Abstract
Circulating ATP possesses unique vasomotor properties in humans and has been hypothesized to play a role in vascular control under a variety of physiological conditions. However, the primary downstream signalling mechanisms underlying ATP-mediated vasodilatation remain unclear. The purpose of the present experiment was to determine whether ATP-mediated vasodilatation is independent of nitric oxide (NO) and prostaglandin (PG) synthesis and occurs primarily via the activation of Na+/K+-ATPase and inwardly rectifying potassium (KIR) channels in humans. In all protocols, young healthy adults were studied and forearm vascular conductance (FVC) was calculated from forearm blood flow (measured via venous occlusion plethysmography) and intra-arterial blood pressure to quantify local vasodilatation. Vasodilator responses (%ΔFVC) during intra-arterial ATP infusions were unchanged following combined inhibition of NO and PGs (n= 8; P > 0.05) whereas the responses to KCl were greater (P < 0.05). Combined infusion of ouabain (to inhibit Na+/K+-ATPase) and barium chloride (BaCl2; to inhibit KIR channels) abolished KCl-mediated vasodilatation (n= 6; %ΔFVC = 134 ± 13 vs. 4 ± 5%; P < 0.05), demonstrating effective blockade of direct vascular hyperpolarization. The vasodilator responses to three different doses of ATP were inhibited on average 56 ± 5% (n= 16) following combined ouabain plus BaCl2 infusion. In follow-up studies, BaCl2 alone inhibited the vasodilator responses to ATP on average 51 ± 3% (n= 6), which was not different than that observed for combined ouabain plus BaCl2 administration. Our novel results indicate that the primary mechanism of ATP-mediated vasodilatation is vascular hyperpolarization via activation of KIR channels. These observations translate in vitro findings to humans in vivo and may help explain the unique vasomotor properties of intravascular ATP in the human circulation.
Key points
ATP is a substance in the blood vessels that can cause vasodilatation and increase blood flow and oxygen delivery in humans.
The exact signalling pathways that ATP stimulates to cause vasodilatation are not well known.
We show that a large portion of ATP-mediated vasodilatation occurs through the activation of inwardly rectifying potassium channels (Kir).
Our results lend insight into the vasodilator mechanisms of ATP, a substance that is important for vascular control.
Further, our results may stimulate additional investigations in humans regarding the activation of Kir channels and subsequent vascular hyperpolarization during other physiologically relevant conditions.
Introduction
Accumulating evidence indicates that circulating adenosine triphosphate (ATP) plays an important role in the regulation of local vascular tone in cerebral (Horiuchi et al. 2003), coronary (Farias et al. 2005) and skeletal muscle circulations (Gonzalez-Alonso et al. 2002). Specifically, data indicate that ATP is involved in the vascular response to physiological stimuli that require the matching of blood flow and oxygen supply to the metabolic demands of the tissue (Gonzalez-Alonso et al. 2002). In humans, intravascular ATP can cause profound vasodilatation and moreover may be important for vascular control in that it has the unique ability to limit sympathetically mediated vasoconstriction, similar to what occurs in contracting skeletal muscle (Kirby et al. 2008). Thus, given the role of ATP in vasomotor control, there has been substantial interest in understanding the primary downstream signalling mechanisms underlying ATP-mediated vasodilatation (Rongen et al. 1994; van Ginneken et al. 2004; Crecelius et al. 2011a).
In vitro studies have demonstrated that intravascular ATP binds to purinergic 2 (P2) receptors on the endothelium, resulting in an increase in intracellular endothelial cell [Ca2+] which then can stimulate multiple vasoactive pathways that ultimately cause relaxation of the vascular smooth muscle (Duza & Sarelius, 2003; Ellsworth et al. 2009). In this context, elevations in endothelial cell [Ca2+] can increase the synthesis of nitric oxide (NO) and arachidonic acid metabolites such as vasodilating prostaglandins (PGs) and, consistent with this, some in vitro preparations have shown that these substances may contribute to the vasodilator action of ATP (Wihlborg et al. 2003; Ellsworth et al. 2009). In contrast, the majority of studies in humans indicate that NO and PGs are not the primary mediators and at best have only a modest role in the local dilatory response to intravascular ATP (Rongen et al. 1994; van Ginneken et al. 2004; Mortensen et al. 2009; Crecelius et al. 2011a). Thus, it appears that ATP-mediated vasodilatation involves mechanisms beyond these traditional endothelial cell signalling pathways in humans.
In addition to stimulating the synthesis of NO and PGs, increases in intracellular endothelial cell [Ca2+] can activate small- and intermediate-conductance Ca2+-activated potassium channels (SKCa and IKCa, respectively) resulting in endothelial cell hyperpolarization which then can be electrically communicated via gap junctions to other adjacent endothelial cells as well as the underlying smooth muscle cells (Edwards et al. 1998; Segal, 2005). Further, K+ efflux from these channels increases K+ concentrations in the myoendothelial space which stimulates both Na+/K+-ATPase and inwardly rectifying potassium (KIR) channels evoking smooth muscle cell hyperpolarization (Edwards et al. 1998). In addition to the activation of KIR channels via increases in [K+], there is evidence that hyperpolarization of the cellular membrane can activate KIR channels and thus facilitate amplification of a hyperpolarizing stimulus which spreads through the vascular wall and translates to a robust conducted or spreading vasodilator response (Smith et al. 2008). Interestingly, studies in vitro have demonstrated that application of ATP stimulates hyperpolarization of both endothelial and vascular smooth muscle cells (Malmsjo et al. 1999; Sheng & Braun, 2007), evokes local and conducted vasodilatation that is substantially reduced via inhibition of SKCa and IKCa channels (Winter & Dora, 2007), and that vasodilatation to direct P2 receptor stimulation is blunted by inhibition of Na+/K+-ATPase (via ouabain) (Ralevic, 2001) and KIR channels (via barium chloride, BaCl2) (Smith et al. 2008).
Recently, Dawes and colleagues were able to successfully and safely administer ouabain and BaCl2 via brachial artery catheter which nearly abolished potassium chloride (KCl)-mediated vasodilatation in the human forearm (Dawes et al. 2002). KCl mimics many of the vascular effects of ATP in vitro in that it causes endothelial and vascular smooth muscle cell hyperpolarization and conducted vasodilatation (Edwards et al. 1998; Horiuchi et al. 2002). Importantly, these responses are inhibited via ouabain and BaCl2 (Edwards et al. 1998; Horiuchi et al. 2002; Smith et al. 2008). Here, we first determined the efficacy of ouabain and BaCl2 to inhibit direct hyperpolarization via KCl, and then determined whether ATP-mediated vasodilatation occurs as a result of vascular hyperpolarization in vivo. Accordingly, in the present investigation we directly tested the hypothesis that ATP-mediated vasodilatation is largely independent of NO and PG synthesis and occurs via Na+/K+-ATPase and KIR channel activation in humans.
Methods
Subjects
With Institutional Review Board approval and after obtaining written informed consent, a total of 33 young healthy adults (Protocol 1: 5 men, 3 women; age, 22 ± 1 years; weight, 71.8 ± 3.6 kg; height, 174 ± 3 cm; body mass index, 23.7 ± 0.9 kg m−2; forearm volume (FAV), 966 ± 64 ml; Protocol 2: 19 men, 6 women; age, 23 ± 1 years; weight, 73.7 ± 1.7 kg; height, 175 ± 1 cm; body mass index, 24.0 ± 0.5 kg m−2; FAV, 960 ± 42 ml; Protocol 3: 4 men, 2 women; age, 23 ± 2 years; weight, 73.3 ± 2.8 kg; height, 172 ± 2 cm; body mass index, 24.9 ± 1.1 kg m−2; FAV, 792 ± 16 ml; means ± SEM) participated in the present study. Six subjects participated in multiple protocols and all subject groups were similar in their characteristics except that those participants in Protocol 1 had a slightly larger FAV than those in Protocol 3 (P < 0.05).
All subjects were sedentary to moderately active, non-smokers, non-obese, normotensive (resting blood pressure <140/90 mmHg), and not taking any medications. Studies were performed after a 4 h fast and 24 h abstention from caffeine and exercise. The subjects were in the supine position with the experimental arm abducted to 90 deg and slightly elevated above heart level upon a tilt-adjustable table. Female subjects were studied during the early follicular phase of their menstrual cycle or placebo phase of oral contraceptive use to minimize any potential cardiovascular effects of sex-specific hormones. All studies were performed according to the Declaration of Helsinki.
Arterial and venous catheterization
A 20 gauge, 7.6 cm catheter was placed in the brachial artery of the non-dominant arm under aseptic conditions after local anaesthesia (2% lidocaine) for local administration of study drugs and blood sampling. The catheter was connected to a 3-port connector as well as a pressure transducer for mean arterial pressure (MAP) measurement and continuously flushed at 3 ml h−1 with heparinized saline. The two side ports were used for drug infusions (Kirby et al. 2008; Crecelius et al. 2010). In addition, an 18 gauge, 3.8 cm catheter was inserted into an antecubital vein of the non-experimental arm for venous blood samples (Crecelius et al. 2011b) to be used for systemic electrolyte monitoring via clinical blood gas analyser (Siemens Rapid Point 400 Series Automatic Blood Gas System, Los Angeles, CA, USA). Saline was continuously infused through this catheter at a rate of approximately 3 ml min−1 for the duration of the study to keep it patent.
Forearm blood flow and vascular conductance
Forearm blood flow (FBF) was measured via venous occlusion plethysmography (VOP) using mercury-in-salistic strain gauges and techniques as previously described (Greenfield et al. 1963; Crecelius et al. 2011a) and was expressed as milliliters per deciliter of tissue per minute (ml dl−1 min−1). As an index of forearm vasodilatation and to account for individual differences in baseline vascular tone, forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100 expressed as ml dl−1 min−1 (100 mmHg−1). In an effort to minimize the contribution of cutaneous blood flow to FBF measurements, a fan was directed at the experimental arm throughout the experimental protocol.
Vasoactive drug infusion
All drug infusions were through the brachial artery catheter to create a local effect in the forearm. Vasodilator infusions were either ATP (P2 receptor agonist; Sigma A7699, St Louis, MO, USA), potassium chloride (KCl; direct hyperpolarizing stimulus; Hospira, Lake Forest, IL, USA) or acetylcholine (ACh; muscarinic receptor agonist; Novartis, East Hanover, IL, USA) in the doses provided in the respective protocol (see below). Our experimental question primarily focused on ATP-mediated vasodilatation and KCl and ACh were used to test efficacy and specificity of the inhibitors employed.
To assess the contribution of NO and PGs to ATP and KCl-mediated vasodilatation, NG-monomethyl-l-arginine (l-NMMA; nitric oxide synthase (NOS) inhibitor; Clinalfa/Bachem, Weil am Rhein, Germany) was administered to inhibit the production of NO and ketorolac (non-selective cyclooxygenase (COX) inhibitor; Hospira) was administered to inhibit the synthesis of PGs. Loading doses of l-NMMA and ketorolac were 25 mg (5 mg min−1 for 5 min) and 6 mg (600 μg min−1 for 10 min), respectively, and maintenance doses of 1.25 mg min−1 (l-NMMA) and 150 μg min−1 (ketorolac) were infused for the duration of the protocol to ensure continuous blockade (Crecelius et al. 2010, 2011b).
In order to determine the role of Na+/K+-ATPase and KIR channels, ouabain octahydrate (Sigma 03125) was infused at 2.7 nmol min−1 for 15 min as a loading dose and continued throughout vasodilator infusion (4 additional minutes) at the same dose to inhibit Na+/K+-ATPase (Dawes et al. 2002; Dwivedi et al. 2005). Barium chloride (10% w/v BDH3238, EMD Chemicals, Gibbstown, NJ, USA) was infused at 4 μmol min−1 for 3 min as a loading dose and continued throughout vasodilator infusion (4 additional minutes) at the same dose to inhibit KIR channels (Dawes et al. 2002; Dwivedi et al. 2005). Importantly, Dawes and colleagues (2002) determined that this same dose of BaCl2 for 6 min caused an increase in venous plasma [Ba2+] to 50.00 ± 8.00 μmol l−1, a concentration that is within the range (<100 μmol l−1) of BaCl2 to specifically inhibit KIR channels (Jackson, 2005).
ATP, ouabain and BaCl2 were prepared in saline and confirmed sterile and free of fungus/endotoxin and particulate matter with a standard microbiology report (JCB-Analytical Research Labs, Wichita, KS, USA) prior to use. FAV used for normalization for specific vasoactive drugs was determined from regional analysis of whole-body dual-energy X-ray absorptiometry scans (QDR series software, Hologic, Inc., Bedford, MA, USA).
Experimental protocols
In general, 2 min of resting data were acquired prior to the start of all vasodilator infusions and 15 min of rest separated each trial, as in our experience, this is more than a sufficient length of time for baseline FBF to return to pre-vasodilator levels. Saline was used as a control infusate at matched rates to the vasodilators and inhibitors.
Protocol 1: effect of combined NOS and COX inhibition
In eight subjects, dose–response trials were performed as 2 min infusions at each progressive dose of either ATP (1.25, 2.50 and 5.00 μg (dl FAV)−1 min−1) or KCl (0.05, 0.10 and 0.20 mmol min−1), thus a total of 6 min of vasodilator infusion. Dose–response was performed in control conditions and during combined l-NMMA and ketorolac administration. The order of ATP and KCl was balanced between subjects. A subgroup of these subjects (n= 4) also received progressive doses of acetylcholine (ACh; 4, 8 and 16 μg (dl FAV)−1 min−1) in control and blockade conditions to test the efficacy of the combined NOS + COX inhibition.
Protocol 2: effect of Na+/K+-ATPase and KIR channel inhibition
Due to safety concerns with BaCl2 administration (Dawes et al. 2002), subjects received either KCl or ATP infusions. Two doses of KCl (n= 6; 0.10 and 0.20 mmol min−1) or ATP (n= 8; 1.25 and 5.00 μg (dl FAV)−1 min−1) were administered for 2 min each, thus a total of 4 min of vasodilator infusion. Dose–response was performed in control conditions and with combined ouabain and BaCl2 administration. Additional subjects (n= 8) were studied at a lower range of ATP doses (0.625 and 1.25 μg (dl FAV)−1 min−1).
A small group of subjects (n= 4) received ACh (2 μg (dl FAV)−1 min−1) for 2 min before and after combined ouabain and BaCl2 administration in order to confirm previous findings that the effects of ouabain and BaCl2 are selective and thus do not impair vasodilator responses in a non-specific manner (Dawes et al. 2002; Dwivedi et al. 2005). We chose to administer ACh because, similar to ATP, it is an endothelium-dependent agonist; however, ACh is primarily dependent on the NOS and COX vasodilator pathways in humans (see Results). The dose of ACh (2 μg (dl FAV)−1 min−1) was based on an anticipated vasodilator response to match that observed with our lower doses of ATP.
Protocol 3: effect of independent KIR channel inhibition
Given our findings from Protocol 2 (see Results) and the ability for KIR channel activation to amplify hyperpolarization that occurs independent of Na+/K+-ATPase activity (Smith et al. 2008), we were interested in the independent role of KIR channels. Therefore, in six subjects, we determined the influence of BaCl2 alone on ATP-mediated vasodilatation. Two doses of ATP (0.625 and 1.25 μg (dl FAV)−1 min−1) were given for 2 min each in control (saline) conditions or with concomitant administration of BaCl2.
Data acquisition and analysis
Data were collected and stored on a computer at 250 Hz and were analysed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH, USA). MAP was determined from the arterial pressure waveform and heart rate (HR) was determined via the standard three-lead ECG. FBF was determined from the derivative of the forearm plethysmogram signal, resulting in one FBF measurement every 15 s. FBF, HR and MAP represent an average of the last minute of steady-state conditions (i.e. 4 FBF measures). FVC was used as our standard index of forearm vascular tone, and we present both absolute FVC and percentage changes in FVC (%ΔFVC) in response to the vasoactive drug infusions. Given the existence of individual differences in baseline vascular tone, individual differences in forearm vascular tone during vasodilator infusion, as well the potential influence of the pharmacological inhibitors on baseline vascular tone, we were especially interested in the %ΔFVC as this tracks changes in blood vessel radius independent of the initial level of vascular tone and is therefore the most appropriate index of changes in vasomotor tone (Buckwalter & Clifford, 2001). The percentage increase in FVC due to vasodilator infusion in each trial was calculated as:
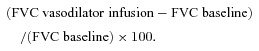 |
The magnitude of inhibition of vasodilator responses was calculated as:
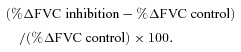 |
Statistics
Data are presented as mean ± SEM. Differences within and between conditions for each vasodilator were determined via two-way (dose and condition (control, inhibition)) repeated-measures analysis of variance (ANOVA). When significance was observed, the Fisher's LSD method was used to make individual comparisons. To compare the effect of combined ouabain and BaCl2 administration vs. BaCl2 alone as well as compare subject characteristics between protocols, unpaired Student's t tests were used. Significance was set a priori at P < 0.05.
Results
Protocol 1: effect of combined NOS and COX inhibition on ATP and K+-mediated vasodilatation
No significant changes in HR or MAP were observed across all conditions within Protocol 1 (Supplementary Tables S1 and S2). All three doses of ATP caused significant increases in FVC from rest and combined NOS–COX inhibition significantly reduced FVC at rest (Fig. 1A; P < 0.05), but not during ATP infusion (P= 0.20). Similarly, the vasodilator responses when quantified as a per cent change from rest were unaffected with combined l-NMMA and ketorolac infusion (Fig. 1B).
Figure 1. Protocol 1: effect of l-NMMA + ketorolac on ATP- and KCl-mediated vasodilatation.
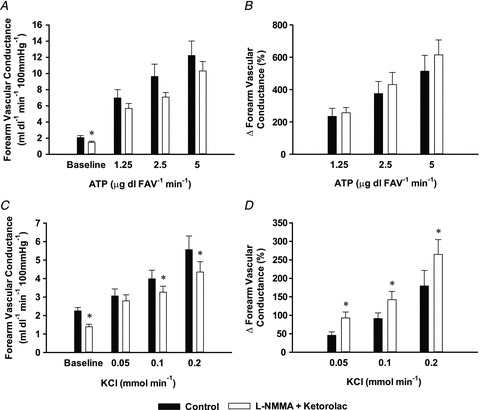
In 8 subjects, combined l-NMMA and ketorolac (open bars) to inhibit the synthesis of NO and PGs, respectively, significantly reduced absolute forearm vascular conductance (FVC) at baseline (A) but had no effect on absolute FVC or the per cent change in FVC (B) to progressive intrabrachial doses of ATP as compared with control (saline; filled bars) conditions. l-NMMA and ketorolac similarly decreased baseline absolute FVC (C) as well as two of three progressive intrabrachial doses of KCl. However, given the magnitude of the reduction in baseline FVC, the per cent change (D) was slightly augmented at all doses. *P < 0.05 vs. control.
All three doses of KCl also caused significant increases in FVC from rest and combined NOS–COX inhibition significantly reduced FVC at rest and minimally, but significantly, during the highest two doses of KCl (Fig. 1C; P < 0.05) but not the lowest dose of KCl (P= 0.32). The vasodilator responses when quantified as a per cent change from rest were slightly augmented with combined l-NMMA and ketorolac infusion at all doses of KCl (Fig. 1D).
ACh infusion resulted in significant increases in FVC from rest (2.7 ± 0.6 vs. 13.4 ± 3.1, 15.3 ± 3.7 and 18.3 ± 4.3 ml dl−1 min−1 (100 mmHg)−1; P < 0.05) and combined NOS–COX inhibition significantly reduced FVC at rest (1.7 ± 0.3 ml dl−1 min−1 (100 mmHg)−1; P < 0.05) and at all doses of ACh (4.1 ± 0.6, 3.9 ± 0.8 and 5.4 ± 1.0 ml dl−1 min−1 (100 mmHg)−1; P < 0.05). On average, the vasodilator response (%ΔFVC; Supplementary Table S2B) was reduced ∼60%, consistent with effective blockade of NOS (Lauer et al. 2001). In all conditions in this protocol, changes in FBF paralleled those observed for FVC (Supplementary Tables S1 and S2).
Protocol 2: effect of Na+/ K+-ATPase and KIR channel inhibition
No significant changes in HR or MAP were observed across all conditions within Protocol 2 (Supplementary Tables S3 and S4). Combined ouabain and BaCl2 administration abolished forearm vasodilatation in response to KCl infusion (P < 0.05 vs. control; Fig. 2). At all doses of ATP, ouabain and BaCl2 significantly attenuated the forearm vasodilator responses (mean effect of all doses pooled: −56 ± 4%; range: 40–70%; P < 0.05 vs. control; Fig. 3). In those subjects in which the vasodilator response to ACh was determined before and after inhibition of Na+/K+-ATPase and KIR channels (n= 4), forearm vasodilatation was unchanged, demonstrating the selectivity of the blockers to KCl and ATP (Table 1). In all conditions in this protocol, changes in FBF paralleled those observed for FVC (Supplementary Tables S3 and S4).
Figure 2. Protocol 2: efficacy of ouabain + BaCl2 to block KCl-mediated vasodilatation.
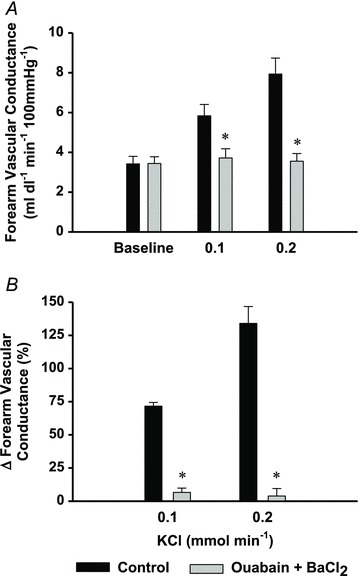
KCl-mediated vasodilatation (absolute FVC (A) and per cent change forearm vascular conductance (B)) was abolished with combined ouabain + BaCl2 infusion (grey bars; to inhibit Na+/K+-ATPase and KIR channels, respectively), indicating successful inhibition of a direct hyperpolarizing stimulus. *P < 0.05 vs. control.
Figure 3. Protocol 2: effect of ouabain + BaCl2 on ATP-mediated vasodilatation.
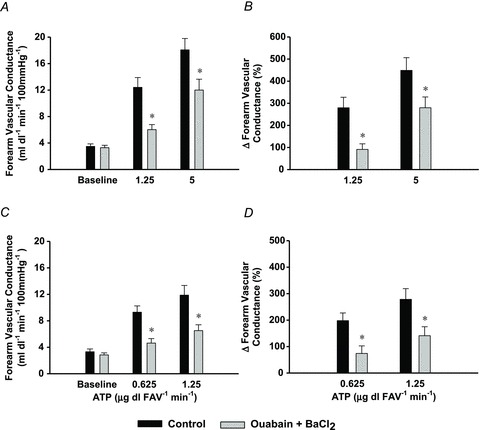
Combined ouabain + BaCl2 infusion (grey bars), significantly reduced absolute (A) and per cent changes (B) in forearm vascular conductance (n= 8; 1.25 μg dl FAV−1 min−1: −69 ± 6%; 5.0 μg dl FAV−1 min−1: −40 ± 6%). Similar findings were observed for FVC (C) and %ΔFVC (D) at two lower doses of ATP (n= 8; 0.625 μg dl FAV−1 min−1: −66 ± 12%; 1.25 μg dl FAV−1 min−1: −50 ± 8%). P < 0.05 vs. control.
Table 1.
Protocol 2 subgroup: effect of ouabain + BaCl2 on vasodilator response to ACh infusion
| Baseline FVC | ACh infusion FVC (2 μg dl FAV−1 min−1) | ΔFVC (%) | Magnitude of inhibition from control (%) | |
|---|---|---|---|---|
| Control | 2.3 ± 0.5 | 8.5 ± 1.8 | 274 ± 47 | |
| Ouabain + BaCl2 | 2.0 ± 0.2 | 8.0 ± 1.5 | 305 ± 60 | +11 ± 9 |
n= 4; ACh, acetylcholine; FAV, forearm volume; FVC, forearm vascular conductance (ml dl-1 min-1 100 mmHg-1).
Protocol 3: independent effect of KIR channel inhibition
No significant changes in HR or MAP were observed across all conditions within Protocol 3 (Supplementary Table S5) where the impact of BaCl2 alone on ATP-mediated vasodilatation was investigated. The ability of BaCl2 alone to attenuate vasodilatation in response to exogenous ATP (mean effect of both doses pooled: −51 ± 3%; P < 0.05 vs. control; Fig. 4) was similar to that of combined ouabain and BaCl2 (P= 0.50). Similar to other protocols, changes in FBF paralleled those observed for FVC (Supplementary Table S5).
Figure 4. Protocol 3: independent effect of BaCl2 on ATP-mediated vasodilatation.
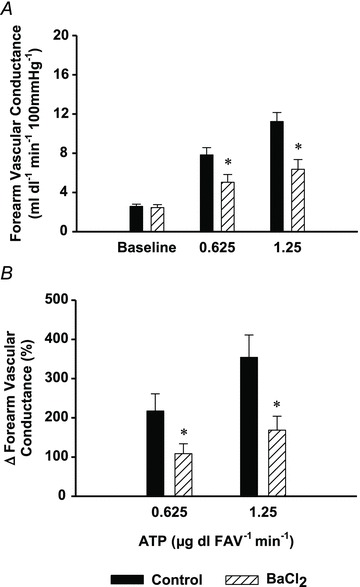
Inhibition of KIR channels (via BaCl2 infusion (striped bars)), significantly reduced absolute (A) and per cent changes (B) in forearm vascular conductance (n= 6; 0.625 μg dl FAV−1 min−1: −50 ± 5%; 1.25 μg dl FAV−1 min−1: −54 ± 4%) to a similar extent as observed with combined infusion of ouabain and BaCl2. *P < 0.05 vs. control.
Discussion
The primary novel finding from the current study is that ATP-mediated vasodilatation is largely independent of NO and PG synthesis and is significantly attenuated during combined inhibition of Na+/K+-ATPase and KIR channels. Further, BaCl2 alone reduced forearm vasodilator responses to a similar extent as combined ouabain and BaCl2, thus implicating a primary role for vascular hyperpolarization via KIR channel activation in ATP-mediated vasodilatation in humans.
Historically, investigations into the mechanisms of endothelium-dependent vasodilatation have focused primarily on NO and PGs, the synthesis of which can increase with elevations in intracellular endothelial cell [Ca2+]. In the present study, we first aimed to determine whether KCl and ATP-mediated vasodilatation occur independently of NO and PGs in the human forearm. Our results from Protocol 1 (Fig. 1) demonstrate that combined inhibition of NO and PG synthesis does not impair the vasodilator response (%Δ) to KCl or ATP, although there is a modest reduction in absolute FVC for KCl. To the best of our knowledge, we are the first to show this type of data for KCl in humans. The lack of a statistical effect of combined NOS and COX inhibition on ATP-mediated vasodilatation is in accordance with previous findings from our own laboratory (Crecelius et al. 2011a) and others (Rongen et al. 1994; van Ginneken et al. 2004). While we believe that %ΔFVC is the most appropriate presentation of these type of data, particularly given the reduction in baseline vascular tone (Buckwalter & Clifford, 2001) and this quantification suggests no role for NO and PGs in the vasodilator response to ATP and KCl, we acknowledge that the absolute FVC data would not completely rule out a potential role for these vasodilator pathways. Despite the slight difference in interpretation based on the method of quantification, within the present study design our data clearly indicate that the majority of the vasodilator response to intravascular ATP is beyond the traditional endothelial cell signalling pathways (e.g. NO and PGs) in humans.
Use of ouabain and BaCl2 to address hyperpolarizing mechanisms of vasodilatation
On vascular smooth muscle cells, activation of the Na+/K+ pump leads to a hyperpolarization of the cellular membrane (net effect of efflux of 3 Na+ ions and influx of 2 K+ ions), as does K+ efflux via opening of KIR channels (Nelson & Quayle, 1995; Edwards et al. 1998). Increases in interstitial [K+] via exogenous KCl or endothelial-cell K+ efflux from SKCa and IKCa channels has been shown to stimulate both Na+/K+-ATPase and KIR channels (Nelson & Quayle, 1995; Edwards et al. 1998), whereas increased [K+] does not directly activate other potassium channels such as calcium-activated (KCa) and ATP-sensitive (KATP) potassium channels (Jackson, 2005). Further, KIR channels are sensitive to changes in vascular smooth muscle cell membrane potential and are directly activated by hyperpolarization (Nelson & Quayle, 1995). Thus, inhibition of Na+/K+-ATPase and KIR channels via ouabain and BaCl2, respectively, can be used as a means to inhibit K+-induced hyperpolarization and the spread/amplification of hyperpolarization (Smith et al. 2008) in experimental models where changes in membrane potential can be directly measured. Previously, Dawes and colleagues (2002) established that ouabain and BaCl2 can be administered intra-arterially in the human forearm without adverse effect and can significantly inhibit KCl-mediated vasodilatation in vivo. Here, we have performed similar experiments in our own laboratory with the addition of another dose of KCl and demonstrate that combined ouabain and BaCl2 administration abolishes KCl-mediated vasodilatation (Fig. 2). Further, we show that the effects of this pharmacological inhibition are not a general reduction in vasodilator capability as the responses to acetylcholine were unchanged during ouabain and BaCl2 infusion (Table 1). These findings are consistent with previous studies which have also shown that vasodilator responses to acetylcholine, verapamil (L-type calcium channel antagonist), sodium nitroprusside (NO donor) and albuterol (β2-adrenergic receptor agonist) are unaffected by ouabain and BaCl2 (Dawes et al. 2002; Dwivedi et al. 2005).
ATP-mediated vasodilatation: role of Na+/K+-ATPase and KIR channels
Certain vasoactive stimuli can evoke ‘conducted vasodilatation’ or vasodilatation that occurs remotely from the site of agonist application (Segal, 2005; Winter & Dora, 2007). Also called ‘spreading’ or ‘ascending’ vasodilatation, this physiological mechanism is thought to provide a means for robust dilatation that arises from the microcirculation and decreases resistance to flow at the level of the upstream vasculature (Segal, 2005). It is recognized that ATP is capable of producing conducted dilatation (Duza & Sarelius, 2003; Winter & Dora, 2007; Dietrich et al. 2009) that can be attenuated by specific inhibition of SKCa and IKCa channels in vitro (Winter & Dora, 2007). Activation of these KCa channels leads to endothelial and vascular smooth muscle cell hyperpolarization that can be blocked by inhibition of Na+/K+-ATPase and KIR channels (Edwards et al. 1998). Thus, we aimed to use ouabain and BaCl2 which can be administered to humans in order to inhibit Na+/K+-ATPase and KIR channels, respectively, to determine whether these hyperpolarizing pathways contribute to ATP-mediated vasodilatation in vivo in humans.
The data from the present study clearly indicate that the local vasodilatation observed in response to all doses of ATP administered was significantly attenuated by combined ouabain and BaCl2 (Fig. 3), and as such, represent the first data in humans to demonstrate vascular hyperpolarization via these pathways as a primary mechanism of ATP-mediated dilatation. Given one previous study in humans that demonstrated no effect of ouabain on ATP-mediated vasodilatation (van Ginneken et al. 2004), we then questioned whether KIR channel activation alone is the predominant hyperpolarizing pathway involved in the dilatory response. Indeed, our data obtained from studies in Protocol 3 demonstrate a similar magnitude of inhibition in ATP-mediated vasodilatation with BaCl2 alone (Fig. 4) as observed with combined ouabain and with BaCl2. Taken together, these novel observations implicate vascular hyperpolarization via KIR channel activation as a primary mechanism of vasodilatation in response to intravascular ATP.
In the present study, we did not attempt to inhibit SKCa and IKCa channels, as there are no specific inhibitors of these channels approved for use in humans. Van Ginneken and colleagues (2004) recently demonstrated that the KCa channel inhibitor tetraethylammonium chloride (TEA) did not impact ATP-mediated vasodilatation in the human forearm. However, at lower concentrations, TEA may not have effectively blocked SKCa and IKCa channels but rather large-conductance KCa channels (Langton et al. 1991; Ledoux et al. 2006) which are predominantly on smooth muscle cells (Jackson, 2005), and thus may not be involved in the endothelium-dependent vasodilatation evoked via intravascular ATP. Nevertheless, our results clearly indicate that combined inhibition of Na+/K+-ATPase and KIR channels, and KIR channel inhibition alone, significantly reduced ATP-mediated vasodilatation. These data are consistent with in vitro data indicating that vasodilatation to luminal perfusion of ATP is substantially reduced via inhibition of SKCa and IKCa channels (Winter & Dora, 2007), and that vasodilatation to direct P2 receptor stimulation is blunted by inhibition of Na+/K+-ATPase (via ouabain) (Ralevic, 2001) and KIR channels (via BaCl2) (Smith et al. 2008).
Experimental considerations
It should be acknowledged that while administration of combined ouabain and BaCl2, or BaCl2 alone, did block a substantial portion of ATP-mediated vasodilatation, the response was not entirely abolished. Therefore, we suggest that the remaining vasodilatation after ouabain and BaCl2 infusion indicates that (1) we did not achieve complete inhibition of these pathways during ATP infusions (see below) or (2) ATP may evoke some vasodilatation independent of activation of Na+/K+-ATPase and KIR channels. In this context, it is possible that in the present study in the forearm using VOP as our method of blood flow measurement, we may have underestimated a potential modest role for NO and PGs in the vasodilator response to exogenous ATP (Mortensen et al. 2009; Crecelius et al. 2011a). However, VOP is a reliable and valid technique for the measurement of limb blood flow, particularly for studies involving pharmacological responses under resting conditions (Joyner et al. 2001).
Further, regarding our use of VOP to measure forearm blood flow, it is important to acknowledge that this technique is reflective of total tissue blood flow and cannot be confined to the skeletal muscle vasculature which is our primary interest. Within our laboratory, we take steps to limit the amount of cutaneous blood flow (cool (18–21°C) environment with a fan directed at the experimental arm) and this is reflected in our blood flow measures that are typically on the low end of predicted total flow based on tissue mass of the forearm (2–4 ml (100 g−1)) (Rowell, 1993). Additionally, the hand circulation is occluded throughout all trials (see Methods), thus reducing the potential impact of additional tissue of mixed type and vascularization (e.g. cutaneous, muscle, fat, bone). It is possible that a different technique to measure limb blood flow such as dye dilution (Jorfeldt & Wahren, 1971) may have strengthened our ability to make conclusions specifically regarding skeletal muscle vasculature; however, we do not believe that this would have altered our primary conclusions regarding the vasodilator mechanisms of exogenous ATP.
The contribution of Na+/K+-ATPase and KIR channel activation to ATP-mediated vasodilatation may appear to be somewhat dose dependent (Fig. 3) and it is possible that the highest dose of ATP was able to override the effectiveness of BaCl2 during this stimulus (Armstrong & Taylor, 1980). Although we were able to abolish KCl-mediated vasodilatation with combined ouabain and BaCl2, the amount of dilatation was markedly less than that for ATP, and we are unable to test the efficacy of our inhibition with KCl at higher doses due to issues regarding subject comfort and safety (Dawes et al. 2002). It would be of interest to increase the dose of BaCl2 (Jantzi et al. 2006) during ATP infusions to test this directly, but again, there are issues regarding subject safety that limit the amount of BaCl2 exposure to each subject (Dawes et al. 2002). Given that we show on average ∼50% inhibition of ATP-mediated vasodilatation with BaCl2, we do not feel that this changes our primary conclusions.
Finally, based on the previous work by Dawes and colleagues (2002), we believe that our current dosing approach of BaCl2 allows us to be within the range of selectivity for KIR channel inhibition by [Ba2+] (<100 μmol l−1). Higher concentrations of BaCl2 can act on other potassium channels, specifically KATP channels (Jackson, 2005). If we were somehow in this range, we do not believe that inhibition of KATP channels explains our results as previously there was no effect of glibenclamide (KATP antagonist) on ATP-mediated vasodilatation (van Ginneken et al. 2004). Further, the relative concentration of Ba2+ would predictably decrease during ATP infusions (as FBF increases). Nevertheless, given that we have no direct evidence for specificity of inhibition of KIR channels by BaCl2, our results should be interpreted with this in mind.
Conclusions
The collective data from the present in vivo investigation are the first to identify vascular hyperpolarization via KIR channel activation as the primary pathway underlying the vasodilator mechanisms of intravascular ATP in humans. Our novel finding of a critical role for KIR channel activation in this regard align with in vitro studies that suggest vascular smooth muscle cell hyperpolarization via KIR channel activation mediates vasomotor responses to a variety of physiological and pharmacological stimuli (Jantzi et al. 2006; Armstrong et al. 2007). Circulating ATP plays a unique and important role in the regulation of vascular control during mismatches in oxygen delivery and demand (Gonzalez-Alonso et al. 2002), and recent evidence suggests that endothelium-dependent ATP-mediated vasodilatation may be impaired in type II diabetics, a population at risk for cardiovascular disease (Thaning et al. 2010). Thus, identification of the downstream signalling pathways of ATP may prove to be of clinical interest as a means for improving blood flow and oxygen delivery in specific patient populations.
Acknowledgments
We thank the subjects who volunteered to participate and Jennifer C. Richards and Leora J. Garcia for their assistance in conducting these studies. This research was supported by the National Institutes of Health award HL102720 (F.A.D.).
Glossary
- ACh
acetylcholine
- COX
cyclooxygenase
- FAV
forearm volume
- FBF
forearm blood flow
- FVC
forearm vascular conductance
- HR
heart rate
- IKCa
intermediate-conductance calcium-activated potassium channel
- KATP
ATP-sensitive potassium channel
- KCa
calcium-activated potassium channel
- KIR
inwardly rectifying potassium channel
- MAP
mean arterial pressure
- NOS
nitric oxide synthase
- P
purinergic
- PG
prostaglandin
- SKCa
small-conductance calcium-activated potassium channel
- VOP
venous occlusion plethysmography
Author contributions
A.R.C. contributed to the conception and design of the experiment, collection, analysis and interpretation of the data, and writing of this article. B.S.K. contributed to the conception and design of the experiment, collection and interpretation of the data, and critical revision of this article. G.J.L and D.G.L. contributed to the experimental design, provided invasive methodology for data collection, and critical revision of this article. F.A.D. contributed to the conception and design of the experiment, collection, analysis and interpretation of the data and writing of this article. All authors gave final approval of the article. All experiments were performed in the Human Cardiovascular Physiology Laboratory at Colorado State University.
Supplementary material
Supplementary Tables S1 and S2
Supplementary Tables S3 and S4
Supplementary Table S5
References
- Armstrong CM, Taylor SR. Interaction of barium ions with potassium channels in squid giant axons. Biophys J. 1980;30:473–488. doi: 10.1016/S0006-3495(80)85108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol. 2007;581:841–852. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Voyles WF, Larson DG, Luckasen GJ, Dinenno FA. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol. 2011a;301:H1302–H1310. doi: 10.1152/ajpheart.00469.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol. 2010;299:H1633–H1641. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol. 2011b;589:3671–3683. doi: 10.1113/jphysiol.2011.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes M, Sieniawska C, Delves T, Dwivedi R, Chowienczyk PJ, Ritter JM. Barium reduces resting blood flow and inhibits potassium-induced vasodilation in the human forearm. Circulation. 2002;105:1323–1328. doi: 10.1161/hc1102.105651. [DOI] [PubMed] [Google Scholar]
- Dietrich HH, Horiuchi T, Xiang C, Hongo K, Falck JR, Dacey RG., Jr Mechanism of ATP-induced local and conducted vasomotor responses in isolated rat cerebral penetrating arterioles. J Vasc Res. 2009;46:253–264. doi: 10.1159/000167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duza T, Sarelius IH. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+ Am J Physiol Heart Circ Physiol. 2003;285:H26–H37. doi: 10.1152/ajpheart.00788.2002. [DOI] [PubMed] [Google Scholar]
- Dwivedi R, Saha S, Chowienczyk PJ, Ritter JM. Block of inward rectifying K+ channels (KIR) inhibits bradykinin-induced vasodilatation in human forearm resistance vasculature. Arterioscler Thromb Vasc Biol. 2005;25:e7–e9. doi: 10.1161/01.ATV.0000152610.40086.31. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias M, 3rd, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol. 2005;288:H1586–H1590. doi: 10.1152/ajpheart.00983.2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- Greenfield AD, Whitney RJ, Mowbray JF. Methods for the investigation of peripheral blood flow. Br Med Bull. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Hongo K, Dacey RG., Jr Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke. 2002;33:2692–2699. doi: 10.1161/01.str.0000034791.52151.6b. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Hongo K, Dacey RG., Jr Comparison of P2 receptor subtypes producing dilation in rat intracerebral arterioles. Stroke. 2003;34:1473–1478. doi: 10.1161/01.STR.0000071527.10129.65. [DOI] [PubMed] [Google Scholar]
- Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2006;291:H1319–H1328. doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol. 2001;91:2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol. 2008;586:4305–4316. doi: 10.1113/jphysiol.2008.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton PD, Nelson MT, Huang Y, Standen NB. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol Heart Circ Physiol. 1991;260:H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- Malmsjo M, Erlinge D, Hogestatt ED, Zygmunt PM. Endothelial P2Y receptors induce hyperpolarisation of vascular smooth muscle by release of endothelium-derived hyperpolarising factor. Eur J Pharmacol. 1999;364:169–173. doi: 10.1016/s0014-2999(98)00848-6. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Bune L, Saltin B, Pilegaard H, Hellsten Y. ATP induced vasodilatation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1140–R1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Ralevic V. Mechanism of prolonged vasorelaxation to ATP in the rat isolated mesenteric arterial bed. Br J Pharmacol. 2001;132:685–692. doi: 10.1038/sj.bjp.0703868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongen GA, Smits P, Thien T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation. 1994;90:1891–1898. doi: 10.1161/01.cir.90.4.1891. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- Sheng JZ, Braun AP. Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293:C458–C467. doi: 10.1152/ajpcell.00036.2007. [DOI] [PubMed] [Google Scholar]
- Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, Welsh DG. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008;586:1147–1160. doi: 10.1113/jphysiol.2007.145474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaning P, Bune LT, Hellsten Y, Pilegaard H, Saltin B, Rosenmeier JB. Attenuated purinergic receptor function in patients with type 2 diabetes. Diabetes. 2010;59:182–189. doi: 10.2337/db09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginneken EE, Meijer P, Verkaik N, Smits P, Rongen GA. ATP-induced vasodilation in human skeletal muscle. Br J Pharmacol. 2004;141:842–850. doi: 10.1038/sj.bjp.0705589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wihlborg AK, Malmsjo M, Eyjolfsson A, Gustafsson R, Jacobson K, Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol. 2003;138:1451–1458. doi: 10.1038/sj.bjp.0705186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582:335–347. doi: 10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


