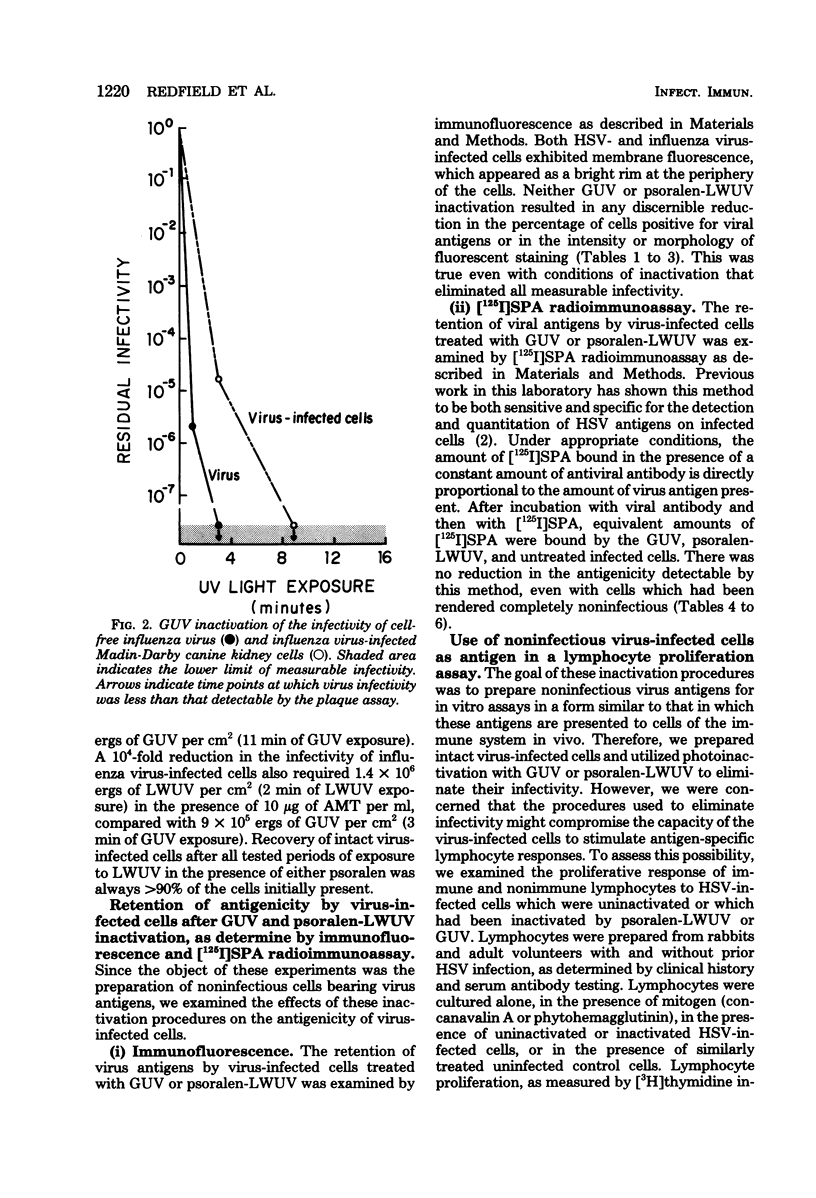
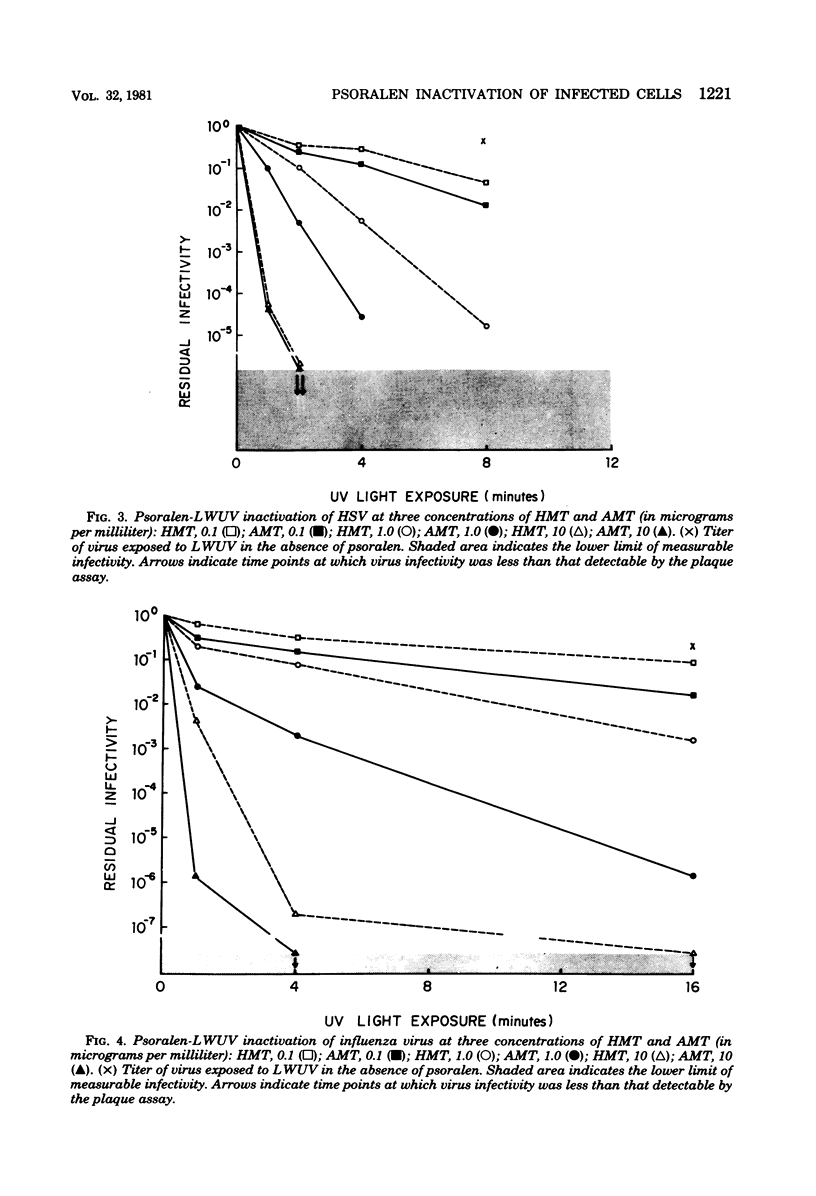
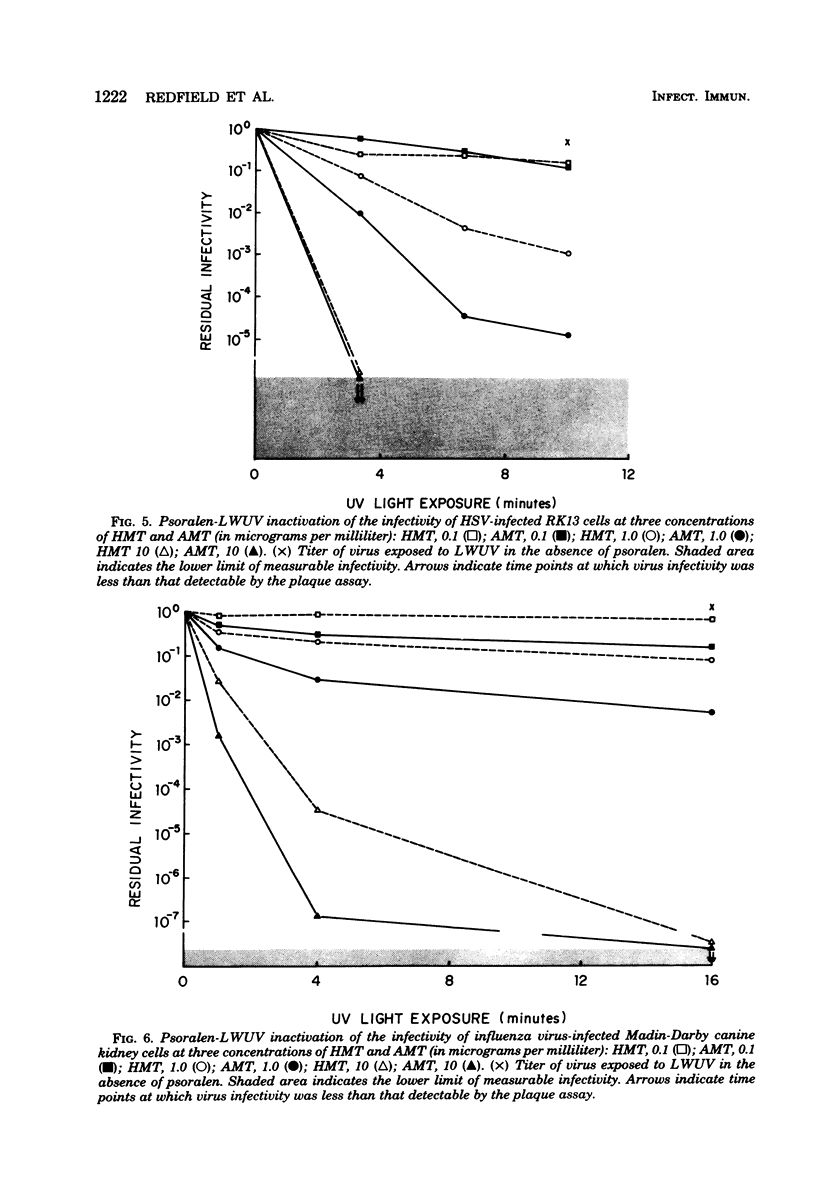
Psoralen compounds covalently bind to nucleic acids when irradiated with long-wavelength ultraviolet light. This treatment can destroy the infectivity of deoxyribonucleic acid and ribonucleic acid viruses. Two psoralen compounds, 4'-hydroxymethyltrioxsalen and 4'-aminomethyltrioxsalen, were used with long-wavelength ultraviolet light to inactivate cell-free herpes simplex and influenza viruses and to render virus-infected cells noninfectious. This method of inactivation was compared with germicidal (short-wavelength) ultraviolet light irradiation. The antigenicity of the treated, virus-infected, antigen-bearing cells was examined by immunofluorescence and radioimmunoassay and by measuring the capacity of the herpes simplex virus-infected cells to stimulate virus-specific lymphocyte proliferation. The infectivity of the virus-infected cells could be totally eliminated without altering their viral antigenicity. The use of psoralen plus long-wavelength ultraviolet light is well suited to the preparation of noninfectious virus antigens and virus antigen-bearing cells for immunological assays.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Cleveland P. H., Richman D. D., Oxman M. N., Wickham M. G., Binder P. S., Worthen D. M. Immobilization of viral antigens on filter paper for a [125I]staphylococcal protein A immunoassay: a rapid and sensitive technique for detection of herpes simplex virus antigens and antiviral antibodies. J Immunol Methods. 1979;29(4):369–386. doi: 10.1016/0022-1759(79)90008-5. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Hallick L. M., Yokota H. A., Bartholomew J. C., Hearst J. E. Photochemical addition of the cross-linking reagent 4,5', 8-trimethylpsoralen (trioxaslen) to intracellular and viral simian virus 40 DNA-histone complexes. J Virol. 1978 Jul;27(1):127–135. doi: 10.1128/jvi.27.1.127-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C. V., Riggs J. L., Lennette E. H. Photochemical inactivation of DNA and RNA viruses by psoralen derivatives. J Gen Virol. 1978 Aug;40(2):345–358. doi: 10.1099/0022-1317-40-2-345. [DOI] [PubMed] [Google Scholar]
- Hearst J. E., Thiry L. The photoinactivation of an RNA animal virus, vesicular stomatitis virus, with the aid of newly synthesized psoralen derivatives. Nucleic Acids Res. 1977;4(5):1339–1347. doi: 10.1093/nar/4.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochkeppel H. K., Gordon J. Evidence for cross-linking of polyribonucleotides with 4'-aminomethyl-4,5',8-trimethylpsoralen hydrochloride. Biochemistry. 1979 Jun 26;18(13):2905–2910. doi: 10.1021/bi00580a035. [DOI] [PubMed] [Google Scholar]
- Hodes H. L., Lavin G. I., Webster L. T. ANTIRABIC IMMUNIZATION WITH CULTURE VIRUS RENDERED AVIRULENT BY ULTRA-VIOLET LIGHT. Science. 1937 Nov 12;86(2237):447–448. doi: 10.1126/science.86.2237.447. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Lin P. F. Genetic recombination induced by DNA cross-links in repressed phage lambda. Genetics. 1973 Apr;73(Suppl):85–90. [PubMed] [Google Scholar]
- Hyde J. E., Hearst J. E. Binding of psoralen derivatives to DNA and chromatin: influence of the ionic environment on dark binding and photoreactivity. Biochemistry. 1978 Apr 4;17(7):1251–1257. doi: 10.1021/bi00600a019. [DOI] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- MUSAJO L., RODIGHIERO G., COLOMBO G., TORLONE V., DALLACQUA F. PHOTOSENSITIZING FUROCOUMARINS: INTERACTION WITH DNA AND PHOTO-INACTIVATION OF DNA CONTAINING VIRUSES. Experientia. 1965 Jan 15;21:22–24. doi: 10.1007/BF02136362. [DOI] [PubMed] [Google Scholar]
- Musajo L., Bordin F., Caporale G., Marciani S., Rigatti G. Photoreactions at 3655 Angstrom between pyrimidine bases and skin-photosensitizing furocoumarins. Photochem Photobiol. 1967 Oct;6(10):711–719. doi: 10.1111/j.1751-1097.1967.tb08736.x. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Chanda P. K., Deutsch V., Banerjee A. K., Shatkin A. J. Inactivation of influenza and vesicular stomatitis virion RNA polymerase activities by photoreaction with 4'-substituted psoralens. J Virol. 1979 Dec;32(3):838–844. doi: 10.1128/jvi.32.3.838-844.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K., Shatkin A. J. Photochemical cross-linking of reovirus genome RNA in situ and inactivation of viral transcriptase. J Biol Chem. 1978 Dec 25;253(24):8680–8682. [PubMed] [Google Scholar]
- Notkins A. L. Immune mechanisms by which the spread of viral infections is stopped. Cell Immunol. 1974 Mar 30;11(1-3):478–483. doi: 10.1016/0008-8749(74)90045-8. [DOI] [PubMed] [Google Scholar]
- Plaeger-Marshall S., Smith J. W. Inhibition of mitogen- and antigen-induced lymphocyte blastogenesis by herpes simplex virus. J Infect Dis. 1978 Oct;138(4):506–511. doi: 10.1093/infdis/138.4.506. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. S. Cell-mediated immunity to herpes simplex virus in man. Am J Clin Pathol. 1973 Dec;60(6):826–830. doi: 10.1093/ajcp/60.6.826. [DOI] [PubMed] [Google Scholar]
- Scott B. R., Pathak M. A., Mohn G. R. Molecular and genetic basis of furocoumarin reactions. Mutat Res. 1976;39(1):29–74. doi: 10.1016/0165-1110(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Psoralen-crosslinked secondary structure map of single-stranded virus DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2649–2653. doi: 10.1073/pnas.73.8.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. K., Hsieh T. S., Wang J. C., Hearst J. E. Photochemical cross-linking of DNA-RNA helices by psoralen derivatives. J Mol Biol. 1977 Nov;116(4):661–679. doi: 10.1016/0022-2836(77)90265-0. [DOI] [PubMed] [Google Scholar]
- Sissons J. G., Oldstone M. B. Killing of virus-infected cells by cytotoxic lymphocytes. J Infect Dis. 1980 Jul;142(1):114–119. doi: 10.1093/infdis/142.1.114. [DOI] [PubMed] [Google Scholar]
- Song P. S., Tapley K. J., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979 Jun;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Oxman M. N. Antibody to varicella-zoster virus-induced membrane antigen: immunofluorescence assay using monodisperse glutaraldehyde-fixed target cells. J Infect Dis. 1977 Oct;136(4):519–530. doi: 10.1093/infdis/136.4.519. [DOI] [PubMed] [Google Scholar]



